Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder
Abstract
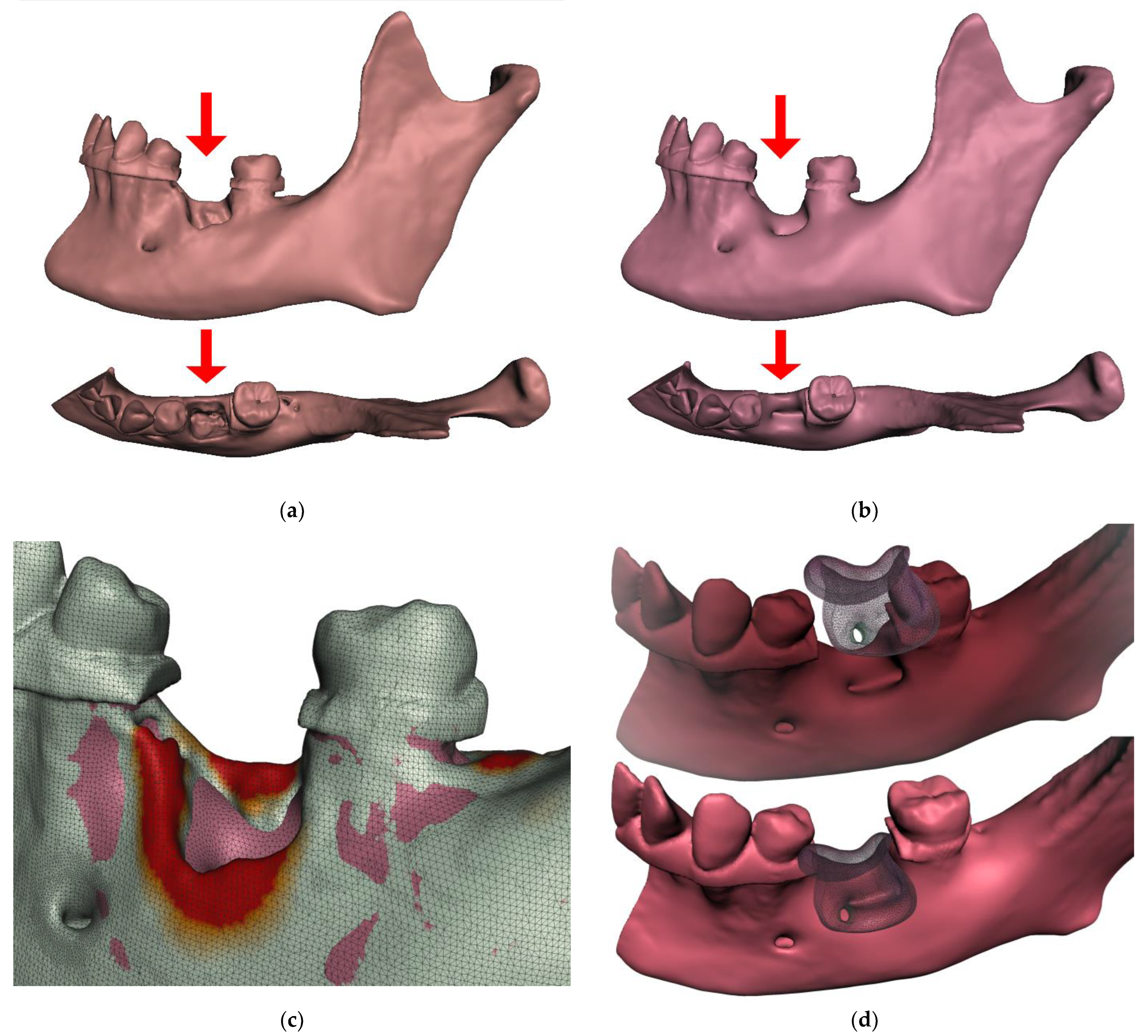
:1. Introduction
2. Results
2.1. MTT Assay
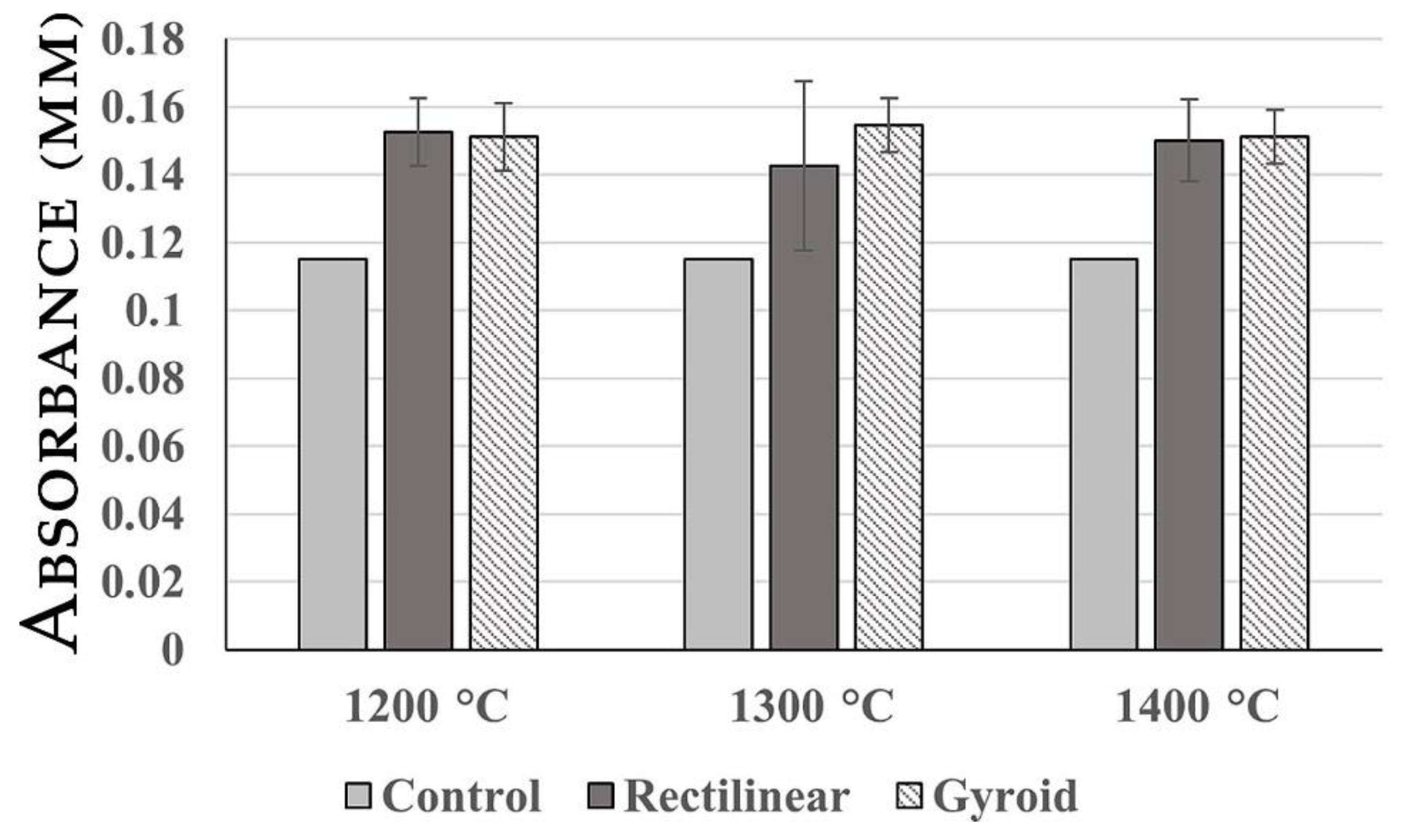
2.2. LDH Release Assay
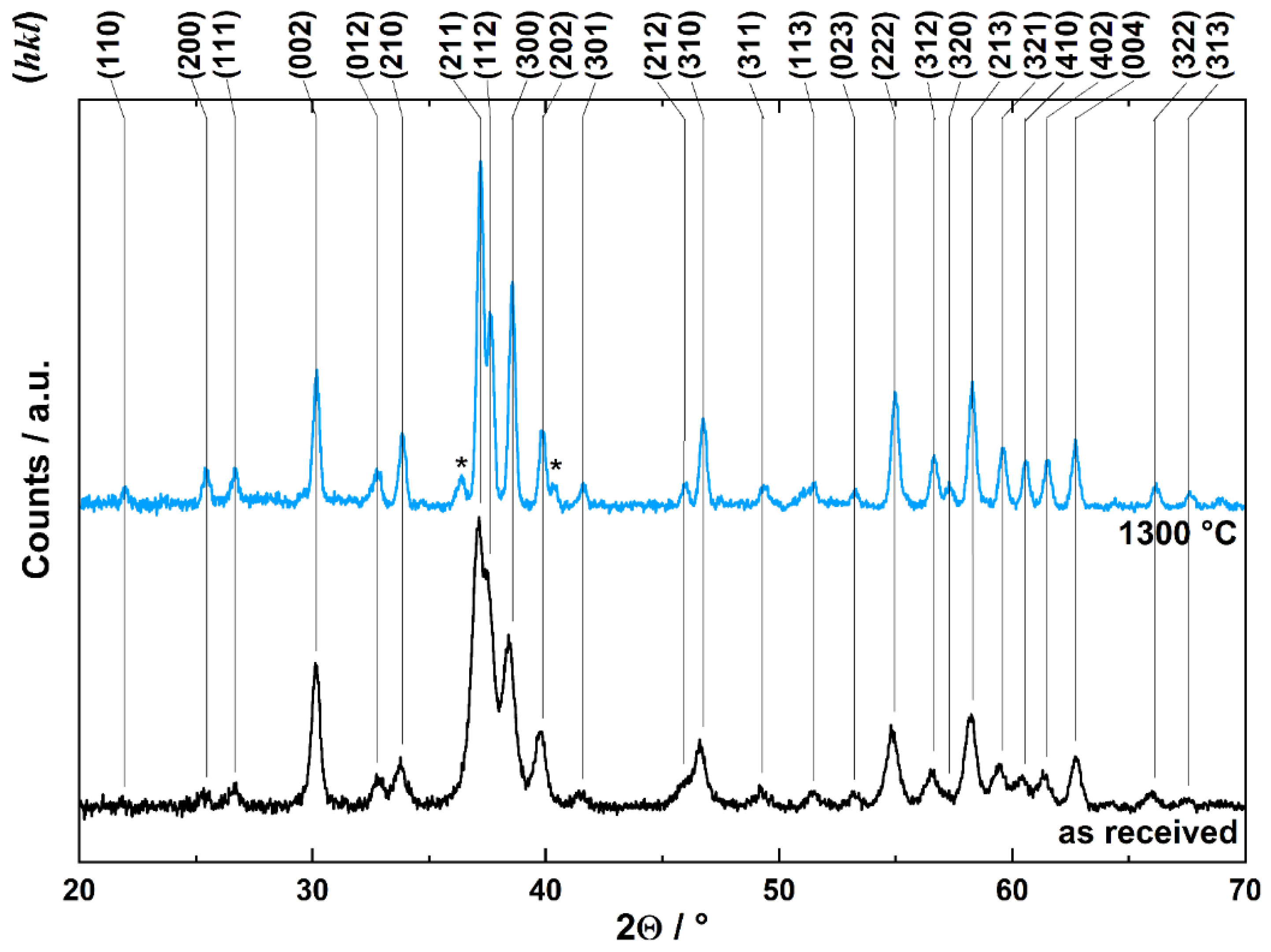
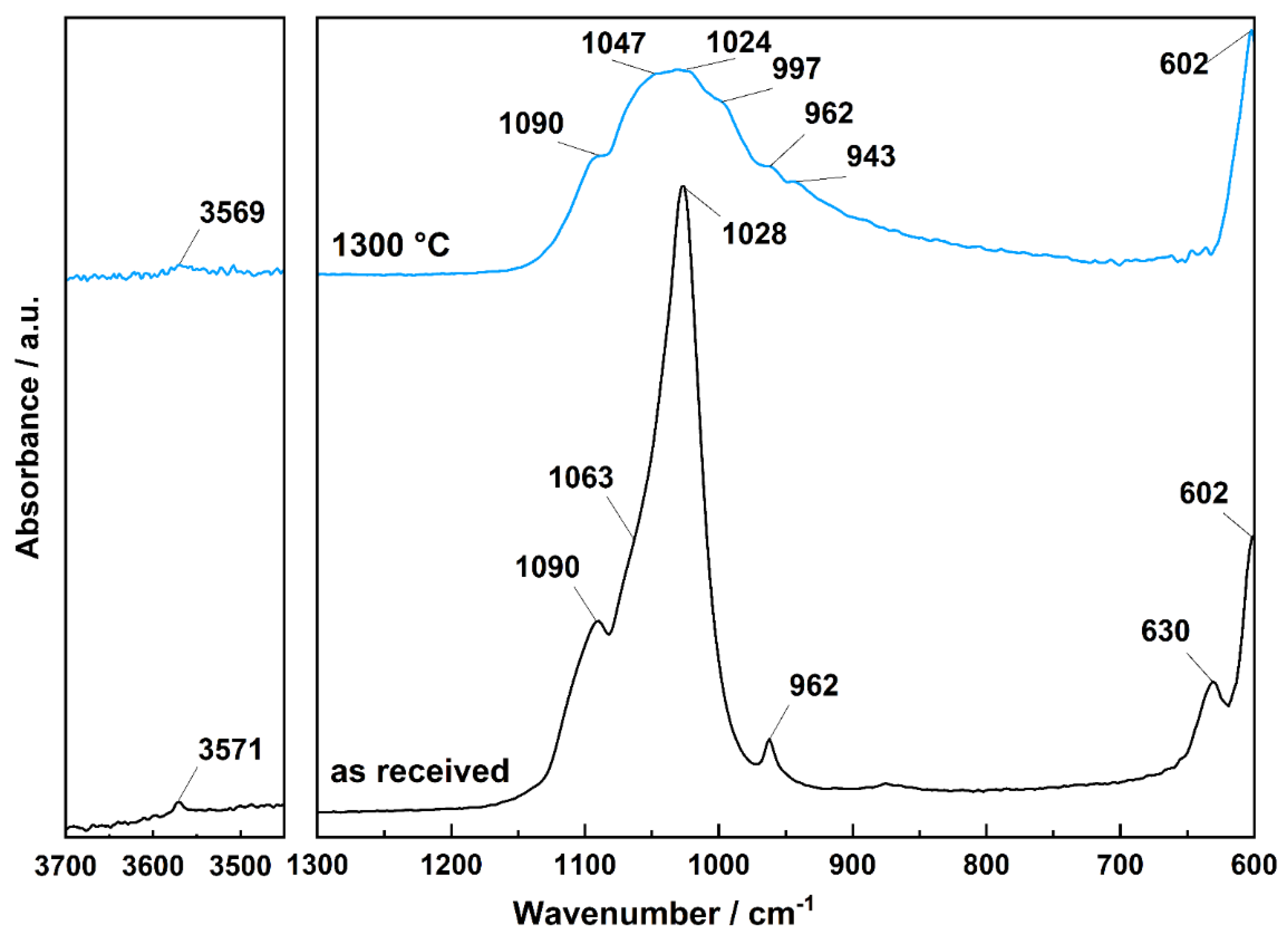
2.3. Characteristics of Used Material
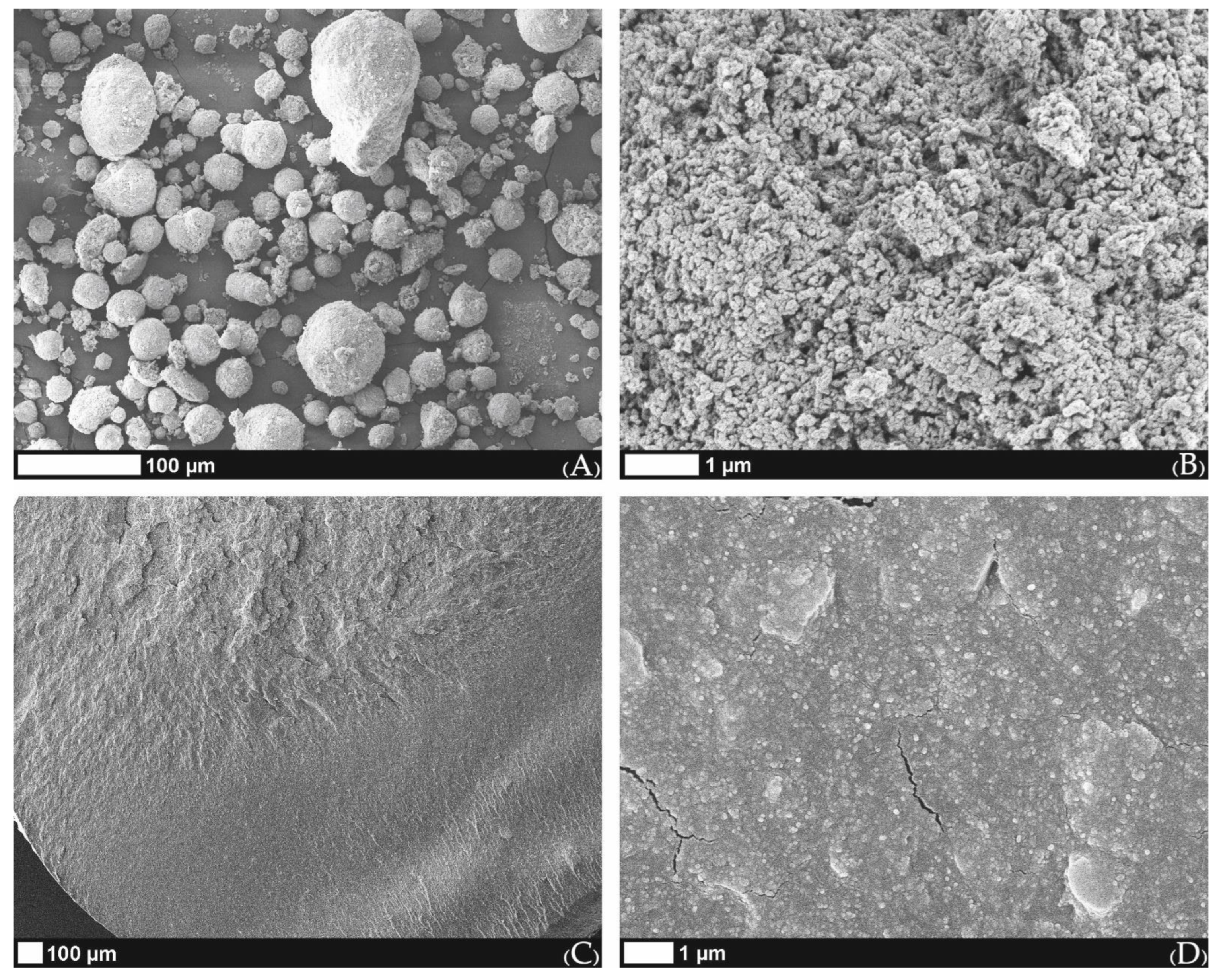
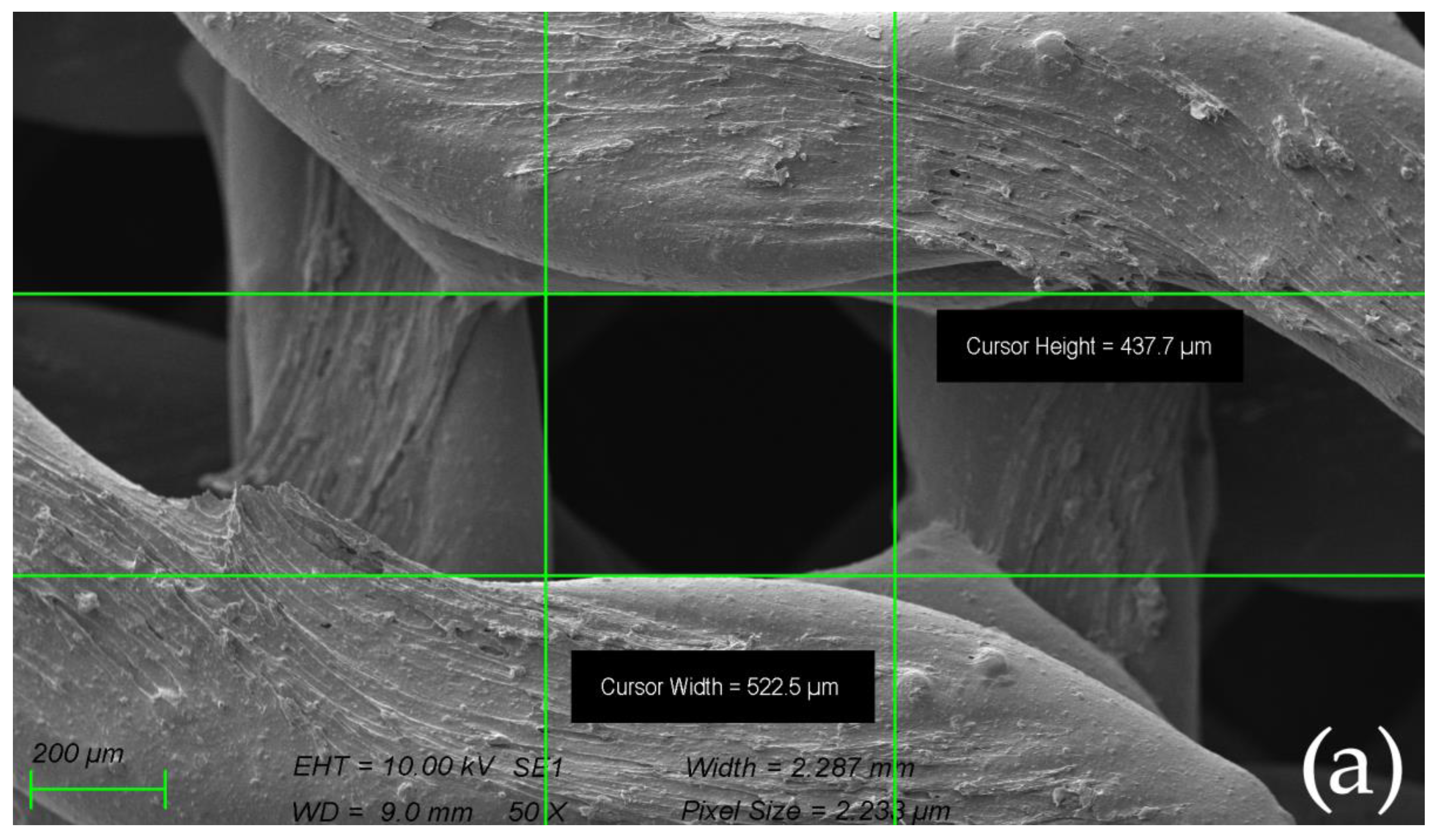
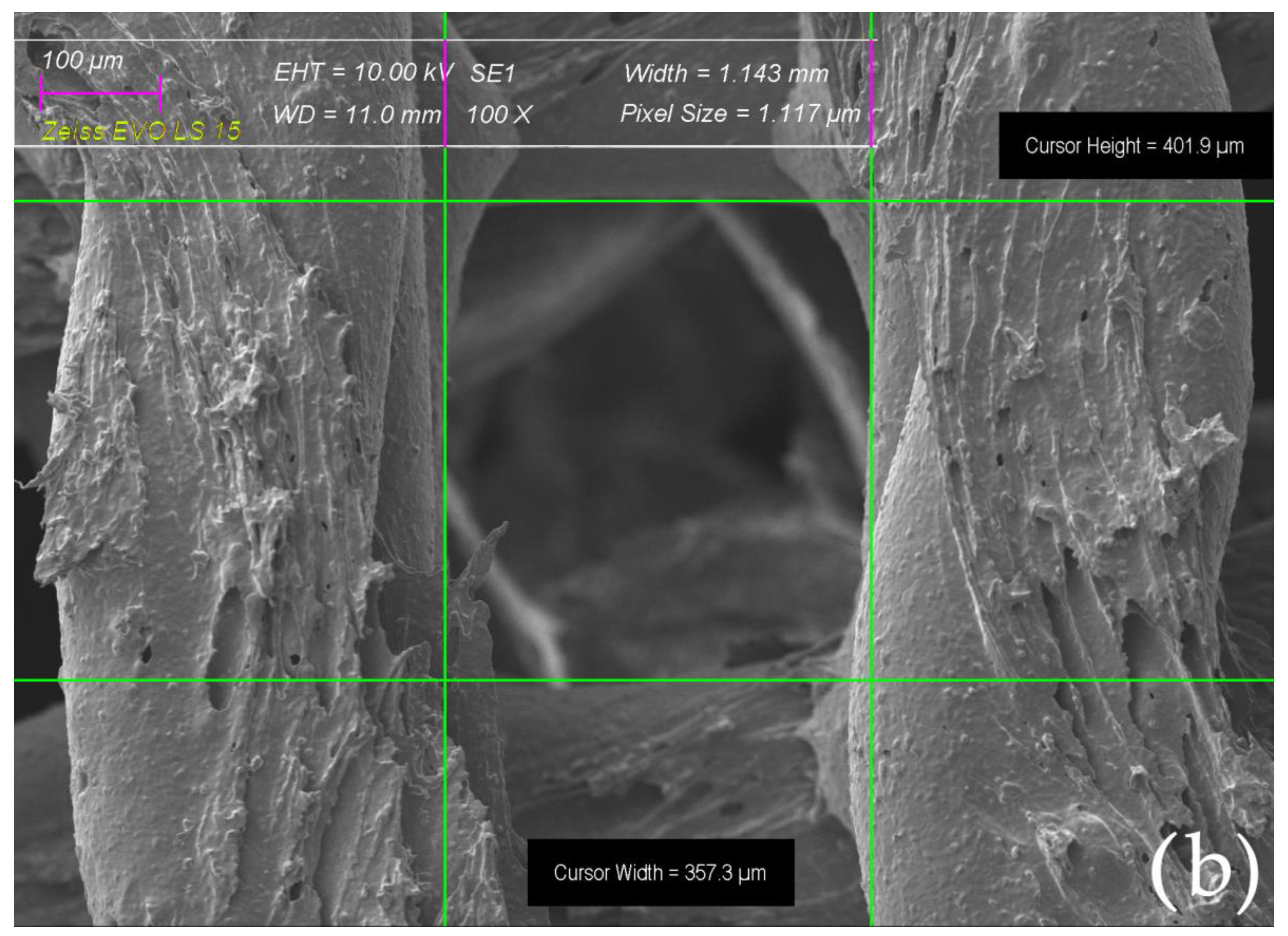
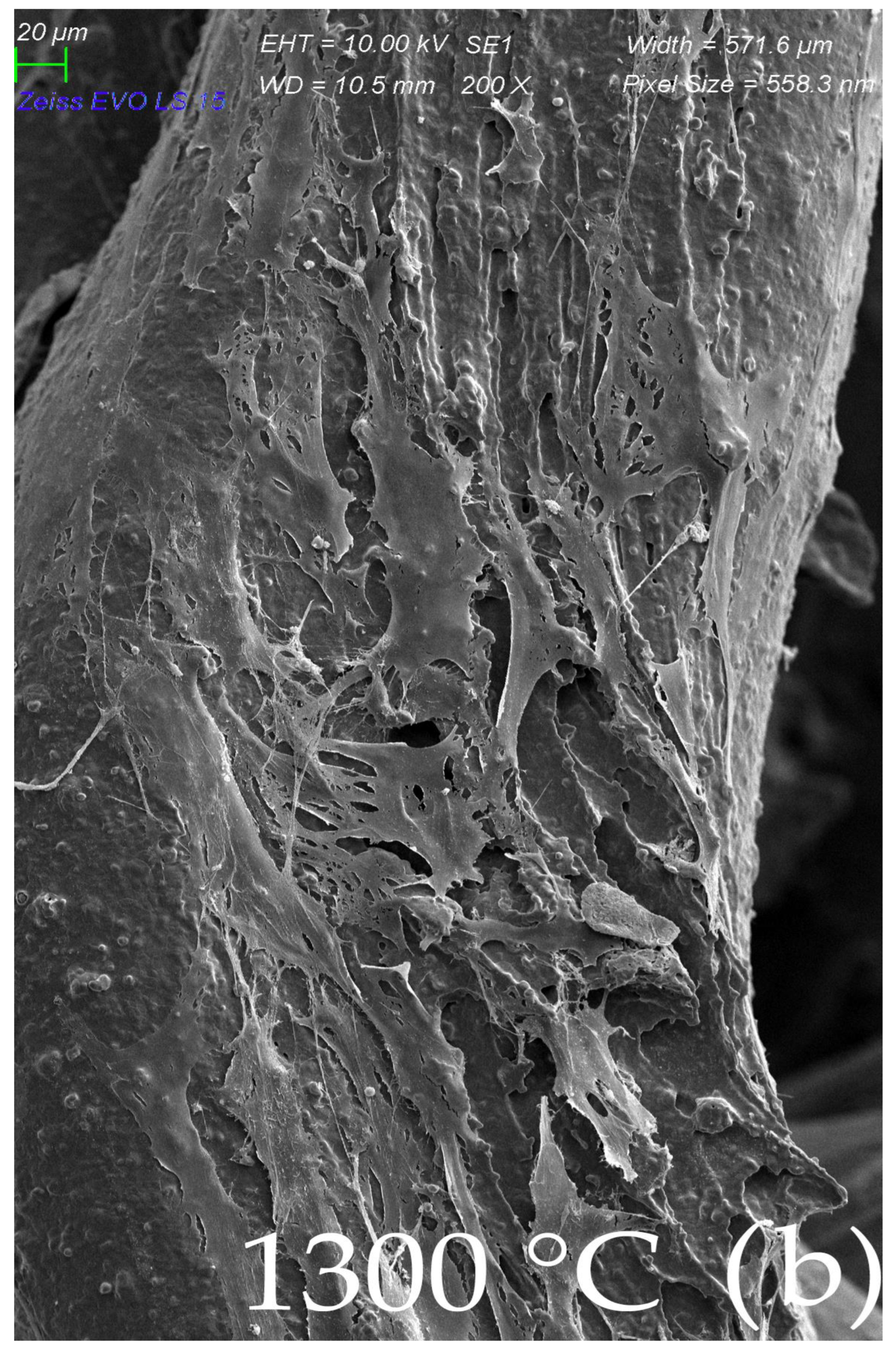
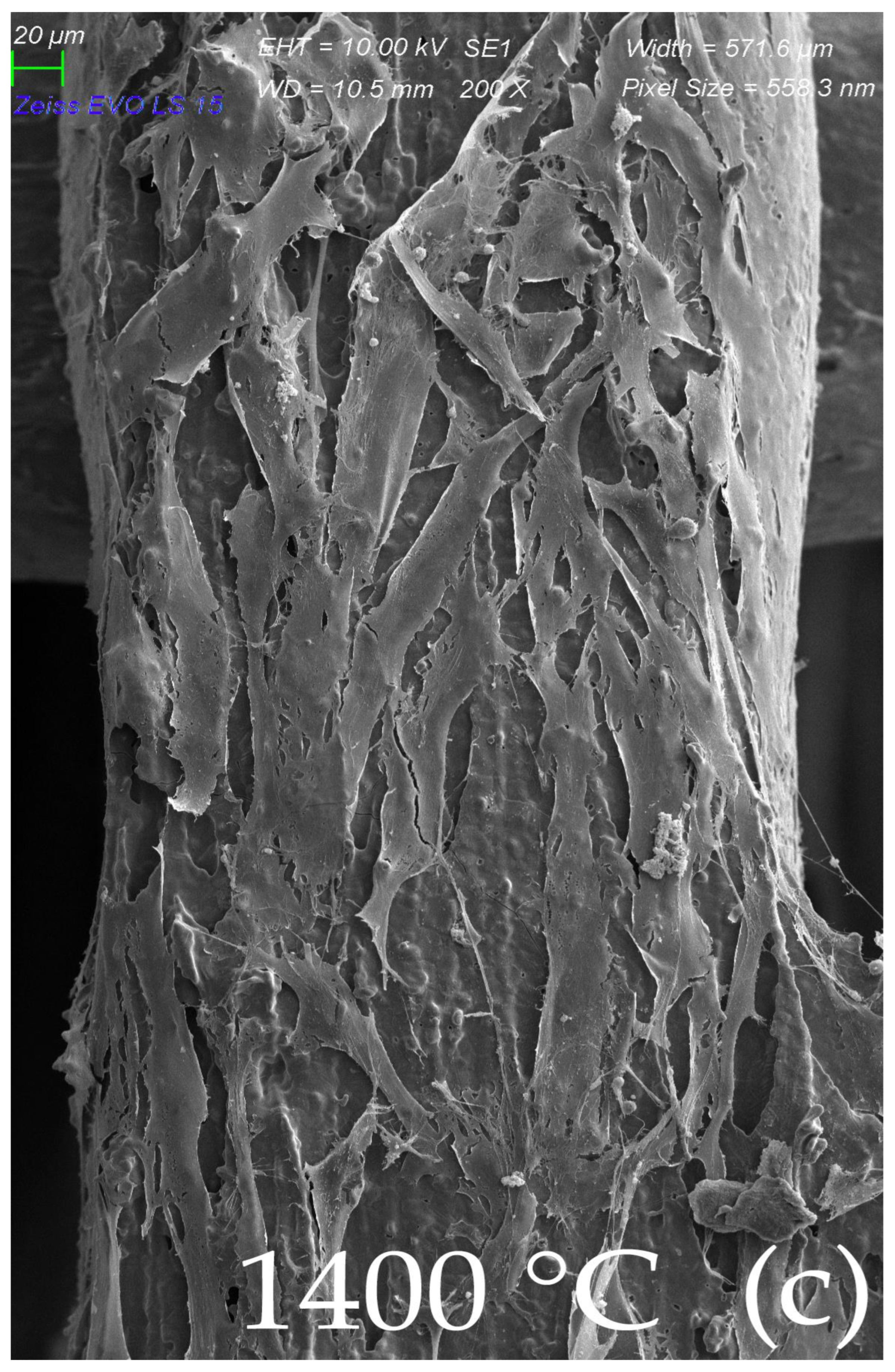
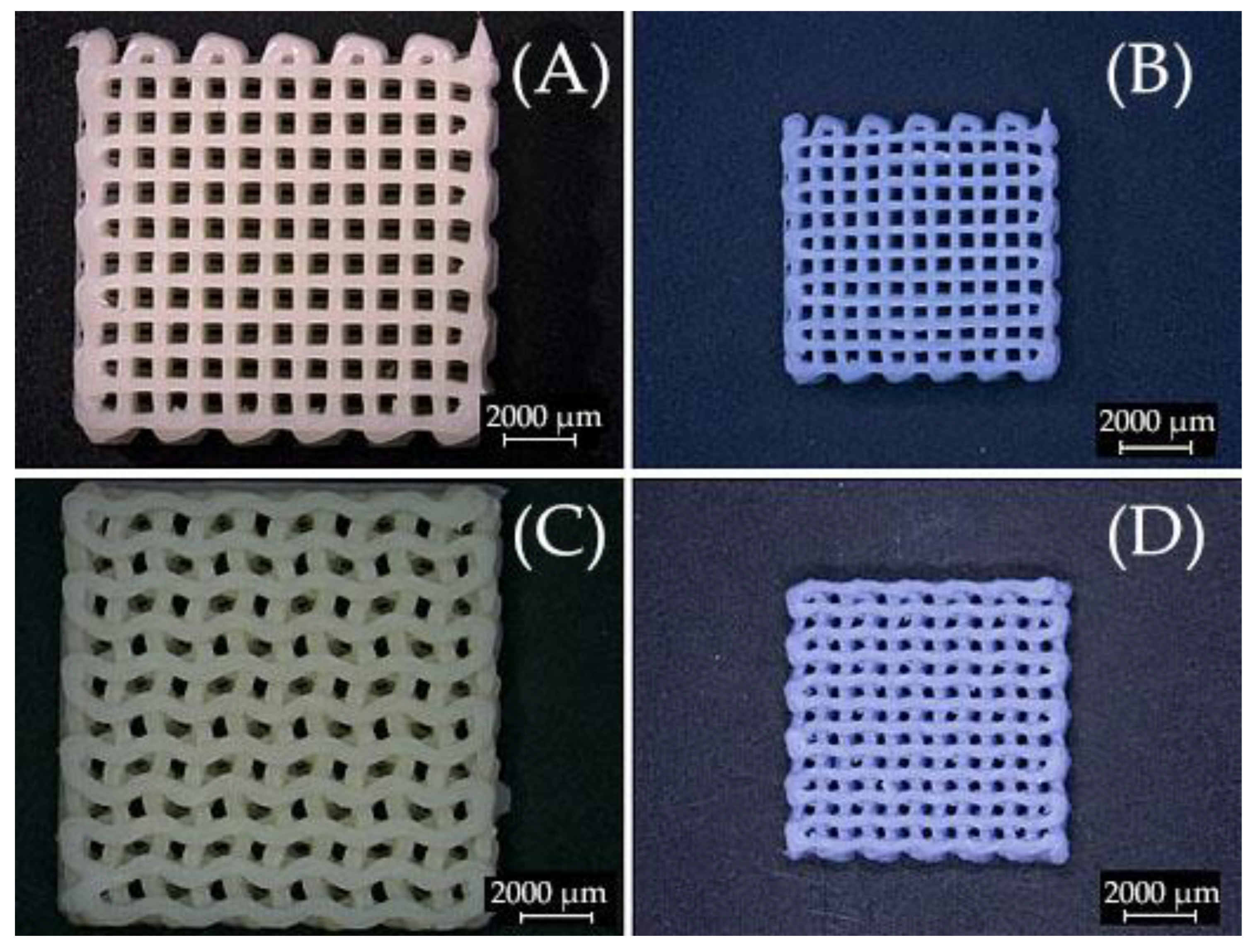
2.4. Surface Morphology of Scaffolds Sintered at Elevated Temperatures
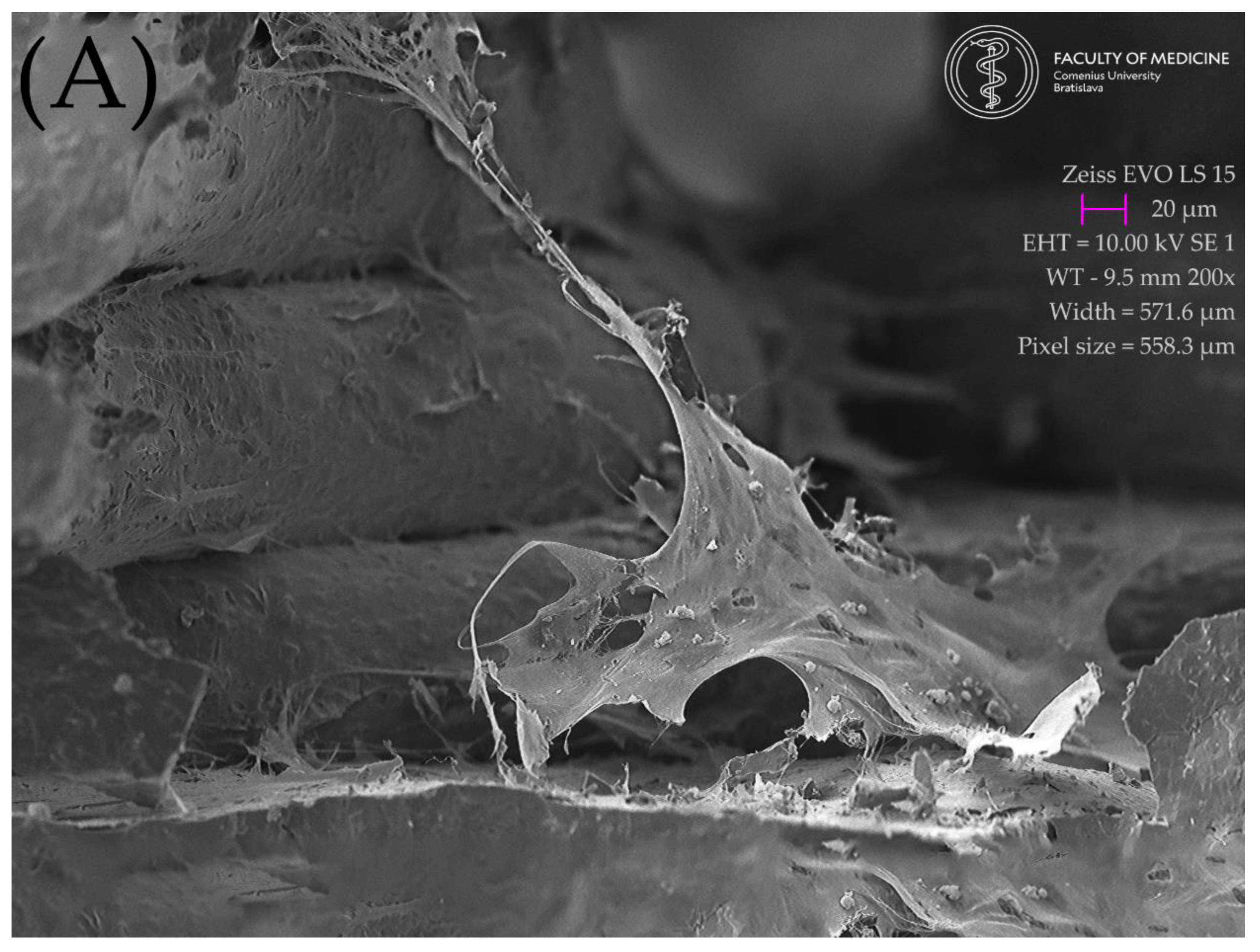
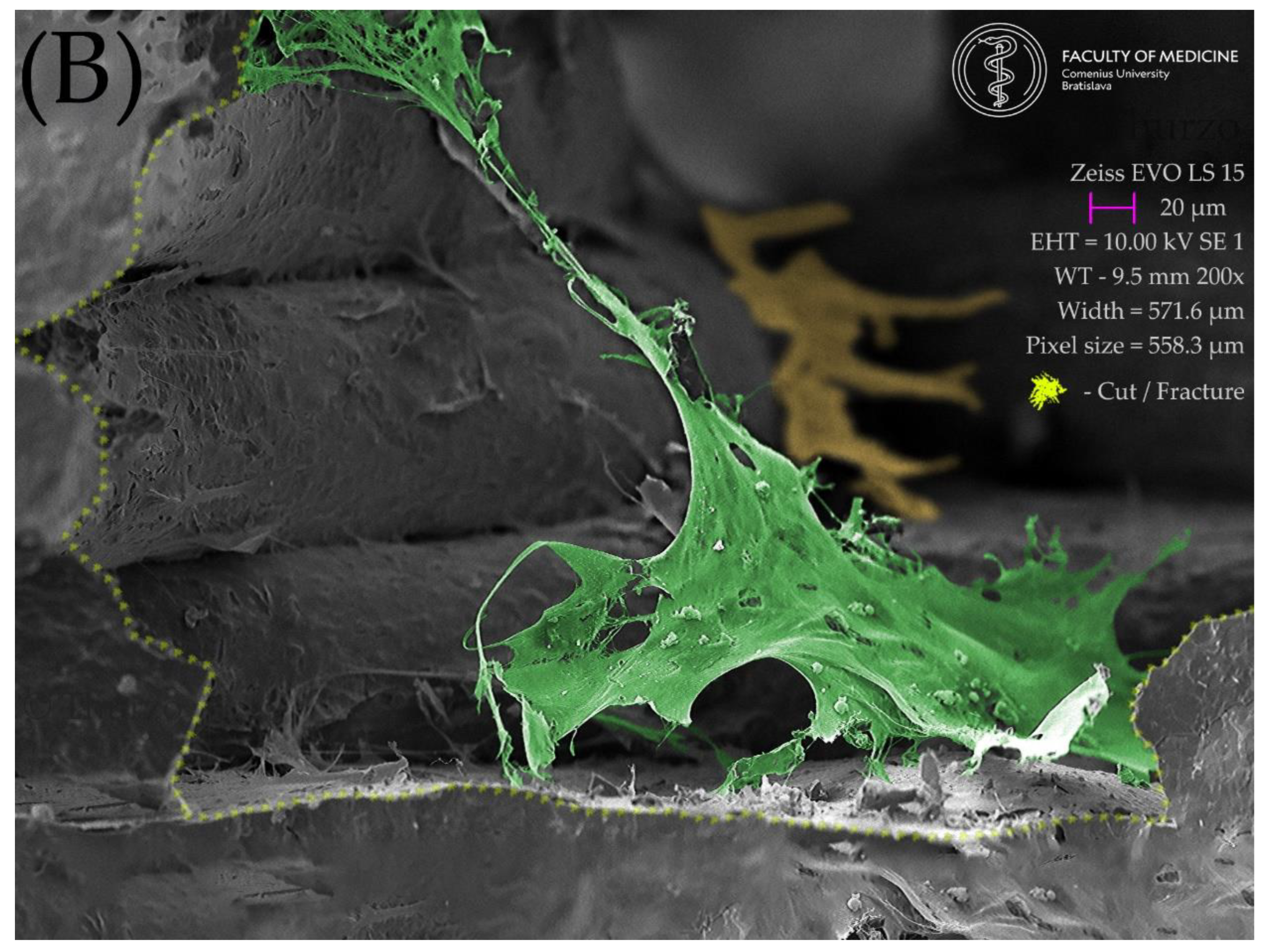
2.5. Resulting Scaffolds after Biocolonization with MSC
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Direct Contact Cytotoxicity Assay
4.2.2. MTT Assay
4.2.3. LDH Release Assay
4.2.4. Methods of Sample Preparation for Scanning Electron Microscopy
4.2.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrows, T. Degradable Implant Materials: A Review of Synthetic Absorbable Polymers and Their Applications. Clin. Mater. 1986, 1, 233–257. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired by Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, S.; Pascadopoli, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Zampetti, P.; Spadari, F.; Maiorana, C.; Scribante, A. Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review. Bioengineering 2022, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Devlin, H.; Ferguson, M.W. Alveolar Ridge Resorption and Mandibular Atrophy. A Review of the Role of Local and Systemic Factors. Br. Dent. J. 1991, 170, 101–104. [Google Scholar] [CrossRef]
