Antibodies against HSV-1 and Curli Show the Highest Correlation in Parkinson’s Disease Patients in Comparison to Healthy Controls
Abstract
1. Introduction
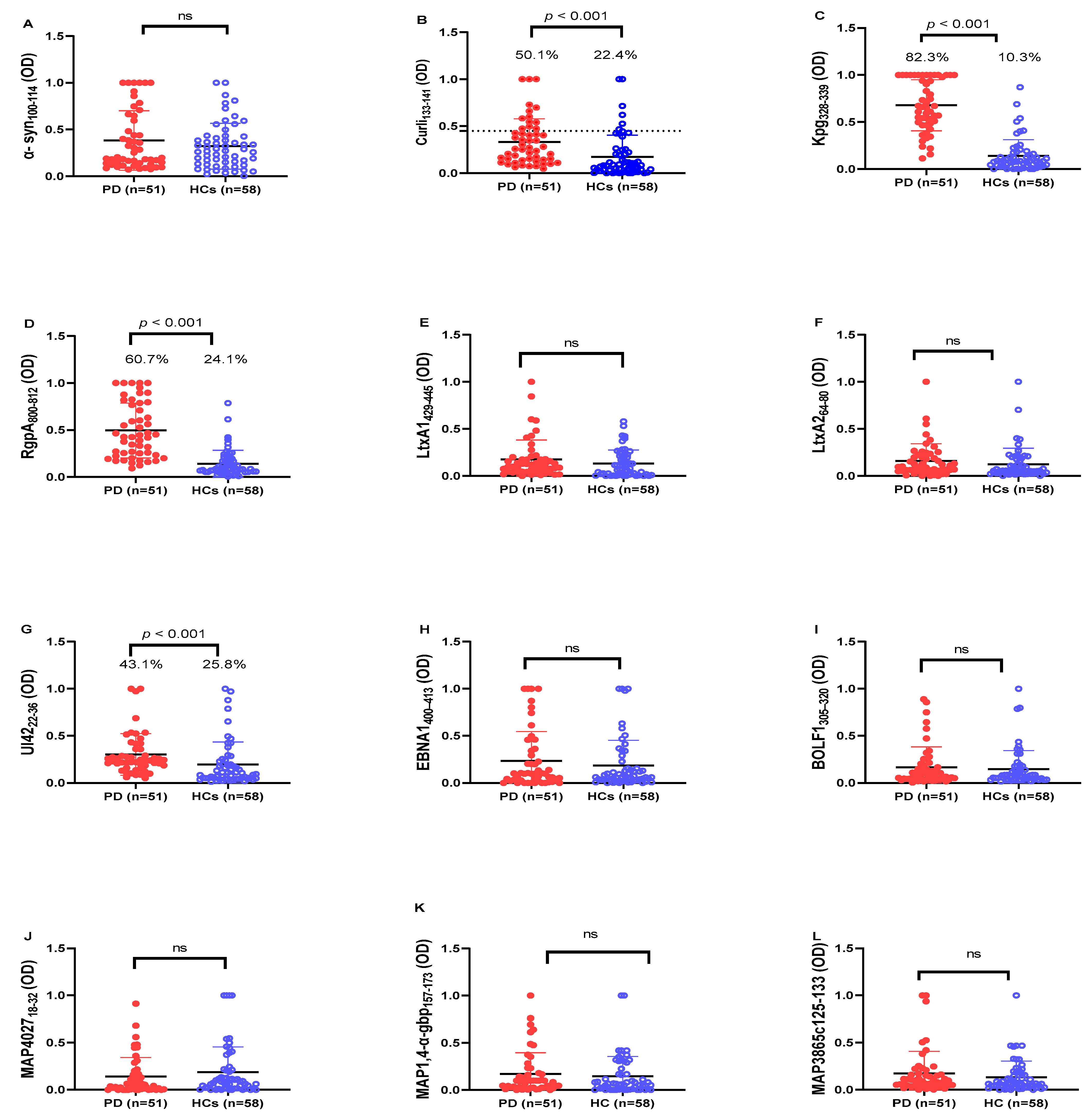
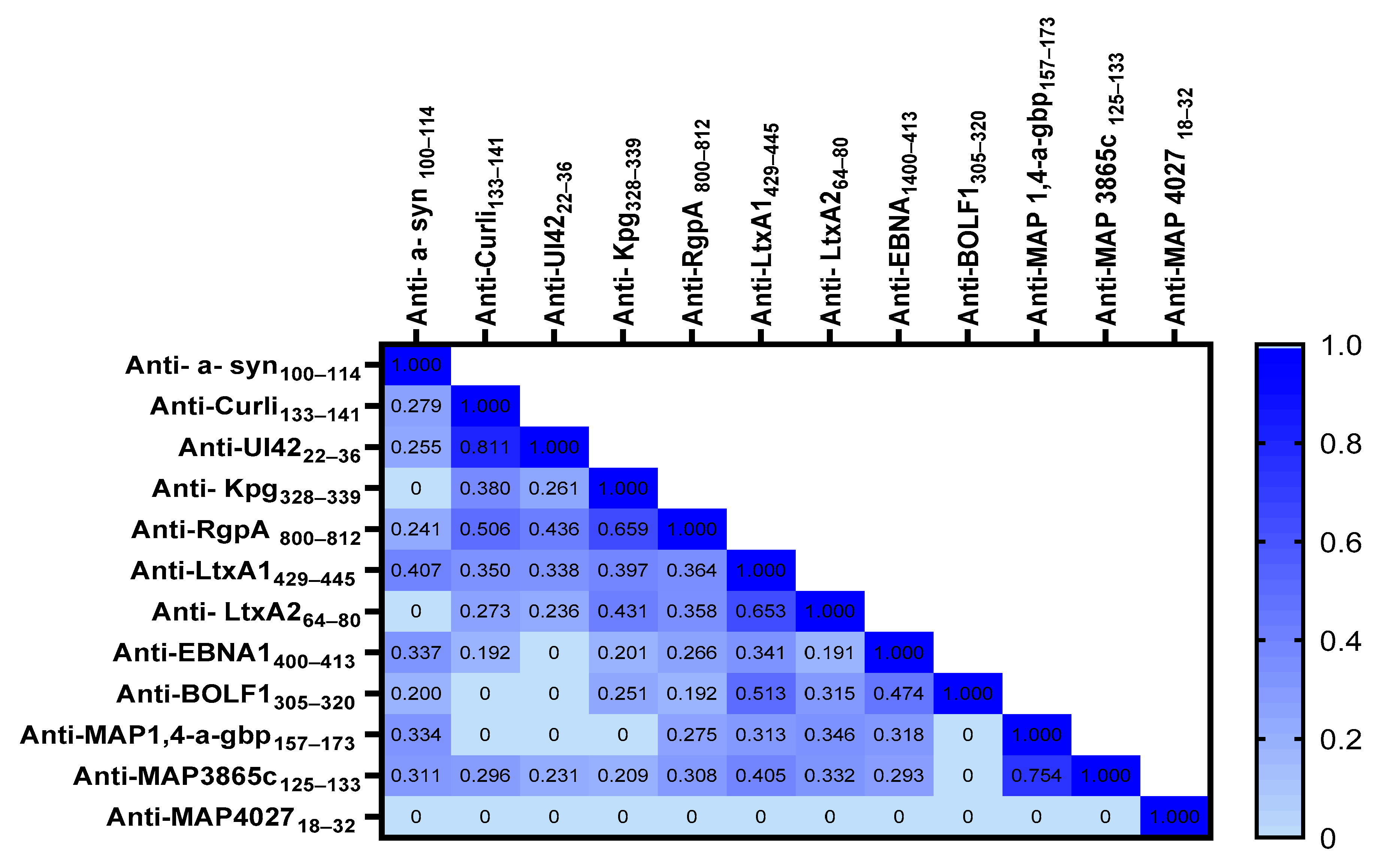
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population and Blood Collection
4.2. Peptides
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gouda, N.A.; Elkamhawy, A.; Cho, J. Emerging Therapeutic Strategies for Parkinson’s Disease and Future Prospects: A 2021 Update. Biomedicines 2022, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.-Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef]
- Li, D.; Ren, T.; Li, H.; Liao, G.; Zhang, X. Porphyromonas gingivalis: A key role in Parkinson’s disease with cognitive impairment? Front. Neurol. 2022, 13, 945523. [Google Scholar] [CrossRef]
- Barnhart, M.M.; Chapman, M.R. Curli Biogenesis and Function. Annu. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef]
- Han, M.N.; Finkelstein, D.I.; McQuade, R.M.; Diwakarla, S. Gastrointestinal Dysfunction in Parkinsons Disease: Current and Potential Therapeutics. J. Pers. Med. 2022, 12, 144. [Google Scholar] [CrossRef]
- Lotz, S.K.; Blackhurst, B.M.; Reagin, K.L.; Funk, K.E. Microbial Infections Are a Risk Factor for Neurodegenerative Diseases. Front. Cell. Neurosci. 2021, 15, 691136. [Google Scholar] [CrossRef] [PubMed]
- Smeyne, R.J.; Noyce, A.J.; Byrne, M.; Savica, R.; Marras, C. Infection and Risk of Parkinson’s Disease. J. Park. Dis. 2021, 11, 31–43. [Google Scholar] [CrossRef]
- Arru, G.; Caggiu, E.; Paulus, K.; Sechi, G.P.; Mameli, G.; Sechi, L.A. Is there a role for Mycobacterium avium subspecies paratuberculosis in Parkinson’s disease? J. Neuroimmunol. 2016, 293, 86–90. [Google Scholar] [CrossRef]
- Caggiu, E.; Paulus, K.; Galleri, G.; Arru, G.; Manetti, R.; Sechi, G.P.; Sechi, L.A. Homologous HSV-1 and alpha-synuclein peptides stimulate a T cell response in Parkinson’s disease. J. Neuroimmunol. 2017, 310, 26–31. [Google Scholar] [CrossRef]
