Sex Steroid Hormone Analysis in Human Tear Fluid Using a Liquid Chromatography—Mass Spectrometry Method
Abstract
:1. Introduction
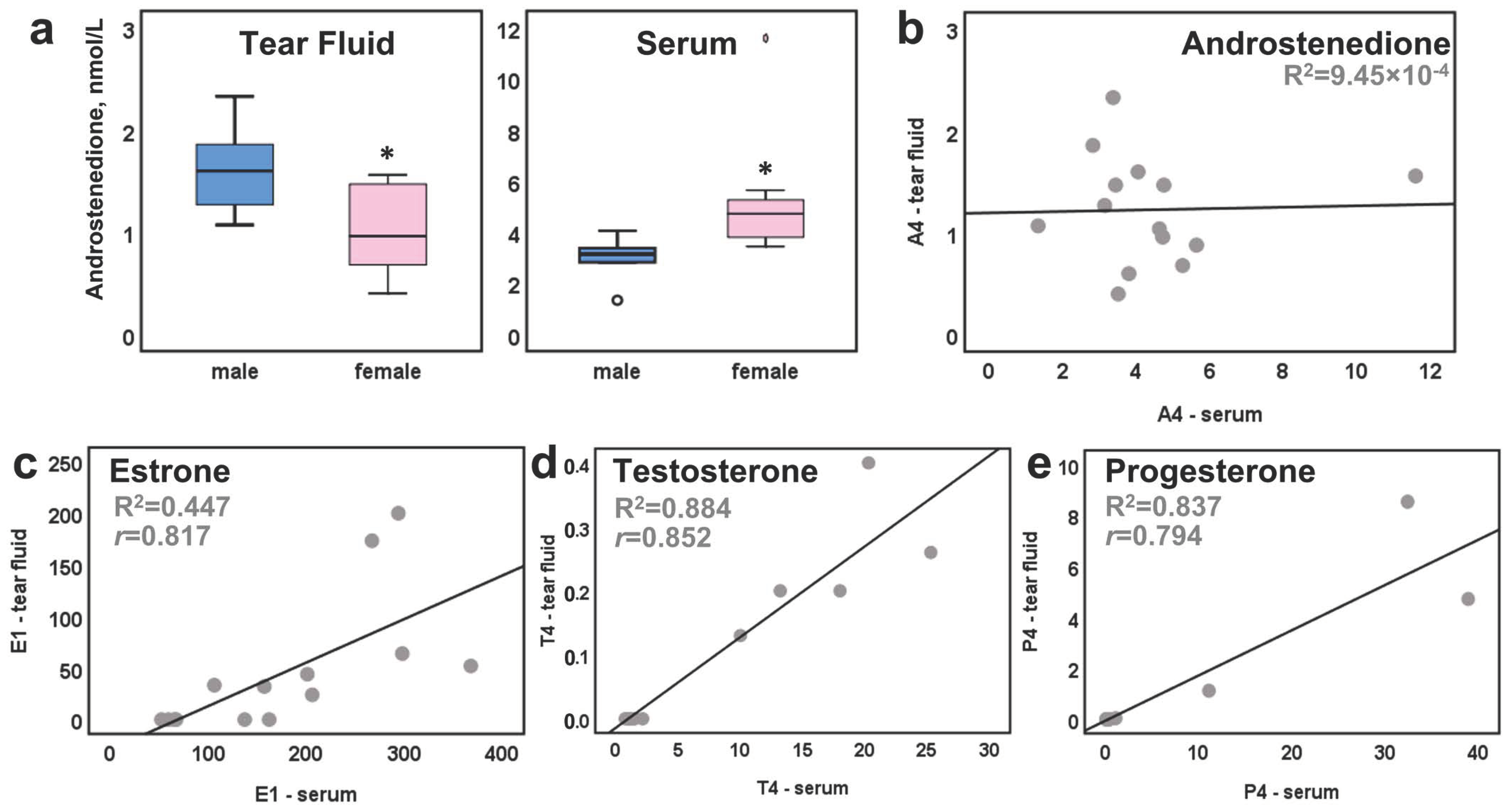
2. Results
3. Discussion
4. Methods and Materials
4.1. Tear Fluid Sample Collection and Preparation
4.2. LC–MS/MS Analysis
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, D.A.; Rocha, E.M.; Aragona, P.; Clayton, J.A.; Ding, J.; Golebiowski, B.; Hampel, U.; McDermott, A.M.; Schaumberg, D.A.; Srinivasan, S.; et al. Willcox M.D.P. TFOS DEWS II Sex, gender, and hormone report. Ocul. Surf. 2017, 15, 284–333. [Google Scholar] [CrossRef] [PubMed]
- Gayton, J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 4, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Truong, S.; Cole, N.; Stapleton, F.; Golebiowski, B. Sex hormones and the dry eye. Clin. Exp. Optom. 2014, 4, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Schirra, F.; Suzuki, T.; Dickinson, D.P.; Townsend, D.J.; Gipson, I.K.; Sullivan, D.A. Identification of steroidogenic enzyme mRNAs in the human lacrimal gland, meibomian gland, cornea, and conjunctiva. Cornea 2006, 25, 438–442. [Google Scholar] [CrossRef]
- Wickham, L.A.; Gao, J.; Toda, I.; Rocha, E.M.; Ono, M.; Sullivan, D.A. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand. 2000, 78, 146–153. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Edwards, J.A.; Wickham, L.A.; Pena, J.D.; Gao, J.; Ono, M.; Kelleher, R.S. Identification and endocrine control of sex steroid binding sites in the lacrimal gland. Curr. Eye Res. 1996, 15, 279–291. [Google Scholar] [CrossRef]
- Labrie, F.; Belanger, A.; Simard, J.; Luu-The, V.A.N.; Labrie, C. DHEA and peripheral androgen and estrogen formation: Intracinology. Ann. N. Y. Acad. Sci. 1995, 774, 16–28. [Google Scholar] [CrossRef]
- Do Rego, J.L.; Seong, J.Y.; Burel, D.; Leprince, J.; van Luu-The, T.K.; Tonon, M.-C.; Pelletier, G.; Vaudry, H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front. Neuroendocr. 2009, 3, 259–301. [Google Scholar] [CrossRef]
- Compston, J. Local Biosynthesis of Sex Steroids in Bone. J. Clin. Endocrinol. Metab. 2002, 12, 5398–5400. [Google Scholar] [CrossRef]
- Inoue, T.; Miki, Y.; Abe, K.; Hatori, M.; Hosaka, M.; Kariya, Y.; Kakuo, S.; Fujimura, T.; Hachiya, A.; Honma, S.; et al. Sex steroid synthesis in human skin in situ: The roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol. Cell. Endocrinol. 2012, 362, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.J.; Stapleton, F.; Wolffsohn, J.S.; Golebiowski, B. Local synthesis of sex hormones: Are there consequences for the ocular surface and dry eye? Br. J. Ophthalmol. 