- Hansson, S.; Halldin, A. Alveolar Ridge Resorption after Tooth Extraction: A Consequence of a Fundamental Principle of Bone Physiology. J. Dent. Biomech. 2012, 3, 1758736012456543. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Zhao, D.W.; Fan, X.C.; Zhao, Y.X.; Zhao, W.; Zhang, Y.Q.; Zhang, R.H.; Cheng, L. Biocompatible Nano-Hydroxyapatites Regulate Macrophage Polarization. Materials 2022, 15, 6986. [Google Scholar] [CrossRef]
- Azmah Hanim, M.A.; Calin, R.; Jung, D.W. PLA-Based Bionanocomposites in Tissue Engineering and Regenerative Medicine. In Bionanocomposites in Tissue Engineering and Regenerative Medicine; Woodhead Publishing—Elsevier: Cambridge, MA, USA, 2021; pp. 481–497. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; de Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics 2022, 7, 112. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.J.; Kotula, A.P.; Takagi, S.; Chow, L.; Alimperti, S. Engineering 3D Printed Scaffolds with Tunable Hydroxyapatite. J. Funct. Biomater. 2022, 13, 34. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.J.; Davydov, A.V.; Frukhtbeyen, S.; Seppala, J.E.; Takagi, S.; Chow, L.; Alimperti, S. Biofabrication of 3D Printed Hydroxyapatite Composite Scaffolds for Bone Regeneration. Biomed. Mater. 2021, 16, 045002. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Urbanová, W.; Neuschlová, I.; Paouris, D.; Čverha, M. Use of Optical Scanning and 3D Printing to Fabricate Customized Appliances for Patients with Craniofacial Disorders. Semin. Orthod. 2022, 1–11. [Google Scholar] [CrossRef]
