β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis
Abstract
1. Introduction
2. Results
2.1. β-Caryophyllene (BCP) Alleviated 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis-like Skin Lesions in BALB/c Mice
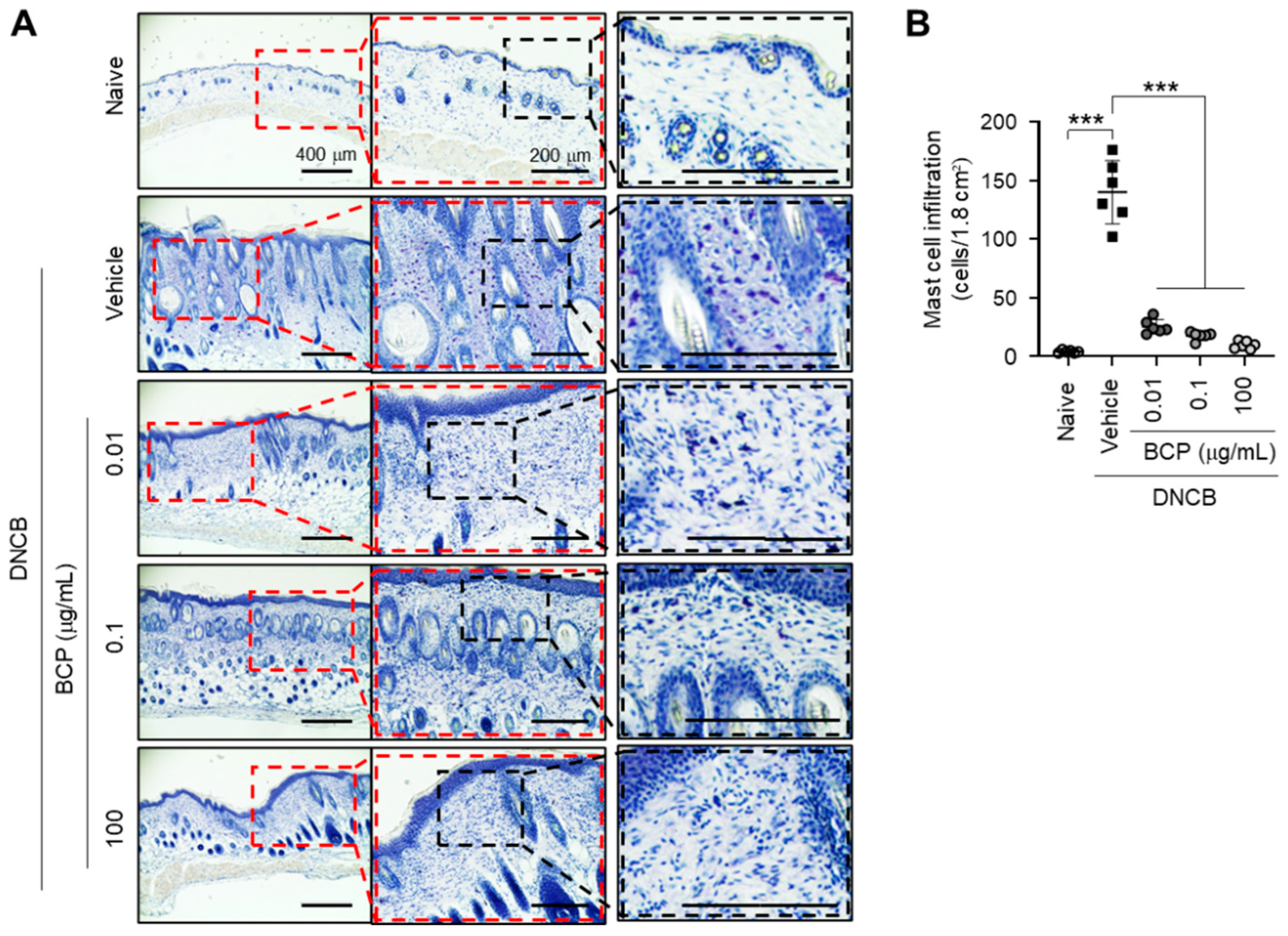
2.2. Topical Application of BCP Inhibits DNCB-Induced Infiltration of Inflammatory Cells in AD-like Skin Lesions
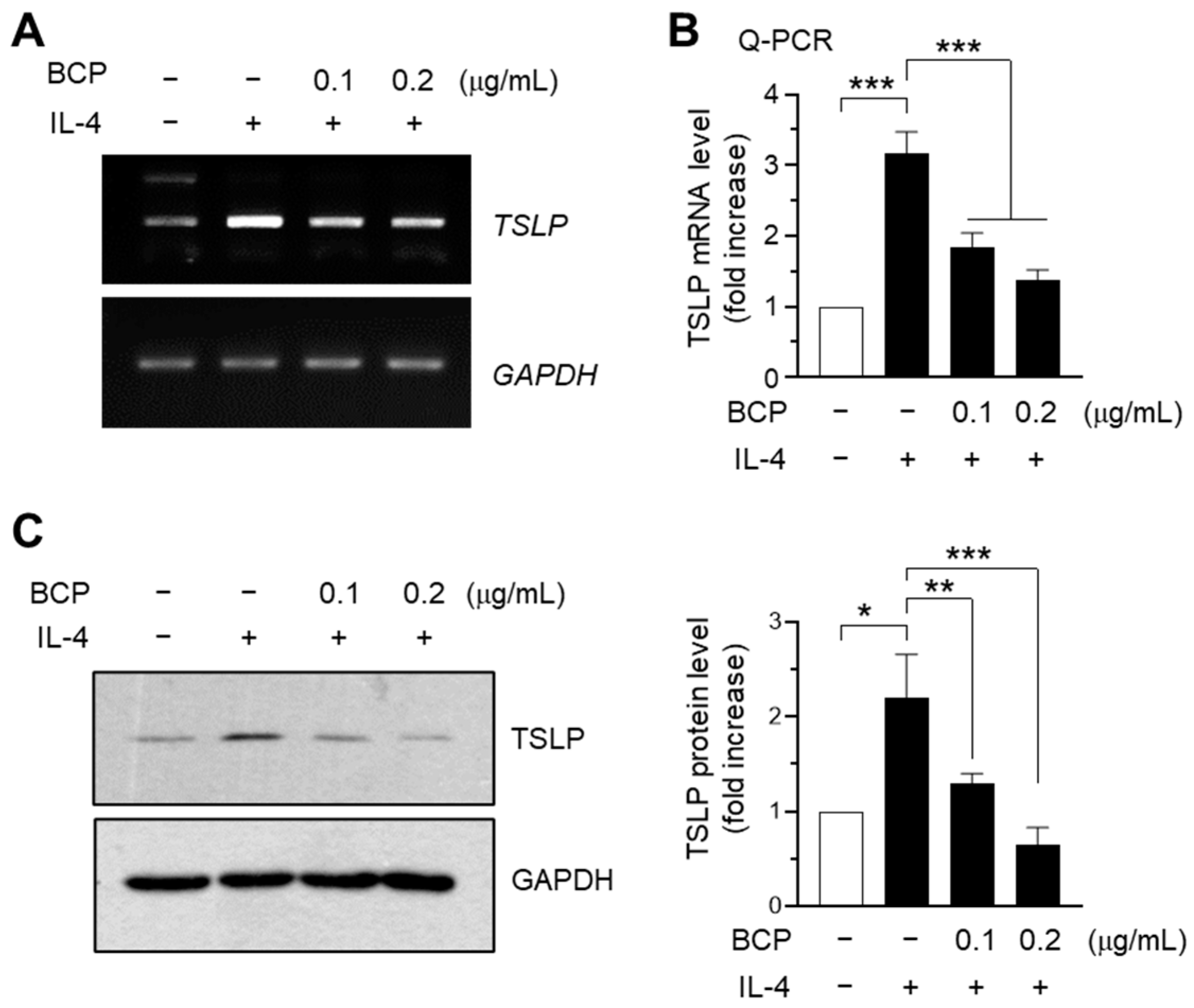
2.3. IL-4-Induced TSLP Expression Is Attenuated by BCP at the mRNA Level in HaCaT Keratinocytes
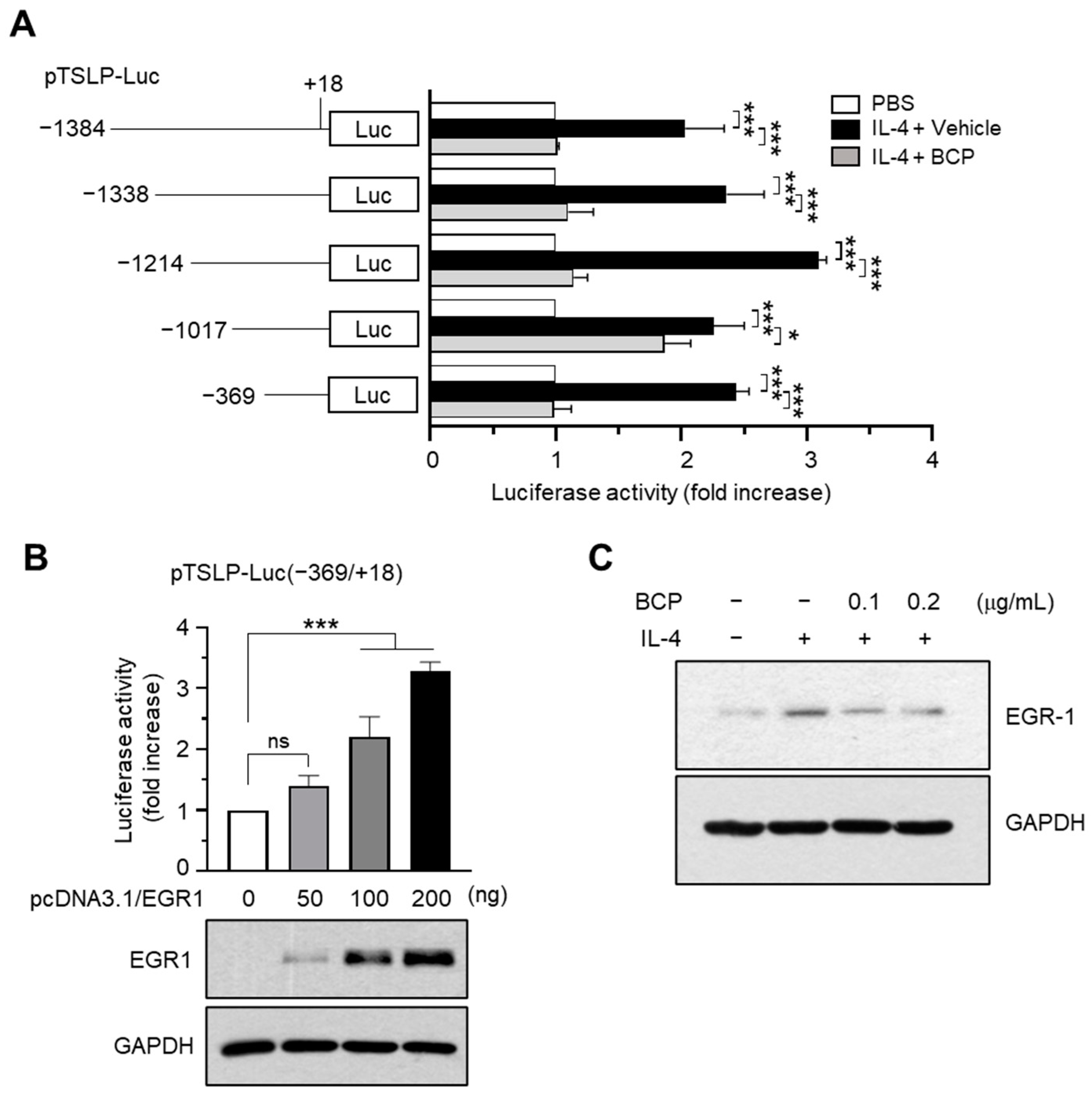
2.4. Chrysin EGR1 Is Involved in the BCP-Induced Suppression of TSLP Expression Evoked by IL-4
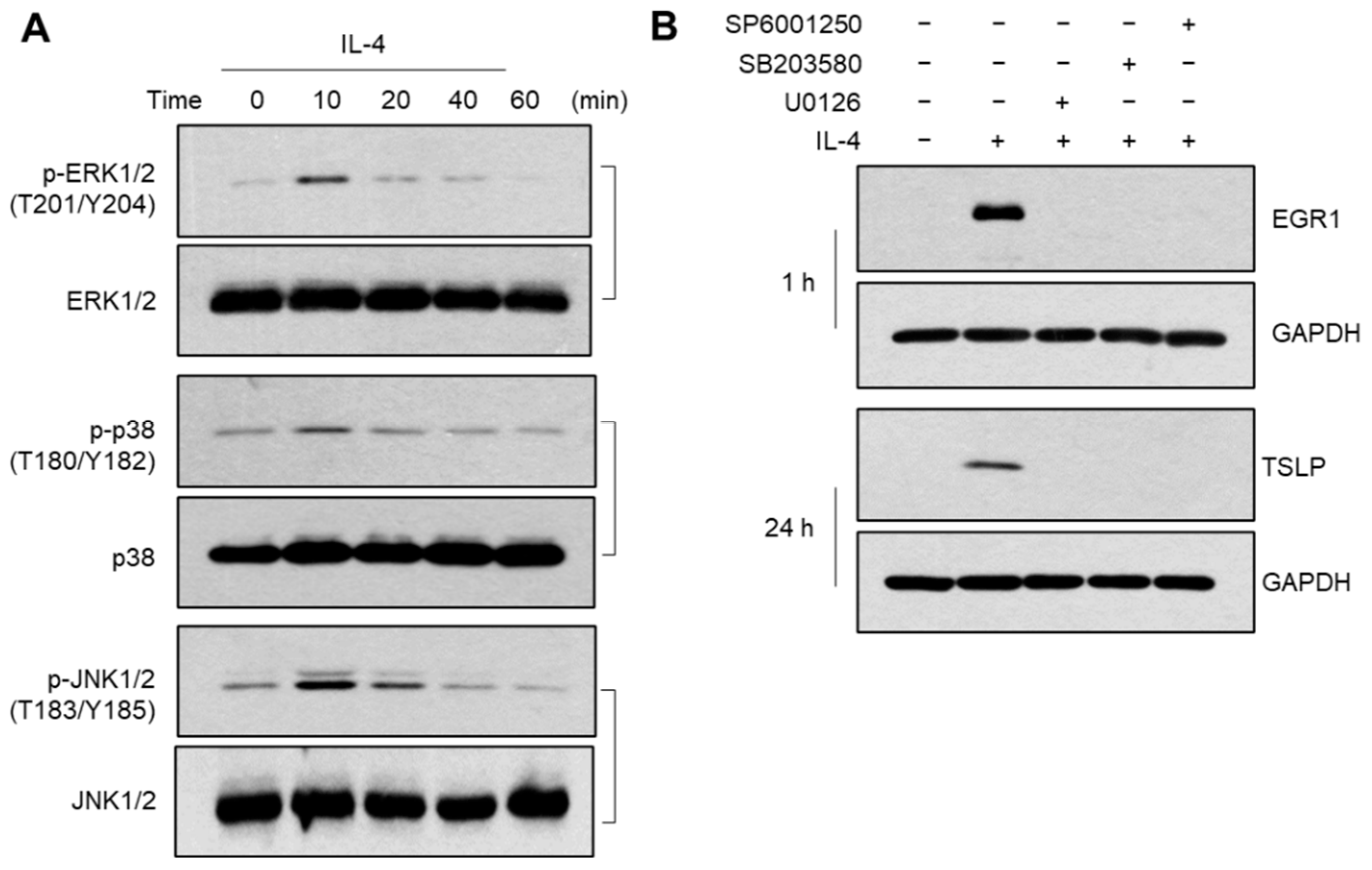
2.5. Role of MAPK Pathways in IL-4-Induced EGR1 Expression
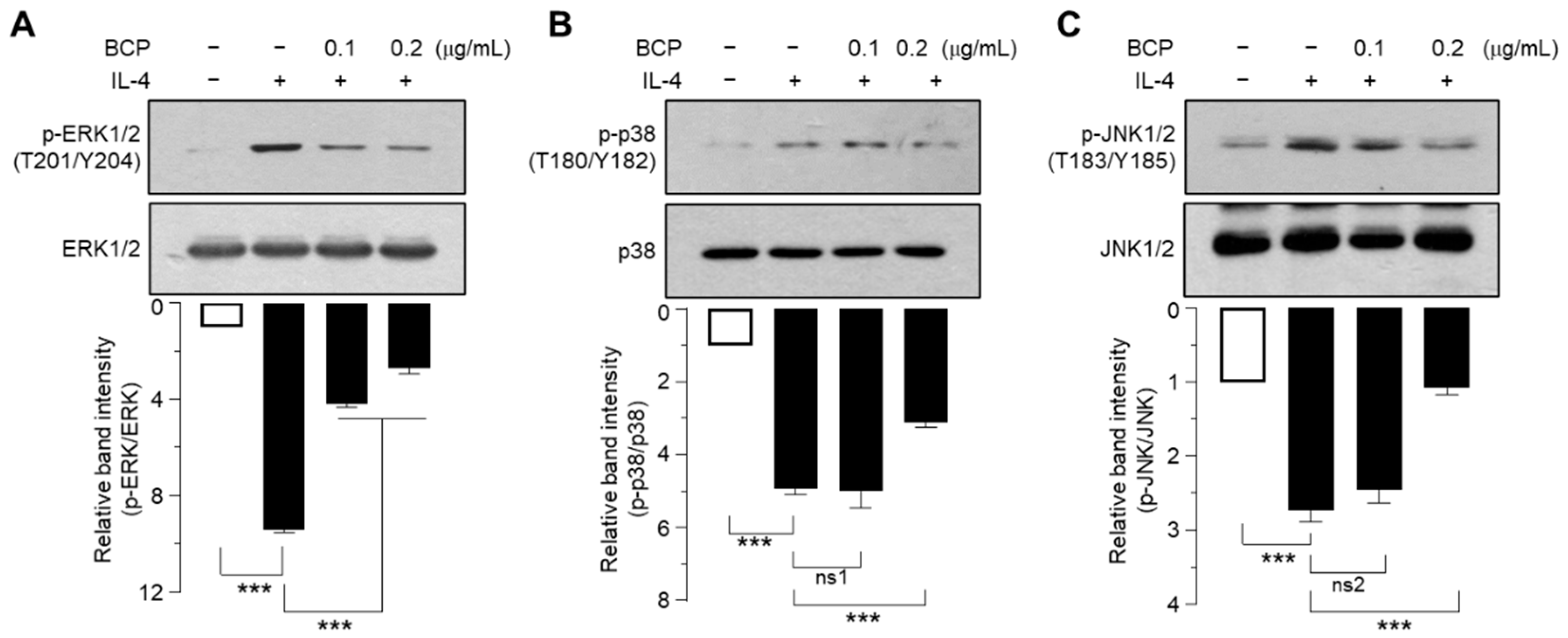
2.6. BCP Inhibits All Three MAPKs Activated by IL-4
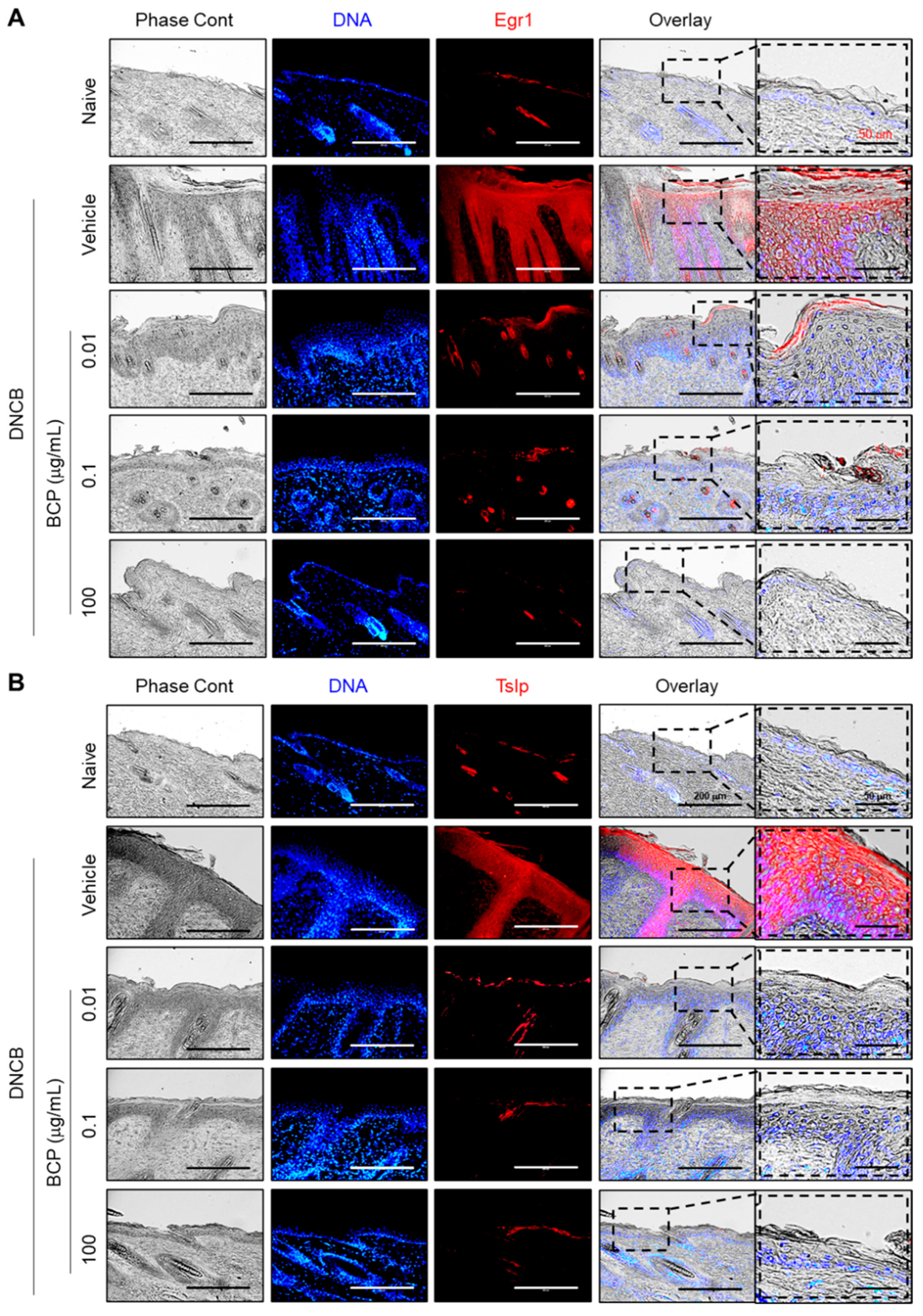
2.7. The Topical Application of BCP Inhibits EGR1 and TSLP Expression Induced by IL-4 in AD-like Skin Lesions In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Induction of Atopic Dermatitis-like Skin Inflammation on the Dorsal Skin of Mice
4.3. Histological Analysis
4.4. Cells
4.5. Reverse Transcription-PCR (RT-PCR)
4.6. Quantitative Real-Time PCR (Q-PCR)
4.7. TSLP Promoter-Reporter Assay
4.8. Immunoblot Analysis
4.9. Immunofluorescence Staining
4.10. Statistical Analysis
