ThSCSP_12: Novel Effector in Tilletia horrida That Induces Cell Death and Defense Responses in Non-Host Plants
Abstract
1. Introduction
2. Results
2.1. ThSCSP_12 from T. horrida Induces Cell Death in N. benthamiana
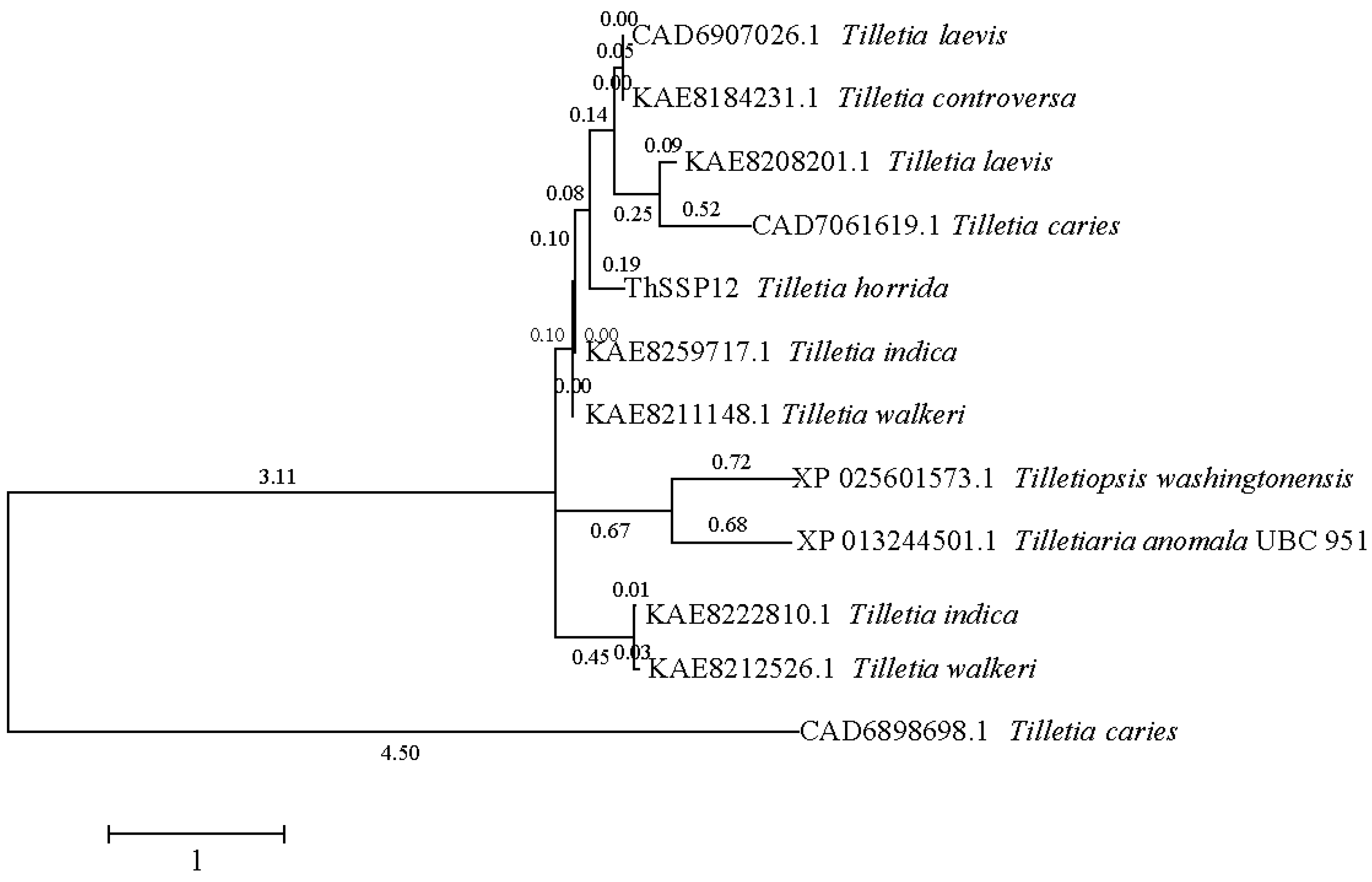
2.2. ThSCSP_12 Is Conserved in Tilletia Fungi
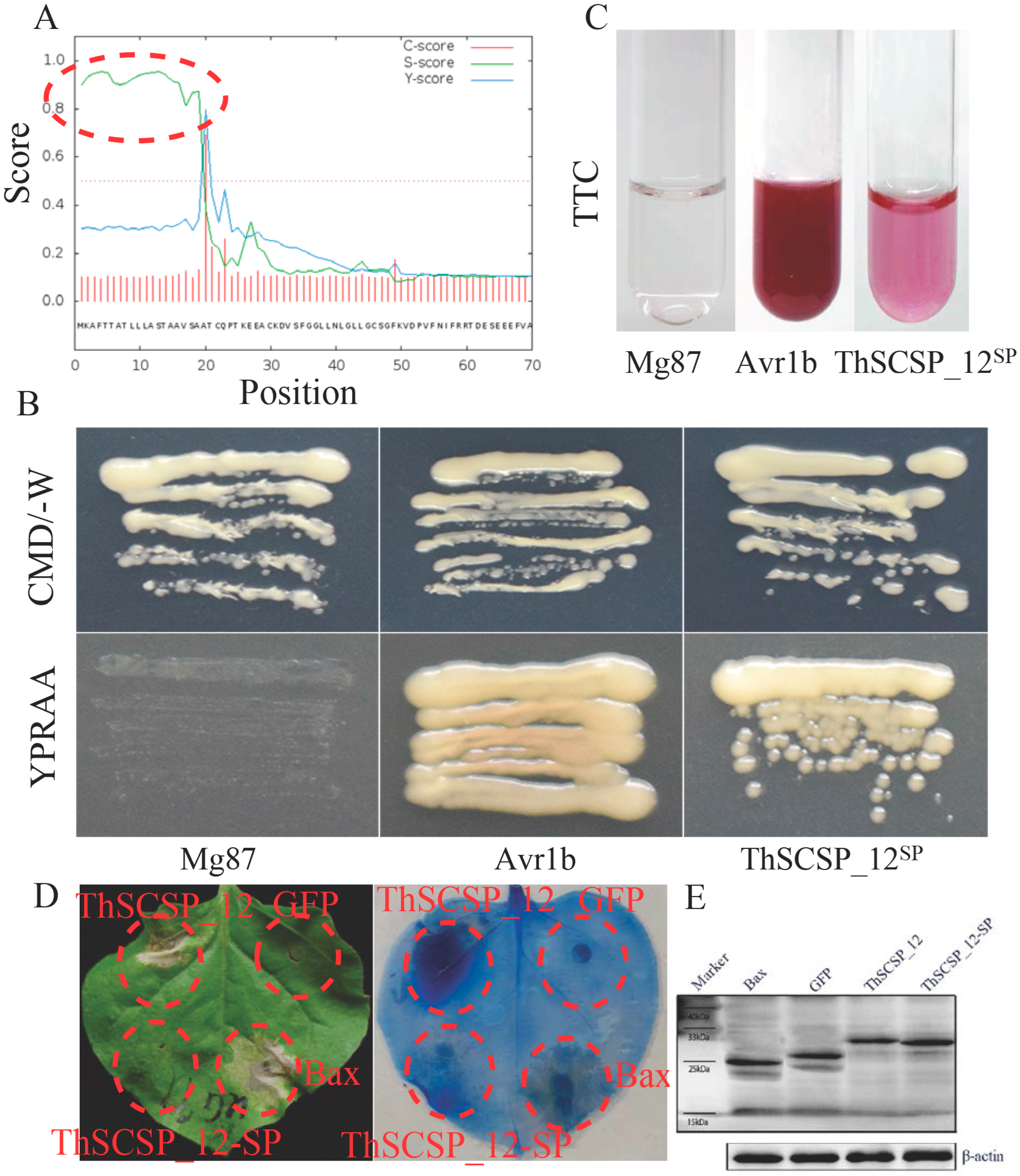
2.3. Functional Analyses of the SP Predicted in ThSCSP_12
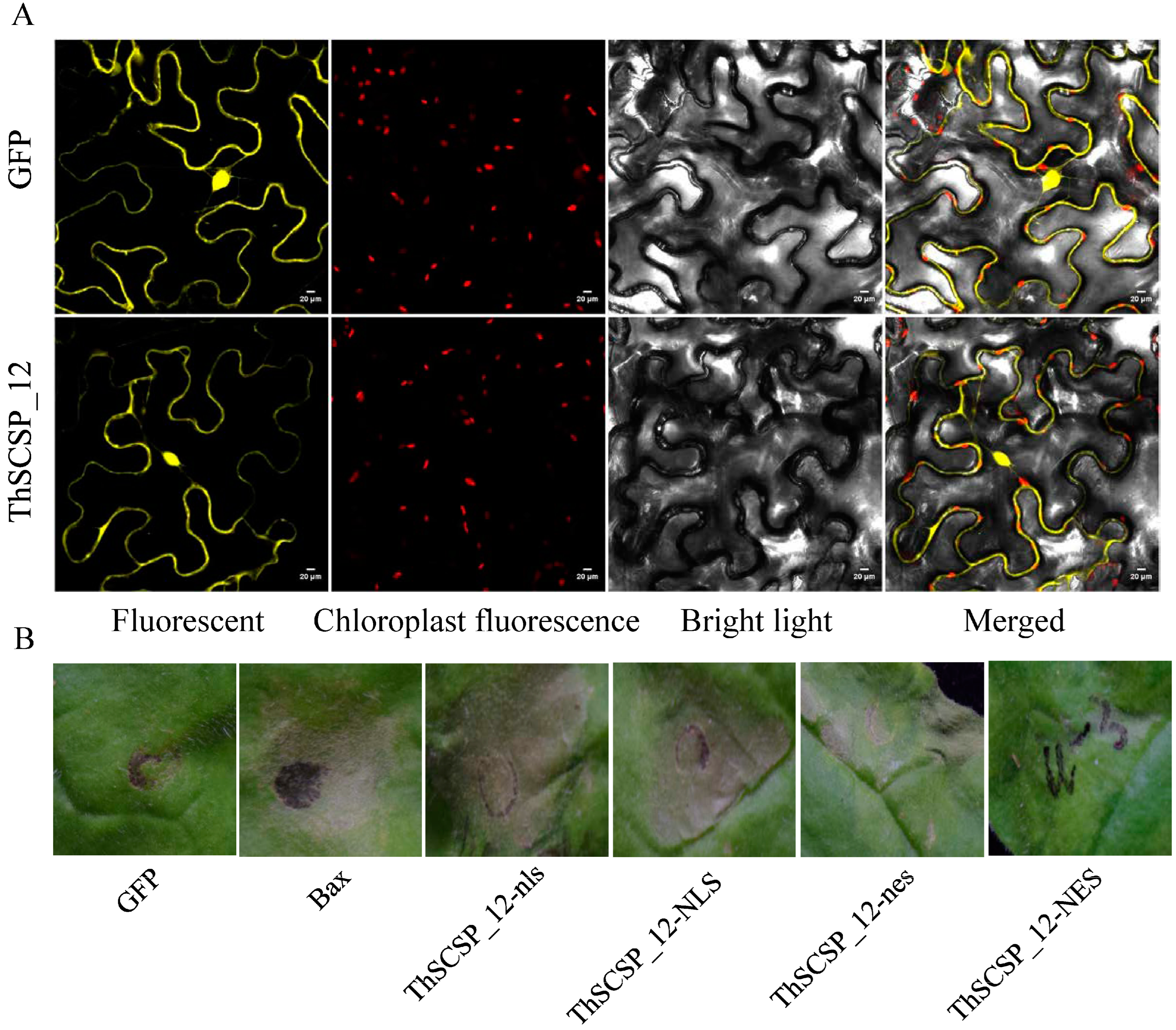
2.4. Nuclear Localization of ThSCSP_12 Is Required to Induce Cell Death
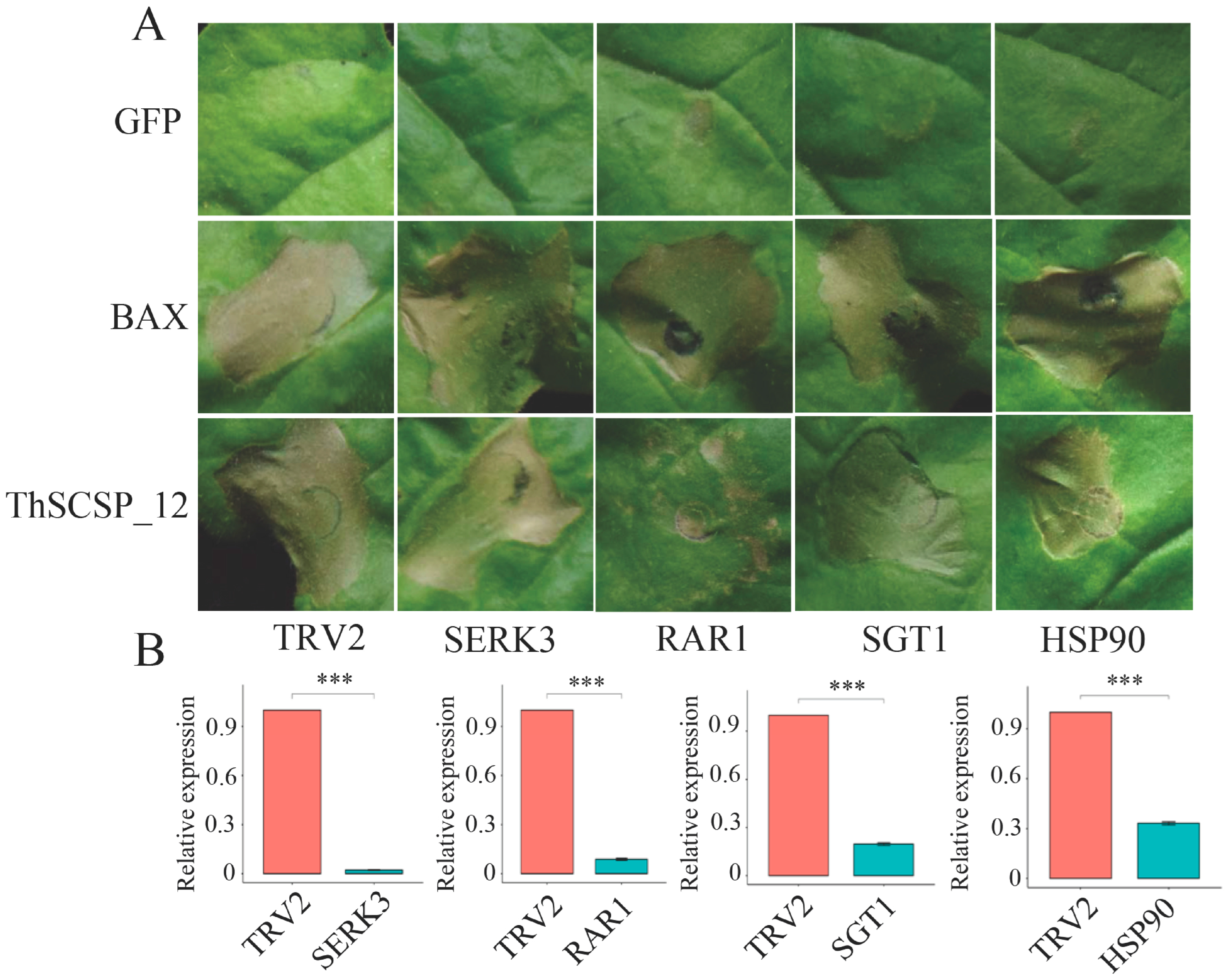
2.5. ThSCSP_12-Triggered Cell Death in N. Benthamiana Depends on RAR1 but Not SGT1, HSP90, or SERK3/Bak1
2.6. The 1-189 Amino Acid Fragment of ThSCSP_12 Is Sufficient for Its Cell-Death-Inducing Activity
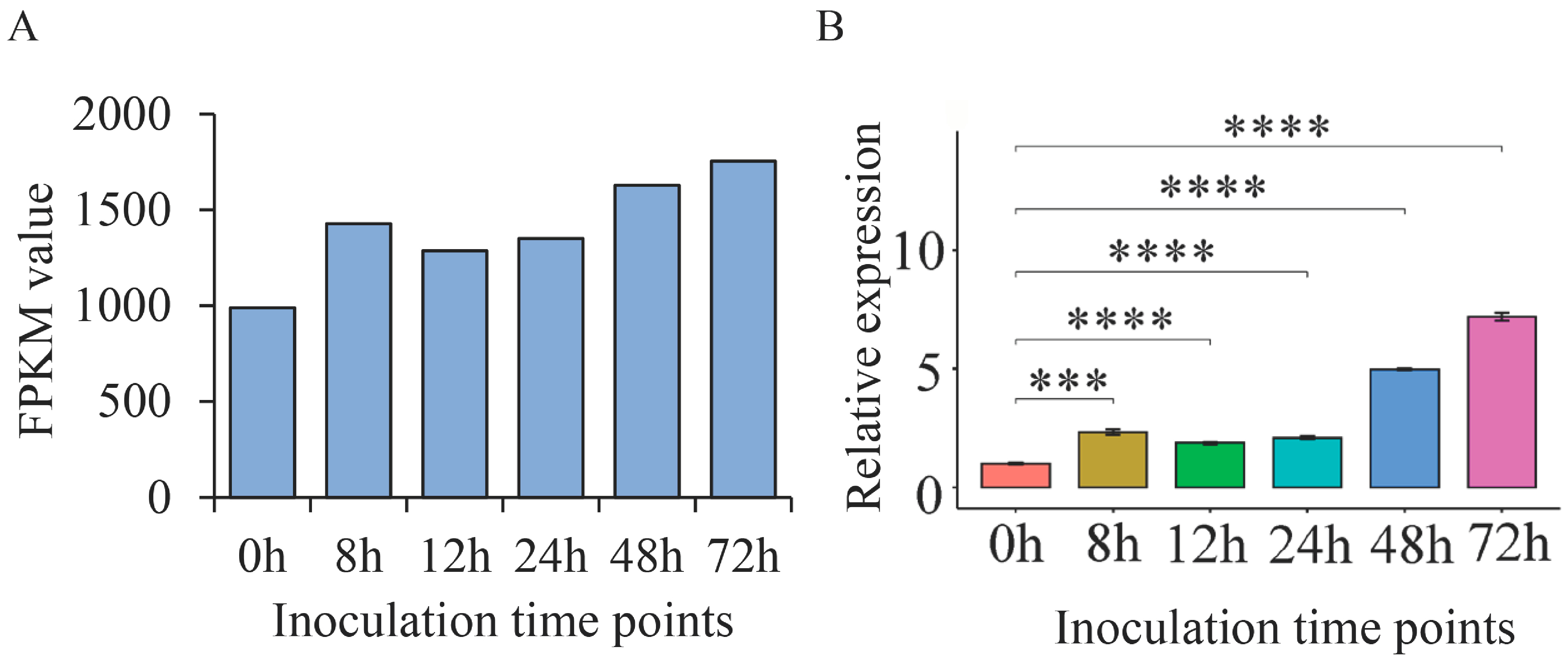
2.7. ThSCSP_12 Expression during T. horrida Infection
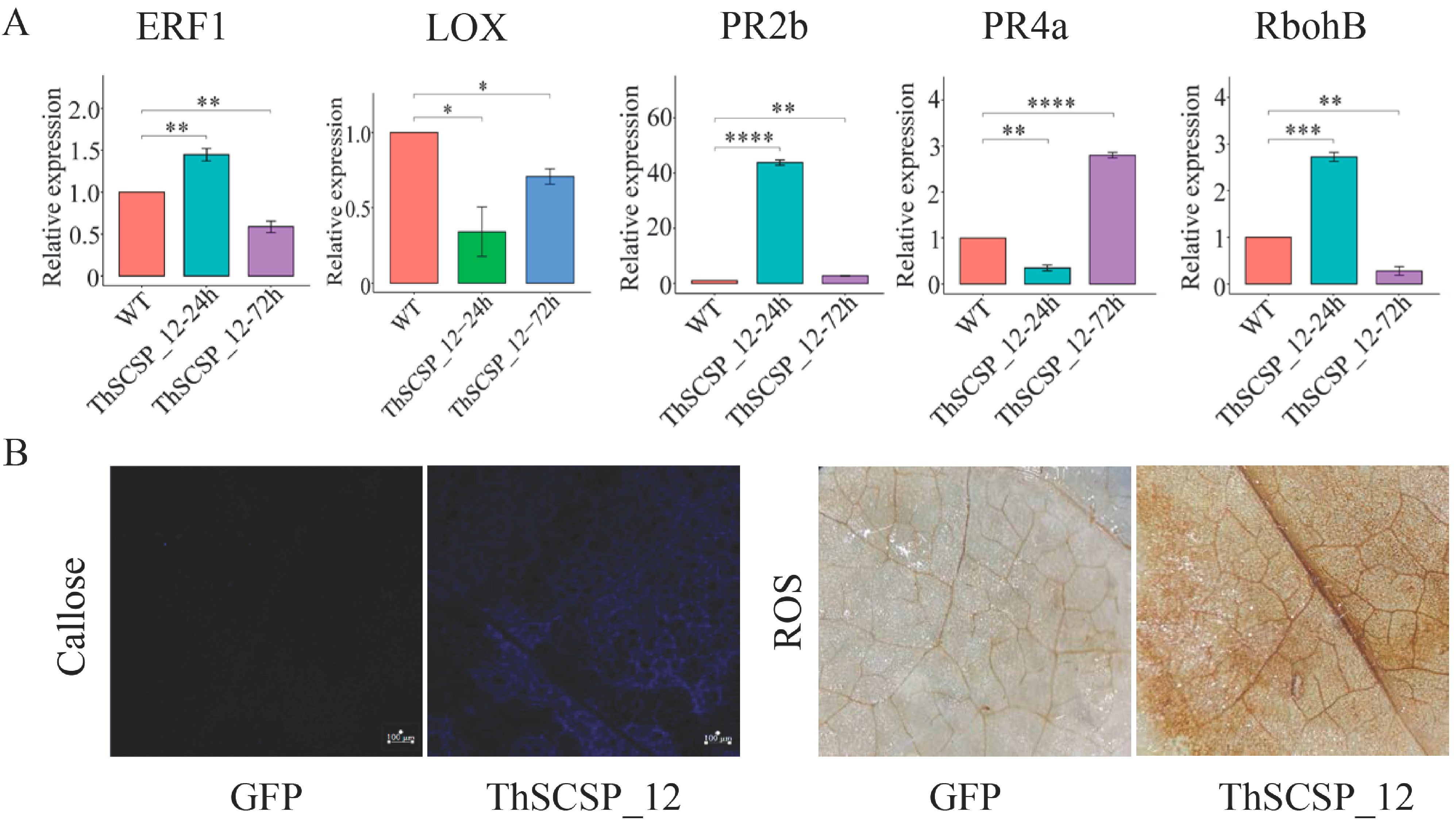
2.8. ThSCSP_12 Triggers Immunity Responses in N. benthamiana
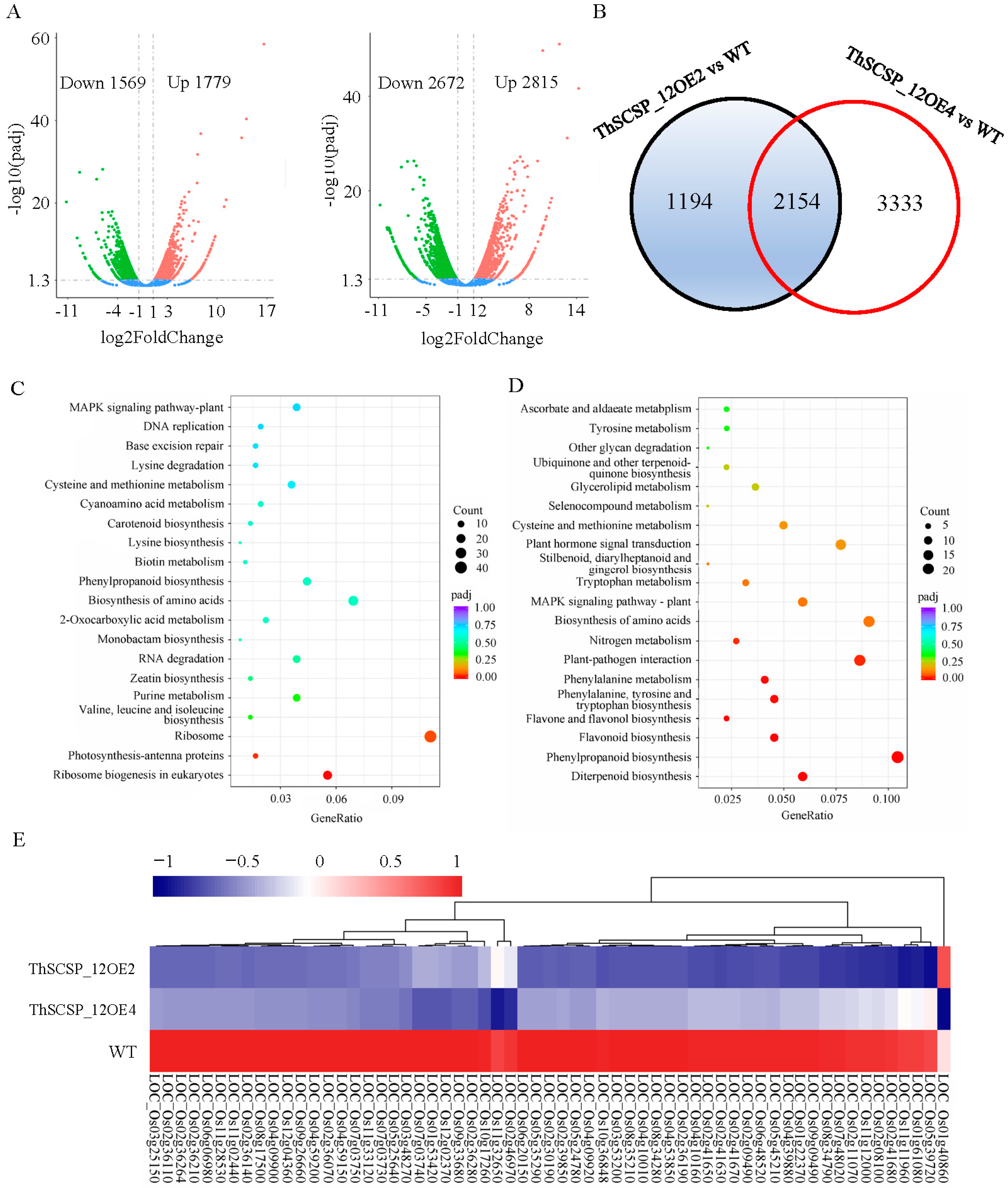
2.9. Transcriptome Analysis of ThSCSP_12 Transgenic Rice
3. Discussion
4. Materials and Methods
4.1. Fungal Strains, Plant Materials, and Growth Conditions
4.2. Screening of Effectors
4.3. RNA Isolation and Plasmid Construction of T. horrida Candidate Effector Genes
4.4. A. grobacterium–Mediated Transient Expression
4.5. Function Validation of the SP
4.6. Protein Extraction and Western Blotting
4.7. Deletion Mutagenesis of ThSCSP_12
4.8. Subcellular Localization
4.9. VIGS Assay in N. benthamiana
4.10. Oxygen Burst and Callose Deposition Observation
4.11. Genetic Transformation of Rice
4.12. RNA-Seq and Data Analyses
4.13. Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Yang, X.; Yao, J.; Kyaw, E.P.; Zhang, A.F.; Li, Y.F.; Gu, C.Y.; Zang, H.Y.; Gao, T.C. Simple and rapid detection of Tilletia horrida causing rice kernel smut in rice seeds. Sci. Rep. 2016, 6, 33258. [Google Scholar] [CrossRef] [PubMed]
