Role of Transposable Elements in Genome Stability: Implications for Health and Disease
Abstract
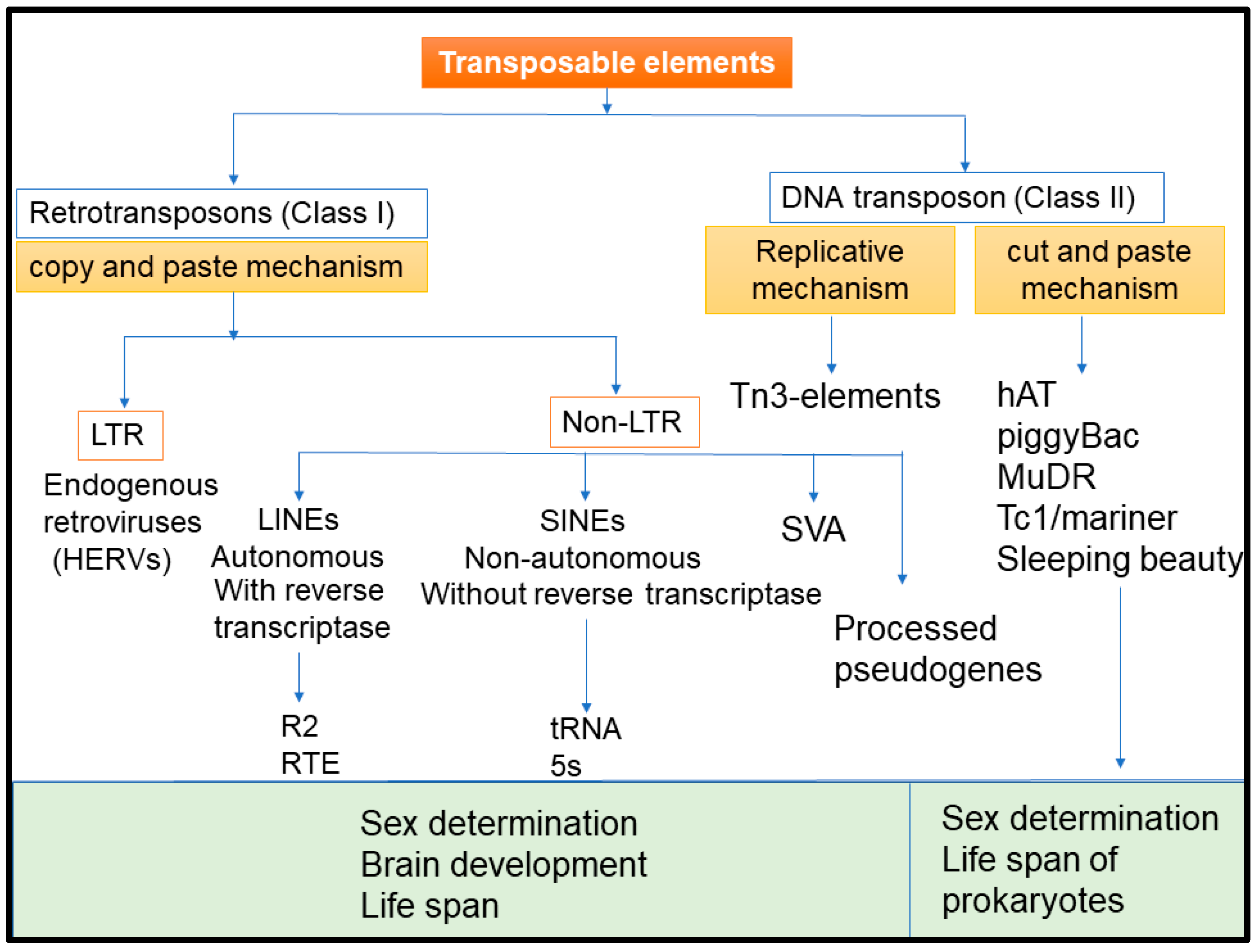
1. Introduction
2. Transposable Element Induced Genomic/Epigenomic Instability and Tumorogenesis
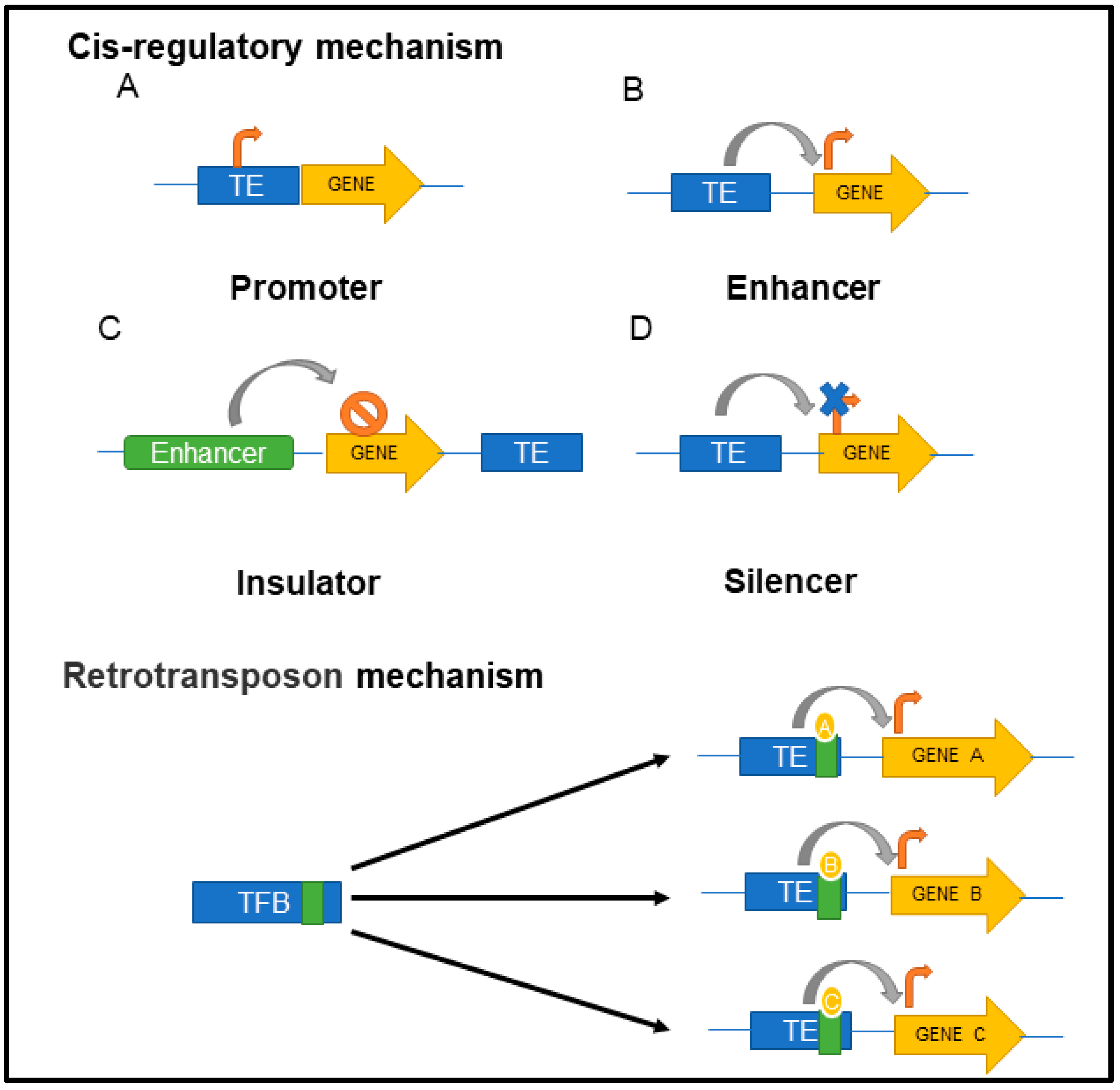
3. Physiological Functions of TEs in the Host Cells
4. Regulation of TEs in the Host Cell
4.1. DNA Methylation
4.2. Histone Modifications
4.3. Silencing of Retroelements in Germ Cells
4.4. Silencing of TEs by miRNA and Other Mechanisms
5. TEs Associated with Other Diseases
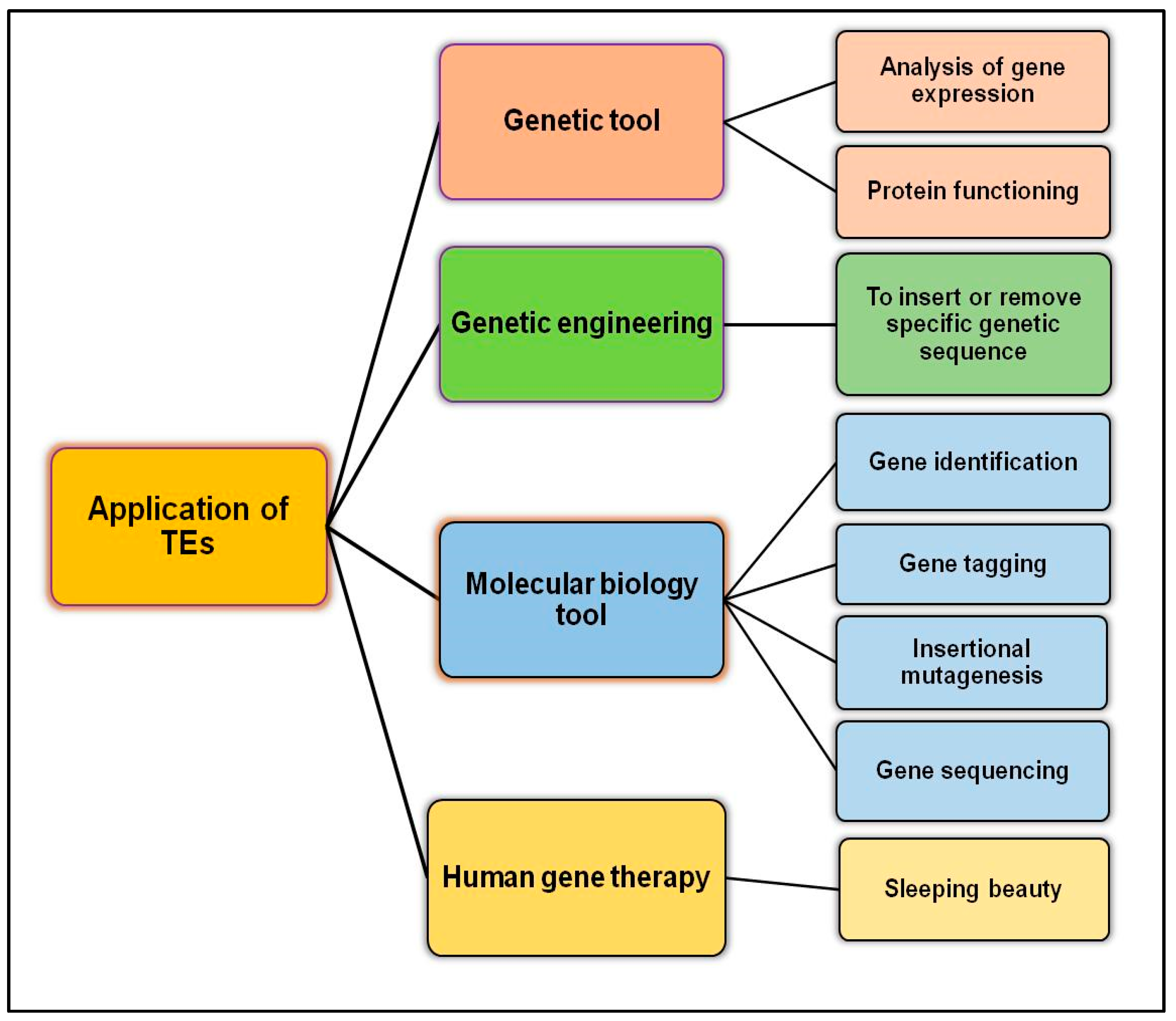
6. Futuristic Therapeutics Involving TEs
7. Clinical Trial
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClintock, B. The origin and behavior of mutable loci in maize. Proc. Natl. Acad. Sci. USA 1950, 36, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.E.; Bennett, E.A.; Iskow, R.C.; Devine, S.E. Which transposable elements are active in the human genome? Trends Genet. 2007, 23, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Munoz-Lopez, M.; Garcia-Perez, J.L. DNA transposons: Nature and applications in genomics. Curr. Genom. 2010, 11, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.K. Human transposable elements in Repbase: Genomic footprints from fish to humans. Mob. DNA 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.K., 2nd; Feschotte, C. The evolutionary history of human DNA transposons: Evidence for intense activity in the primate lineage. Genome Res. 2007, 17, 422–432. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.; Han, K. Transposable Elements: No More ‘Junk DNA’. Genom. Inform. 2012, 10, 226–233. [Google Scholar] [CrossRef]
- Belancio, V.P.; Hedges, D.J.; Deininger, P. Mammalian non-LTR retrotransposons: For better or worse, in sickness and in health. Genome Res. 2008, 18, 343–358. [Google Scholar] [CrossRef]
- Chen, J.M.; Stenson, P.D.; Cooper, D.N.; Ferec, C. A systematic analysis of LINE-1 endonuclease-dependent retrotranspositional events causing human genetic disease. Hum. Genet. 2005, 117, 411–427. [Google Scholar] [CrossRef]
- Deininger, P.L.; Batzer, M.A. Alu repeats and human disease. Mol. Genet. Metab. 1999, 67, 183–193. [Google Scholar] [CrossRef]
- Kazazian, H.H.; Wong, C.; Youssoufian, H.; Scott, A.F.; Phillips, D.G.; Antonarakis, S.E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature 1988, 332, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Ardeljan, D.; Taylor, M.S.; Ting, D.T.; Burns, K.H. The Human Long Interspersed Element-1 Retrotransposon: An Emerging Biomarker of Neoplasia. Clin. Chem. 2017, 63, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Kazazian, H.H., Jr. Mobile elements: Drivers of genome evolution. Science 2004, 303, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Belancio, V.P.; Deininger, P.L.; Roy-Engel, A.M. LINE dancing in the human genome: Transposable elements and disease. Genome Med. 2009, 1, 97. [Google Scholar] [CrossRef]
- Guffanti, G.; Gaudi, S.; Fallon, J.H.; Sobell, J.; Potkin, S.G.; Pato, C.; Macciardi, F. Transposable elements and psychiatric disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014, 165, 201–216. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukhopadhyay, A.; Banerjee, D.; Chandak, G.R.; Ray, K. Molecular pathology of haemophilia B: Identification of five novel mutations including a LINE 1 insertion in Indian patients. Haemophilia 2004, 10, 259–263. [Google Scholar] [CrossRef]
- Tighe, P.J.; Stevens, E.S.; Dempsey, S.; Le Deist, F.; Rieux-Laucat, F.; Edgar, J.D.M. Inactivation of the Fas gene by Alu insertion: Retrotransposition in an intron causing splicing variation and autoimmune lymphoproliferative syndrome. Genes Immun. 2002, 3 (Suppl. S1), S66–S70. [Google Scholar] [CrossRef]
- Wallace, M.R.; Andersen, L.B.; Saulino, A.M.; Gregory, P.E.; Glover, T.W.; Collins, F.S. A de novo Alu insertion results in neurofibromatosis type 1. Nature 1991, 353, 864–866. [Google Scholar] [CrossRef]
- Miki, Y.; Katagiri, T.; Kasumi, F.; Yoshimoto, T.; Nakamura, Y. Mutation analysis in the BRCA2 gene in primary breast cancers. Nat. Genet. 1996, 13, 245–247. [Google Scholar] [CrossRef]
- Miki, Y.; Nishisho, I.; Horii, A.; Miyoshi, Y.; Utsunomiya, J.; Kinzler, K.W.; Vogelstein, B.; Nakamura, Y. Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res. 1992, 52, 643–645. [Google Scholar]
- Molaro, A.; Malik, H.S. Hide and seek: How chromatin-based pathways silence retroelements in the mammalian germline. Curr. Opin. Genet. Dev. 2016, 37, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hancks, D.C.; Kazazian, H.H., Jr. Roles for retrotransposon insertions in human disease. Mob. DNA 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, J.; Theurkauf, W.; Weng, Z. TEMP: A computational method for analyzing transposable element polymorphism in populations. Nucleic Acids Res. 2014, 42, 6826–6838. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, S.L.; Su, S.; Dong, X.; Zappia, L.; Ritchie, M.E.; Gouil, Q. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 2020, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rishishwar, L.; Marino-Ramirez, L.; Jordan, I.K. Human population-specific gene expression and transcriptional network modification with polymorphic transposable elements. Nucleic Acids Res. 2017, 45, 2318–2328. [Google Scholar] [CrossRef]
- Hedges, D.J.; Deininger, P.L. Inviting instability: Transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat. Res. 2007, 616, 46–59. [Google Scholar] [CrossRef]
- Lerman, D.N.; Feder, M.E. Naturally occurring transposable elements disrupt hsp70 promoter function in Drosophila melanogaster. Mol. Biol. Evol. 2005, 22, 776–783. [Google Scholar] [CrossRef]
- Prak, E.T.; Kazazian, H.H., Jr. Mobile elements and the human genome. Nat. Rev. Genet. 2000, 1, 134–144. [Google Scholar] [CrossRef]
- Henssen, A.G.; Koche, R.; Zhuang, J.; Jiang, E.; Reed, C.; Eisenberg, A.; Still, E.; MacArthur, I.C.; Rodríguez-Fos, E.; Gonzalez, S.; et al. PGBD5 promotes site-specific oncogenic mutations in human tumors. Nat. Genet. 2017, 49, 1005–1014. [Google Scholar] [CrossRef]
- Rommel, P.C.; Oliveira, T.Y.; Nussenzweig, M.C.; Robbiani, D.F. RAG1/2 induces genomic insertions by mobilizing DNA into RAG1/2-independent breaks. J. Exp. Med. 2017, 214, 815–831. [Google Scholar] [CrossRef]
- Majumdar, S.; Singh, A.; Rio, D.C. The human THAP9 gene encodes an active P-element DNA transposase. Science 2013, 339, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Gasior, S.L.; Wakeman, T.P.; Xu, B.; Deininger, P.L. The human LINE-1 retrotransposon creates DNA double-strand breaks. J. Mol. Biol. 2006, 357, 1383–1393. [Google Scholar] [CrossRef]