- Zhang, N.; Zuo, Y.; Jiang, L.; Peng, Y.; Huang, X.; Zuo, L. Epstein-Barr Virus and Neurological Diseases. Front. Mol. Biosci. 2022, 8, 816098. [Google Scholar] [CrossRef] [PubMed]
- Patrick, K.L.; Bell, S.L.; Weindel, C.G.; Watson, R.O. Exploring the “Multiple-Hit Hypothesis” of Neurodegenerative Disease: Bacterial Infection Comes Up to Bat. Front. Cell. Infect. Microbiol. 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.C.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Visanji, N.P.; Marras, C.; Hazrati, L.-N.; Liu, L.W.C.; Lang, A.E. Alimentary, my dear Watson? The challenges of enteric α-synuclein as a Parkinson’s disease biomarker. Mov. Disord. 2014, 29, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Bessho, S.; Grando, K.; Tükel, Ç. Microbiome or Infections: Amyloid-Containing Biofilms as a Trigger for Complex Human Diseases. Front. Immunol. 2021, 12, 638867. [Google Scholar] [CrossRef]
- Tükel, Ç.; Nishimori, J.H.; Wilson, R.P.; Winter, M.G.; Keestra, A.M.; Van Putten, J.P.M.; Bäumler, A.J. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell. Microbiol. 2010, 12, 1495–1505. [Google Scholar] [CrossRef]
- Tursi, S.A.; Tükel, Ç. Curli-containing enteric biofilms inside and out: Matrix composition, immune recognition, and disease implications. Microbiol. Mol. Biol. Rev. 2018, 82, e00028-18. [Google Scholar] [CrossRef]
- Noli, M.; Meloni, G.; Manca, P.; Cossu, D.; Palermo, M.; Sechi, L.A. HERV-W and Mycobacterium avium subspecies paratuberculosis Are at Play in Pediatric Patients at Onset of Type 1 Diabetes. Pathogens 2021, 10, 1135. [Google Scholar] [CrossRef]
- Woulfe, J.M.; Duke, R.; Middeldorp, J.M.; Stevens, S.; Vervoort, M.; Hashimoto, M.; Masliah, E.; Chan, P.; Di Monte, D.A.; Langston, J.W.; et al. Absence of elevated anti–α-synuclein and anti-EBV latent membrane protein antibodies in PD. Neurology 2002, 58, 1435. [Google Scholar] [CrossRef]
