Impairment of Vitamin D Nutritional Status and Metabolic Profile Are Associated with Worsening of Obesity According to the Edmonton Obesity Staging System
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Size
4.2. Evaluation of Anthropometric Parameters and Physical Activity
4.3. Evaluation of Clinical, Biochemical and Metabolic Parameters
4.4. Diagnosis of Metabolic Syndrome (MS)
4.5. Diagnosis of MAFLD—Liver Biopsy
4.5.1. Macrovesicular Steatosis
4.5.2. Necroinflammatory Activity (NASH)
4.6. Vitamin D Status
4.7. Edmonton Obesity Staging System (EOSS)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 22 October 2018).
- Ministério da Saúde. Vigitel Brasil 2019: Vigilância de Fatores de Risco para Doenças Crônicas por Inquérito Telefônico. Update 2019. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2019_vigilancia_fatores_risco.pdf (accessed on 22 October 2020).
- Sharma, A.M.; Kushner, R.F. A proposed clinical staging system for obesity. Int. J. Obes. 2009, 33, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Pataky, Z.; Bobbioni-Harsch, E.; Golay, A. Déficiences micronutritionnelles chez les patients obèses. Rev. Med. Suisse 2013, 9, 664–669. [Google Scholar]
- Krzizek, E.C.; Briz, J.M.; Herz, C.T.; Kopp, H.P.; Schernthaner, G.-H.; Schernthaner, G.; Ludvik, B. Prevalence of Micronutrient Deficiency in Patients with Morbid Obesity Before Bariatric Surgery. Obes. Surg. 2018, 28, 643–648. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, S.; Stier, C.; Squillante, S.; Theodoridou, S.; Weiner, R.A. The importance of the Edmonton Obesity Staging System in predicting postoperative outcome and 30-day mortality after metabolic surgery Manuscript type: Original contribution. Surg. Obes. Relat. Dis. 2016, 12, 1847–1855. [Google Scholar] [CrossRef]
- Ramuth, H.; Hunma, S.; Ramessur, V.; Ramuth, M.; Monnard, C.; Montani, J.P.; Schutz, Y.; Joonas, N.; Dulloo, A.G. Body composition-derived BMI cut-offs for overweight and obesity in ethnic Indian and Creole urban children of Mauritius. Br. J. Nutr. 2020, 124, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Sahebolamri, M.; Cheema, B.S.; Williams, K. Usefulness of the Edmonton Obesity Staging System for stratifying the presence and severity of weight-related health problems in clinical and community settings: A rapid review of observational studies. Obes. Rev. 2020, 21, 1–26. [Google Scholar] [CrossRef]
- Canning, K.L.; Brown, R.E.; Wharton, S.; Sharma, A.M.; Kuk, J.L. Edmonton Obesity Staging System Prevalence and Association with Weight Loss in a Publicly Funded Referral-Based Obesity Clinic. J. Obes. 2015, 2015, 619734. [Google Scholar] [CrossRef] [PubMed]