- Han, K.; Sen, S.K.; Wang, J.; Callinan, P.A.; Lee, J.; Cordaux, R.; Liang, P.; Batzer, M.A. Genomic rearrangements by LINE-1 insertion-mediated deletion in the human and chimpanzee lineages. Nucleic Acids Res. 2005, 33, 4040–4052. [Google Scholar] [CrossRef]
- Gilbert, N.; Lutz-Prigge, S.; Moran, J.V. Genomic deletions created upon LINE-1 retrotransposition. Cell 2002, 110, 315–325. [Google Scholar] [CrossRef]
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Belancio, V.P.; Hedges, D.J.; Deininger, P. LINE-1 RNA splicing and influences on mammalian gene expression. Nucleic Acids Res. 2006, 34, 1512–1521. [Google Scholar] [CrossRef]
- Lev-Maor, G.; Ram, O.; Kim, O.; Sela, N.; Goren, A.; Levanon, E.Y.; Ast, G. Intronic Alus influence alternative splicing. PLoS Genet. 2008, 4, e1000204. [Google Scholar] [CrossRef]
- Landry, J.R.; Medstrand, P.; Mager, D.L. Repetitive elements in the 5′ untranslated region of a human zinc-finger gene modulate transcription and translation efficiency. Genomics 2001, 76, 110–116. [Google Scholar] [CrossRef]
- Konkel, M.K.; Batzer, M.A. A mobile threat to genome stability: The impact of non-LTR retrotransposons upon the human genome. Semin. Cancer Biol. 2010, 20, 211–221. [Google Scholar] [CrossRef]
- Hsu, P.S.; Yu, S.-H.; Tsai, Y.-T.; Chang, J.-Y.; Tsai, L.-K.; Ye, C.-H.; Song, N.-Y.; Yao, L.-C.; Lin, S.-P. More than causing (epi)genomic instability: Emerging physiological implications of transposable element modulation. J. Biomed. Sci. 2021, 28, 58. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, G.; Molloy, P.L.; Jones, P.A. DNA methylation enables transposable element-driven genome expansion. Proc. Natl. Acad. Sci. USA 2020, 117, 19359–19366. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.; Bacolla, A.; Zhao, J.; Spitser, S.; Vasquez, K.M. Short Inverted Repeats Are Hotspots for Genetic Instability: Relevance to Cancer Genomes. Cell Rep. 2015, 10, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Voineagu, I.; Narayanan, V.; Lobachev, K.S.; Mirkin, S.M. Replication stalling at unstable inverted repeats: Interplay between DNA hairpins and fork stabilizing proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 9936–9941. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Lee, J.; Meyer, T.J.; Remedios, P.; Goodwin, L.; Batzer, M.A. L1 recombination-associated deletions generate human genomic variation. Proc. Natl. Acad. Sci. USA 2008, 105, 19366–19371. [Google Scholar] [CrossRef]
- Sen, S.K.; Han, K.; Wang, J.; Lee, J.; Wang, H.; Callinan, P.A.; Dyer, M.; Cordaux, R.; Liang, P.; Batzer, M.A. Human genomic deletions mediated by recombination between Alu elements. Am. J. Hum. Genet. 2006, 79, 41–53. [Google Scholar] [CrossRef]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef]
- Bojang, P., Jr.; Ramos, K.S. Epigenetic reactivation of LINE-1 retrotransposon disrupts NuRD corepressor functions and induces oncogenic transformation in human bronchial epithelial cells. Mol. Oncol. 2018, 12, 1342–1357. [Google Scholar] [CrossRef]
- Teugels, E.; De Brakeleer, S.; Goelen, G.; Lissens, W.; Sermijn, E.; De Grève, J. De novo Alu element insertions targeted to a sequence common to the BRCA1 and BRCA2 genes. Hum. Mutat. 2005, 26, 284. [Google Scholar] [CrossRef]
- Rodríguez-Martín, C.; Cidre, F.; Fernández-Teijeiro, A.; Gómez-Mariano, G.; De La Vega, L.; Ramos, P.; Zaballos, A.; Monzón, S.; Alonso, J. Familial retinoblastoma due to intronic LINE-1 insertion causes aberrant and noncanonical mRNA splicing of the RB1 gene. J. Hum. Genet. 2016, 61, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Daskalos, A.; Nikolaidis, G.; Xinarianos, G.; Savvari, P.; Cassidy, A.; Zakopoulou, R.; Kotsinas, A.; Gorgoulis, V.; Field, J.K.; Liloglou, T. Hypomethylation of retrotransposable elements correlates with genomic instability in non-small cell lung cancer. Int. J. Cancer 2009, 124, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.L.; Wulaningsih, W.; Lehmann, U. Transposable Elements in Human Cancer: Causes and Consequences of Deregulation. Int. J. Mol. Sci. 2017, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Na Seo, A.; Jung, H.Y.; Gwak, J.M.; Jung, N.; Cho, N.-Y.; Kang, G.H. Alu and LINE-1 hypomethylation is associated with HER2 enriched subtype of breast cancer. PLoS ONE 2014, 9, e100429. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, E.M.; Aispuro, I.; Tavera-Garcia, M.A.; Field, M.; Moore, S.; Ramos, I.; Ramos, K.S. LINE-1 couples EMT programming with acquisition of oncogenic phenotypes in human bronchial epithelial cells. Oncotarget 2017, 8, 103828–103842. [Google Scholar] [CrossRef] [PubMed]
- Suter, C.M.; Martin, D.I.; Ward, R.L. Hypomethylation of L1 retrotransposons in colorectal cancer and adjacent normal tissue. Int. J. Colorectal Dis. 2004, 19, 95–101. [Google Scholar] [CrossRef]
- Wilson, A.S.; Power, B.E.; Molloy, P.L. DNA hypomethylation and human diseases. Biochim. Biophys. Acta 2007, 1775, 138–162. [Google Scholar] [CrossRef]
- Ramos, K.S.; Bojang, P.; Bowers, E. Role of long interspersed nuclear element-1 in the regulation of chromatin landscapes and genome dynamics. Exp. Biol. Med. 2021, 246, 2082–2097. [Google Scholar] [CrossRef]
- Yoder, J.A.; Walsh, C.P.; Bestor, T.H. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997, 13, 335–340. [Google Scholar] [CrossRef]
- Dupressoir, A.; Lavialle, C.; Heidmann, T. From ancestral infectious retroviruses to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta 2012, 33, 663–671. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Wen, F.; Yang, F.; Li, X.; Geng, D.; Li, L.; Chen, J.; Zheng, J. Upregulation of syncytin-1 promotes invasion and metastasis by activating epithelial-mesenchymal transition-related pathway in endometrial carcinoma. Oncotargets Ther. 2019, 12, 31–40. [Google Scholar] [CrossRef]
- Larsson, L.I.; Holck, S.; Christensen, I.J. Prognostic role of syncytin expression in breast cancer. Hum. Pathol. 2007, 38, 726–731. [Google Scholar] [CrossRef]
- Sun, Y.; Ouyang, D.-Y.; Pang, W.; Tu, Y.-Q.; Li, Y.-Y.; Shen, X.-M.; Tam, S.C.; Yang, H.-Y.; Zheng, Y.-T. Expression of syncytin in leukemia and lymphoma cells. Leuk. Res. 2010, 34, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Christensen, I.J.; Nielsen, H.J.; Hansen, U.; Bjerregaard, B.; Talts, J.F.; Larsson, L.-I. Syncytin immunoreactivity in colorectal cancer: Potential prognostic impact. Cancer Lett. 2009, 280, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Bannert, N.; Hofmann, H.; Block, A.; Hohn, O. HERVs New Role in Cancer: From Accused Perpetrators to Cheerful Protectors. Front. Microbiol. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, M.; Wei, Y.; Lin, K.; Lu, Y.; Shen, J.; Johanning, G.L.; Wang-Johanning, F. Activation of HERV-K Env protein is essential for tumorigenesis and metastasis of breast cancer cells. Oncotarget 2016, 7, 84093–84117. [Google Scholar] [CrossRef] [PubMed]