2017, 12, 1596–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vehof, J.; Hysi, P.G.; Hammond, C.J. A metabolome-wide study of dry eye disease. Ophthalmology 2017, 124, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Versura, P.; Giannaccare, G.; Campos, E.C. Sex-steroid imbalance in females and dry eye. Curr. Eye Res. 2015, 40, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, U.; Hämäläinen, E.; Haanpää, M.; Dunkel, L. Determination of salivary testosterone and androstendione by liquid chromatography–tandem mass spectrometry. Clin. Chim. Acta 2012, 413, 594–599. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Haanpää, M.; Turpeinen, U.; Hämäläinen, E.; Seuri, R.; Tyrväinen, E.; Sankilampi, U.; Dunkel, L. Postnatal ovarian activation has effects in estrogen target tissues in infant girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716. [Google Scholar] [CrossRef]
- Vihma, V.; Wang, F.; Savolainen-Peltonen, H.; Turpeinen, U.; Hämäläinen, E.; Leidenius, M.; Mikkola, T.S.; Tikkanen, M.J. Quantitative determination of estrone by liquid chromatography-tandem mass spectrometry in subcutaneous adipose tissue from the breast in postmenopausal women. J. Steroid Biochem. Mol. Biol. 2016, 155, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colldén, H.; Nilsson, M.E.; Norlén, A.-K.; Landin, A.; Windahl, S.H.; Wu, J.; Gustafsson, K.L.; Poutanen, M.; Ryberg, H.; Vandenput, L.; et al. Comprehensive Sex Steroid Profiling in Multiple Tissues Reveals Novel Insights in Sex Steroid Distribution in Male Mice. Endocrinology 2022, 163, bqac001. [Google Scholar] [CrossRef] [PubMed]
- Hobo, Y.; Nishikawa, J.; Miyashiro, Y.; Fujikata, A. Analysis of hair steroid hormone concentrations at different parts of the head by liquid chromatography-tandem mass spectrometry. Clin. Chim. Acta 2021, 523, 260–266. [Google Scholar] [CrossRef]
- Rao, G.S. MOde of entry of steroid and thyroid hormones into cells. Mol. Cell. Endocrinol. 1981, 21, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Banbury, L.K. Stress markers in tear fluid. Ph.D. Thesis, Submitted at Southern Cross University, Lismore, NSW, Australia, 2009. [Google Scholar]
- Fortunati, N.; Fissore, F.; Fazzari, A.; Berta, L.; Giudici, M.; Frairia, R. Sex steroid binding protein interacts with a specific receptor on human premenopausal endometrium membranes: Modulating effect of estradiol. Steroids 1991, 56, 341. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.R.; Ho, R.H. Organic Anion Transporting Polypeptides: Emerging Roles in Cancer Pharmacology. Mol. Pharm. 2019, 95, 490–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, E.J.; Bucknallb, M.P.; Golebiowskia, B.; Stapleton, F. Comparative limitations and benefits of liquid chromatography–mass spectrometry techniques for analysis of sex steroids in tears. Exp. Eye Res. 2019, 179, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Dartt, A.D. Tear lipocalin: Structure and function. Ocul. Surf. 2011, 9, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrie, F. Intracrinology. Mol. Cell. Endocrinol. 1991, 78, C113–C118. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Gibson, E.; Golebiowski, B.; Stapleton, F.; Jenner, A.M.; Bucknall, M.P. Analysis of sex steroids in human tears using LC-MS and GC-MS: Considerations and developments to improve method sensitivity and accuracy. Exp. Eye Res. 2022, 225, 109283. [Google Scholar] [CrossRef]
