In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A
Abstract
:1. Introduction
2. Results
2.1. Sperm Motility
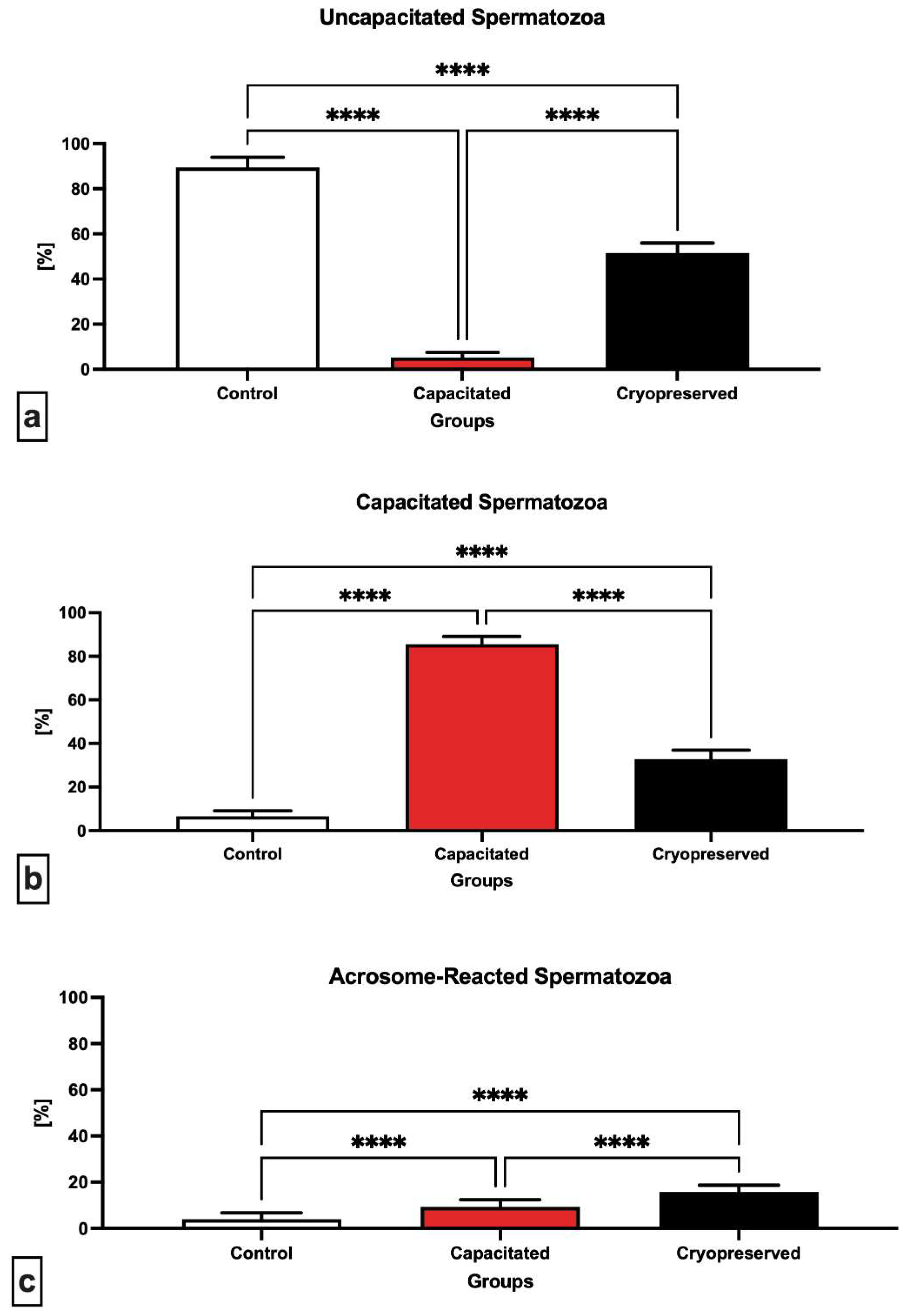
2.2. Capacitation Status
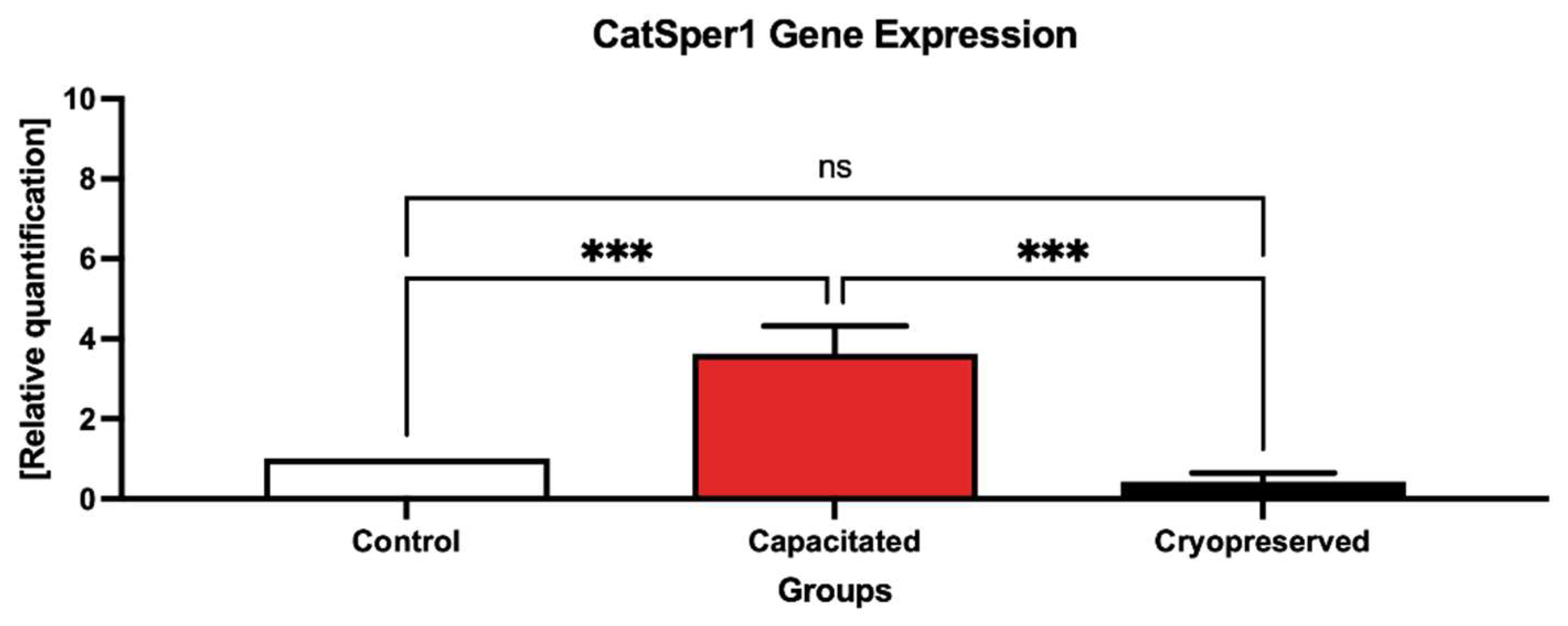
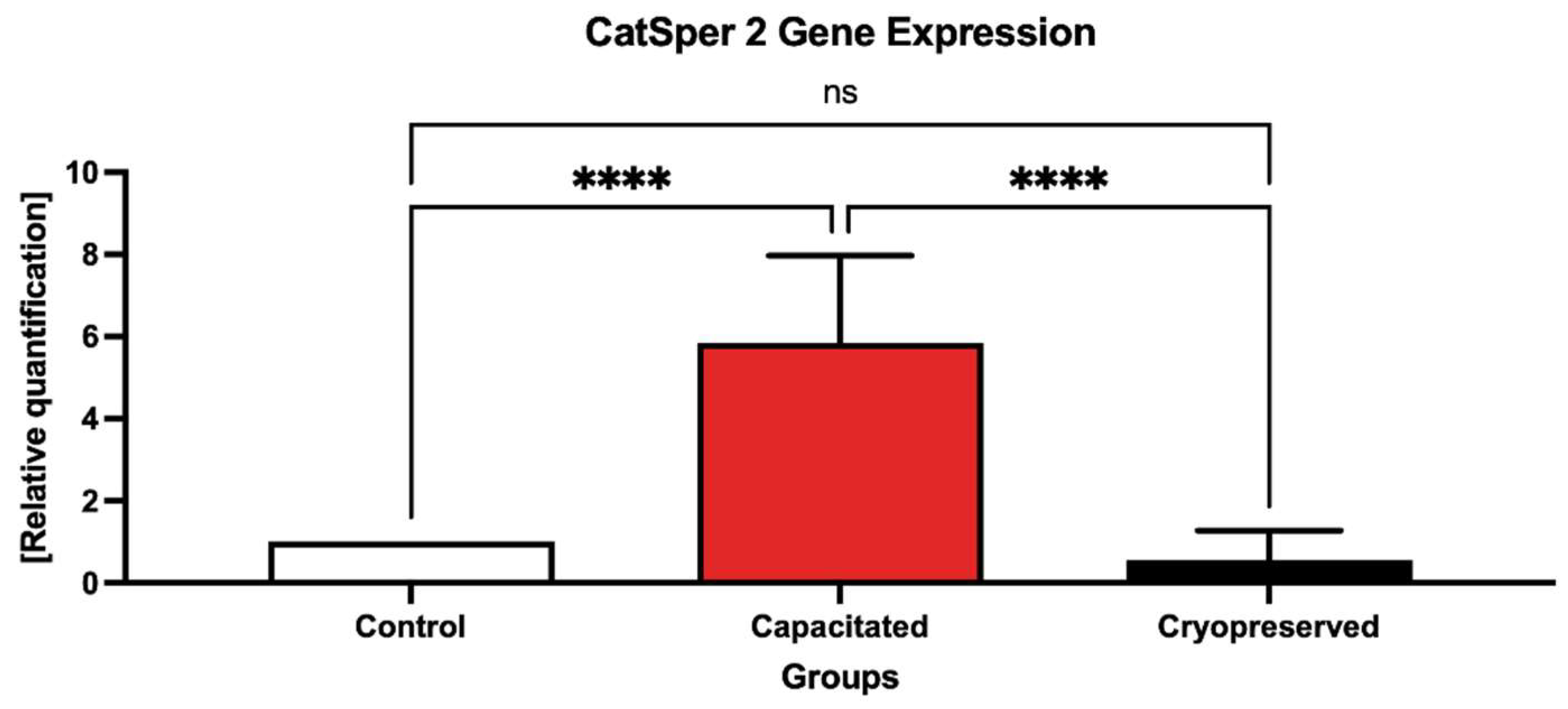
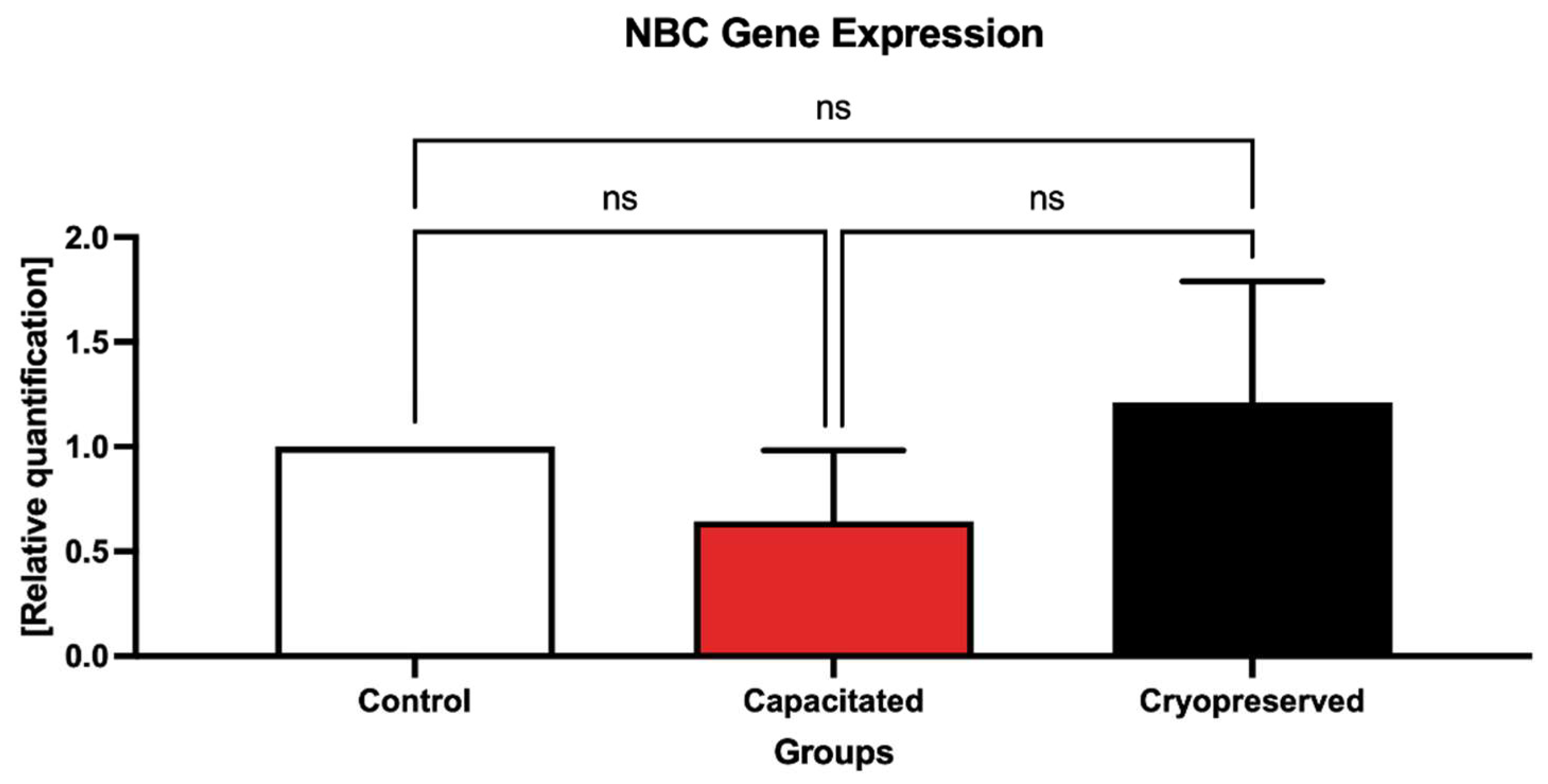