- Galli, U.M.; Sauter, M.; Lecher, B.; Maurer, S.; Herbst, H.; Roemer, K.; Mueller-Lantzsch, N. Human endogenous retrovirus rec interferes with germ cell development in mice and may cause carcinoma in situ, the predecessor lesion of germ cell tumors. Oncogene 2005, 24, 3223–3228. [Google Scholar] [CrossRef]
- Hirsch, C.D.; Springer, N.M. Transposable element influences on gene expression in plants. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 157–165. [Google Scholar] [CrossRef]
- Burns, K.H. Transposable elements in cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Satish, L.; Kalendar, R.; Narayanan, M.; Kandasamy, S.; Sharma, A.; Emamverdian, A.; Wei, Q.; Zhou, M. The Dynamism of Transposon Methylation for Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2021, 22, 11387. [Google Scholar] [CrossRef]
- Lié, O.; Renault, S.; Augé-Gouillou, C. SETMAR, a case of primate co-opted genes: Towards new perspectives. Mob. DNA 2022, 13, 9. [Google Scholar] [CrossRef]
- Lee, S.-H.; Oshige, M.; Durant, S.T.; Rasila, K.K.; Williamson, E.A.; Ramsey, H.; Kwan, L.; Nickoloff, J.A.; Hromas, R. The SET domain protein Metnase mediates foreign DNA integration and links integration to nonhomologous end-joining repair. Proc. Natl. Acad. Sci. USA 2005, 102, 18075–18080. [Google Scholar] [CrossRef]
- Miao, B.; Fu, S.; Lyu, C.; Gontarz, P.; Wang, T.; Zhang, B. Tissue-specific usage of transposable element-derived promoters in mouse development. Genome Biol. 2020, 21, 255. [Google Scholar] [CrossRef] [PubMed]
- Nikolaienko, O.; Patil, S.; Eriksen, M.S.; Bramham, C.R. Arc protein: A flexible hub for synaptic plasticity and cognition. Semin. Cell Dev. Biol. 2018, 77, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Petri, R.; Brattås, P.L.; Sharma, Y.; Jönsson, M.E.; Pircs, K.; Bengzon, J.; Jakobsson, J. LINE-2 transposable elements are a source of functional human microRNAs and target sites. PLoS Genet. 2019, 15, e1008036. [Google Scholar] [CrossRef]
- Fort, V.; Khelifi, G.; Hussein, S.M.I. Long non-coding RNAs and transposable elements: A functional relationship. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118837. [Google Scholar] [CrossRef]
- Grandi, N.; Tramontano, E. Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses. Front. Immunol. 2018, 9, 2039. [Google Scholar] [CrossRef] [PubMed]
- Reik, W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 2007, 447, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Guelen, L.; Pagie, L.; Brasset, E.; Meuleman, W.; Faza, M.B.; Talhout, W.; Eussen, B.H.; de Klein, A.; Wessels, L.; de Laat, W.; et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 2008, 453, 948–951. [Google Scholar] [CrossRef]
- Martens, J.; O’Sullivan, R.J.; Braunschweig, U.; Opravil, S.; Radolf, M.; Steinlein, P.; Jenuwein, T. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 2005, 24, 800–812. [Google Scholar] [CrossRef]
- Göke, J.; Lu, X.; Chan, Y.-S.; Ng, H.-H.; Ly, L.-H.; Sachs, F.; Szczerbinska, I. Dynamic transcription of distinct classes of endogenous retroviral elements marks specific populations of early human embryonic cells. Cell Stem Cell 2015, 16, 135–141. [Google Scholar] [CrossRef]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Supe.erior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Egger, G.; Jeong, S.; Escobar, S.G.; Cortez, C.C.; Li, T.W.H.; Saito, Y.; Yoo, C.B.; Jones, P.A.; Liang, G. Identification of DNMT1 (DNA methyltransferase 1) hypomorphs in somatic knockouts suggests an essential role for DNMT1 in cell survival. Proc. Natl. Acad. Sci. USA 2006, 103, 14080–14085. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.V.; Dunleavy, E.; Almouzni, G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 2009, 10, 192–206. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fu, X.; Zhang, M.; He, F.; Li, W.; Abdul, M.; Zhou, J.; Sun, L.; Chang, C.; Li, Y.; et al. Transposable elements are regulated by context-specific patterns of chromatin marks in mouse embryonic stem cells. Nat. Commun. 2019, 10, 34. [Google Scholar] [CrossRef]
- Bourc’his, D.; Bestor, T.H. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 2004, 431, 96–99. [Google Scholar] [CrossRef]
- Zamudio, N.; Barau, J.; Teissandier, A.; Walter, M.; Borsos, M.; Servant, N.; Bourc’his, D. DNA methylation restrains transposons from adopting a chromatin signature permissive for meiotic recombination. Genes Dev. 2015, 29, 1256–1270. [Google Scholar] [CrossRef]
- Bestor, T.H. DNA methylation: Evolution of a bacterial immune function into a regulator of gene expression and genome structure in higher eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 326, 179–187. [Google Scholar]
- Rose, N.R.; Klose, R.J. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim. Biophys. Acta 2014, 1839, 1362–1372. [Google Scholar] [CrossRef]
- Walter, M.; Teissandier, A.; Perez-Palacios, R.; Bourc’his, D. An epigenetic switch ensures transposon repression upon dynamic loss of DNA methylation in embryonic stem cells. eLife 2016, 5, e11418. [Google Scholar] [CrossRef]
- Rothbart, S.B.; Krajewski, K.; Nady, N.; Tempel, W.; Xue, S.; I Badeaux, A.; Barsyte-Lovejoy, D.; Martínez-Márquez, J.; Bedford, M.T.; Fuchs, S.; et al. Association of UHRF1 with methylated H3K9 directs the maintenance of DNA methylation. Nat. Struct. Mol. Biol. 2012, 19, 1155–1160. [Google Scholar] [CrossRef]
- Jacobs, F.M.; Greenberg, D.; Nguyen, N.; Haeussler, M.; Ewing, A.D.; Katzman, S.; Paten, B.; Salama, S.R.; Haussler, D. An evolutionary arms race between KRAB zinc-finger genes ZNF91/93 and SVA/L1 retrotransposons. Nature 2014, 516, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Quenneville, S.; Verde, G.; Corsinotti, A.; Kapopoulou, A.; Jakobsson, J.; Offner, S.; Baglivo, I.; Pedone, B.V.; Grimaldi, G.; Riccio, A.; et al. In embryonic stem cells, ZFP57/KAP1 recognize a methylated hexanucleotide to affect chromatin and DNA methylation of imprinting control regions. Mol. Cell 2011, 44, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Matsui, Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat. Rev. Genet. 2008, 9, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P.; Stein, P.; Anger, M.; Bernstein, E.; Hannon, G.J.; Schultz, R.M. RNAi and expression of retrotransposons MuERV-L and IAP in preimplantation mouse embryos. Dev. Biol. 2004, 269, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Kabayama, Y.; Toh, H.; Katanaya, A.; Sakurai, T.; Chuma, S.; Kuramochi-Miyagawa, S.; Saga, Y.; Nakano, T.; Sasaki, H. Roles of MIWI, MILI and PLD6 in small RNA regulation in mouse growing oocytes. Nucleic Acids Res. 2017, 45, 5387–5398. [Google Scholar] [CrossRef]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef]