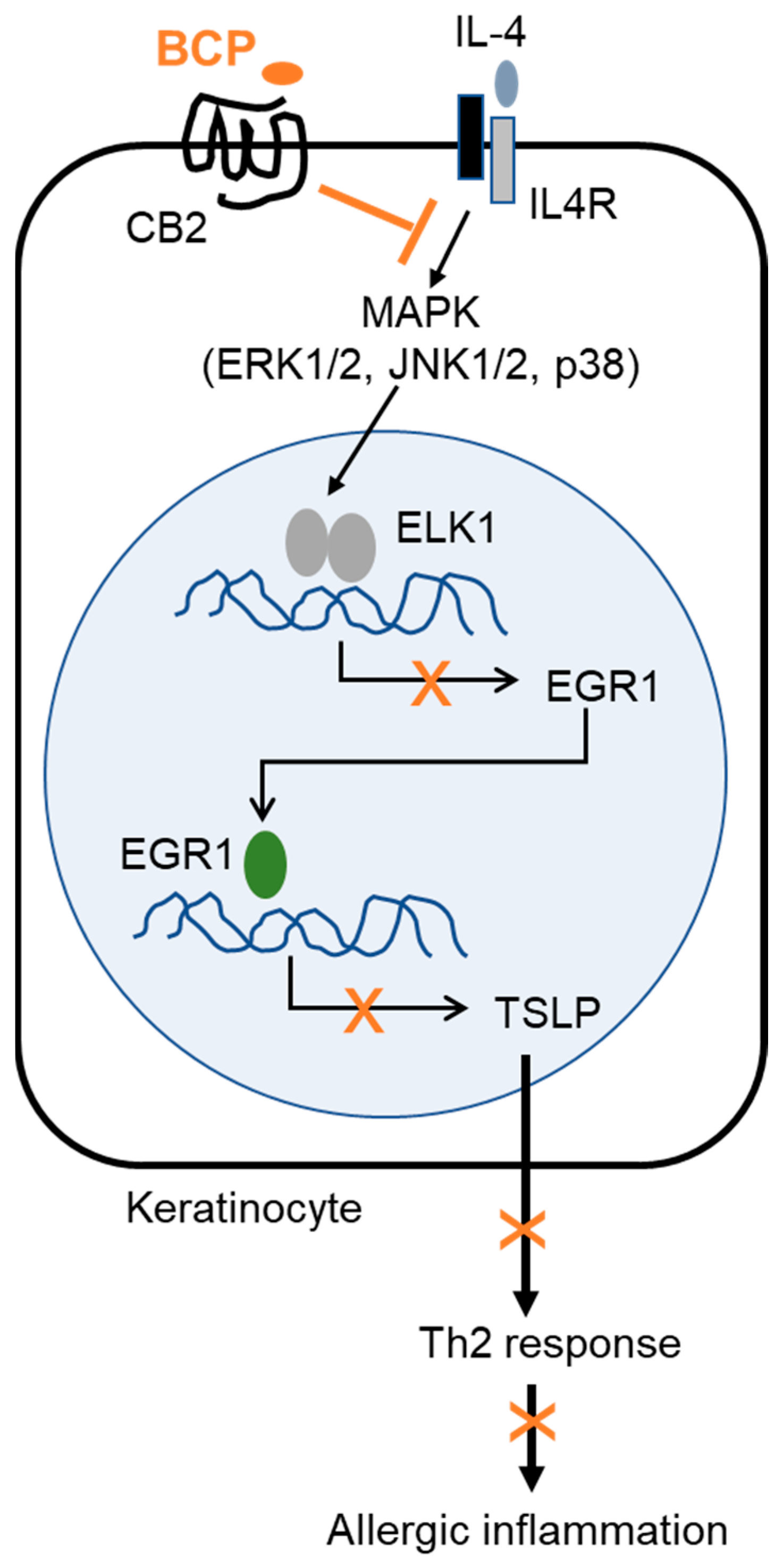
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| BCP | β-caryophyllene |
| CB | cannabinoid receptor |
| DNCB | 2,4-dinitrochlorobenzene |
| EGR1 | early growth response 1 |
| ERK | extracellular signal-regulated kinases |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| H&E | hematoxylin and eosin |
| IL | interleukin |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinases |
| Q-PCR | quantitative real-time PCR |
| RT-PCR | reverse transcription polymerase chain reaction |
| TB | toluidine blue |
| Th2 | T helper cell 2 |
| TNFα | tumor necrosis factor-alpha |
| TSLP | thymic stromal lymphopoietin |
References
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Werfel, T.; Allam, J.-P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.-U.; Wollenberg, A. Cellular and Molecular Immunologic Mechanisms in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef]
- Chan, L.S.; Robinson, N.; Xu, L. Expression of Interleukin-4 in the Epidermis of Transgenic Mice Results in a Pruritic Inflammatory Skin Disease: An Experimental Animal Model to Study Atopic Dermatitis. J. Investig. Dermatol. 2001, 117, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Trier, A.M.; Kim, B.S. Cytokine Modulation of Atopic Itch. Curr. Opin. Immunol. 2018, 54, 7–12. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human Epithelial Cells Trigger Dendritic Cell Mediated Allergic Inflammation by Producing Tslp. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Takai, T. Tslp Expression: Cellular Sources, Triggers, and Regulatory Mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef]
- Watanabe, N.; Hanabuchi, S.; Soumelis, V.; Yuan, W.; Ho, S.; de Waal Malefyt, R.; Liu, Y.-J. Human Thymic Stromal Lymphopoietin Promotes Dendritic Cell–Mediated Cd4+ T Cell Homeostatic Expansion. Nat. Immunol. 2004, 5, 426–434. [Google Scholar] [CrossRef]
- Nakajima, S.; Kabata, H.; Kabashima, K.; Asano, K. Anti-Tslp Antibodies: Targeting a Master Regulator of Type 2 Immune Responses. Allergol. Int. 2020, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial Cell–Derived Cytokines: More Than Just Signaling the Alarm. J. Clin. Investig. 2019, 129, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Oyoshi, M.K.; Garibyan, L.; Kumar, L.; Ziegler, S.F.; Geha, R.S. Tslp Acts on Infiltrating Effector T Cells to Drive Allergic Skin Inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 11875–11880. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.C.; Rusta-Sallehy, S.; Heroux, D.; Denburg, J.A. Thymic stromal lymphopoietin promotes human eosinophil-basophil lineage commitment: A key role for tumor necrosis factor-alpha. All. Asth. Clin. Immun. 2014, 10 (Suppl. 