- Thurzo, A.; Šufliarsky, B.; Urbanová, W.; Čverha, M.; Strunga, M.; Varga, I. Pierre Robin Sequence and 3D Printed Personalized Composite Appliances in Interdisciplinary Approach. Polymers 2022, 14, 3858. [Google Scholar] [CrossRef]
- Thurzo, A.; Urbanová, W.; Novák, B.; Waczulíková, I.; Varga, I. Utilization of a 3D Printed Orthodontic Distalizer for Tooth-Borne Hybrid Treatment in Class II Unilateral Malocclusions. Materials 2022, 15, 1740. [Google Scholar] [CrossRef]
- Thurzo, A.; Kočiš, F.; Novák, B.; Czako, L.; Varga, I. Three-Dimensional Modeling and 3D Printing of Biocompatible Orthodontic Power-Arm Design with Clinical Application. Appl. Sci. 2021, 11, 9693. [Google Scholar] [CrossRef]
- Csöbönyeiová, M.; Beerová, N.; Klein, M.; Debreová-Čeháková, M.; Uboš Danišovič, Ľ. Cell-Based and Selected Cell-Free Therapies for Myocardial Infarction: How Do They Compare to the Current Treatment Options? Int. J. Mol. Sci. 2022, 23, 10314. [Google Scholar] [CrossRef] [PubMed]
- Osouli-Bostanabad, K.; Masalehdan, T.; Kapsa, R.M.I.; Quigley, A.; Lalatsa, A.; Bruggeman, K.F.; Franks, S.J.; Williams, R.J.; Nisbet, D.R. Traction of 3D and 4D Printing in the Healthcare Industry: From Drug Delivery and Analysis to Regenerative Medicine. ACS Biomater. Sci. Eng. 2022, 8, 2764–2797. [Google Scholar] [CrossRef]
- Thurzo, A.; Kurilová, V.; Varga, I. Artificial Intelligence in Orthodontic Smart Application for Treatment Coaching and Its Impact on Clinical Performance of Patients Monitored with AI-Telehealth System. Healthcare 2021, 9, 1695. [Google Scholar] [CrossRef]
- Thurzo, A.; Urbanová, W.; Novák, B.; Czako, L.; Siebert, T.; Stano, P.; Mareková, S.; Fountoulaki, G.; Kosnáčová, H.; Varga, I. Where is the Artificial Intelligence Applied in Dentistry? Systematic Review and Literature Analysis. Healthcare 2022, 10, 1269. [Google Scholar] [CrossRef]