- Aravin, A.A.; Sachidanandam, R.; Bourc’His, D.; Schaefer, C.; Pezic, D.; Toth, K.F.; Bestor, T.; Hannon, G.J. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell 2008, 31, 785–799. [Google Scholar] [CrossRef]
- Carmell, M.A.; Girard, A.; van de Kant, H.J.; Bourc’His, D.; Bestor, T.H.; de Rooij, D.G.; Hannon, G.J. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 2007, 12, 503–514. [Google Scholar] [CrossRef]
- Ernst, C.; Odom, D.T.; Kutter, C. The emergence of piRNAs against transposon invasion to preserve mammalian genome integrity. Nat. Commun. 2017, 8, 1411. [Google Scholar] [CrossRef]
- Molaro, A.; Falciatori, I.; Hodges, E.; Aravin, A.A.; Marran, K.; Rafii, S.; McCombie, W.R.; Smith, A.D.; Hannon, G.J. Two waves of de novo methylation during mouse germ cell development. Genes Dev. 2014, 28, 1544–1549. [Google Scholar] [CrossRef]
- Zoch, A.; Auchynnikava, T.; Berrens, R.V.; Kabayama, Y.; Schopp, T.; Heep, M.; Vasiliauskaite, L.; Perez-Rico, Y.A.; Cook, A.G.; Shkumatava, A.; et al. SPOCD1 is an essential executor of piRNA-directed de novo DNA methylation. Nature 2020, 584, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Cui, X.; Yuan, Z.; Qi, H.; Lin, H. MIWI2 targets RNAs transcribed from piRNA-dependent regions to drive DNA methylation in mouse prospermatogonia. EMBO J. 2018, 37, e95329. [Google Scholar] [CrossRef] [PubMed]
- Berrens, R.V.; Andrews, S.; Spensberger, S.; Santos, F.; Dean, W.; Gould, P.; Sharif, J.; Olova, N.; Chandra, T.; Koseki, H.; et al. An endosiRNA-Based Repression Mechanism Counteracts Transposon Activation during Global DNA Demethylation in Embryonic Stem Cells. Cell Stem Cell 2017, 21, 694–703.e7. [Google Scholar] [CrossRef] [PubMed]
- Hamdorf, M.; Idica, A.; Zisoulis, D.G.; Gamelin, L.; Martin, C.; Sanders, K.J.; Pedersen, I.M. miR-128 represses L1 retrotransposition by binding directly to L1 RNA. Nat. Struct. Mol. Biol. 2015, 22, 824–831. [Google Scholar] [CrossRef]
- Marchetto, M.C.N.; Narvaiza, I.; Denli, A.M.; Benner, C.; Lazzarini, T.; Nathanson, J.L.; Paquola, A.C.M.; Desai, K.N.; Herai, R.; Weitzman, M.D.; et al. Differential L1 regulation in pluripotent stem cells of humans and apes. Nature 2013, 503, 525–529. [Google Scholar] [CrossRef]
- Volkmann, B.; Wittmann, S.; Lagisquet, J.; Deutschmann, J.; Eissmann, K.; Ross, J.J.; Biesinger, B.; Gramberg, T. Human TRIM5alpha senses and restricts LINE-1 elements. Proc. Natl. Acad. Sci. USA 2020, 117, 17965–17976. [Google Scholar] [CrossRef]
- Kassiotis, G. Endogenous retroviruses and the development of cancer. J. Immunol. 2014, 192, 1343–1349. [Google Scholar] [CrossRef]
- Lower, R.; Lower, J.; Kurth, R. The viruses in all of us: Characteristics and biological significance of human endogenous retrovirus sequences. Proc. Natl. Acad. Sci. USA 1996, 93, 5177–5184. [Google Scholar] [CrossRef]
- He, Q.; Kim, H.; Huang, R.; Lu, W.; Tang, M.; Shi, F.; Yang, D.; Zhang, X.; Huang, J.; Liu, D.; et al. The Daxx/Atrx Complex Protects Tandem Repetitive Elements during DNA Hypomethylation by Promoting H3K9 Trimethylation. Cell Stem Cell 2015, 17, 273–286. [Google Scholar] [CrossRef]
- Yu, W.; McIntosh, C.; Lister, R.; Zhu, I.; Han, Y.; Ren, J.; Landsman, D.; Lee, E.; Briones, V.; Terashima, M.; et al. Genome-wide DNA methylation patterns in LSH mutant reveals de-repression of repeat elements and redundant epigenetic silencing pathways. Genome Res. 2014, 24, 1613–1623. [Google Scholar] [CrossRef]
- Fukuda, K.; Shinkai, Y. SETDB1-Mediated Silencing of Retroelements. Viruses 2020, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Pandita, R.K.; Hambarde, S.; Mattoo, A.R.; Charaka, V.; Ahmed, K.M.; Iyer, S.P.; Hunt, C.R.; Pandita, T.K. SMARCAD1 Phosphorylation and Ubiquitination Are Required for Resection during DNA Double-Strand Break Repair. iScience 2018, 2, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Singh, M.; Pandita, R.K.; Singh, V.; Lo, C.S.; Leonard, F.; Horikoshi, N.; Moros, E.G.; Guha, D.; Hunt, C.R.; et al. Heat-induced SIRT1-mediated H4K16ac deacetylation impairs resection and SMARCAD1 recruitment to double strand breaks. iScience 2022, 25, 104142. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, A.; Marconi, S.; Specchia, V.; Bozzetti, M.P.; Ivics, Z.; Caizzi, R.; Marsano, R.M. Functional characterization of the Bari1 transposition system. PLoS ONE 2013, 8, e79385. [Google Scholar] [CrossRef]
- Callinan, P.A.; Batzer, M.A. Retrotransposable elemen.nts and human disease. Genome Dyn. 2006, 1, 104–115. [Google Scholar]
- Payer, L.M.; Burns, K.H. Transposable elements in human genetic disease. Nat. Rev. Genet. 2019, 20, 760–772. [Google Scholar] [CrossRef]
- Ayarpadikannan, S.; Kim, H.S. The impact of transposable elements in genome evolution and genetic instability and their implications in various diseases. Genom. Inform. 2014, 12, 98–104. [Google Scholar] [CrossRef]
- Babaian, A.; Mager, D.L. Endogenous retroviral promoter exaptation in human cancer. Mob. DNA 2016, 7, 24. [Google Scholar] [CrossRef]
- Kapusta, A.; Kronenberg, Z.; Lynch, V.; Zhuo, X.; Ramsay, L.; Bourque, G.; Yandell, M.; Feschotte, C. Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet. 2013, 9, e1003470. [Google Scholar] [CrossRef]
- Scott, E.C.; Gardner, E.; Masood, A.; Chuang, N.T.; Vertino, P.M.; Devine, S.E. A hot L1 retrotransposon evades somatic repression and initiates human colorectal cancer. Genome Res. 2016, 26, 745–755. [Google Scholar] [CrossRef]
- Kondo-Iida, E.; Kobayashi, K.; Watanabe, M.; Sasaki, J.; Kumagai, T.; Koide, H.; Saito, K.; Osawa, M.; Nakamura, Y.; Toda, T. Novel mutations and genotype-phenotype relationships in 107 families with Fukuyama-type congenital muscular dystrophy (FCMD). Hum. Mol. Genet. 1999, 8, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Nakao, H.; Katoh, T.; Sasaki, H.; Hiroshima, M.; Tanaka, T.; Matsunaga, T.; Hanaoka, T.; Tsugane, S.; Ikenoue, T. Association between endometriosis and genetic polymorphisms of the estradiol-synthesizing enzyme genes HSD17B1 and CYP19. Hum. Reprod. 2005, 20, 974–978. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beck, C.R.; Garcia-Perez, J.L.; Badge, R.M.; Moran, J.V. LINE-1 elements in structural variation and disease. Annu. Rev. Genom. Hum. Genet. 2011, 12, 187–215. [Google Scholar] [CrossRef] [PubMed]
- Garay, I.M.; Ballesta, M.J.; Oltra, S.; Orellana, C.; Palomeque, A.; Molto, M.D.; Prieto, F.; Martinez, F. Intronic L1 insertion and F268S, novel mutations in RPS6KA3 (RSK2) causing Coffin-Lowry syndrome. Clin. Genet. 2003, 64, 491–496. [Google Scholar] [CrossRef]