- Webster, P.K.; Gunnell, P.S. Compendium of Rice Diseases; American Phytopathological Society Press: St. Paul, MN, USA, 1992; p. 110. [Google Scholar]
- Wang, N.; Ai, P.; Tang, Y.F.; Zhang, J.F.; Dai, X.J.; Li, P.; Zheng, A.P. Draft Genome Sequence of the Rice Kernel Smut Tilletia horrida Strain QB-1. Genome Announc. 2016, 3, e00621-15. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Pang, L.X.; Wang, N.; Ai, P.; Yin, D.S.; Li, S.C.; Deng, Q.M.; Zhu, J.; Liang, Y.; Zhu, J.; et al. The pathogenic mechanisms of Tilletia horrida as revealed by comparative and functional genomics. Sci. Rep. 2018, 8, 15413. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Zhou, J.M. Phytopathogen effectors subverting host immunity: Different foes, similar battleground. Cell Host Microbe 2012, 12, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Howden, A.J.; Huitema, E. Effector-triggered post-translational modifications and their role in suppression of plant immunity. Front. Plant Sci. 2012, 3, 160. [Google Scholar] [CrossRef]
- Quentin, M.; Abad, P.; Favery, B. Plant parasitic nematode effectors target host defense and nuclear functions to establish feeding cells. Front. Plant Sci. 2013, 4, 53. [Google Scholar] [CrossRef]
- Wang, A.J.; Pan, L.X.; Niu, X.Y.; Shu, X.Y.; Yi, X.Q.; Yamamoto, N.; Li, S.C.; Deng, Q.M.; Zhu, J.; Liang, Y.Y.; et al. Comparative secretome analysis of different smut fungi and identification of plant cell death-inducing secreted proteins from Tilletia horrida. BMC Plant Biol. 2019, 19, 360. [Google Scholar] [CrossRef]