- Smith, L.M.; Schiess, M.C.; Coffey, M.P.; Klaver, A.C.; Loeffler, D.A. α-Synuclein and Anti-α-Synuclein Antibodies in Parkinson’s Disease, Atypical Parkinson Syndromes, REM Sleep Behavior Disorder, and Healthy Controls. PLoS ONE 2012, 7, e52285. [Google Scholar] [CrossRef]
- Yanamandra, K.; Gruden, M.A.; Casaite, V.; Meskys, R.; Forsgren, L.; Morozova-Roche, L.A. α-Synuclein Reactive Antibodies as Diagnostic Biomarkers in Blood Sera of Parkinson’s Disease Patients. PLoS ONE 2011, 6, e18513. [Google Scholar] [CrossRef]
- Li, Q.-X.; Mok, S.S.; Laughton, K.M.; McLean, C.A.; Cappai, R.; Masters, C.L.; Culvenor, J.G.; Horne, M.K. Plasma α-synuclein is decreased in subjects with Parkinson’s disease. Exp. Neurol. 2007, 204, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 6, 1469–1480. [Google Scholar] [CrossRef]
- Pachucki, R.J.; Corradetti, C.; Kohler, L.; Ghadiali, J.; Gallo, P.M.; Nicastro, L.; Tursi, S.A.; Gallucci, S.; Tükel, Ç.; Caricchio, R. Persistent Bacteriuria and Antibodies Recognizing Curli/eDNA Complexes From Escherichia coli Are Linked to Flares in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020, 72, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Makiura, N.; Ojima, M.; Kou, Y.; Furuta, N.; Okahashi, N.; Shizukuishi, S.; Amano, A. Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol. Immunol. 2008, 23, 348–351. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 2017, 66, 872–885. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimers disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kobayashi, T.; Ito, S.; Yokoyama, T.; Abe, A.; Murasawa, A.; Yoshie, H. Periodontal Treatment Decreases Levels of Antibodies to Porphyromonas gingivalis and Citrulline in Patients With Rheumatoid Arthritis and Periodontitis. J. Periodontol. 2013, 84, e74–e84. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, S.; Erre, G.L.; Cadoni, M.L.; Bo, M.; Sechi, L.A. Humoral Response to Microbial Biomarkers in Rheumatoid Arthritis Patients. J. Clin. Med. 2021, 10, 5153. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Links between atherosclerotic and periodontal disease. Exp. Mol. Pathol. 2016, 100, 220–235. [Google Scholar] [CrossRef]