- Thurzo, A.; Urbanová, W.; Waczulíková, I.; Kurilová, V.; Mriňáková, B.; Kosnáčová, H.; Gális, B.; Varga, I.; Matajs, M.; Novák, B. Dental Care and Education Facing Highly Transmissible SARS-CoV-2 Variants: Prospective Biosafety Setting: Prospective, Single-Arm, Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 7693. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Christopoulou, I.; Papadopoulou, E.; Papaioannou, W.; Alexiou, K.-E.; Lyros, I.; Rontogianni, A.; Souliou, C.-E.; Tsolakis, A.I. Applications of Biotechnology to the Craniofacial Complex: A Critical Review. Bioengineering 2022, 9, 640. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2014, 3, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3D Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef] [PubMed]
- Quigley, A.F.; Razal, J.M.; Thompson, B.C.; Moulton, S.E.; Kita, M.; Kennedy, E.L.; Clark, G.M.; Wallace, G.G.; Kapsa, R.M.I. A Conducting-Polymer Platform with Biodegradable Fibers for Stimulation and Guidance of Axonal Growth. Adv. Mater. 2009, 21, 4393–4397. [Google Scholar] [CrossRef]
- Wiatrak, B.; Sobierajska, P.; Szandruk-Bender, M.; Jawien, P.; Janeczek, M.; Dobrzynski, M.; Pistor, P.; Szelag, A.; Wiglusz, R.J. Bionanocomposites in Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2021; Volume 22, ISBN 9780128212806. [Google Scholar]
- Miranda, C.C.; Gomes, M.R.; Moço, M.; Cabral, J.M.S.; Ferreira, F.C.; Sanjuan-Alberte, P.A.; Zarrabi, A.; Miranda, C.C.; Gomes, M.R.; Moço, M.; et al. A Concise Review on Electrospun Scaffolds for Kidney Tissue Engineering. Bioengineering 