Heterogeneity of Patient-Derived Acute Myeloid Leukemia Cells Subjected to SYK In Vitro Inhibition
Abstract
1. Introduction
2. Results
2.1. Dose–Response Studies of AML Cell Proliferation after Exposure to SYK Inhibitors
2.2. SYK Inhibition Demonstrated the Antiproliferative Effect in a Larger AML Patient Cohort
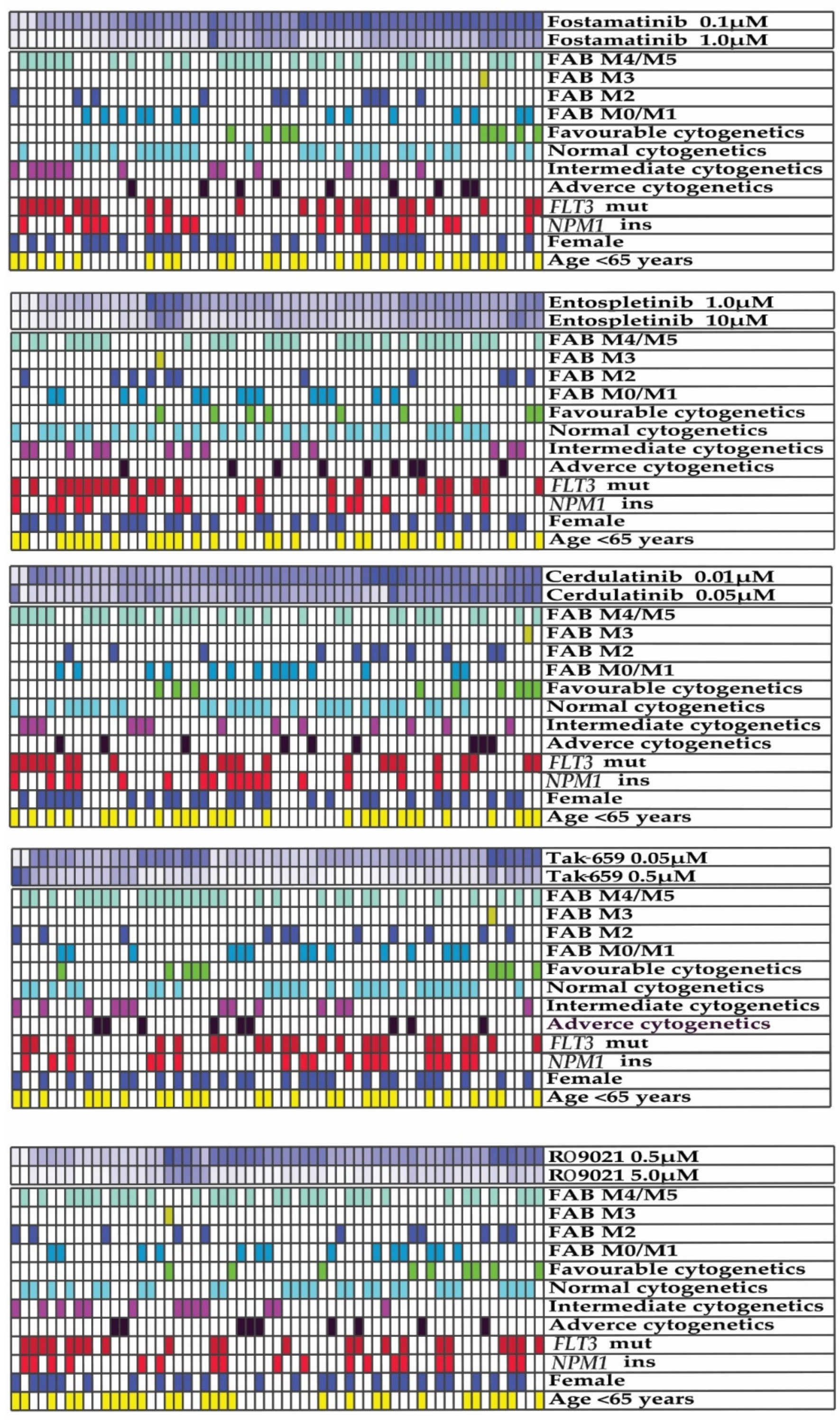
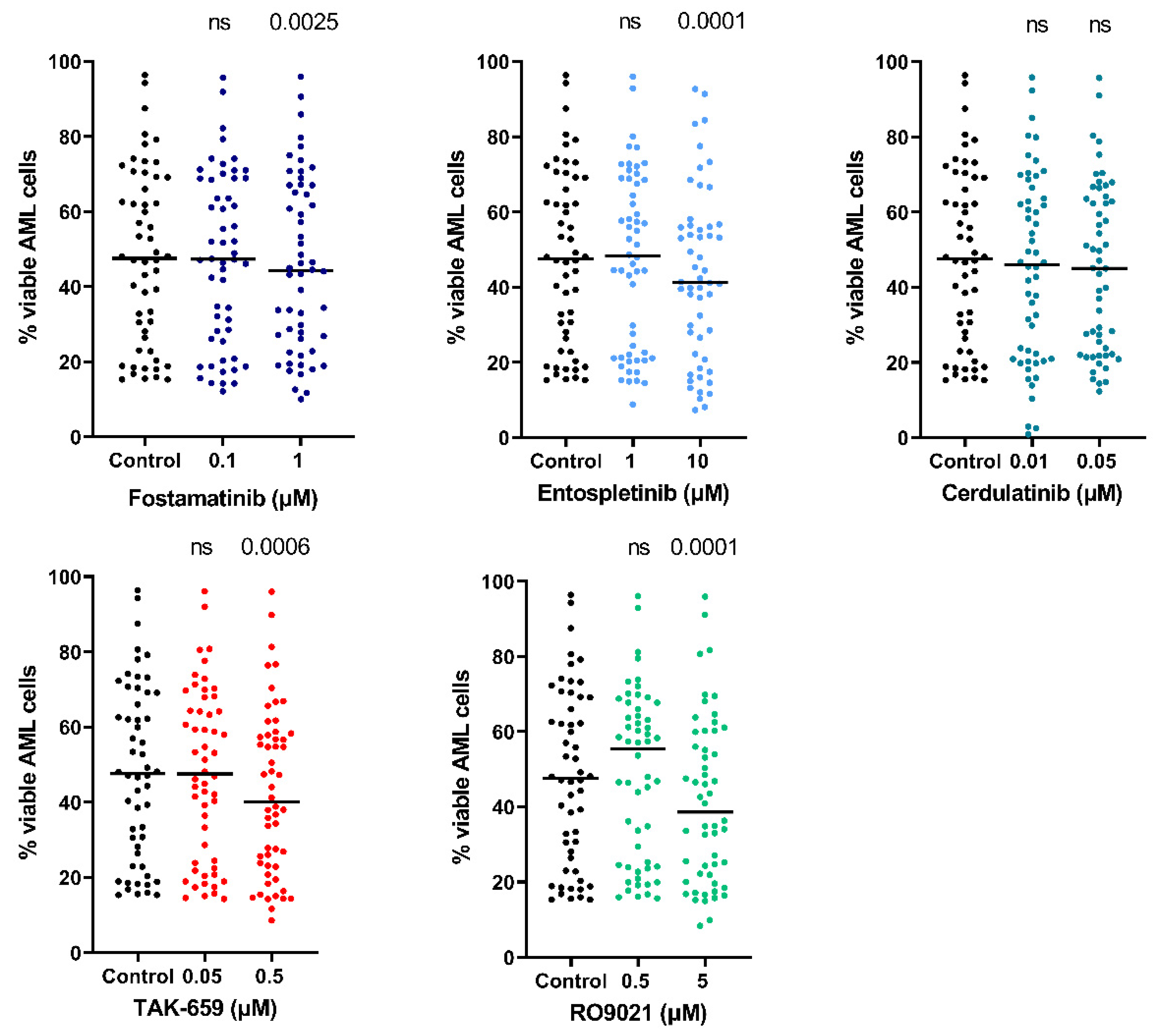
2.3. Antiproliferative Effects of SYK Inhibition Varied between AML Patients
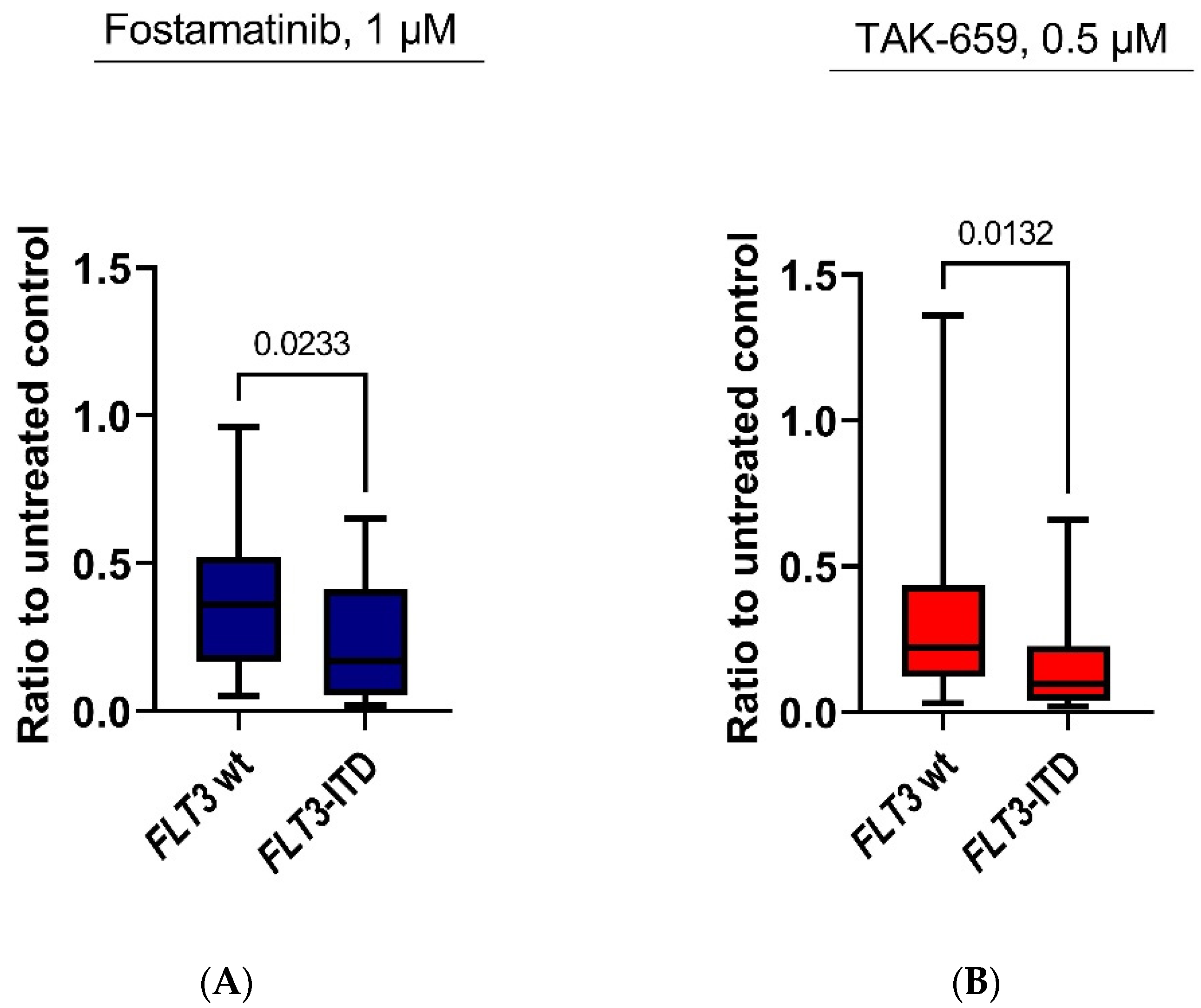
2.4. Antiproliferative Effects of Fostamatinib and TAK-659 Were Significantly Higher in FLT3 Mutated AML Patients
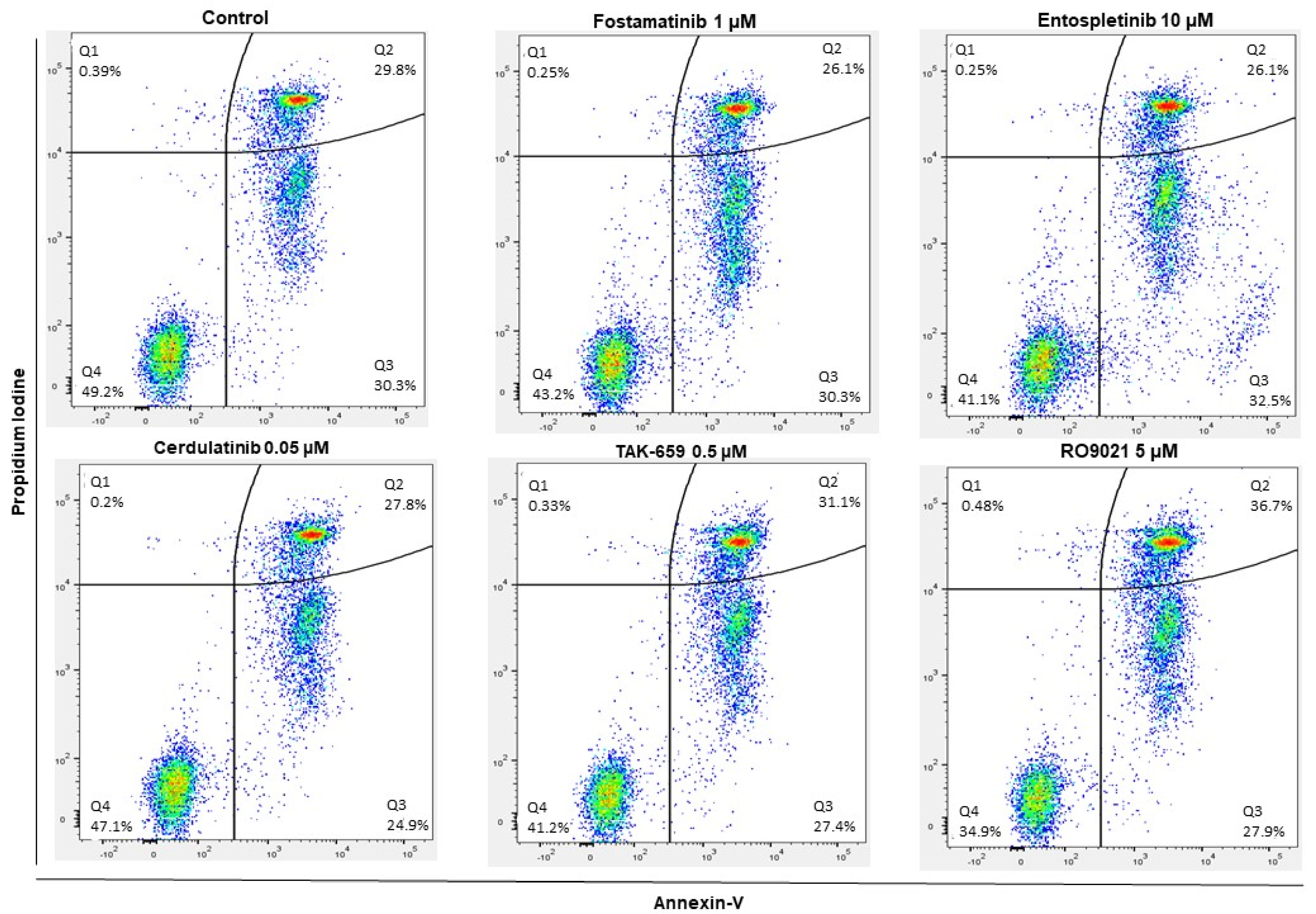
2.5. SYK Inhibitors Decreased AML Cell Viability and Induced Apoptosis
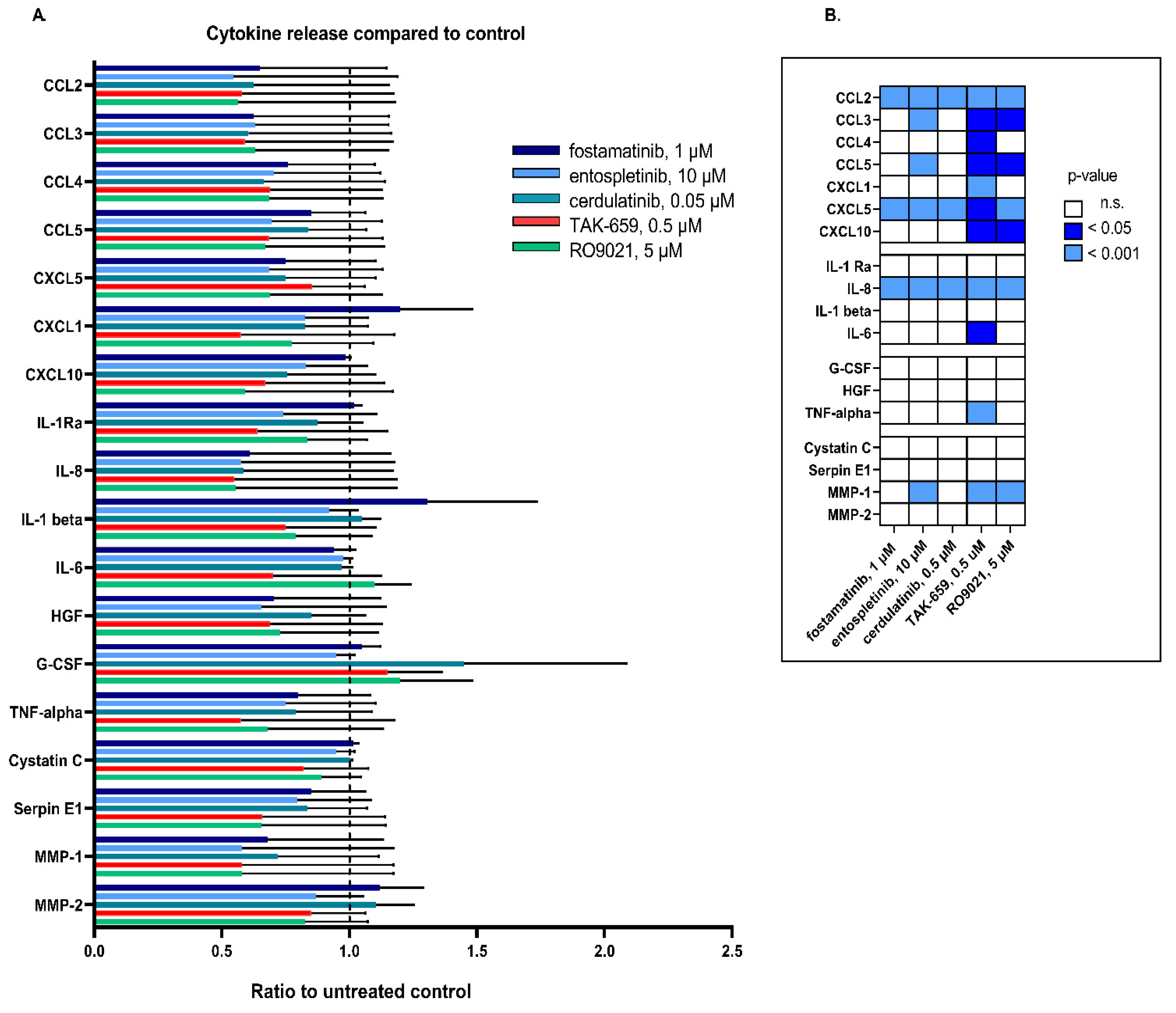
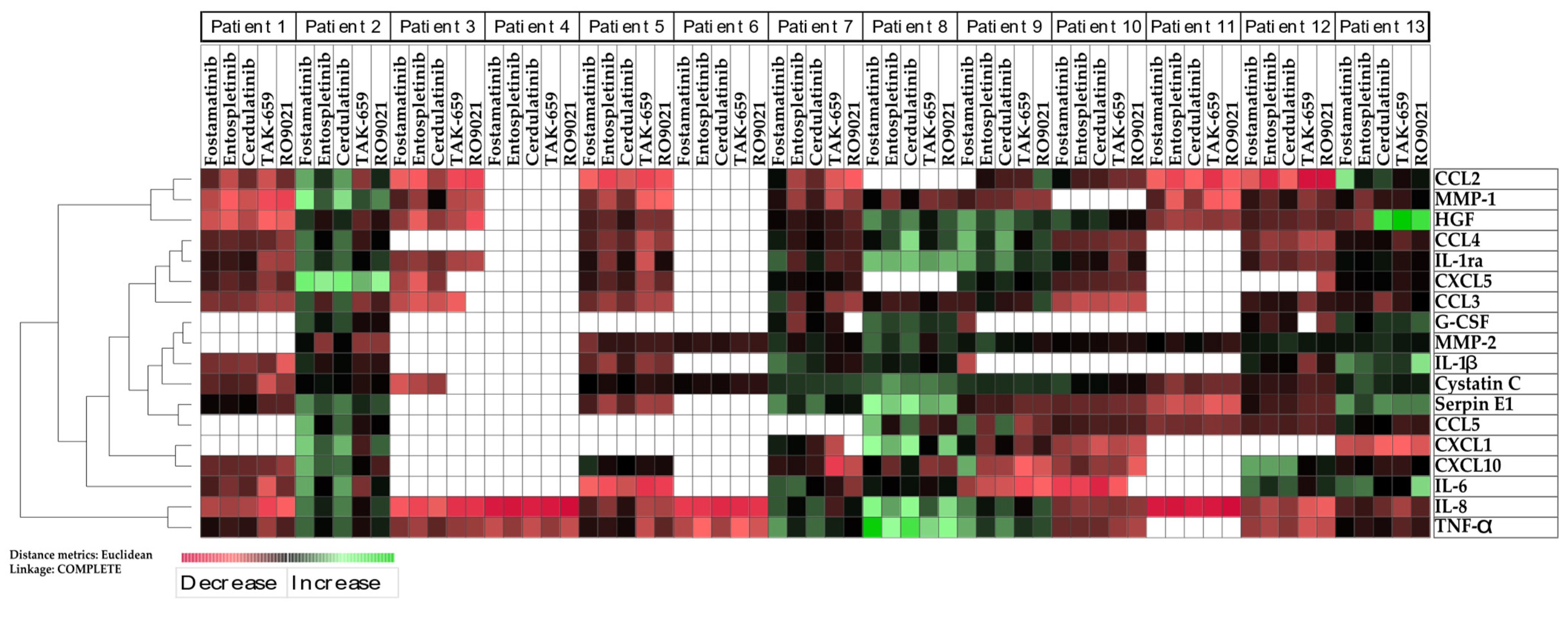
2.6. SYK Inhibition Resulted in Reduced Release of Cytokines
3. Discussion
4. Materials and Methods
4.1. Patients and Primary Human AML Cells
4.2. Reagents
4.3. AML Cell Proliferation Assay
4.4. Apoptosis Assay
4.5. Cytokine Release
4.6. Statistics and Bioinformatics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 ELN Recommendations from an International Expert Panel. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.K.; Berchuck, J.E.; Ross, K.N.; Kakoza, R.M.; Clauser, K.; Schinzel, A.C.; Ross, L.; Galinsky, I.; Davis, T.N.; Silver, S.J.; et al. Proteomic and Genetic Approaches Identify Syk as an AML Target. Cancer Cell 2009, 16, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Hartmut Döhner, M.D.; Daniel, J.; Weisdorf, M.D.; Clara, D.; Bloomfield, M.D. Acute Myeloid Leukemia. NEJM 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Cremer, A.; Ellegast, J.M.; Alexe, G.; Frank, E.S.; Ross, L.; Chu, S.H.; Pikman, Y.; Robichaud, A.; Goodale, A.; Häupl, B.; et al. Resistance Mechanisms to SYK Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2020, 10, 214–231. [Google Scholar] [CrossRef]
- Liu, D.; Mamorska-Dyga, A. Syk Inhibitors in Clinical Development for Hematological Malignancies. J. Hematol. Oncol. 2017, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Doebele, C.; Comoglio, F.; Berg, T.; Beck, J.; Bohnenberger, H.; Alexe, G.; Corso, J.; Ströbel, P.; Wachter, A.; et al. Hoxa9 and Meis1 Cooperatively Induce Addiction to Syk Signaling by Suppressing MiR-146a in Acute Myeloid Leukemia. Cancer Cell 2017, 31, 549–562.e11. [Google Scholar] [CrossRef]
- Polak, A.; Bialopiotrowicz, E.; Krzymieniewska, B.; Wozniak, J.; Stojak, M.; Cybulska, M.; Kaniuga, E.; Mikula, M.; Jablonska, E.; Gorniak, P.; et al. SYK Inhibition Targets Acute Myeloid Leukemia Stem Cells by Blocking Their Oxidative Metabolism. Cell Death Dis. 2020, 11, 956. [Google Scholar] [CrossRef]