- Deshpande, R.G.; Khan, M.; Genco, C.A. Invasion strategies of the oral pathogen Porphyromonas gingivalis: Implications for cardiovascular disease. Invasion Metastasis 1998, 18, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Zúñiga, J.; Muñoz, Y.; Melgar-Rodríguez, S.; More, J.; Bruna, B.; Lobos, P.; Monasterio, G.; Vernal, R.; Paula-Lima, A. Serotype b of Aggregatibacter actinomycetemcomitans triggers pro-inflammatory responses and amyloid beta secretion in hippocampal cells: A novel link between periodontitis and Alzheimer’s disease? J. Oral Microbiol. 2019, 11, 1586423. [Google Scholar] [CrossRef] [PubMed]
- Agostini, S.; Mancuso, R.; Costa, A.S.; Citterio, L.A.; Guerini, F.R.; Meloni, M.; Navarro, J.; Clerici, M. A Possible Role for HSV-1-Specific Humoral Response and PILRA rs1859788 Polymorphism in the Pathogenesis of Parkinson’s Disease. Vaccines 2021, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Mendonca, S.; Ruegger, P.M.; Kim, J.H.; Borneman, J.; Cantin, E.M. Herpes simplex virus infection, Acyclovir and IVIG treatment all independently cause gut dysbiosis. PLoS ONE 2020, 15, e0237189. [Google Scholar] [CrossRef] [PubMed]
- Woulfe, J.; Gray, M.T.; Ganesh, M.S.; Middeldorp, J.M. Human serum antibodies against EBV latent membrane protein 1 cross-react with α-synuclein. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e239. [Google Scholar] [CrossRef]
- Woulfe, J.M.; Gray, M.T.; Gray, D.A.; Munoz, D.G.; Middeldorp, J.M. Hypothesis: A role for EBV-induced molecular mimicry in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef]
- Mameli, G.; Cocco, E.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Epstein Barr Virus and Mycobacterium avium subsp. paratuberculosis peptides are recognized in sera and cerebrospinal fluid of MS patients. Sci. Rep. 2016, 6, 22401. [Google Scholar] [CrossRef] [PubMed]