- Miné, M.; Chen, J.-M.; Brivet, M.; Desguerre, I.; Marchant, D.; de Lonlay, P.; Bernard, A.; Férec, C.; Abitbol, M.; Ricquier, D.; et al. A large genomic deletion in the PDHX gene caused by the retrotranspositional insertion of a full-length LINE-1 element. Hum. Mutat. 2007, 28, 137–142. [Google Scholar] [CrossRef]
- Apoil, P.A.; Kuhlein, E.; Robert, A.; Rubie, H.; Blancher, A. HIGM syndrome caused by insertion of an AluYb8 element in exon 1 of the CD40LG gene. Immunogenetics 2007, 59, 17–23. [Google Scholar] [CrossRef]
- Claverie-Martín, F.; Flores, C.; Antón-Gamero, M.; González-Acosta, H.; García-Nieto, V. The Alu insertion in the CLCN5 gene of a patient with Dent’s disease leads to exon 11 skipping. J. Hum. Genet. 2005, 50, 370–374. [Google Scholar] [CrossRef]
- Cruickshanks, H.A.; Tufarelli, C. Isolation of cancer-specific chimeric transcripts induced by hypomethylation of the LINE-1 antisense promoter. Genomics 2009, 94, 397–406. [Google Scholar] [CrossRef]
- Chen, L.L.; Carmichael, G.G. Gene regulation by SINES and inosines: Biological consequences of A-to-I editing of Alu element inverted repeats. Cell Cycle 2008, 7, 3294–3301. [Google Scholar] [CrossRef]
- Ade, C.; Roy-Engel, A.M.; Deininger, P.L. Alu elements: An intrinsic source of human genome instability. Curr. Opin. Virol. 2013, 3, 639–645. [Google Scholar] [CrossRef]
- Zarnack, K.; König, J.; Tajnik, M.; Martincorena, I.; Eustermann, S.; Stévant, I.; Reyes, A.; Anders, S.; Luscombe, N.M.; Ule, J. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell 2013, 152, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Labuda, D.; Fontaine, G.; Saudubray, J.M.; Bonnefont, J.P.; Lyonnet, S.; Brody, L.C.; Steel, G.; Obie, C.; Valle, D. Splice-mediated insertion of an Alu sequence inactivates ornithine delta-aminotransferase: A role for Alu elements in human mutation. Proc. Natl. Acad. Sci. USA 1991, 88, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Murata, M.; Takagi, Y.; Kozuka, T.; Nakata, Y.; Hasebe, R.; Takagi, A.; Kitazawa, J.; Shima, M.; Kojima, M. SVA retrotransposition in exon 6 of the coagulation factor IX gene causing severe hemophilia B. Int. J. Hematol. 2015, 102, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Van der Klift, H.M.; Tops, C.M.; Hes, F.J.; Devilee, P.; Wijnen, J.T. Insertion of an SVA element, a nonautonomous retrotransposon, in PMS2 intron 7 as a novel cause of Lynch syndrome. Hum. Mutat. 2012, 33, 1051–1055. [Google Scholar] [CrossRef]
- Reilly, M.T.; Faulkner, G.J.; Dubnau, J.; Ponomarev, I.; Gage, F.H. The role of transposable elements in health and diseases of the central nervous system. J. Neurosci. 2013, 33, 17577–17586. [Google Scholar] [CrossRef] [PubMed]
- Gulland, A. Role model: Ian Nesbitt. BMJ 2018, 360, k881. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, B.; Cheng, Y.; Wang, Z.; Li, Y.; Chen, L.; Huang, L.; Zhang, W.; Chen, D.; Wu, H.; Tang, B.; et al. DNA N6-methyladenine is dynamically regulated in the mouse brain following environmental stress. Nat. Commun. 2017, 8, 1122. [Google Scholar] [CrossRef]
- Hunter, R.G.; Murakami, G.; Dewell, S.; Seligsohn, M.A.; Baker, M.E.; Datson, N.A.; McEwen, B.S.; Pfaff, D.W. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc. Natl. Acad. Sci. USA 2012, 109, 17657–17662. [Google Scholar] [CrossRef]
- Cappucci, U.; Torromino, G.; Casale, A.M.; Camon, J.; Capitano, F.; Berloco, M.; Mele, A.; Pimpinelli, S.; Rinaldi, A.; Piacentini, L. Stress-induced strain and brain region-specific activation of LINE-1 transposons in adult mice. Stress 2018, 21, 575–579. [Google Scholar] [CrossRef]
- Kerur, N.; Hirano, Y.; Tarallo, V.; Fowler, B.J.; Bastos-Carvalho, A.; Yasuma, T.; Yasuma, R.; Kim, Y.; Hinton, D.R.; Kirschning, C.J.; et al. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7395–7401. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, V.; Hirano, Y.; Gelfand, B.D.; Dridi, S.; Kerur, N.; Kim, Y.; Gil Cho, W.; Kaneko, H.; Fowler, B.J.; Bogdanovich, S.; et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 2012, 149, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Xu, J.; Shi, J.; Li, H.; Li, B. Molecular cloning and characterization of a novel glyoxalase I gene TaGly I in wheat (Triticum aestivum L.). Mol. Biol. Rep. 2010, 37, 729–735. [Google Scholar] [CrossRef]
- Rana, G.; Donizetti, A.; Virelli, G.; Piscopo, M.; Viggiano, E.; De Luca, B.; Fucci, L. Cortical spreading depression differentially affects lysine methylation of H3 histone at neuroprotective genes and retrotransposon sequences. Brain Res. 2012, 1467, 113–119. [Google Scholar] [CrossRef]
- Nelson, P.N.; Lever, A.M.L.; Smith, S.; Pitman, R.; Murray, P.; Perera, S.A.; Westwood, O.M.R.; Hay, F.C.; Ejtehadi, H.D.; Booth, J.C. Molecular investigations implicate human endogenous retroviruses as mediators of anti-retroviral antibodies in autoimmune rheumatic disease. Immunol. Investig. 1999, 28, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Krug, L.; Chatterjee, N.; Borges-Monroy, R.; Hearn, S.; Liao, W.-W.; Morrill, K.; Prazak, L.; Rozhkov, N.; Theodorou, D.; Hammell, M.; et al. Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLoS Genet. 2017, 13, e1006635. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Prazak, L.; Chatterjee, N.; Grüninger, S.; Krug, L.; Theodorou, D.; Dubnau, J. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat. Neurosci. 2013, 16, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lee, M.-H.; Henderson, L.; Tyagi, R.; Bachani, M.; Steiner, J.; Campanac, E.; Hoffman, D.A.; von Geldern, G.; Johnson, K.; et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci. Transl. Med. 2015, 7, 307ra153. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Bayam, E.; Cernilogar, F.; Bonapace, I.M.; Schulze, M.; Riemenschneider, M.J.; Schotta, G.; Götz, M. Loss of Uhrf1 in neural stem cells leads to activation of retroviral elements and delayed neurodegeneration. Genes Dev. 2016, 30, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Habibi, L.; Shokrgozar, M.A.; Tabrizi, M.; Modarressi, M.H.; Akrami, S.M. Mercury specifically induces LINE-1 activity in a human neuroblastoma cell line. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2014, 759, 9–20. [Google Scholar] [CrossRef]