- Fang, A.F.; Han, Y.Q.; Zhang, N.; Zhang, M.; Liu, L.J.; Li, S.; Lu, F.; Sun, W. Identification and characterization of plant cell death-inducing secreted proteins from Ustilaginoidea virens. Mol. Plant-Microbe Interact. 2016, 29, 405–416. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.Y.; Fang, A.F.; Wang, J.Y.; Li, D.Y.; Li, Y.J.; Wang, S.Z.; Cui, F.H.; Yu, J.J.; Liu, Y.F.; et al. The essential effector SCRE1 in Ustilaginoidea virens suppresses rice immunity via a small peptide region. Mol. Plant Pathol. 2020, 21, 445–459. [Google Scholar] [CrossRef]
- Mueller, A.N.; Ziemann, S.; Treitschke, S.; Aßmann, D.; Doehlemann, G. Compatibility in the Ustilago maydis-maize interaction requires inhibition of host cysteine proteases by the fungal effector Pit2. PLoS Pathog. 2013, 9, e1003177. [Google Scholar] [CrossRef]
- Redkar, A.; Villajuana-Bonequi, M.; Doehlemann, G. Conservation of the Ustilago maydis effector See1 in related smuts. Plant Signal. Behav. 2015, 10, e1086855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hemetsberger, C.; Herrberger, C.; Zechmann, B.; Hillmer, M.; Doehlemann, G. The Ustilago maydis effector Pep1 suppresses plant immunity by inhibition of host peroxidase activity. PLoS Pathog. 2012, 8, e1002684. [Google Scholar] [CrossRef] [PubMed]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Brefort, T.; Neidig, N.; Djamei, A.; Kahnt, J.; Vermerris, W.; Koenig, S.; Feussner, K.; Feussner, I.; Kahmann, R. A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. eLife 2014, 3, e01355. [Google Scholar] [CrossRef]
- Yin, X.; Fu, Q.Q.; Shang, B.X.; Wang, Y.L.; Liu, R.Q.; Chen, T.T.; Xiang, G.Q.; Dou, M.R.; Liu, G.T.; Xu, Y. An RxLR effector from Plasmopara viticola suppresses plant immunity in grapevine by targeting and stabilizing VpBPA1. Plant J. 2022, 112, 104–114. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, X.B.; Duan, Y.H.; Pei, Z.X.; Liu, H.; Yin, W.X.; Huang, J.B.; Luo, C.X.; Chen, X.L.; Li, G.T.; et al. A secreted fungal subtilase interferes with rice immunity via degradation of SUPPRESSOR OF G2 ALLELE OF skp1. Plant Physiol. 