2022, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Zeni, E.; Cassari, L.; Zamuner, A.; Gloria, A.; Russo, T.; Boscolo-Berto, R.; Sfriso, M.M.; Macchi, V.; et al. Development of Two-Layer Hybrid Scaffolds Based on Oxidized Polyvinyl Alcohol and Bioactivated Chitosan Sponges for Tissue Engineering Purposes. Int. J. Mol. Sci. 2022, 23, 12059. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, S.-C.; Lim, K.; Ramaswamy, Y.; Jabbarzadeh, A.; Tribological, R.; Mărginean, M.; Cojocaru, V.; Frunzăverde, D.; Mele, A.; et al. Tribological and Rheological Properties of Poly(Vinyl Alcohol)-Gellan Gum Composite Hydrogels. Polymers 2022, 14, 3830. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Khydyakov, Y.; Nesterov, D.; Ippolitov, I.; Ippolitov, Y.; Vongsvivut, J. Development of a Hybrid Biomimetic Enamel-Biocomposite Interface and a Study of Its Molecular Features Using Synchrotron Submicron ATR-FTIR Microspectroscopy and Multivariate Analysis Techniques. Int. J. Mol. Sci. 2022, 23, 11699. [Google Scholar] [CrossRef]
- Cestari, F.; Yang, Y.; Wilbig, J.; Günster, J.; Motta, A.; Sglavo, V.M. Powder 3D Printing of Bone Scaffolds with Uniform and Gradient Pore Sizes Using Cuttlebone-Derived Calcium Phosphate and Glass-Ceramic. Materials 2022, 15, 5139. [Google Scholar] [CrossRef]
- Devi, G.V.Y.; Nagendra, A.H.; Shenoy, P.S.; Chatterjee, K.; Venkatesan, J. Fucoidan-Incorporated Composite Scaffold Stimulates Osteogenic Differentiation of Mesenchymal Stem Cells for Bone Tissue Engineering. Mar. Drugs 2022, 20, 589. [Google Scholar] [CrossRef]
- Baru, O.; Nutu, A.; Braicu, C.; Cismaru, C.A.; Berindan-Neagoe, I.; Buduru, S.; Badea, M. Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy? Int. J. Mol. Sci. 2021, 22, 929. [Google Scholar] [CrossRef] [PubMed]