- Hyland, K.A.; Aronovich, E.L.; Olson, E.R.; Bell, J.B.; Rusten, M.U.; Gunther, R.; Hunter, D.W.; Hackett, P.B.; McIvor, R.S. Transgene Expression in Dogs after Liver-Directed Hydrodynamic Delivery of Sleeping Beauty Transposons Using Balloon Catheters. Hum. Gene Ther. 2017, 28, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Aronovich, E.L.; Hyland, K.A.; Hall, B.C.; Bell, J.B.; Olson, E.R.; Rusten, M.U.; Hunter, D.W.; Ellinwood, N.M.; McIvor, R.S.; Hackett, P.B. Prolonged Expression of Secreted Enzymes in Dogs after Liver-Directed Delivery of Sleeping Beauty Transposons: Implications for Non-Viral Gene Therapy of Systemic Disease. Hum. Gene Ther. 2017, 28, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.F.; Zenthon, J.; Evans, E.P.; Burtenshaw, M.D.; Wareham, K.A.; Williams, E.D. Lack of inactivation of a mouse X-linked gene physically separated from the inactivation centre. J. Embryol. Exp. Morphol. 1986, 97, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.S.; Mavoungou, L.O.; Ronzoni, F.; Zemla, J.; Schmid-Siegert, E.; Antonini, S.; Neff, L.A.; Dorchies, O.M.; Jaconi, M.; Lekka, M.; et al. Autologous Cell Therapy Approach for Duchenne Muscular Dystrophy using PiggyBac Transposons and Mesoangioblasts. Mol. Ther. 2018, 26, 1093–1108. [Google Scholar] [CrossRef]
- Carlson, C.M.; Frandsen, J.L.; Kirchhof, N.; McIvor, R.S.; Largaespada, D.A. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc. Natl. Acad. Sci. USA 2005, 102, 17059–17064. [Google Scholar] [CrossRef]
- Zhao, Y.; Oreskovic, E.; Zhang, Q.; Lu, Q.; Gilman, A.; Lin, Y.S.; He, J.; Zheng, Z.; Lu, J.Y.; Lee, J.; et al. Transposon-triggered innate immune response confers cancer resistance to the blind mole rat. Nat. Immunol. 2021, 22, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Guffanti, G.; Bartlett, A.; DeCrescenzo, P.; Macciardi, F.; Hunter, R. Transposable Elements. Curr. Top. Behav. Neurosci. 2019, 42, 221–246. [Google Scholar]
- Moszczynska, A. Differential Responses of LINE-1 in the Dentate Gyrus, Striatum and Prefrontal Cortex to Chronic Neurotoxic Methamphetamine: A Study in Rat Brain. Genes 2020, 11, 364. [Google Scholar] [CrossRef]
- Valdebenito-Maturana, B.; Guatimosim, C.; Carrasco, M.A.; Tapia, J.C. Spatially Resolved Expression of Transposable Elements in Disease and Somatic Tissue with SpatialTE. Int. J. Mol. Sci. 2021, 22, 13623. [Google Scholar] [CrossRef]
- Padmanabhan Nair, V.; Liu, H.; Ciceri, G.; Jungverdorben, J.; Frishman, G.; Tchieu, J.; Cederquist, G.Y.; Rothenaigner, I.; Schorpp, H.; Klepper, L.; et al. Activation of HERV-K(HML-2) disrupts cortical patterning and neuronal differentiation by increasing NTRK3. Cell Stem Cell 2021, 28, 1566–1581.e8. [Google Scholar] [CrossRef]
- Ivics, Z.; Izsvak, Z. The expanding universe of transposon technologies for gene and cell engineering. Mob. DNA 2010, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Ivics, Z.; Izsvak, Z. Transposons for gene therapy! Curr. Gene Ther. 2006, 6, 593–607. [Google Scholar] [CrossRef] [PubMed]
- VandenDriessche, T.; Ivics, Z.; Izsvak, Z.; Chuah, M.K. Emerging potential of transposons for gene therapy and generation of induced pluripotent stem cells. Blood 2009, 114, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Hudecek, M.; Izsvák, Z.; Johnen, S.; Renner, M.; Thumann, G.; Ivics, Z. Going non-viral: The Sleeping Beauty transposon system breaks on through to the clinical side. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 355–380. [Google Scholar] [CrossRef]
- Ivics, Z.; Hackett, P.B.; Plasterk, R.H.; Izsvák, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997, 91, 501–510. [Google Scholar] [CrossRef]
- Peng, P.D.; Cohen, C.J.; Yang, S.; Hsu, C.; Jones, S.; Zhao, Y.; Zheng, Z.; A Rosenberg, S.; A Morgan, R. Efficient nonviral Sleeping Beauty transposon-based TCR gene transfer to peripheral blood lymphocytes confers antigen-specific antitumor reactivity. Gene Ther. 2009, 16, 1042–1049. [Google Scholar] [CrossRef]
- Chicaybam, L.; Sodré, A.L.; Bonamino, M. Chimeric antigen receptors in cancer immuno-gene therapy: Current status and future directions. Int. Rev. Immunol. 2011, 30, 294–311. [Google Scholar] [CrossRef]
- Kowolik, C.M.; Topp, M.S.; Gonzalez, S.; Pfeiffer, T.; Olivares, S.; Gonzalez, S.; Smith, D.D.; Forman, S.J.; Jensen, M.C.; Cooper, L.J.N. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006, 66, 10995–11004. [Google Scholar] [CrossRef]
- Singh, H.; Figliola, M.J.; Dawson, M.J.; Huls, H.; Olivares, S.; Switzer, K.; Mi, T.; Maiti, S.; Kebriaei, P.; Lee, D.A.; et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 2011, 71, 3516–3527. [Google Scholar] [CrossRef]
- Xue, X.; Huang, X.; Nodland, S.E.; Mátés, L.; Ma, L.; Izsvák, Z.; Ivics, Z.; LeBien, T.W.; McIvor, R.S.; Wagner, J.E.; et al. Stable gene transfer and expression in cord blood-derived CD34+ hematopoietic stem and progenitor cells by a hyperactive Sleeping Beauty transposon system. Blood 2009, 114, 1319–1330. [Google Scholar] [CrossRef]
- Galvan, D.; Nakazawa, Y.; Kaja, A.; Kettlun, C.; Cooper, L.J.N.; Rooney, C.M.; Wilson, M.H. Genome-wide mapping of PiggyBac transposon integrations in primary human T cells. J. Immunother. 2009, 32, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Kimhi, S.; Howard, G.; Eden, A.; Lyko, F. Demethylation of a LINE-1 antisense promoter in the cMet locus impairs Met signalling through induction of illegitimate transcription. Oncogene 2010, 29, 5775–5784. [Google Scholar] [CrossRef]
- Curtin, F.; Bernard, C.; Levet, S.; Perron, H.; Porchet, H.; Médina, J.; Malpass, S.; Lloyd, D.; Simpson, R. A new therapeutic approach for type 1 diabetes: Rationale for GNbAC1, an anti-HERV-W-Env monoclonal antibody. Diabetes Obes. Metab. 2018, 20, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Diebold, M.; Derfuss, T. The monoclonal antibody GNbAC1: Targeting human endogenous retroviruses in multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Zahnow, C.A.; Ahuja, N.; Baylin, S.B. Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer Res. 2016, 76, 1683–1689. [Google Scholar] [CrossRef]
- Strick, R.; Strissel, P.L.; Baylin, S.B.; Chiappinelli, K.B. Unraveling the molecular pathways of DNA-methylation inhibitors: Human endogenous retroviruses induce the innate immune response in tumors. Oncoimmunology 2016, 5, e1122160. [Google Scholar] [CrossRef]
- Stone, M.L.; Chiappinelli, K.B.; Li, H.; Murphy, L.M.; Travers, M.E.; Topper, M.J.; Mathios, D.; Lim, M.; Shih, I.E.; Wang, T.L.; et al. Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden. Proc. Natl. Acad. Sci. USA 2017, 114, E10981–E10990. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, P.; Marhon, S.A.; Ettayebi, I.; Chakravarthy, A.; Hosseini, A.; Wang, Y.; De Castro, F.A.; Yau, H.L.; Ishak, C.; Abelson, S.; et al. Epigenetic therapy induces transcription of inverted SINEs and ADAR1 dependency. Nature 2020, 588, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.; Lichtenberg, J.; Thomsen, P.D.; Larsson, L.I. Spontaneous fusion between cancer cells and endothelial cells. Cell Mol. Life Sci. 2004, 61, 2125–2131. [Google Scholar] [CrossRef]