- Boros, K.; Puissant, A.; Back, M.; Alexe, G.; Bassil, C.F.; Sinha, P.; Tholouli, E.; Stegmaier, K.; Byers, R.J.; Rodig, S.J. Increased SYK Activity Is Associated with Unfavorable Outcome among Patients with Acute Myeloid Leukemia. Oncotarget 2015, 6, 25575–25587. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Fenouille, N.; Alexe, G.; Pikman, Y.; Bassil, C.F.; Mehta, S.; Du, J.; Kazi, J.U.; Luciano, F.; Rönnstrand, L.; et al. SYK Is a Critical Regulator of FLT3 in Acute Myeloid Leukemia. Cancer Cell 2014, 25, 226–242. [Google Scholar] [CrossRef]
- Oellerich, T.; Oellerich, M.F.; Engelke, M.; Münch, S.; Mohr, S.; Nimz, M.; Hsiao, H.-H.; Corso, J.; Zhang, J.; Bohnenberger, H.; et al. B2 Integrin-Derived Signals Induce Cell Survival and Proliferation of AML Blasts by Activating a Syk/STAT Signaling Axis. Blood 2013, 121, 3889–3899. [Google Scholar] [CrossRef] [PubMed]
- Tomasson, M.H.; Xiang, Z.; Walgren, R.; Zhao, Y.; Kasai, Y.; Miner, T.; Ries, R.E.; Lubman, O.; Fremont, D.H.; McLellan, M.D.; et al. Somatic Mutations and Germline Sequence Variants in the Expressed Tyrosine Kinase Genes of Patients with de Novo Acute Myeloid Leukemia. Blood 2008, 111, 4797–4808. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, J.; Ross, L.; Puissant, A.; Banerji, V.; Stone, R.M.; DeAngelo, D.J.; Ross, K.N.; Stegmaier, K. SYK Regulates MTOR Signaling in AML. Leukemia 2013, 27, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Krisenko, M.O.; Geahlen, R.L. Calling in SYK: SYK’s Dual Role as a Tumor Promoter and Tumor Suppressor in Cancer. Biochim. Biophys. Acta 2015, 1853, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.A.; Bei, L.; Wang, H.; Altman, J.K.; Platanias, L.C.; Eklund, E.A. Cooperation between AlphavBeta3 Integrin and the Fibroblast Growth Factor Receptor Enhances Proliferation of Hox-Overexpressing Acute Myeloid Leukemia Cells. Oncotarget 2016, 7, 54782–54794. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zeng, D.; Shen, Z.; Liao, J.; Wang, X.; Liu, Y.; Zhang, X.; Kong, P. Integrin Alphavbeta3 Enhances β-Catenin Signaling in Acute Myeloid Leukemia Harboring Fms-like Tyrosine Kinase-3 Internal Tandem Duplication Mutations: Implications for Microenvironment Influence on Sorafenib Sensitivity. Oncotarget 2016, 7, 40387–40397. [Google Scholar] [CrossRef]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L.J. The SYK Tyrosine Kinase: A Crucial Player in Diverse Biological Functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R.; Byrd, J.C.; Blachly, J.S.; Bhatnagar, B.; Mims, A.S.; Orwick, S.; Lin, T.L.; Crosswell, H.E.; Zhang, D.; Minden, M.D.; et al. Entospletinib in Combination with Induction Chemotherapy in Previously Untreated Acute Myeloid Leukemia: Response and Predictive Significance of HOXA9 and MEIS1 Expression. Clin. Cancer Res. 2020, 26, 5852–5859. [Google Scholar] [CrossRef]