| Epitope Sequence | Epitope Position | |
|---|---|---|
| α-syn100–114 | LGKNEEGAPQEGILE | 100–114 |
| Curli133–141 | NSSVNVTQV | 133–141 |
| UI4222–36 | LGQPEEGAPCQVVLQ | 22–36 |
| RgpA800–812 | ADPVVTTQNIIVT | 800–812 |
| Kpg328–339 | VTDLYYSAVDGD | 328–339 |
| LtxA1429–445 | AWENKYGKNTFENGYDA | 429–445 |
| LtxA264–80 | TALIKAAQKLGIEVYHE | 64–80 |
| MAP3865c125–133 | MIAVALAGL | 125–133 |
| MAP1,4-a-gbp157–173 | GTVELLGGPLAHPFQPL | 157–173 |
| MAP_402718–32 | AVVPVLAYAAARLL | 18–32 |
| EBNA1400–413 | PGRRPFFHPVGEAD | 400–413 |
| BOLF1305–320 | AAVPVLAFDAARLRLLE | 305–320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasemi, S.; Paulus, K.; Noli, M.; Simula, E.R.; Ruberto, S.; Sechi, L.A. Antibodies against HSV-1 and Curli Show the Highest Correlation in Parkinson’s Disease Patients in Comparison to Healthy Controls. Int. J. Mol. Sci. 2022, 23, 14816. https://doi.org/10.3390/ijms232314816
Jasemi S, Paulus K, Noli M, Simula ER, Ruberto S, Sechi LA. Antibodies against HSV-1 and Curli Show the Highest Correlation in Parkinson’s Disease Patients in Comparison to Healthy Controls. International Journal of Molecular Sciences. 2022; 23(23):14816. https://doi.org/10.3390/ijms232314816
Chicago/Turabian StyleJasemi, Seyedesomaye, Kai Paulus, Marta Noli, Elena Rita Simula, Stefano Ruberto, and Leonardo A. Sechi. 2022. "Antibodies against HSV-1 and Curli Show the Highest Correlation in Parkinson’s Disease Patients in Comparison to Healthy Controls" International Journal of Molecular Sciences 23, no. 23: 14816. https://doi.org/10.3390/ijms232314816
APA StyleJasemi, S., Paulus, K., Noli, M., Simula, E. R., Ruberto, S., & Sechi, L. A. (2022). Antibodies against HSV-1 and Curli Show the Highest Correlation in Parkinson’s Disease Patients in Comparison to Healthy Controls. International Journal of Molecular Sciences, 23(23), 14816. https://doi.org/10.3390/ijms232314816