1), A51. [Google Scholar] [CrossRef][Green Version]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.R.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic Stromal Lymphopoietin Is Released by Human Epithelial Cells in Response to Microbes, Trauma, or Inflammation and Potently Activates Mast Cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-Derived Atopic Dermatitis Cytokine Tslp Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous Atopic Dermatitis in Mice Expressing an Inducible Thymic Stromal Lymphopoietin Transgene Specifically in the Skin. J. Exp. Med. 2005, 202, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. The Biology of Thymic Stromal Lymphopoietin (Tslp). Adv. Pharmacol. 2013, 66, 129–155. [Google Scholar]
- Gertsch, J. Antiinflammatory Cannabinoids in Diet–Towards a Better Understanding of Cb2 Receptor Action? Towards a Better Understanding of Cb2 Receptor Action? Commun. Integr. Biol. 2008, 1, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Demuth, D.G.; Molleman, A. Cannabinoid Signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Chang, H.-J.; Lee, S.-K.; Kim, H.-J.; Hwang, J.-K.; Chun, H.S. Amelioration of Dextran Sulfate Sodium-Induced Colitis in Mice by Oral Administration of Β-Caryophyllene, a Sesquiterpene. Life Sci. 2007, 80, 932–939. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Li, Y.; Wang, M.; Qian, F. Β-Caryophyllene Attenuates Lipopolysaccharide-Induced Acute Lung Injury Via Inhibition of the Mapk Signalling Pathway. J. Pharm. Pharmacol. 2021, 73, 1319–1329. [Google Scholar] [CrossRef]