- Tüzün-Antepli, B.; Şeker, Ş.; Elçin, A.E.; Khang, G.; Elçin, Y.M. Evaluation of Human Osteoblasts on NIPS Micro-Patterned PCL Carriers Containing Nanohydroxyapatite and Reduced Graphene Oxide Using PSµM. Molecules 2022, 27, 7091. [Google Scholar] [CrossRef] [PubMed]
- Limraksasin, P.; Kondo, T.; Zhang, M.; Okawa, H.; Osathanon, T.; Pavasant, P.; Egusa, H. In Vitro Fabrication of Hybrid Bone/Cartilage Complex Using Mouse Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 581. [Google Scholar] [CrossRef] [Green Version]
- Smojver, I.; Katalinić, I.; Bjelica, R.; Gabrić, D.; Matišić, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef] [Green Version]
- Fonticoli, L.; della Rocca, Y.; Rajan, T.S.; Murmura, G.; Trubiani, O.; Oliva, S.; Pizzicannella, J.; Marconi, G.D.; Diomede, F. A Narrative Review: Gingival Stem Cells as a Limitless Reservoir for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 4135. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.-C.; Ardelean, L.C.; Mohd, N.; Razali, M.; Ghazali, M.J.; Hayaty, N.; Kasim, A. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Botelho, M. Biphasic Calcium Phosphates (BCP) of Hydroxyapatite (HA) and Tricalcium Phosphate (TCP) as Bone Substitutes: Importance of Physicochemical Characterizations in Biomaterials Studies. Data Brief 2017, 10, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Denry, I.; Kuhn, L.T. Design and Characterization of Calcium Phosphate Ceramic Scaffolds for Bone Tissue Engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, B.O. Infrared Studies of Apatites. I. Vibrational Assignments for Calcium, Strontium, and Barium Hydroxyapatites Utilizing Isotopic Substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Rodríguez-Umanzor, F.E.; Acuña-Ruiz, N.F.; Vera-Rojas, G.E.; Terraza-Inostroza, C.; Cohn-Inostroza, N.A.; Utrera, A.