- Skulsky, S.L.; Dang, J.T.; Battiston, A.; Switzer, N.; Birch, D.; Sharma, A.; Karmali, S. Higher Edmonton Obesity Staging System scores are associated with complications following laparoscopic Roux-en-Y gastric bypass. Surg. Endosc. 2020, 34, 3102–3109. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, F.; Haghighyfard, K.; Salami, M.S.; Ghadiri-Anari, A. Relationship Between Vitamin D Deficiency and Markers of Metabolic Syndrome Among Overweight and Obese Adults. Acta Med. Iran. 2017, 55, 399–403. [Google Scholar]
- Teixeira, J.S.; Campos, A.B.F.; Cordeiro, A.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Vitamin D nutritional status and its relationship with metabolic changes in adolescents and adults with severe obesity. Nutr. Hosp. 2018, 35, 847–853. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Mechanisms Involved in the Relationship between Vitamin D and Insulin Resistance: Impact on Clinical Practice. Nutrients 2021, 13, 3491. [Google Scholar] [CrossRef]
- Ozougwu, J.C.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef]
- Ilinčić, B.; Stokić, E.; Stošić, Z.; Kojić, N.; Katsiki, N.; Mikhailidis, D.; Isenovic, E.R. Vitamin D status and circulating biomarkers of endothelial dysfunction and inflammation in non-diabetic obese individuals: A pilot study. Arch. Med. Sci. 2017, 1, 53–60. [Google Scholar] [CrossRef]
- Xie, D.D.; Chen, Y.H.; Xu, S.; Zhang, C.; Wang, D.M.; Wang, H.; Chen, L.; Zhang, Z.H.; Xia, M.Z.; Xu, D.X.; et al. Low vitamin D status is associated with inflammation in patients with prostate cancer. Oncotarget 2017, 8, 22076–22085. [Google Scholar] [CrossRef]
- Greco, E.A.; Lenzi, A.; Migliaccio, S. Role of hypovitaminosis D in the Pathogenesis of Obesity-Induced Insulin Resistance. Nutrients 2019, 11, 1506. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanian, H.; Djazayery, A.; Javanbakht, M.H.; Eshraghian, M.R.; Mirshafiey, A.; Jahanabadi, S.; Ghadbeigi, S.; Zarei, M.; Alvandi, E.; Djalali, M. Vitamin D downregulates key genes of diabetes complications in cardiomyocyte. J. Cell. Physiol. 2019, 234, 21352–21358. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid. Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Ionica, M.; Aburel, O.M.; Văduva, A.O.; Petrus, A.; Rațiu, S.; Olariu, S.; Sturza, A.; Muntean, M.-D. Vitamin D alleviates oxidative stress in adipose tissue and mesenteric vessels from obese patients with subclinical inflammation. Can. J. Physiol. Pharmacol. 2020, 98, 85–92. [Google Scholar] [CrossRef]