2022, 190, 1474–1489. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Liu, M.X.; Chen, T.T.; Ma, X.; Li, Z.K.; Zheng, Z.; Zheng, S.R.; Chen, L.; Li, Y.Z.; Tang, L.R.; et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci. Adv. 2022, 8, eabq5108. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N.; Gardiner, D.M.; Singh, K.B.; Taylor, J.M. Improved prediction of fungal effector proteins from secretomes with EffectorP 2.0. Mol. Plant Pathol. 2018, 19, 2094–2110. [Google Scholar] [CrossRef]
- Lee, S.J.; Rose, J.K. A yeast secretion trap assay for identification of secreted proteins from eukaryotic phytopathogens and their plant hosts. Methods Mol. Biol. 2012, 835, 519–530. [Google Scholar]
- Saitoh, H.; Fujisawa, S.; Mitsuoka, C.; Ito, A.; Hirabuchi, A.; Ikeda, K.; Irieda, H.; Yoshino, K.; Yoshida, K.; Matsumura, H.; et al. Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLoS Pathog. 2012, 8, e1002711. [Google Scholar] [CrossRef]
- Xiang, J.; Li, X.L.; Yin, L.; Liu, Y.X.; Zhang, Y.L.; Qu, J.J.; Lu, J. A candidate RxLR effector from Plasmopara viticola can elicit immune responses in Nicotiana benthamiana. BMC Plant Biol. 2017, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.H.; Saijo, Y.; Mauch, S.; Biskup, C.; Bieri, S.; Keller, B.; Seki, H.; Ulker, B.; Somssich, I.E.; Schulze-Lefert, P. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 2007, 315, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, K.; Schulze-Lefert, P. Complex formation, promiscuity and multi-functionality: Protein interactions in disease-resistance pathways. Trends Plant Sci. 2003, 8, 252–258. [Google Scholar] [CrossRef]