- Pieragostino, D.; Agnifili, L.; Cicalini, I.; Calienno, R.; Zucchelli, M.; Mastropasqua, L.; Sacchetta, P.; Del Boccio, P.; Rossi, C. Tear film steroid profiling in dry eye disease by liquid chromatography tandem mass spectrometry. Int. J. Mol. Sci. 2017, 18, 1349. [Google Scholar] [CrossRef]

| E2 (pmol/L) | E1 (pmol/L) | P4 (nmol/L) | T4 (nmol/L) | A4 (nmol/L) | DHEA (nmol/L) | |
|---|---|---|---|---|---|---|
| H2O blank | <5 | <5 | <0.05 | <0.01 | <0.01 | <0.1 |
| PBS blank | <5 | <5 | <0.05 | <0.01 | <0.01 | <0.1 |
| Tear fluid | <5 | 11 | 0.9 | <0.01 | 0.5 | 0.6 |
| E2 (pmol/L) |
E1 (pmol/L) |
P4 (nmol/L) |
T4 (nmol/L) |
A4 (nmol/L) |
DHEA (nmol/L) | ||
|---|---|---|---|---|---|---|---|
| blank | <5 | <5 | <0.01 | <0.01 | <0.01 | <0.1 | |
| female | TF 1 n = 5 | <5 | 24 (0–173.4) | 0.04 (0–8.5) | <0.01 | 0.68 (0.4–1.5) | 0.8 (0–2.8) |
| TF 2 n = 4 | <5 | 48 (44–200.1) | 1.12 (0–3.94) | <0.01 | 1.26 (0.8–2.0) | 0.8 (0–1.2) | |
| Serum 1 n = 5 | 131 (15–698) | 162 (52–294) | 0.18 (0.1–1.1) | 1.05 (0.7–1.4) | 4.75 (3.5–5.6) | 16.9 (14.8–26.4) | |
| Serum 2 n = 4 | 324 (248–434) | 282 (201–368) | 21.75 (0.4–38.9) | 1.28 (1–2.1) | 4.67 (3.4–11.6) | 21.1 (14.6–30.1) | |
| male | TF n = 5 | <5 | 33.35 | <0.01 | 0.2 (0.1–0.4) | 1.6 (1.1–2.3) | 2.01 |
| Serum n = 5 | 58 (21–80) | 67 (59–137) | 0.18 (0.1–0.4) | 18 (10–25.3) | 3.37 (1.3–4.1) | 20 (6.1–27.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robciuc, A.; Savolainen-Peltonen, H.; Haanpää, M.; Moilanen, J.A.O.; Mikkola, T.S. Sex Steroid Hormone Analysis in Human Tear Fluid Using a Liquid Chromatography—Mass Spectrometry Method. Int. J. Mol. Sci. 2022, 23, 14864. https://doi.org/10.3390/ijms232314864
Robciuc A, Savolainen-Peltonen H, Haanpää M, Moilanen JAO, Mikkola TS. Sex Steroid Hormone Analysis in Human Tear Fluid Using a Liquid Chromatography—Mass Spectrometry Method. International Journal of Molecular Sciences. 2022; 23(23):14864. https://doi.org/10.3390/ijms232314864
Chicago/Turabian StyleRobciuc, Alexandra, Hanna Savolainen-Peltonen, Mikko Haanpää, Jukka A. O. Moilanen, and Tomi S. Mikkola. 2022. "Sex Steroid Hormone Analysis in Human Tear Fluid Using a Liquid Chromatography—Mass Spectrometry Method" International Journal of Molecular Sciences 23, no. 23: 14864. https://doi.org/10.3390/ijms232314864
APA StyleRobciuc, A., Savolainen-Peltonen, H., Haanpää, M., Moilanen, J. A. O., & Mikkola, T. S. (2022). Sex Steroid Hormone Analysis in Human Tear Fluid Using a Liquid Chromatography—Mass Spectrometry Method. International Journal of Molecular Sciences, 23(23), 14864. https://doi.org/10.3390/ijms232314864