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Fabrication and Testing of Multi-Hierarchical Porous Scaffolds Designed for Bone Regeneration via Additive Manufacturing Processes. Polymers 2022, 14, 4041. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.-C.; Miculescu, F.; Dascălu, C.-A.; Voicu, Ş.I.; Pandele, M.-A.; Ciocoiu, R.-C.; Batalu, D.; Dondea, S.; Mitran, V.; Ciocan, L.-T. Influence of Ceramic Particles Size and Ratio on Surface—Volume Features of the Naturally Derived HA-Reinforced Filaments for Biomedical Applications. J. Funct. Biomater. 2022, 13, 199. [Google Scholar] [CrossRef]
- Janek, M.; Žilinská, V.; Kovár, V.; Hajdúchová, Z.; Tomanová, K.; Peciar, P.; Veteška, P.; Gabošová, T.; Fialka, R.; Feranc, J.; et al. Mechanical Testing of Hydroxyapatite Filaments for Tissue Scaffolds Preparation by Fused Deposition of Ceramics. J. Eur. Ceram. Soc. 2020, 40, 4932–4938. [Google Scholar] [CrossRef]
- Rahyussalim, A.J.; Aprilya, D.; Handidwiono, R.; Whulanza, Y.; Ramahdita, G.; Kurniawati, T. The Use of 3D Polylactic Acid Scaffolds with Hydroxyapatite/Alginate Composite Injection and Mesenchymal Stem Cells as Laminoplasty Spacers in Rabbits. Polymers 2022, 14, 3292. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Kasim, N.H.A. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef]
- Bayart, M.; Dubus, M.; Charlon, S.; Kerdjoudj, H.; Baleine, N.; Benali, S.; Raquez, J.M.; Soulestin, J. Pellet-Based Fused Filament Fabrication (FFF)-Derived Process for the Development of Polylactic Acid/Hydroxyapatite Scaffolds Dedicated to Bone Regeneration. Materials 2022, 15, 5615. [Google Scholar] [CrossRef]