- Gordon, L.I.; Kaplan, J.B.; Popat, R.; Burris, H.A.; Ferrari, S.; Madan, S.; Patel, M.R.; Gritti, G.; El-Sharkawi, D.; Chau, I.; et al. Phase I Study of TAK-659, an Investigational, Dual SYK/FLT3 Inhibitor, in Patients with B-Cell Lymphoma. Clin. Cancer Res. 2020, 26, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.; Hawkins, M.; Kolibaba, K.; Boxer, M.; Klein, L.; Wu, M.; Hu, J.; Abella, S.; Yasenchak, C. An Open-Label Phase 2 Trial of Entospletinib (GS-9973), a Selective Spleen Tyrosine Kinase Inhibitor, in Chronic Lymphocytic Leukemia. Blood 2015, 125, 2336–2343. [Google Scholar] [CrossRef]
- Friedberg, J.W.; Sharman, J.; Sweetenham, J.; Johnston, P.B.; Vose, J.M.; Lacasce, A.; Schaefer-Cutillo, J.; De Vos, S.; Sinha, R.; Leonard, J.P.; et al. Inhibition of Syk with Fostamatinib Disodium Has Significant Clinical Activity in Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia. Blood 2010, 115, 2578–2585. [Google Scholar] [CrossRef]
- Flinn, I.W.; Bartlett, N.L.; Blum, K.A.; Ardeshna, K.M.; LaCasce, A.S.; Flowers, C.R.; Shustov, A.R.; Thress, K.S.; Mitchell, P.; Zheng, F.; et al. A Phase II Trial to Evaluate the Efficacy of Fostamatinib in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL). Eur. J. Cancer 2016, 54, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sender, S.; Sekora, A.; Villa Perez, S.; Chabanovska, O.; Becker, A.; Ngezahayo, A.; Junghanss, C.; Murua Escobar, H. Precursor B-ALL Cell Lines Differentially Respond to SYK Inhibition by Entospletinib. Int. J. Mol. Sci 2021, 22, 592. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Monti, S.; Juszczynski, P.; Daley, J.; Chen, W.; Witzig, T.E.; Habermann, T.M.; Kutok, J.L.; Shipp, M.A. SYK-Dependent Tonic B-Cell Receptor Signaling Is a Rational Treatment Target in Diffuse Large B-Cell Lymphoma. Blood 2008, 111, 2230–2237. [Google Scholar] [CrossRef]
- Buchner, M.; Fuchs, S.; Prinz, G.; Pfeifer, D.; Bartholomé, K.; Burger, M.; Chevalier, N.; Vallat, L.; Timmer, J.; Gribben, J.G.; et al. Spleen Tyrosine Kinase Is Overexpressed and Represents a Potential Therapeutic Target in Chronic Lymphocytic Leukemia. Cancer Res. 2009, 69, 5424–5432. [Google Scholar] [CrossRef] [PubMed]
- Blunt, M.D.; Koehrer, S.; Dobson, R.C.; Larrayoz, M.; Wilmore, S.; Hayman, A.; Parnell, J.; Smith, L.D.; Davies, A.; Johnson, P.W.M.; et al. The Dual Syk/JAK Inhibitor Cerdulatinib Antagonizes B-Cell Receptor and Microenvironmental Signaling in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 2313–2324. [Google Scholar] [CrossRef]
- Ishikawa, C.; Senba, M.; Mori, N. Anti-Adult T-cell Leukemia/Lymphoma Activity of Cerdulatinib, a Dual SYK/JAK Kinase Inhibitor. Int. J. Oncol. 2018, 53, 1681–1690. [Google Scholar] [CrossRef]