- Ando, T.; Matsumoto, K.; Namiranian, S.; Yamashita, H.; Glatthorn, H.; Kimura, M.; Dolan, B.R.; Lee, J.J.; Galli, S.J.; Kawakami, Y.; et al. Mast Cells Are Required for Full Expression of Allergen/Seb-Induced Skin Inflammation. J. Investig. Dermatol. 2013, 133, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, P.P.; Tan, Z.; Page, B.D.G.; Hedtrich, S. Tslp as Druggable Target—A Silver-Lining for Atopic Diseases? Pharmacol. Ther. 2021, 217, 107648. [Google Scholar] [CrossRef]
- Yeo, H.; Lee, Y.H.; Ahn, S.S.; Jung, E.; Lim, Y.; Shin, S.Y. Chrysin Inhibits Tnfα-Induced Tslp Expression through Downregulation of Egr1 Expression in Keratinocytes. Int. J. Mol. Sci. 2021, 22, 4350. [Google Scholar] [CrossRef]
- McMahon, S.B.; Monroe, J.G. The Role of Early Growth Response Gene 1 (Egr-1) in Regulation of the Immune Response. J. Leukoc. Biol. 1996, 60, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Ahn, S.S.; Lee, J.Y.; Jung, E.; Jeong, M.; Kang, G.S.; Ahn, S.; Lee, Y.; Koh, D.; Lee, Y.H. Disrupting the DNA Binding of Egr-1 with a Small-Molecule Inhibitor Ameliorates 2, 4-Dinitrochlorobenzene-Induced Skin Inflammation. J. Investig. Dermatol. 