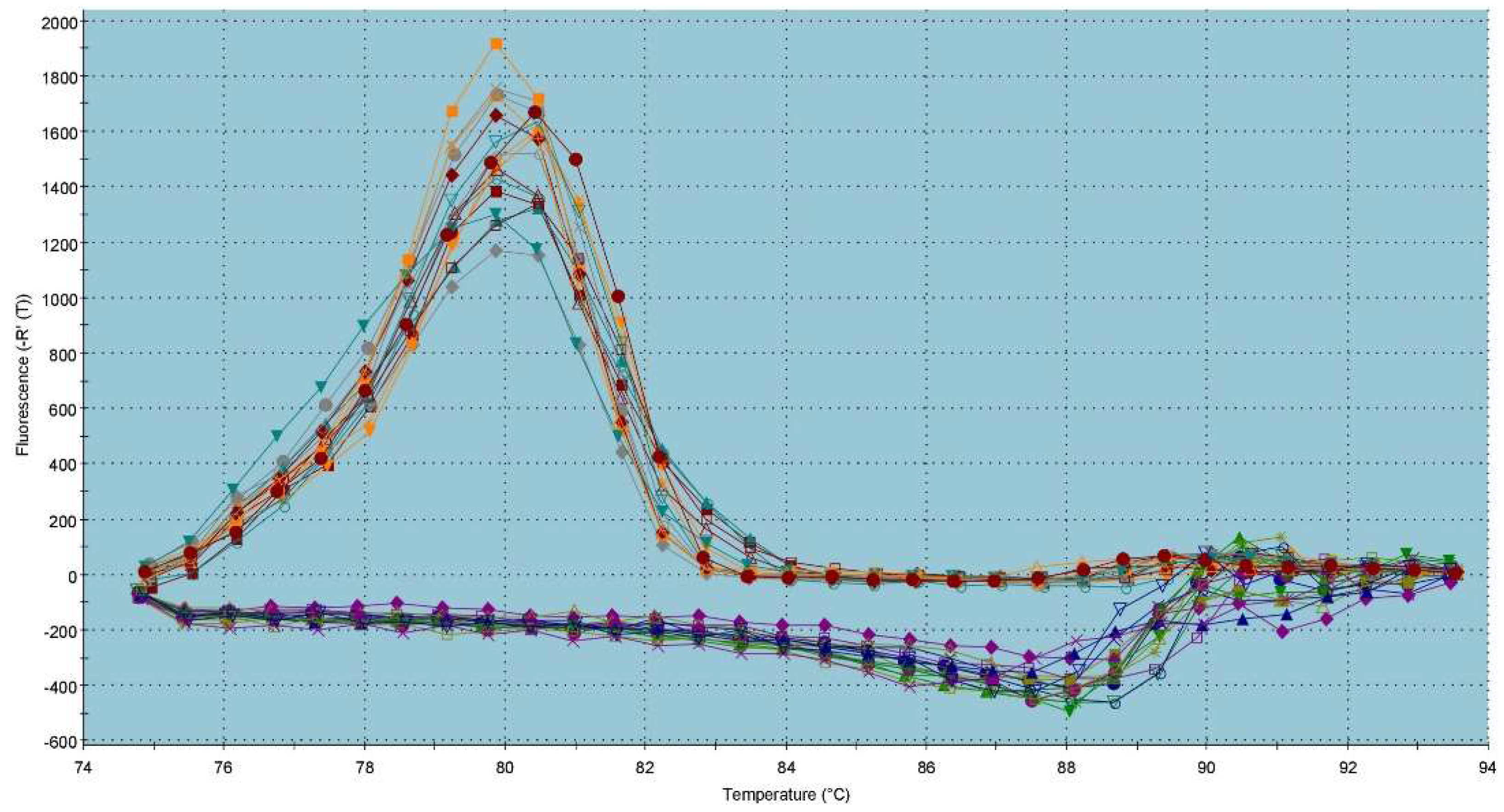
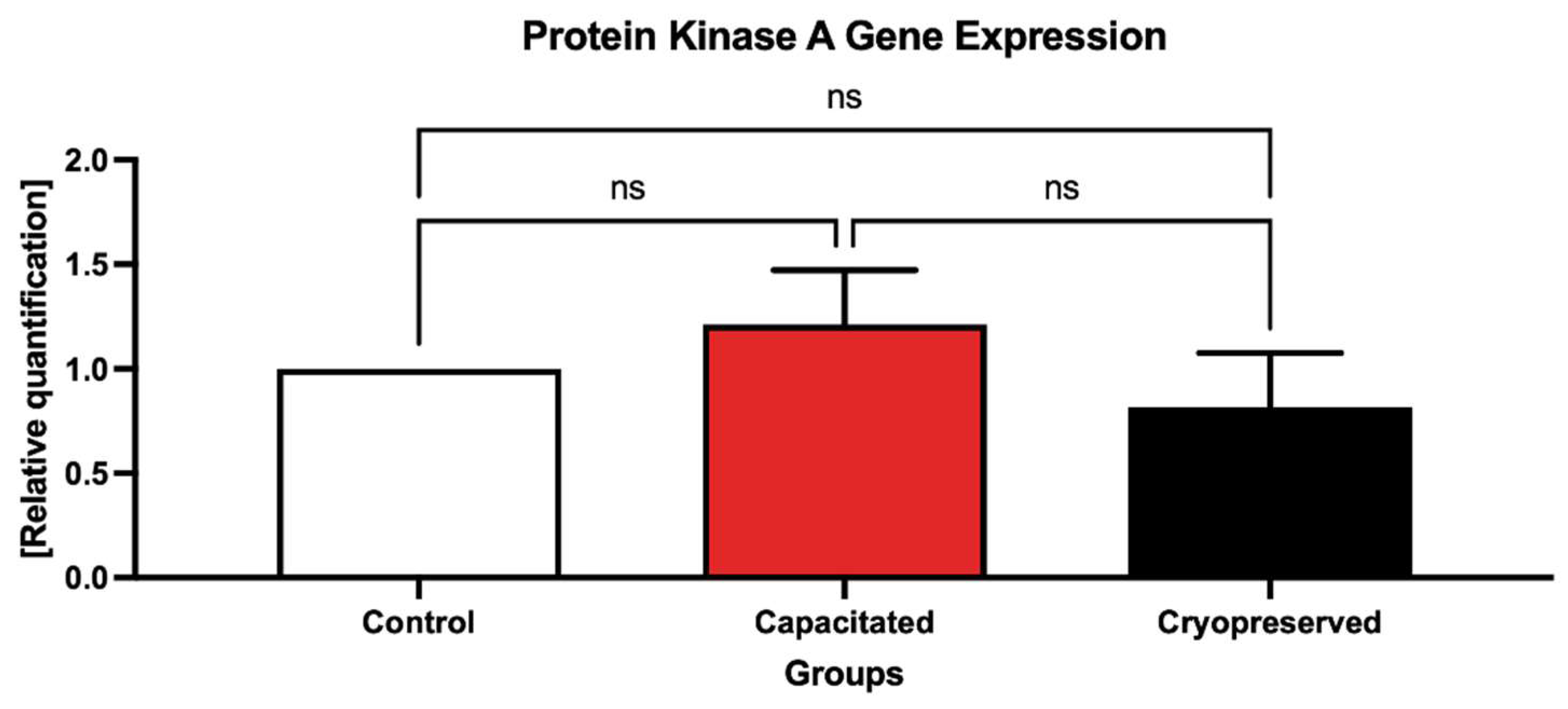
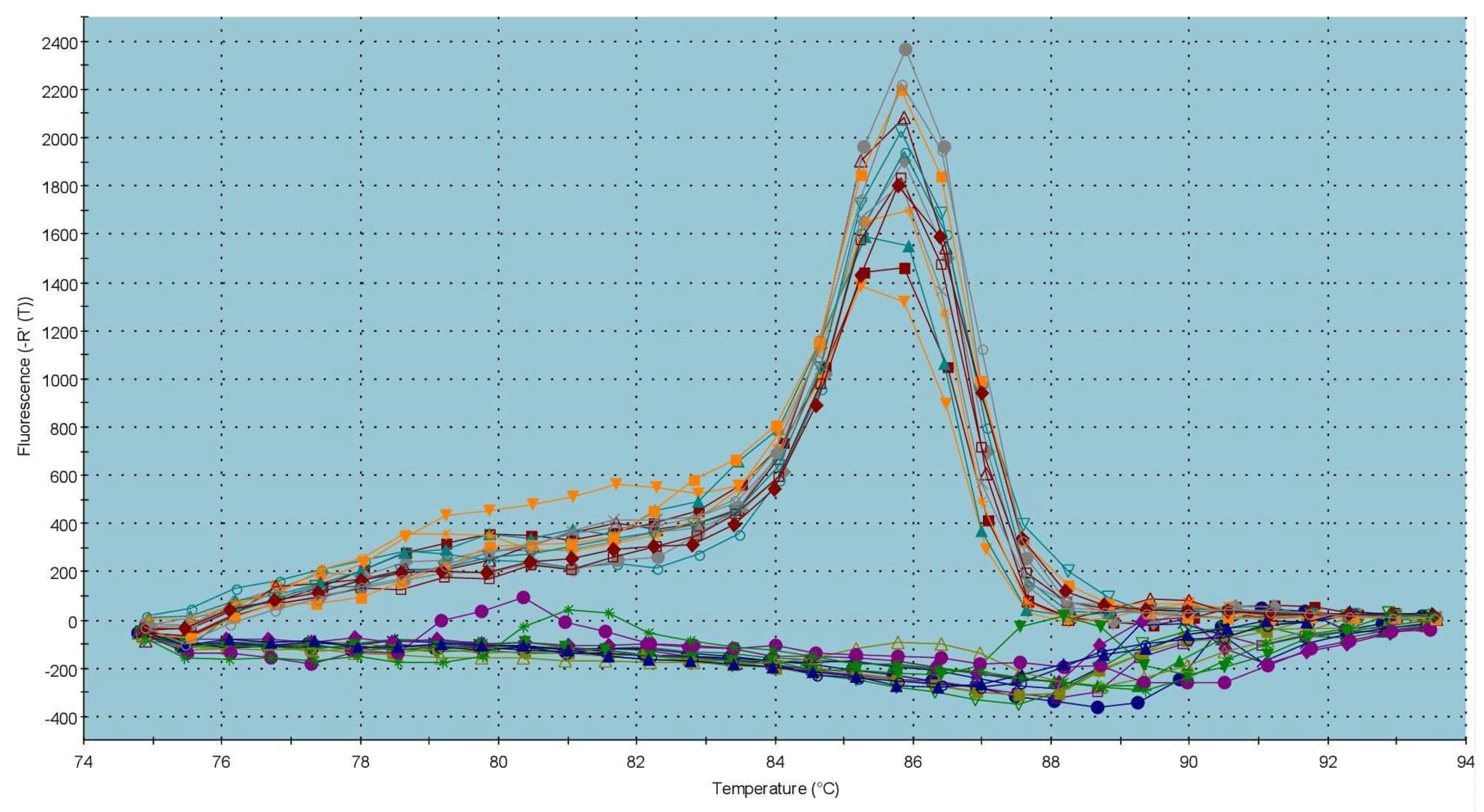
2.3. Gene Expression Patterns
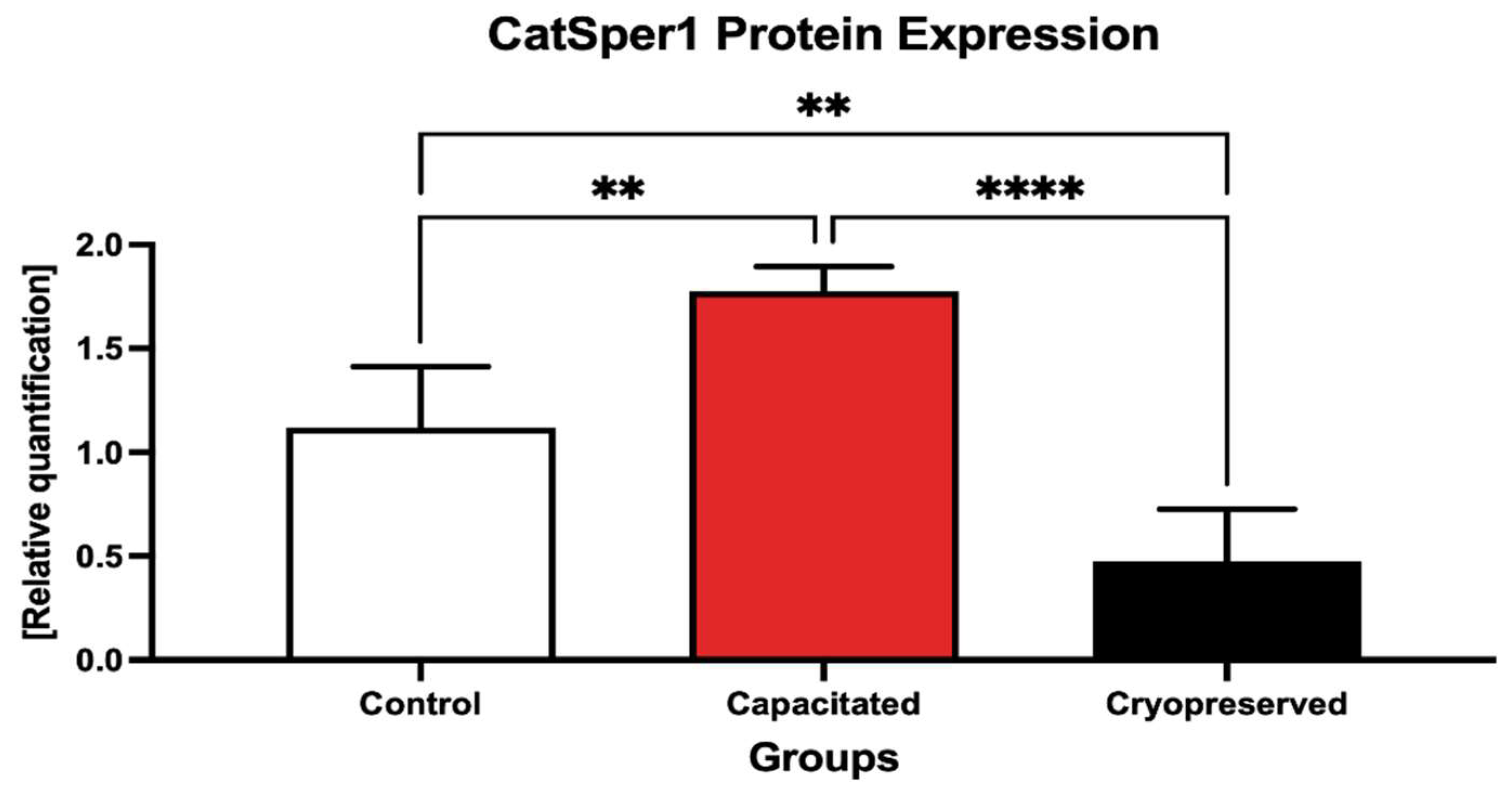
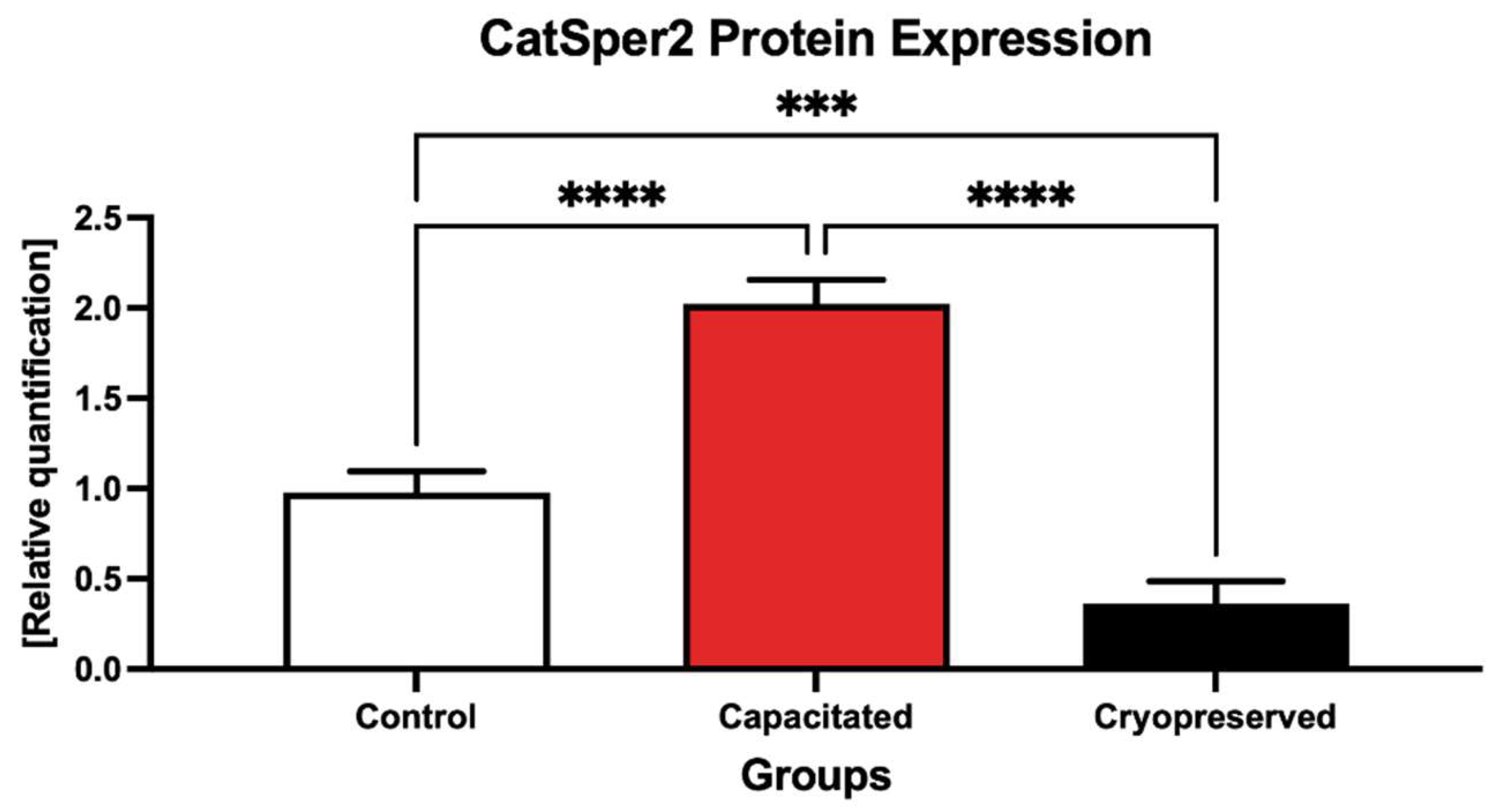
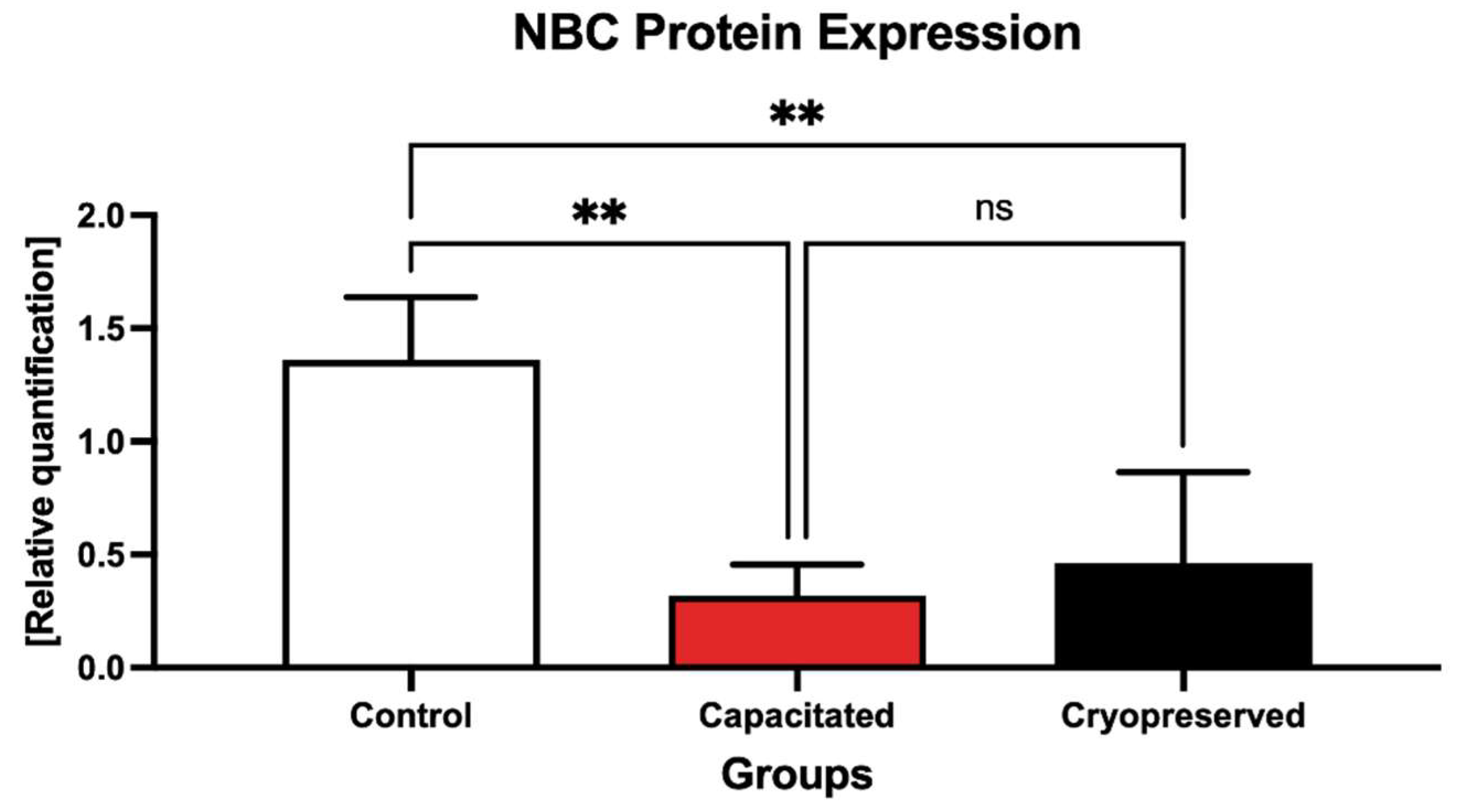
2.4. Protein Expression Patterns
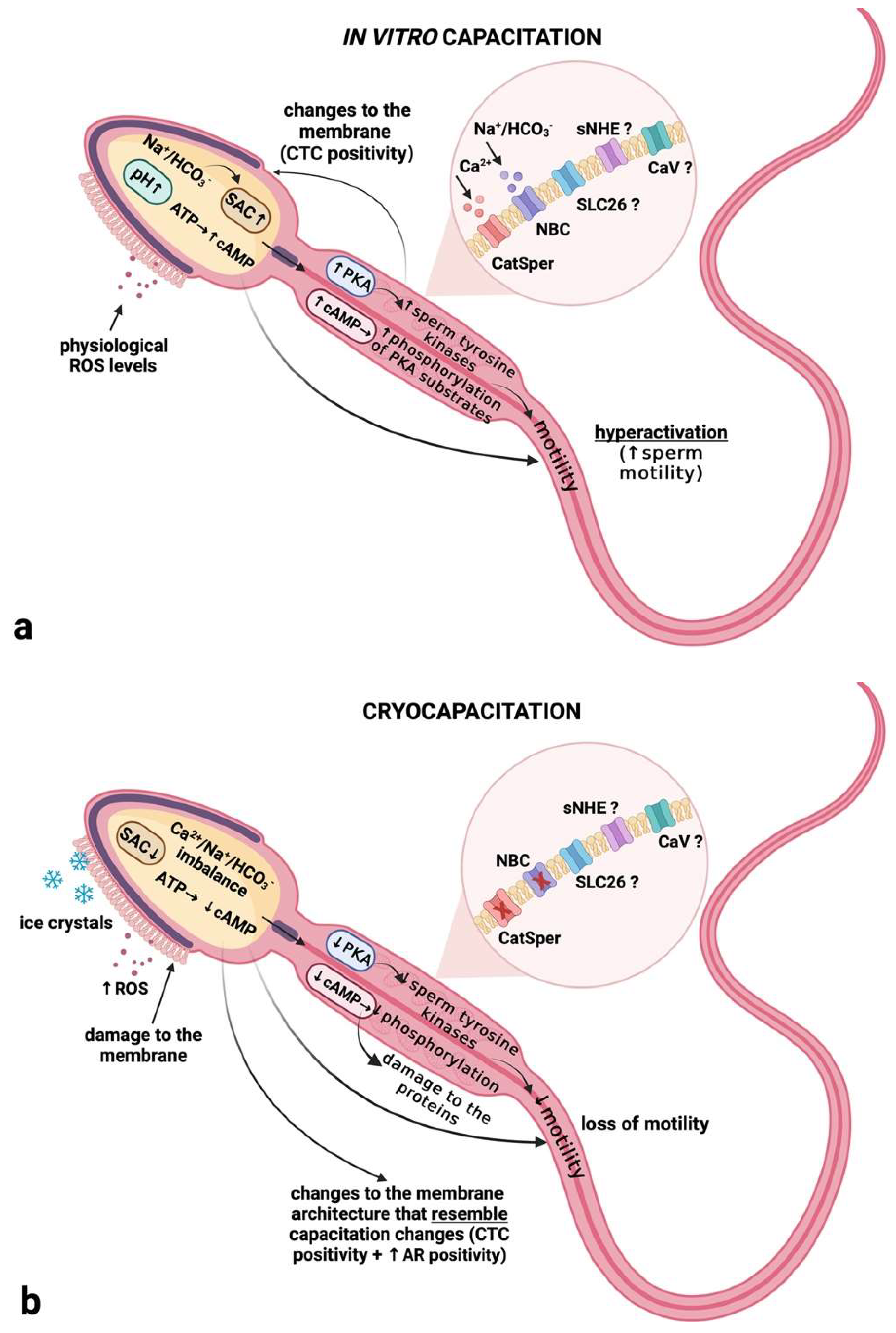