- Purroy, N.; Carabia, J.; Abrisqueta, P.; Egia, L.; Aguiló, M.; Carpio, C.; Palacio, C.; Crespo, M.; Bosch, F. Inhibition of BCR Signaling Using the Syk Inhibitor TAK-659 Prevents Stroma-Mediated Signaling in Chronic Lymphocytic Leukemia Cells. Oncotarget 2017, 8, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Hsu, J.; Kim, Y.; Hu, D.-Q.; Xu, D.; Zhang, J.; Pashine, A.; Menke, J.; Whittard, T.; Romero, N.; et al. Selective Inhibition of Spleen Tyrosine Kinase (SYK) with a Novel Orally Bioavailable Small Molecule Inhibitor, RO9021, Impinges on Various Innate and Adaptive Immune Responses: Implications for SYK Inhibitors in Autoimmune Disease Therapy. Arthritis Res. Ther. 2013, 15, R146. [Google Scholar] [CrossRef]
- Reikvam, H.; Brenner, A.K.; Hagen, K.M.; Liseth, K.; Skrede, S.; Hatfield, K.J.; Bruserud, Ø. The Cytokine-Mediated Crosstalk between Primary Human Acute Myeloid Cells and Mesenchymal Stem Cells Alters the Local Cytokine Network and the Global Gene Expression Profile of the Mesenchymal Cells. Stem Cell Res. 2015, 15, 530–541. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Ryningen, A.; Olsnes, A.M.; Stordrange, L.; Øyan, A.M.; Kalland, K.H.; Gjertsen, B.T. Subclassification of Patients with Acute Myelogenous Leukemia Based on Chemokine Responsiveness and Constitutive Chemokine Release by Their Leukemic Cells. Haematologica 2007, 92, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Grønningsæter, I.S.; Reikvam, H.; Aasebø, E.; Bartaula-Brevik, S.; Tvedt, T.H.; Bruserud, Ø.; Hatfield, K.J. Targeting Cellular Metabolism in Acute Myeloid Leukemia and the Role of Patient Heterogeneity. Cells 2020, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, H.; Nepstad, I.; Sulen, A.; Gjertsen, B.T.; Hatfield, K.J.; Bruserud, Ø. Increased Antileukemic Effects in Human Acute Myeloid Leukemia by Combining HSP70 and HSP90 Inhibitors. Expert. Opin Investig. Drugs 2013, 22, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Day, M.A.; Sergeev, P.; Heckman, C.A.; Schinzel, A.; Obholzer, N.D.; Lin, C.Y.; Kumar, P.; DiMartino, J.; Saffran, D.C. Saffran Preclinical Activity of Selective SYK Inhibitors, Entospletinib and Lanraplenib, Alone or Combined with Targeted Agents in Ex Vivo AML Models with Diverse Mutational Backgrounds. Blood 2021, 138, 3356. [Google Scholar] [CrossRef]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Abel, M.; et al. Functional Genomic Landscape of Acute Myeloid Leukaemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.L.; Puissant, A.; Stone, R.; Sattler, M.; Buhrlage, S.J.; Yang, J.; Manley, P.W.; Meng, C.; Buonopane, M.; Daley, J.F.; et al. Characterization of Midostaurin as a Dual Inhibitor of FLT3 and SYK and Potentiation of FLT3 Inhibition against FLT3-ITD-Driven Leukemia Harboring Activated SYK Kinase. Oncotarget 2017, 8, 52026–52044. [Google Scholar] [CrossRef] [PubMed]