- Chadefaux, C.; Vignaud, C.; Chalmin, E.; Robles-Camacho, J.; Arroyo-Cabrales, J.; Johnson, E.; Reiche, I. Color Origin and Heat Evidence of Paleontological Bones: Case Study of Blue and Gray Bones from San Josecito Cave, Mexico. Am. Mineral. 2009, 94, 27–33. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553. [Google Scholar] [CrossRef] [PubMed]
- Veteška, P.; Hajdúchová, Z.; Feranc, J.; Tomanová, K.; Milde, J.; Kritikos, M.; Bača, Ľ.; Janek, M. Novel Composite Filament Usable in Low-Cost 3D Printers for Fabrication of Complex Ceramic Shapes. Appl. Mater. Today 2021, 22, 100949. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. https://doi.org/10.3390/ijms232314870
Thurzo A, Gálfiová P, Nováková ZV, Polák Š, Varga I, Strunga M, Urban R, Surovková J, Leško Ľ, Hajdúchová Z, et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. International Journal of Molecular Sciences. 2022; 23(23):14870. https://doi.org/10.3390/ijms232314870
Chicago/Turabian StyleThurzo, Andrej, Paulína Gálfiová, Zuzana Varchulová Nováková, Štefan Polák, Ivan Varga, Martin Strunga, Renáta Urban, Jana Surovková, Ľuboš Leško, Zora Hajdúchová, and et al. 2022. "Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder" International Journal of Molecular Sciences 23, no. 23: 14870. https://doi.org/10.3390/ijms232314870
APA StyleThurzo, A., Gálfiová, P., Nováková, Z. V., Polák, Š., Varga, I., Strunga, M., Urban, R., Surovková, J., Leško, Ľ., Hajdúchová, Z., Feranc, J., Janek, M., & Danišovič, Ľ. (2022). Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. International Journal of Molecular Sciences, 23(23), 14870. https://doi.org/10.3390/ijms232314870