- Kraus, B.; Fischer, K.; Büchner, S.M.; Wels, W.S.; Löwer, R.; Sliva, K.; Schnierle, B.S. Vaccination directed against the human endogenous retrovirus-K envelope protein inhibits tumor growth in a murine model system. PLoS ONE 2013, 8, e72756. [Google Scholar] [CrossRef]
- Houede, N.; Piazza, P.V.; Pourquier, P. LINE-1 as a therapeutic target for castration-resistant prostate cancer. Front. Biosci. 2018, 23, 1292–1309. [Google Scholar]
- Sciamanna, I.; De Luca, C.; Spadafora, C. The Reverse Transcriptase Encoded by LINE-1 Retrotransposons in the Genesis, Progression, and Therapy of Cancer. Front. Chem. 2016, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018, 25, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Singh, M.; Santos, G.S.; Guerlavais, V.; Carvajal, L.A.; Aivado, M.; Zhan, Y.; Oliveira, M.M.; Westerberg, L.S.; Annis, D.A.; et al. Pharmacologic Activation of p53 Triggers Viral Mimicry Response Thereby Abolishing Tumor Immune Evasion and Promoting Antitumor Immunity. Cancer Discov. 2021, 11, 3090–3105. [Google Scholar] [CrossRef]
- Harris, C.R.; DeWan, A.; Zupnick, A.; Normart, R.; Gabriel, A.; Prives, C.; Levine, A.J.; Hoh, J. p53 responsive elements in human retrotransposons. Oncogene 2009, 28, 3857–3865. [Google Scholar] [CrossRef]
- Haoudi, A.; Semmes, O.J.; Mason, J.M.; Cannon, R.E. Retrotransposition-Competent Human LINE-1 Induces Apoptosis in Cancer Cells With Intact p53. J. Biomed. Biotechnol. 2004, 2004, 461360. [Google Scholar] [CrossRef]
- Grundy, E.E.; Diab, N.; Chiappinelli, K.B. Transposable element regulation and expression in cancer. FEBS J. 2022, 289, 1160–1179. [Google Scholar] [CrossRef]
- Mita, P.; Sun, X.; Fenyö, D.; Kahler, D.J.; Li, D.; Agmon, N.; Wudzinska, A.; Keegan, S.; Bader, J.S.; Yun, C.; et al. BRCA1 and S phase DNA repair pathways restrict LINE-1 retrotransposition in human cells. Nat. Struct. Mol. Biol. 2020, 27, 179–191. [Google Scholar] [CrossRef]
- Tsai, H.C.; Li, H.; Van Neste, L.; Cai, Y.; Robert, C.; Rasool, F.V.; Shin, J.J.; Harbom, K.M.; Beaty, R.; Pappou, E.; et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell 2012, 21, 430–446. [Google Scholar] [CrossRef]
- Roulois, D.; Loo Yau, H.; Singhania, R.; Wang, Y.; Danesh, A.; Shen, S.Y.; Han, H.; Liang, G.; Jones, P.A.; Pugh, T.J.; et al. DNA-Demethylating Agents Target Colorectal Cancer Cells by Inducing Viral Mimicry by Endogenous Transcripts. Cell 2015, 162, 961–973. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Sote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, E.; Farrell, A.; Abraham, S.; Baird, A.; Hsieh, L.-S.; Lee, S.-L.; Leighton, J.K.; Patel, H.; Rahman, A.; Sridhara, R.; et al. Approval summary: Azacitidine for treatment of myelodysplastic syndrome subtypes. Clin. Cancer Res. 2005, 11, 3604–3608. [Google Scholar] [CrossRef] [PubMed]
- Karpf, A.R.; Peterson, P.W.; Rawlins, J.T.; Dalley, B.K.; Yang, Q.; Albertsen, H.; Jones, D.A. Inhibition of DNA methyltransferase stimulates the expression of signal transducer and activator of transcription 1, 2, and 3 genes in colon tumor cells. Proc. Natl. Acad. Sci. USA 1999, 96, 14007–14012. [Google Scholar] [CrossRef] [PubMed]
- Raslan, O.; Garcia-Horton, A. Azacitidine and its role in the upfront treatment of acute myeloid leukemia. Expert Opin. Pharmacother. 2022, 23, 873–884. [Google Scholar] [CrossRef]
- Liu, M.; Thomas, S.L.; DeWitt, A.K.; Zhou, W.; Madaj, Z.B.; Ohtani, H.; Baylin, S.B.; Liang, G.; Jones, P.A. Dual Inhibition of DNA and Histone Methyltransferases Increases Viral Mimicry in Ovarian Cancer Cells. Cancer Res. 2018, 78, 5754–5766. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ohtani, H.; Zhou, W.; Ørskov, A.D.; Charlet, J.; Zhang, Y.W.; Shen, H.; Baylin, S.B.; Liang, G.; Grønbæk, K.; et al. Vitamin C increases viral mimicry induced by 5-aza-2′-deoxycytidine. Proc. Natl. Acad. Sci. USA 2016, 113, 10238–10244. [Google Scholar] [CrossRef]
- Hahn, S.; Ugurel, S.; Hanschmann, K.M.; Strobel, H.; Tondera, C.; Schadendorf, D.; Lower, J.; Lower, R. Serological response to human endogenous retrovirus K in melanoma patients correlates with survival probability. AIDS Res. Hum. Retrovir. 2008, 24, 717–723. [Google Scholar] [CrossRef]
- Jonkhout, N.; Tran, J.; Smith, M.; Schonrock, N.; Mattick, J.; Novoa, E.M. The RNA modification landscape in human disease. RNA 2017, 23, 1754–1769. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Jung, H.; Mun, S.; Lee, S.; Park, K.; Baek, S.C.; Moon, H.C.; Kim, H.; Kim, B.; Choi, Y.; et al. L1 retrotransposons exploit RNA m(6)A modification as an evolutionary driving force. Nat. Commun. 2021, 12, 880. [Google Scholar] [CrossRef]
- Pacini, C.E.; Bradshaw, C.R.; Garrett, N.J.; Koziol, M.J. Characteristics and homogeneity of N6-methylation in human genomes. Sci. Rep. 2019, 9, 5185. [Google Scholar] [CrossRef]
- Koziol, M.J.; Bradshaw, C.R.; Allen, G.E.; Costa, A.S.; Frezza, C. Identification of Methylated Deoxyadenosines in Genomic DNA by dA(6m) DNA Immunoprecipitation. Bio-Protoc. 2016, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, A.M.; Covre, A.; Finotello, F.; Rieder, D.; Danielli, R.; Sigalotti, L.; Giannarelli, D.; Petitprez, F.; Lacroix, L.; Valente, M.; et al. Guadecitabine Plus Ipilimumab in Unresectable Melanoma: The NIBIT-M4 Clinical Trial. Clin. Cancer Res. 2019, 25, 7351–7362. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, M.; Vernier, M.; Mignacca, L.; Lessard, F.; Huot, G.; Moiseeva, O.; Bourdeau, V.; Ferbeyre, G. A CDK4/6-Dependent Epigenetic Mechanism Protects Cancer Cells from PML-induced Senescence. Cancer Res. 2016, 76, 3252–3264. [Google Scholar] [CrossRef]
- Bourdeau, V.; Ferbeyre, G. CDK4-CDK6 inhibitors induce autophagy-mediated degradation of DNMT1 and facilitate the senescence antitumor response. Autophagy 2016, 12, 1965–1966. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Yau, H.L.; Chakravarthy, A.B.; Wang, B.; Shen, S.Y.; Ettayebi, I.; Ishak, C.A.; Bedard, P.L.; Razak, A.A.; Hansen, A.R.; et al. An open-label, phase II multicohort study of an oral hypomethylating agent CC-486 and durvalumab in advanced solid tumors. J. Immunother. Cancer 2020, 8, e000883. [Google Scholar] [CrossRef]
- Magnani, C.F.; Gaipa, G.; Lussana, F.; Belotti, D.; Gritti, G.; Napolitano, S.; Matera, G.; Cabiati, B.; Buracchi, C.; Borleri, G.; et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J. Clin. Investig. 2020, 130, 6021–6033. [Google Scholar] [CrossRef]
- Cingoz, O.; Goff, S.P. Cyclin-dependent kinase activity is required for type I interferon production. Proc. Natl. Acad. Sci. USA 2018, 115, E2950–E2959. [Google Scholar] [CrossRef]