- Richine, B.M.; Virts, E.L.; Bowling, J.D.; Ramdas, B.; Mali, R.; Naoye, R.; Liu, Z.; Zhang, Z.-Y.; Boswell, H.S.; Kapur, R.; et al. Syk Kinase and Shp2 Phosphatase Inhibition Cooperate to Reduce FLT3-ITD-Induced STAT5 Activation and Proliferation of Acute Myeloid Leukemia. Leukemia 2016, 30, 2094–2097. [Google Scholar] [CrossRef]
- Lam, B.; Arikawa, Y.; Cramlett, J.; Dong, Q.; de Jong, R.; Feher, V.; Grimshaw, C.E.; Farrell, P.J.; Hoffman, I.D.; Jennings, A.; et al. Discovery of TAK-659 an Orally Available Investigational Inhibitor of Spleen Tyrosine Kinase (SYK). Bioorg. Med. Chem. Lett. 2016, 26, 5947–5950. [Google Scholar] [CrossRef]
- Bruserud, O.; Gjertsen, B.T.; von Volkman, H.L. In Vitro Culture of Human Acute Myelogenous Leukemia (AML) Cells in Serum-Free Media: Studies of Native AML Blasts and AML Cell Lines. J. Hematother Stem Cell Res. 2000, 9, 923–932. [Google Scholar] [CrossRef]
- Bertho, J.M.; Demarquay, C.; Mouiseddine, M.; Douenat, N.; Stefani, J.; Prat, M.; Paquet, F. Bone Marrow Stromal Cells Spontaneously Produce Flt3-Ligand: Influence of Ionizing Radiations and Cytokine Stimulation. Int. J. Radiat. Biol. 2008, 84, 659–667. [Google Scholar] [CrossRef]
- Solanilla, A.; Grosset, C.; Lemercier, C.; Dupouy, M.; Mahon, F.X.; Schweitzer, K.; Reiffers, J.; Weksler, B.; Ripoche, J. Expression of Flt3-Ligand by the Endothelial Cell. Leukemia 2000, 14, 153–162. [Google Scholar] [CrossRef]
- Ryningen, A.; Ersvaer, E.; Øyan, A.M.; Kalland, K.-H.; Vintermyr, O.K.; Gjertsen, B.T.; Bruserud, Ø. Stress-Induced in Vitro Apoptosis of Native Human Acute Myelogenous Leukemia (AML) Cells Shows a Wide Variation between Patients and Is Associated with Low BCL-2:Bax Ratio and Low Levels of Heat Shock Protein 70 and 90. Leuk. Res. 2006, 30, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Koerber, R.-M.; Held, S.A.E.; Heine, A.; Kotthoff, P.; Daecke, S.N.; Bringmann, A.; Brossart, P. Analysis of the Anti-Proliferative and the pro-Apoptotic Efficacy of Syk Inhibition in Multiple Myeloma. Exp. Hematol. Oncol. 2015, 4, 21. [Google Scholar] [CrossRef]
- Candido, J.; Hagemann, T. Cancer-Related Inflammation. J. Clin. Immunol. 2013, 33 (Suppl. S1), S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, K.M.; Ferwerda, G.; Faro-Trindade, I.; Pyz, E.; Willment, J.A.; Taylor, P.R.; Kerrigan, A.; Tsoni, S.V.; Gordon, S.; Meyer-Wentrup, F.; et al. Syk Kinase Is Required for Collaborative Cytokine Production Induced through Dectin-1 and Toll-like Receptors. Eur. J. Immunol. 2008, 38, 500–506. [Google Scholar] [CrossRef]
- Jacobs, C.F.; Eldering, E.; Kater, A.P. Kinase Inhibitors Developed for Treatment of Hematologic Malignancies: Implications for Immune Modulation in COVID-19. Blood Adv. 2021, 5, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Aasebø, E.; Berven, F.S.; Hovland, R.; Døskeland, S.O.; Bruserud, Ø.; Selheim, F.; Hernandez-Valladares, M. The Progression of Acute Myeloid Leukemia from First Diagnosis to Chemoresistant Relapse: A Comparison of Proteomic and Phosphoproteomic Profiles. Cancers 2020, 12, 1466. [Google Scholar] [CrossRef]