- National Kidney Foundation. Clinical Practice Guidelines: For Chronic Kidnet Disease: Evaluation, Classification and Stratification. Updated 1 January 2013. Available online: https://www.kidney.org/professionals/guidelines/guidelines_commentaries/chronic-kidney-disease-classification (accessed on 22 October 2019).
- World Health Organization. Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation. Updated December 2008. Available online: http://whqlibdoc.who.int/publications/2011/9789241501491_eng.pdf (accessed on 22 October 2018).
- Zeng, Q.; He, Y.; Dong, S.; Zhao, X.; Chen, Z.; Song, Z.; Chang, G.; Yang, F.; Wang, Y. Optimal cut-off values of BMI, waist circumference and waist: Height ratio for defining obesity in Chinese adults. Br. J. Nutr. 2014, 112, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Brellenthin, A.G.; Lee, D.; Bennie, J.A.; Sui, X.; Blair, S.N. Resistance exercise, alone and in combination with aerobic exercise, and obesity in Dallas, Texas, US: A prospective cohort study. PLoS Med. 2021, 18, e1003687. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- The Oxford Centre for Diabetes. Endocrinology & Metabolism. Diabetes Trial Unit. HOMA Calculator. Updated March 2009. Available online: http://www.dtu.ox.ac.uk/ (accessed on 22 October 2018).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.Á.; Gordon, C.; Hanley, D.; Heaney, R.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Hanwell, H.E.; Vieth, R.; Cole, D.E.; Scillitani, A.; Modoni, S.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun exposure questionnaire predicts circulating 25-hydroxyvitamin D concentrations in Caucasian hospital workers in southern Italy. J. Steroid. Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
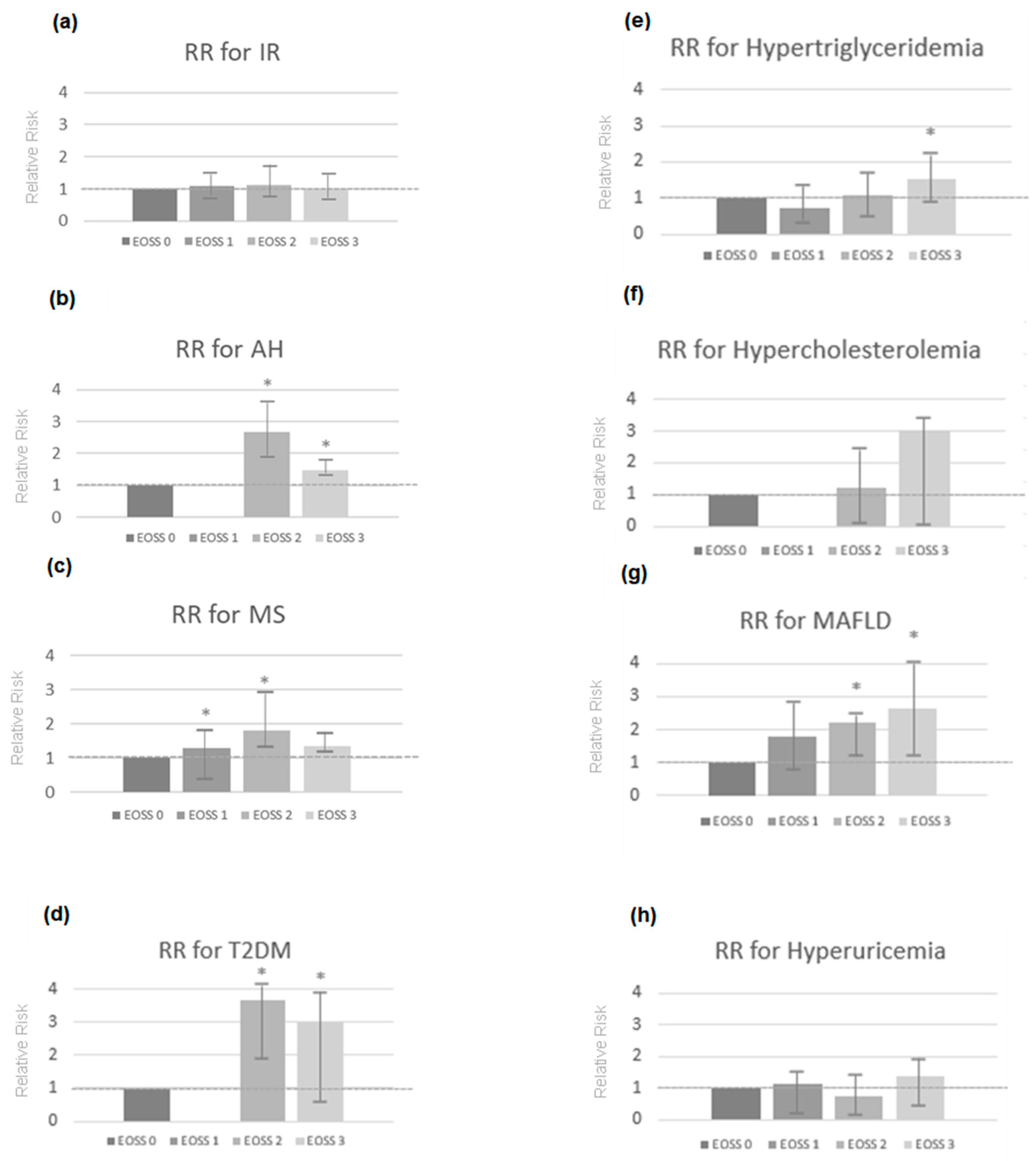

| Metabolic Changes and Comorbidities | EOSS 0 n = 3 | EOSS 1 n = 50 | EOSS 2 n = 142 | EOSS 3 n = 31 | TOTAL | ||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | p | n (%) | p | n (%) | p | n (%) | p | n | |
| IR | 0 (0.0%) | 0.202 | 36 (20.5%) | 0.147 | 115 (65.3%) | 0.127 | 25 (14.2%) | 0.887 | 176 |
| AH | 0 (0.0%) | 0.002 | 0 (0.0%) | ≤0.001 | 133 (81.6%) | ≤0.001 | 30 (18.4%) | ≤0.001 | 163 |
| MS | 0 (0.0%) | 0.242 | 12 (7.6%) | 0.004 | 118 (75.2%) | ≤0.001 | 29 (18.5%) | 0.007 | 159 |
| T2DM | 0 (0.0%) | 0.331 | 0 (0.0%) | 0.135 | 43 (86.0%) | ≤0.001 | 7 (14.0%) | 0.959 | 50 |
| Hypertriglyceridemia | 0 (0.0%) | 0.111 | 15 (17.1%) | 0.277 | 56 (63.6%) | 0.401 | 17 (19.3%) | 0.026 | 88 |
| Hypercholesterolemia | 0 (0.0%) | 0.817 | 0 (0.0%) | 0.335 | 2 (66.7%) | 0.881 | 1 (33.3%) | 0.340 | 3 |