2021, 141, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Hipskind, R.A.; Büscher, D.; Nordheim, A.; Baccarini, M. Ras/Map Kinase-Dependent and-Independent Signaling Pathways Target Distinct Ternary Complex Factors. Genes Dev. 1994, 8, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, C.; Yanagihara, S.; Otsuka, A. Innovation in the Treatment of Atopic Dermatitis: Emerging Topical and Oral Janus Kinase Inhibitors. Allergol. Int. 2022, 71, 40–46. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. Β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Shin, S.Y.; Koh, D.; Lim, Y.; Lee, Y.H. Inhibition of Egr-1-Dependent Mmp1 Transcription by Ethanol Extract of Ageratum Houstonianum in Hacat Keratinocytes. Mol. Biol. Rep. 2021, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.C.; Diop, M.M.; Harman, C.L.; Hayashi, S.-m.; Krammer, G. Dietary Administration of Β-Caryophyllene and Its Epoxide to Sprague-Dawley Rats for 90 Days. Food Chem. Toxicol. 2020, 135, 110876. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Amjad Kamal, M.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of Β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, P.; Barry, B. Sesquiterpene Components of Volatile Oils as Skin Penetration Enhancers for the Hydrophilic Permeant 5-Fluorouracil. J. Pharm. Pharmacol. 