- Reikvam, H.; Hemsing, A.L.; Smith, C. Therapy for Acute Myelogenous Leukemia Revisited: Moving Away from a One-Size-Fits-All Approach. Expert Rev. Anticancer Ther. 2021, 21, 5–8. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab Ozogamicin for de Novo Acute Myeloid Leukemia: Final Efficacy and Safety Updates from the Open-Label, Phase III ALFA-0701 Trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Cortes, J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.; Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O.; et al. Randomized Comparison of Low Dose Cytarabine with or without Glasdegib in Patients with Newly Diagnosed Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndrome. Leukemia 2019, 33, 379–389. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Kantarjian, H.M.; Levis, M.; Guerra, V.; Borthakur, G.; Alvarado, Y.; DiNardo, C.D.; Kadia, T.; Garcia-Manero, G.; Ohanian, M.; et al. A Phase I/II Study of the Combination of Quizartinib with Azacitidine or Low-Dose Cytarabine for the Treatment of Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. Haematologica 2021, 106, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in Mutant IDH2 Relapsed or Refractory Acute Myeloid Leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef]
- Newland, A.; McDonald, V. Fostamatinib: A Review of Its Clinical Efficacy and Safety in the Management of Chronic Adult Immune Thrombocytopenia. Immunotherapy 2020, 12, 1325–1340. [Google Scholar] [CrossRef]
- Burke, J.M.; Shustov, A.; Essell, J.; Patel-Donnelly, D.; Yang, J.; Chen, R.; Ye, W.; Shi, W.; Assouline, S.; Sharman, J. An Open-Label, Phase II Trial of Entospletinib (GS-9973), a Selective Spleen Tyrosine Kinase Inhibitor, in Diffuse Large B-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2018, 18, e327–e331. [Google Scholar] [CrossRef]
- Boccia, R.; Cooper, N.; Ghanima, W.; Boxer, M.A.; Hill, Q.A.; Sholzberg, M.; Tarantino, M.D.; Todd, L.K.; Tong, S.; Bussel, J.B.; et al. Fostamatinib Is an Effective Second-Line Therapy in Patients with Immune Thrombocytopenia. Br. J. Haematol. 2020, 190, 933–938. [Google Scholar] [CrossRef]
- Hamlin, P.A.; Flinn, I.W.; Wagner-Johnston, N.; Burger, J.A.; Coffey, G.P.; Conley, P.B.; Michelson, G.; Leeds, J.M.; Der, K.; Kim, Y.; et al. Efficacy and Safety of the Dual SYK/JAK Inhibitor Cerdulatinib in Patients with Relapsed or Refractory B-Cell Malignancies: Results of a Phase I Study. Am. J. Hematol. 2019, 94, E90–E93. [Google Scholar] [CrossRef]