3. Discussion
4. Materials and Methods
4.1. Biological Material and Culture
4.2. Cryopreservation Procedure
4.3. Thawing and Washing Procedure
4.4. Computer-Assisted Sperm Analysis
4.5. Capacitation Patterns
4.6. RNA Isolation and cDNA Synthesis
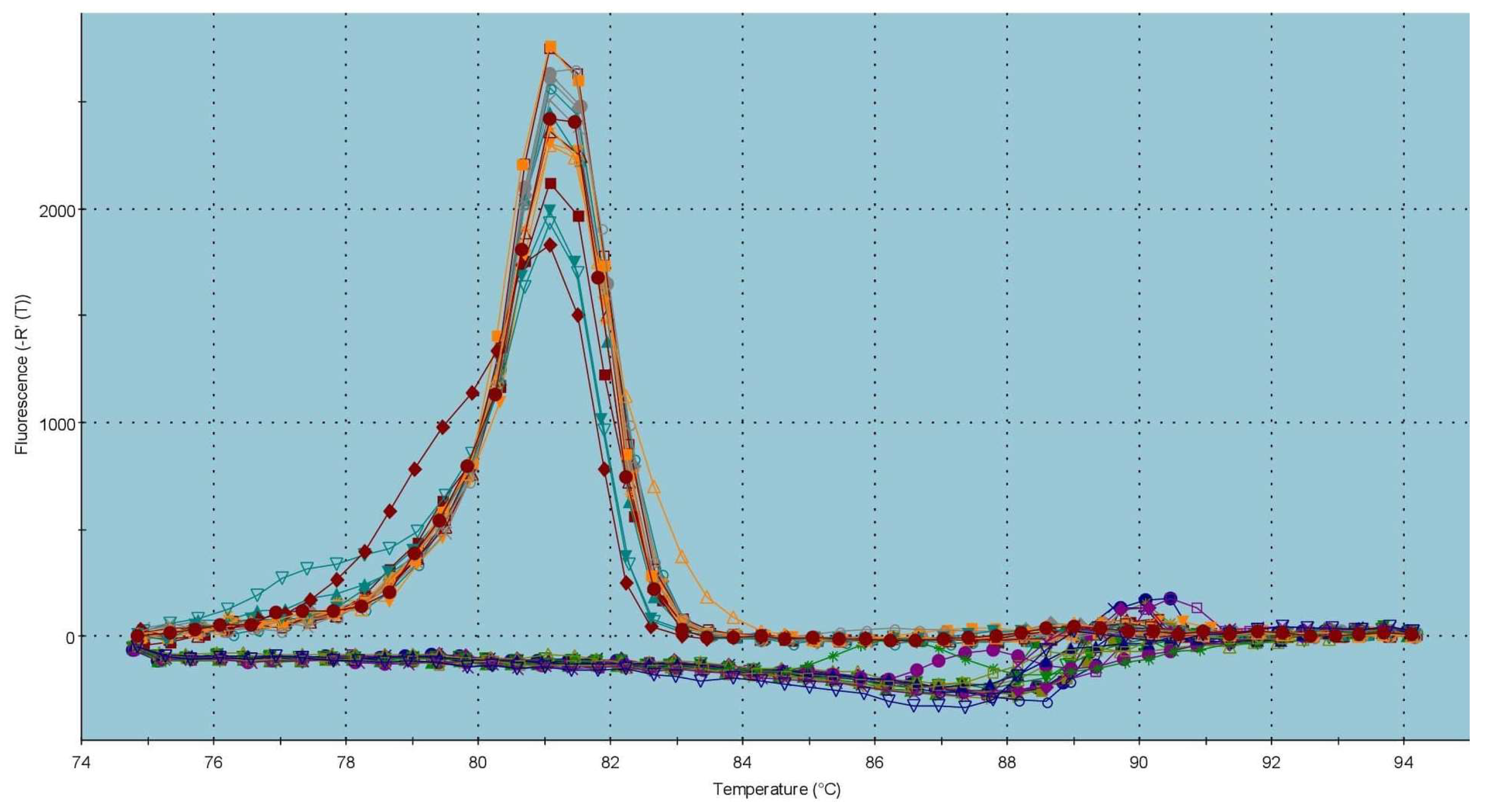
4.7. Real-Time qPCR