- DePinto, W.; Chu, X.-J.; Yin, X.; Smith, M.; Packman, K.; Goelzer, P.; Lovey, A.; Chen, Y.; Qian, H.; Hamid, R.; et al. In Vitro and In Vivo activity of R547: A potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol. Cancer Ther. 2006, 5, 2644–2658. [Google Scholar] [CrossRef]
- Palazzo, A.; Marsano, R.M. Transposable elements: A jump toward the future of expression vectors. Crit. Rev. Biotechnol. 2021, 41, 792–808. [Google Scholar] [CrossRef]

| Type of TE | Genetic Disorder/Disease | References |
|---|---|---|
| LINE-1 promotor hypomethylation. | Lung, Colon, Pancreatic, Ovarian Cancer | [120] |
| LINE-1 insertion in exon 14 of factor VIII gene | Hemophilia A | [11] |
| LINE-1 insertion | Familial Retinoblastoma | [22] |
| LINE-1 insertion in 3′noncoding region of fukutin gene | Fukuyama type congenital muscular dystrophy | [121] |
| LINE-1 insertion in DMD gene | Duchene muscular dystrophy | [122] |
| LINE-1 intronic insertion in RP2 gene | Retinis pigmentosa | [123] |
| LINE-1 insertion | Coffin-Lowry Syndrome | [124] |
| LINE-1 insertion in PDHX gene | Pyruvate dehydrogenase complex deficiency. | [125] |
| Alu insertion in exon 1 of CD40LG gene | Higm Syndrome | [126] |
| Alu insertion in CLCN5 gene | Dent’s Disease | [127] |
| Alu intronic insertion in NF1 gene | Neurofibromatosis type1 | [18] |
| Alu insertions | Colon, Breast, Ovarian Cancer | [20,128,129] |
| Alu insertion in APC gene | Leukemia | [130] |
| Alu insertion in QAT gene | OAT deficiency | [131] |
| Alu insertion in COL4A3 gene | Alport Syndrome | [132] |
| SVA insertion in exon 6 of factor VIII gene | Hemophilia B | [133] |
| SVA insertion in intron 7 of PMS gene | Lynch syndrome | [134] |
| Sr. No. | Transposon | Animal Model | Delivery | Disease | References |
|---|---|---|---|---|---|
| 1. | Sleeping Beauty Transposons | Dogs | Liver-Directed Hydrodynamic Delivery | [150] | |
| 2. | Sleeping Beauty Transposons | Dogs | Liver-Directed Delivery | [151] | |
| 3. | Retrotransposon activation in Alzheimer’s disease | Mouse | Alzheimer’s disease | [152] | |
| 4. | PiggyBac Transposons | Mice | Duchenne Muscular Dystrophy | [153] | |
| 5. | Sleeping Beauty (SB) | C57BL/6 J mice | Hydrodynamic Tail Vein Injection | Hepatocellular Carcinoma | [154] |
| 6. | Transposon-triggered innate immune response confers cancer resistance | blind mole rat | Cancer | [155] | |
| 7. | Corticosterone dynamically regulates retrotransposable element expression | Rat | Stress condition | [156] | |
| 8. | Differential Responses of LINE-1 | Rat | Psychomotor impairments | [157] | |
| 9. | Spatially Resolved Expression of Transposable Elements | Mice | Neurodegenerative disease amyotrophic lateral sclerosis | [158] | |
| 10. | Activation of HERV-K(HML-2) | Human pluripotent stem cells | Disrupts cortical patterning and neuronal differentiation | [159] |
| Sr. No. | Study | Disease | Intervention/ Treatment | Phase | Clinical Trial Gov. Identifier |
|---|---|---|---|---|---|
| 1. | MT2018-18: Sleeping Beauty Transposon-Engineered Plasmablasts for Hurler Syndrome Post Allo HSCT | Mucopolysaccharidosis Type IH (MPS IH, Hurler Syndrome), Mucopolysaccharidosis Type IH MPS IH, Hurler Syndrome | Autologous Plasmablasts | 1/2 | NCT04284254 |
| 2. | Analysis of Transposon Control Pathways in Germinal Cancers of the Testicle | Germinal Cancers of the Testicle | Genetic: Extraction of total RNA from healthy and tumor tissues | NCT02873793 | |
| 3. | Transposon-manipulated Allogeneic CARCIK-CD19 Cells in Paediatric and Adult Patients With r/r ALL Post HSCT (CARCIK) | Acute Lymphoblastic Leukemia in Relapse | Biological: CARCIK-CD19 | 1/2 | NCT03389035 |
| 4. | Anti-CD19 CAR in PiggyBac Transposon-Engineered T Cells for Relapsed/Refractory B-cell Lymphoma or B-cell Acute Lymphoblastic Leukaemia | B Cell Lymphoma, B-cell Acute Lymphoblastic Leukemia | Biological: Anti-CD19 CAR-T Cells Injection | 1 | NCT04289220 |
| 5. | Mechanisms and Factors Responsible for the Inhibition of Transposons During Fatal Gonad Development in Humans | Medical Termination of Pregnancy, Voluntary Termination of Pregnancy | Other: surgical biopsies | NCT02171845 | |
| 6. | Measurable Residual Disease Driven Strategy for One or Two Infusions of Non- Viral, Transposon-manipulated CARCIK (CD19) Cells: A Phase II Study in Paediatric and Adult Patients with Relapsed/Refractory B Cell Precursor ALL (BCP-ALL) | Acute Lymphoblastic Leukemia | Genetic: PTG-CARCIK-CD19 | 2 | NCT05252403 |
| 7. | A Phase II Study Using the Administration of Autologous T-Cells Engineered Using the Sleeping Beauty Transposon/Transposase System to Express T-Cell Receptors Reactive Against Mutated Neoantigens in Patients with Metastatic Cancer | Endocrine/ Neuroendocrine, Non-Small Cell Lung Cancer, Breast Cancer, Gastrointestinal/ Genitourinary Cancers, Ovarian Cancer | Drug: Fludarabine Drug: Cyclophosphamide Drug: Aldesleukin Biological: Sleeping Beauty Transposed PBL | 2 | NCT04102436 |
| 8. | Phase I/II Study of Autologous T Cells Engineered Using the Sleeping Beauty System to Express T-Cell Receptors (TCRs) Reactive Against Cancer-specific Mutations in Subjects with Solid Tumors | Gynecologic Cancer, Colorectal Cancer, Pancreatic Cancer, Non-small Cell Lung Cancer, Cholangiocarcinoma, Ovarian Cancer | Biological: Neoantigen specific TCR-T cell drug product Biological: Aldesleukin (IL-2) | 1/2 | NCT05194735 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, A.; Ghatage, T.; Bhan, S.; Lahane, G.P.; Dhar, A.; Kumar, R.; Pandita, R.K.; Bhat, K.M.; Ramos, K.S.; Pandita, T.K. Role of Transposable Elements in Genome Stability: Implications for Health and Disease. Int. J. Mol. Sci. 2022, 23, 7802. https://doi.org/10.3390/ijms23147802
Bhat A, Ghatage T, Bhan S, Lahane GP, Dhar A, Kumar R, Pandita RK, Bhat KM, Ramos KS, Pandita TK. Role of Transposable Elements in Genome Stability: Implications for Health and Disease. International Journal of Molecular Sciences. 2022; 23(14):7802. https://doi.org/10.3390/ijms23147802
Chicago/Turabian StyleBhat, Audesh, Trupti Ghatage, Sonali Bhan, Ganesh P. Lahane, Arti Dhar, Rakesh Kumar, Raj K. Pandita, Krishna M. Bhat, Kenneth S. Ramos, and Tej K. Pandita. 2022. "Role of Transposable Elements in Genome Stability: Implications for Health and Disease" International Journal of Molecular Sciences 23, no. 14: 7802. https://doi.org/10.3390/ijms23147802
APA StyleBhat, A., Ghatage, T., Bhan, S., Lahane, G. P., Dhar, A., Kumar, R., Pandita, R. K., Bhat, K. M., Ramos, K. S., & Pandita, T. K. (2022). Role of Transposable Elements in Genome Stability: Implications for Health and Disease. International Journal of Molecular Sciences, 23(14), 7802. https://doi.org/10.3390/ijms23147802