- Zeng, A.G.X.; Bansal, S.; Jin, L.; Mitchell, A.; Chen, W.C.; Abbas, H.A.; Chan-Seng-Yue, M.; Voisin, V.; van Galen, P.; Tierens, A.; et al. A Cellular Hierarchy Framework for Understanding Heterogeneity and Predicting Drug Response in Acute Myeloid Leukemia. Nat. Med. 2022, 28, 1212–1223. [Google Scholar] [CrossRef]
- Schiller, G. How Thinly Can One Slice the AML Diagnostic Pie? Blood 2022, 140, 1330–1331. [Google Scholar] [CrossRef] [PubMed]
- FlowJoTM Software, version 10.8.0; Becton, Dickinson and Company: East Rutherford, NJ, USA, 2021.
- STATA Statistical Software, version 17; StataCorp. LLC.: College Station, TX, USA, 2021.
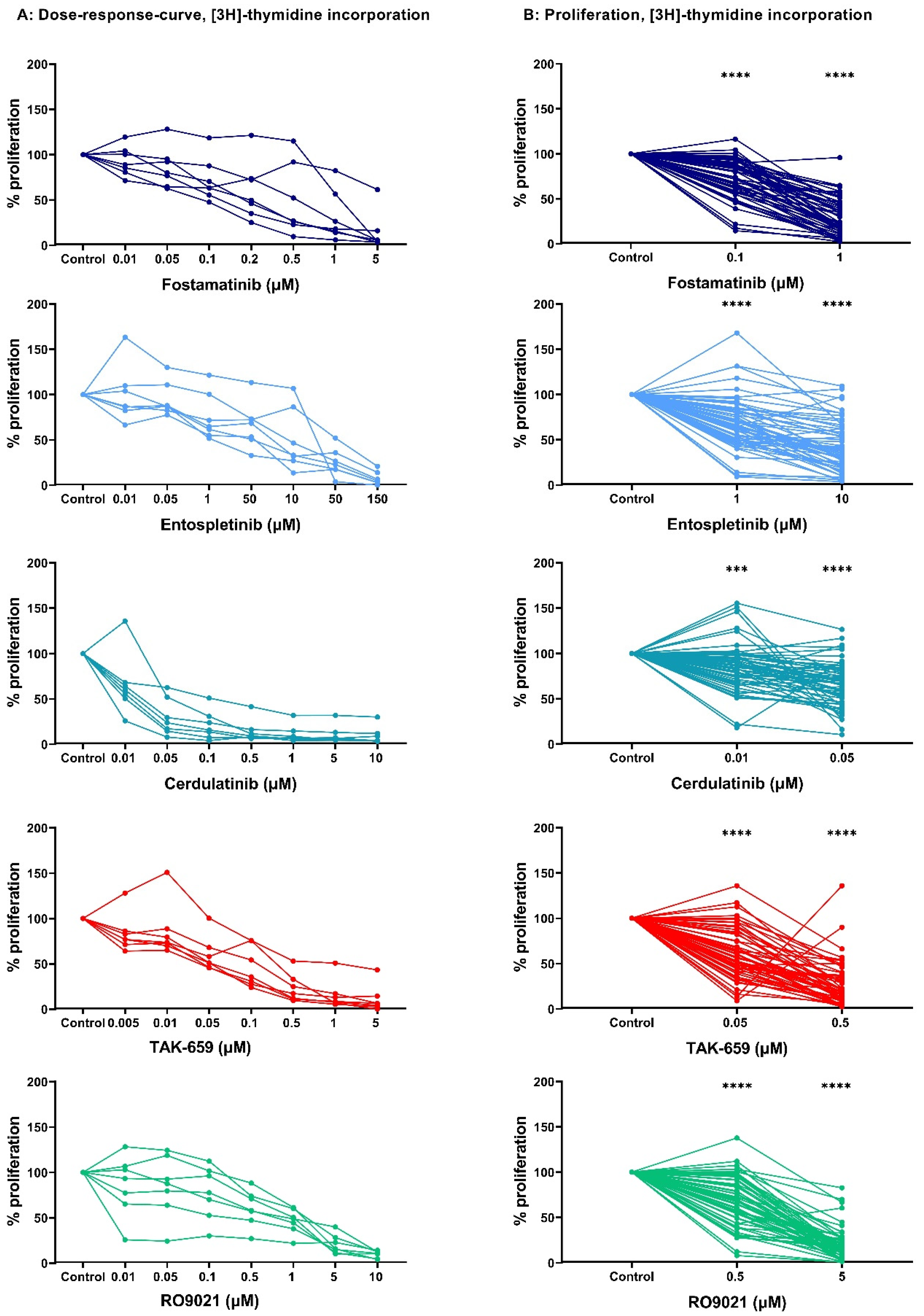
| Inhibitor, Concentration | Median Percent Proliferation Compared to Control (Range) |
|---|---|
| Fostamatinib, 1 µM Fostamatinib, 0.1 µM | 27% (2–96) 74% (14–116) |
| Entospletinib, 10 µM Entospletinib, 1 µM | 36% (4–109) 65% (9–101) |
| Cerdulatinib, 0.05 µM Cerdulatinib, 0.01 µM | 66% (10–126) 86% (18–105) |
| TAK-659, 0.5 µM TAK-659, 0.05 µM | 19% (2–112) 56% (9–103) |
| RO9021, 5 µM RO9021, 0.5 µM | 20% (3–88) 66% (8–103) |
| Cytokine | Number of Patients with Detectable Release | Median Levels (pg/mL) | Variation Range (pg/mL) |
|---|---|---|---|
| Chemokines | |||
| CCL 2 | 8 | 1478 | 35.1–10,521 |
| CCL3 | 11 | 1745 | 75–17,933 |
| CCL4 | 11 | 980 | 91–12,622 |
| CCL5 | 10 | 106 | 6.0–675 |
| CXCL1 | 7 | 1007 | 59–6965 |
| CXCL5 | 10 | 1064 | 178–16,771 |
| CXCL10 | 11 | 8.9 | 0.2–290 |
| Interleukins | |||
| IL-1 Ra | 11 | 2453 | 134–29,599 |
| IL-8 | 12 | 11,266 | 164–111,476 |
| IL-1β | 9 | 24.6 | 6.4–458 |
| IL-6 | 9 | 55 | 8.9–956 |
| Growth factors | |||
| G-CSF | 8 | 28.1 | 5.6–141 |
| HGF | 11 | 65 | 14–806 |
| TNF-α | 13 | 11.6 | 1–495 |
| Protease system | |||
| Cystatin C | 12 | 5302 | 1379–13,223 |
| Serpin-E1 | 10 | 370 | 37.2–12,243 |
| MMP-1 | 11 | 139 | 19.5–519 |
| MMP-2 | 10 | 2808 | 1079–8714 |
| Patient Characteristics | Observations | |
|---|---|---|
| Demographic Data and Disease History | ||
| Gender (number, percent) | Female/male | 31/37 (46/54) |
| Age, years (median, range) | 62 (17–87) | |
| Disease History (number, percent) | De novo | 51 (75) |
| Secondary | 15 (22) | |
| Relapse | 2 (3) | |
| Hematology (mean, range) | ||
| Hemoglobin, g/dL | 9.9 (6.3–15.4) | |
| Platelets, ×109/L | 77 (5–258) | |
| AML cell differentiation (number, percent) | ||
| FAB | M0-1 | 13 (19) |
| M2 | 13 (19) | |
| M3 | 1 (2) | |
| M4-5 | 38 (56) | |
| CD34 | Unclassified Positive (≥30%) Negative (≤30%) Unknown | 3 (4) 37 (54) 27 (40) 4 (6) |
| Cytogenetic and genetic abnormalities (number, percent) | ||
| Cytogenetics * | Favorable | 9 (13) |
| Intermediate | 15 (22) | |
| Normal | 31 (46) | |
| Adverse | 11 (16) | |
| Unknown | 2 (3) | |
| FLT3 | Wild type | 39 (57) |
| ITD TKD | 24 (37) 1 (2) | |
| Unknown | 4 (6) | |
| NPM1 | Wild type | 44 (65) |