| MAFLD | 0 (0.0%) | 0.432 | 3 (6.0%) | 0.065 | 19 (38.0%) | 0.008 | 28 (56.0%) | 0.004 | 50 |
| Hyperuricemia | 0 (0.0%) | 0.739 | 17 (23.8%) | 0.231 | 42 (57.5%) | 0.112 | 14 (19.2%) | 0.201 | 73 |
| EOSS Stage | Conceptual EOSS Definition | Features Definition |
|---|---|---|
| 0 | No apparent obesity-related risk factors, physical symptoms, psychopathology, functional limitations, and/or impairments of well-being. | No EOSS factors were reported. |
| 1 | Presence of obesity-related subclinical risk factors, mild physical symptoms, mild psychopathology, mild functional limitations, and/or impairment of well-being. | Any of the following:
|
| 2 | Presence of established obesity-related chronic disease, moderate limitations in activities of daily living, and/or well-being. | Any of the following:
|
| 3 | Established end-organ damage, significant psychopathology, significant functional limitations, and/or impairment of well-being. | Any of the following:
|
| 4 | Severe (potentially end-stage) disabilities from obesity-related chronic diseases, disabling psychopathology, functional limitations, and/or impairment of well-being. | No data on these factors available to evaluate this stage. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, A.; Luna, M.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Impairment of Vitamin D Nutritional Status and Metabolic Profile Are Associated with Worsening of Obesity According to the Edmonton Obesity Staging System. Int. J. Mol. Sci. 2022, 23, 14705. https://doi.org/10.3390/ijms232314705
Cordeiro A, Luna M, Pereira SE, Saboya CJ, Ramalho A. Impairment of Vitamin D Nutritional Status and Metabolic Profile Are Associated with Worsening of Obesity According to the Edmonton Obesity Staging System. International Journal of Molecular Sciences. 2022; 23(23):14705. https://doi.org/10.3390/ijms232314705
Chicago/Turabian StyleCordeiro, Adryana, Mariana Luna, Silvia Elaine Pereira, Carlos José Saboya, and Andrea Ramalho. 2022. "Impairment of Vitamin D Nutritional Status and Metabolic Profile Are Associated with Worsening of Obesity According to the Edmonton Obesity Staging System" International Journal of Molecular Sciences 23, no. 23: 14705. https://doi.org/10.3390/ijms232314705
APA StyleCordeiro, A., Luna, M., Pereira, S. E., Saboya, C. J., & Ramalho, A. (2022). Impairment of Vitamin D Nutritional Status and Metabolic Profile Are Associated with Worsening of Obesity According to the Edmonton Obesity Staging System. International Journal of Molecular Sciences, 23(23), 14705. https://doi.org/10.3390/ijms232314705