- Shirasu, K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 2009, 60, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Heese, A.; Hann, D.R.; Gimenez-Ibanez, S.; Jones, A.M.; He, K.; Li, J.; Schroeder, J.I.; Peck, S.C.; Rathjen, J.P. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 2007, 104, 12217–12222. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; de Wit, P.J. Fungal effector proteins. Annu. Rev. Phytopathol. 2009, 47, 233–263. [Google Scholar] [CrossRef]
- Wang, C.; Zien, C.A.; Afitlhile, M.; Welti, R.; Hildebrand, D.F.; Wang, X. Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in arabidopsis. Plant Cell 2000, 12, 2237–2246. [Google Scholar] [CrossRef]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. Ethylene response factor1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef]
- Lee, S.; Ishiga, Y.; Clermont, K.; Mysore, K.S. Coronatine inhibits stomatal closure and delays hypersensitive response cell death induced by nonhost bacterial pathogens. PeerJ 2013, 1, e34. [Google Scholar] [CrossRef]
- Cui, H.T.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Zhang, D.D.; Song, J.; Song, S.S.; Yin, C.M.; Zhou, L.; Liu, Y.; Wang, B.L.; Kong, Z.Q.; et al. Functional analyses of small secreted cysteine-rich proteins identified candidate effectors in Verticillium dahliae. Mol. Plant Pathol. 2020, 21, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Segovia, V.; MacLean, D.; Bayles, R.; Chen, X.; Kamoun, S.; Dubcovsky, J.; Saunders, D.G.; Uauy, C. Genome analyses of the wheat yellow (stripe) rust pathogen Puccinia striiformis f. sp. tritici reveal polymorphic and haustorial expressed secreted proteins as candidate effectors. BMC Genom. 2013, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- Hane, J.K.; Anderson, J.P.; Williams, A.H.; Sperschneider, J.; Singh, K.B. Genome sequencing and comparative genomics of the broad host-range pathogen Rhizoctonia solani AG8. PLoS Genet. 2014, 10, e1004281. [Google Scholar] [CrossRef]