| Insertion | 22 (32) | |
| Unknown | 2 (3) | |
| Inhibitor | Molecular Weight | Pharmacological Properties | Company | Chemical Structure | Cas Number |
|---|---|---|---|---|---|
| Fostamatinib Disodium (R788) | 624.42 | Oral prodrug of the active compound R406. Competitive SYK/FLT3 inhibitor. Demonstrated in vitro antiproliferative effect in concentrations of 0.8–8.1 µM to DLBCL [23] and 41 nM to CLL [24]. | MedChemExpress (Monmouth Junction, NJ, USA) | C23H24FN6Na2O9P | 1025687-58-4 |
| Entospletinib (GS-9973) | 411.46 | Orally bioavailable, selective SYK inhibitor. Induced antiproliferative and proapoptotic effects in vitro in pre-B-ALL and pro-B-ALL cell lines with concentrations of 0.001–20 µM [22]. | MedChemExpress (Monmouth Junction, NJ, USA) | C23H21N7O | 1229208-44-9 |
| Cerdulatinib (PRT062070; PRT2070) | 482 | Orally active, multi-targeted tyrosine kinase inhibitor to JAK1/JAK2/JAK3/TYK2 and SYK. Induced apoptosis of CLL cells in vitro with concentrations of 0.3–1 µM [25], suppressed cell proliferation, and reduced cell viability in T-cell lines with a concentration of 10 µM [26]. | Selleckchem (Houston, TX, USA) | C20H27N7O3S | 1198300-79-6 |
| TAK-659 | 380.85 | Orally bioavailable, selective SYK inhibitor. Selective against most other kinases, but potent toward SYK and FLT3. Induced in vitro apoptosis of CLL cells in concentrations of 0.1–10 µM [27]. | Selleckchem (Houston, TX, USA) | C17H21FN6HCl | 1952251-28-3 |
| RO9021 | 355.44 | Potent SYK inhibitor. Suppresses BCR signaling and B-cell proliferation at 1 µM [28]. | Selleckchem (Houston, TX, USA) | C18H25N7O | 1446790-62-0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brattås, M.K.; Hemsing, A.L.; Rye, K.P.; Hatfield, K.J.; Reikvam, H. Heterogeneity of Patient-Derived Acute Myeloid Leukemia Cells Subjected to SYK In Vitro Inhibition. Int. J. Mol. Sci. 2022, 23, 14706. https://doi.org/10.3390/ijms232314706
Brattås MK, Hemsing AL, Rye KP, Hatfield KJ, Reikvam H. Heterogeneity of Patient-Derived Acute Myeloid Leukemia Cells Subjected to SYK In Vitro Inhibition. International Journal of Molecular Sciences. 2022; 23(23):14706. https://doi.org/10.3390/ijms232314706
Chicago/Turabian StyleBrattås, Marte Karen, Anette Lodvir Hemsing, Kristin Paulsen Rye, Kimberley Joanne Hatfield, and Håkon Reikvam. 2022. "Heterogeneity of Patient-Derived Acute Myeloid Leukemia Cells Subjected to SYK In Vitro Inhibition" International Journal of Molecular Sciences 23, no. 23: 14706. https://doi.org/10.3390/ijms232314706
APA StyleBrattås, M. K., Hemsing, A. L., Rye, K. P., Hatfield, K. J., & Reikvam, H. (2022). Heterogeneity of Patient-Derived Acute Myeloid Leukemia Cells Subjected to SYK In Vitro Inhibition. International Journal of Molecular Sciences, 23(23), 14706. https://doi.org/10.3390/ijms232314706