1994, 46, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Lee, Y.H.; Yeo, H.; Jung, E.; Lim, Y.; Shin, S.Y. Saikosaponin a and Saikosaponin C Reduce Tnf-Alpha-Induced Tslp Expression through Inhibition of Mapk-Mediated Egr1 Expression in Hacat Keratinocytes. Int. J. Mol. Sci. 2022, 23, 4857. [Google Scholar] [CrossRef]
- Matacchione, G.; Gurău, F.; Silvestrini, A.; Tiboni, M.; Mancini, L.; Valli, D.; Rippo, M.R.; Recchioni, R.; Marcheselli, F.; Carnevali, O. Anti-Sasp and Anti-Inflammatory Activity of Resveratrol, Curcumin and Β-Caryophyllene Association on Human Endothelial and Monocytic Cells. Biogerontology 2021, 22, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Chen, Y.; Yang, M. Β-Caryophyllene Inhibits High Glucose-Induced Oxidative Stress, Inflammation and Extracellular Matrix Accumulation in Mesangial Cells. Int. Immunopharmacol. 2020, 84, 106556. [Google Scholar] [CrossRef]
- Sousa, L.F.B.; Oliveira, H.B.M.; das Neves Selis, N.; Morbeck, L.L.B.; Santos, T.C.; da Silva, L.S.C.; Viana, J.C.S.; Reis, M.M.; Sampaio, B.A.; Campos, G.B. Β-Caryophyllene and Docosahexaenoic Acid, Isolated or Associated, Have Potential Antinociceptive and Anti-Inflammatory Effects in Vitro and in Vivo. Sci. Rep. 2022, 12, 19199. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; De Souza, P.F.; Campos, J.C.L.; Massaro, T.N.C.; Hussni, C.A.; Takahira, R.K. Beta-Caryophyllene as an Antioxidant, Anti-Inflammatory and Re-Epithelialization Activities in a Rat Skin Wound Excision Model. Oxid. Med. Cell. Longev. 2022, 2022, 9004014. [Google Scholar] [CrossRef]
- Yeo, H.; Ahn, S.S.; Jung, E.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Transcription Factor Egr1 Regulates the Expression of the Clock Gene Per2 under Il-4 Stimulation in Human Keratinocytes. J. Investig. Dermatol. 2022, 142, 2677–2686.e9. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.S.; Yeo, H.; Jung, E.; Ou, S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis. Int. J. Mol. Sci. 2022, 23, 14861. https://doi.org/10.3390/ijms232314861
Ahn SS, Yeo H, Jung E, Ou S, Lee YH, Lim Y, Shin SY. β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis. International Journal of Molecular Sciences. 2022; 23(23):14861. https://doi.org/10.3390/ijms232314861
Chicago/Turabian StyleAhn, Sung Shin, Hyunjin Yeo, Euitaek Jung, Sukjin Ou, Young Han Lee, Yoongho Lim, and Soon Young Shin. 2022. "β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis" International Journal of Molecular Sciences 23, no. 23: 14861. https://doi.org/10.3390/ijms232314861
APA StyleAhn, S. S., Yeo, H., Jung, E., Ou, S., Lee, Y. H., Lim, Y., & Shin, S. Y. (2022). β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis. International Journal of Molecular Sciences, 23(23), 14861. https://doi.org/10.3390/ijms232314861