- Wei, M.M.; Wang, A.J.; Liu, Y.; Ma, L.; Niu, X.Y.; Zheng, A.P. Identification of the novel effector RsIA_NP8 in Rhizoctonia solani AG1 IA that induces cell death and triggers defense responses in non-host plants. Front. Microbiol. 2020, 11, 1115. [Google Scholar] [CrossRef]
- Niu, X.Y.; Yang, G.J.; Lin, H.; Liu, Y.; Li, P.; Zheng, A.P. A novel, small cysteine-rich effector, RsSCR10 in Rhizoctonia solani is sufficient to trigger plant cell death. Front. Microbiol. 2021, 12, 684923. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.; Kamoun, S. RXLR effectors of plant pathogenic oomycetes. Curr. Opin. Microbiol. 2007, 10, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.J.; Yin, Z.Y.; Li, Z.P.; Wu, Y.X.; Huang, L.L. A small cysteine-rich protein from two kingdoms of microbes is recognized as a novel pathogen-associated molecular pattern. New Phytol. 2019, 222, 995–1011. [Google Scholar] [CrossRef]
- Taylor, K.W.; Kim, J.G.; Su, X.B.; Aakre, C.D.; Roden, J.A.; Adams, C.M.; Mudgett, M.B. Tomato TFT1 is required for PAMP-triggered immunity and mutations that prevent T3S effector XopN from binding to TFT1 attenuate Xanthomonas virulence. PLoS Pathog. 2012, 8, e1002768. [Google Scholar] [CrossRef]
- Azevedo, C.; Sadanandom, A.; Kitagawa, K.; Freialdenhoven, A.; Shirasu, K.; Schulze-Lefert, P. The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 2002, 295, 2073–2076. [Google Scholar] [CrossRef]
- Situ, J.J.; Jiang, L.Q.; Fan, X.N.; Yang, W.S.; Li, W.; Xi, P.G.; Deng, Y.Z.; Kong, G.H.; Jiang, Z.D. An RXLR effector PlAvh142 from Peronophythora litchii triggers plant cell death and contributes to virulence. Mol. Plant Pathol. 2020, 21, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Kim, H.; Choi, D. Rpi-blb2-mediated late blight resistance in Nicotiana benthamiana requires SGT1 and salicylic acid-mediated signaling but not RAR1 or HSP90. FEBS Lett. 2014, 588, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ren, Y.J.; Zhou, J.; Du, J.; Hou, J.; Jiang, R.; Wang, H.X.; Tian, Z.D.; Xie, C.H. The cell death triggered by the nuclear localized RxLR effector PITG_22798 from Phytophthora infestans is suppressed by the effector AVR3b. Int. J. Mol. Sci. 2017, 18, 409. [Google Scholar] [CrossRef]
- Xu, G.Y.; Yuan, M.; Ai, C.R.; Liu, L.J.; Zhuang, E.; Karapetyan, S.; Wang, S.P.; Dong, X.N. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature 2017, 545, 491–494. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Zha, Z.P.; Yin, D.S.; Shu, X.Y.; Ma, L.; Wang, L.X.; Li, P.; Zheng, A.P. Comparative transcriptome analysis of Tilletia horrida infection in resistant and susceptible rice (Oryza sativa L.) male sterile lines reveals potential candidate genes and resistance mechanisms. Genomics 2020, 112, 5214–5226. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, J.; Xing, D. Phosphatidylinositol 3-kinase plays a vital role in regulation of rice seed vigor via altering NADPH oxidase activity. PLoS ONE 2012, 7, e33817. [Google Scholar] [CrossRef]
- Yoshie, Y.; Goto, K.; Takai, R.; Iwano, M.; Takayama, S.; Isogai, A.; Che, F.S. Function of the rice gp91phox homologs OsrbohA and OsrbohE genes in ROS-dependent plant immune responses. Plant Biotechnol. 2005, 22, 127–135. [Google Scholar] [CrossRef]
- Li, G.; He, J.; Wu, J.; Wang, H.; Zhang, X.; Liu, J.; Hu, X.; Zhu, Y.; Shen, S.; Bai, Y.; et al. Enhanced production of OsRACK1A, an effector-targeted scaffold protein that promotes OsRBOHB-mediated ROS production, confers rice floral resistance to false smut disease without yield penalty. Mol. Plant 2022, 10, 009. [Google Scholar]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Hans, T.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar]
- Li, S.C.; Gao, F.Y.; Xie, K.L.; Zeng, X.H.; Cao, Y.; Zeng, J.; He, Z.S.; Ren, Y.; Li, W.B.; Deng, Q.M.; et al. The OsmiR396c-OsGRF4-OsGIF1 regulatory module determines grain size and yield in rice. Plant Biotechnol. J. 2016, 14, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level-the DESeq Package; European Molecular Biology Laborator: Heidelberg, Germany, 2013. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B-Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, X.; Yin, D.; Liang, J.; Xu, D.; Jiang, Y.; Xiang, T.; Wang, Y.; Jiao, C.; Li, P.; Zheng, A.; et al. ThSCSP_12: Novel Effector in Tilletia horrida That Induces Cell Death and Defense Responses in Non-Host Plants. Int. J. Mol. Sci. 2022, 23, 14752. https://doi.org/10.3390/ijms232314752
Shu X, Yin D, Liang J, Xu D, Jiang Y, Xiang T, Wang Y, Jiao C, Li P, Zheng A, et al. ThSCSP_12: Novel Effector in Tilletia horrida That Induces Cell Death and Defense Responses in Non-Host Plants. International Journal of Molecular Sciences. 2022; 23(23):14752. https://doi.org/10.3390/ijms232314752
Chicago/Turabian StyleShu, Xinyue, Desuo Yin, Juan Liang, Deze Xu, Yuqi Jiang, Ting Xiang, Yuxuan Wang, Chunhai Jiao, Ping Li, Aiping Zheng, and et al. 2022. "ThSCSP_12: Novel Effector in Tilletia horrida That Induces Cell Death and Defense Responses in Non-Host Plants" International Journal of Molecular Sciences 23, no. 23: 14752. https://doi.org/10.3390/ijms232314752
APA StyleShu, X., Yin, D., Liang, J., Xu, D., Jiang, Y., Xiang, T., Wang, Y., Jiao, C., Li, P., Zheng, A., & Wang, A. (2022). ThSCSP_12: Novel Effector in Tilletia horrida That Induces Cell Death and Defense Responses in Non-Host Plants. International Journal of Molecular Sciences, 23(23), 14752. https://doi.org/10.3390/ijms232314752