4.8. Western Blot
- CATSPER1 Polyclonal Antibody (#PA5-75788; Invitrogen, Waltham, MA, USA), source: rabbit, dilution 1:1000 in 5% milk/TBS/0.1% Tween-20;
- CATSPER2 Polyclonal Antibody (#PA5-41072; Invitrogen, Waltham, MA, USA), source: rabbit, dilution 1:1000 in 5% milk/TBS/0.1% Tween-20;
- PKA alpha Antibody (#PA5-17626; Invitrogen, Waltham, MA, USA), source: rabbit, dilution 1:1000 in 5% milk/TBS/0.1% Tween-20;
- Anti-Na+/HCO3− Contransporter Polyclonal Antibody (#AB3212-I; EMD Milipore Corporation; Temecula, CA, USA), source: rabbit, dilution 1:500 in 5% milk/TBS/0.1% Tween-20.
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.M.; Cao, Z.; Liu, H.; Khan, A.; Rahman, S.U.; Khan, M.Z.; Sathanawongs, A.; Zhang, Y. Impact of Cryopreservation on Spermatozoa Freeze-Thawed Traits and Relevance OMICS to Assess Sperm Cryo-Tolerance in Farm Animals. Front. Vet. Sci. 2021, 8, 609180. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wu, G.; Hong, Q.; Quan, G. Spermatozoa Cryopreservation: State of Art and Future in Small Ruminants. Biopreserv. Biobank. 2019, 17, 171–182. [Google Scholar] [CrossRef]
- Mustapha, A.R.; Ghosh, S.K.; Prasad, J.K.; Katiyar, R.; Kumar, A.; Amin, B.Y.; Bag, S.; Bhure, S.K.; Sharma, G.T.; Verma, M.R. Optimization of dissolved oxygen levels in extender prevents development of cryocapacitation like changes, oxidative stress and augments zona binding capacity of crossbred bull spermatozoa. Andrologia 2022, 54, e14331. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, N.K.; Ghosh, S.K.; Kumar, A.; Perumal, P.; Jerome, A. Acrosome membrane integrity and cryocapacitation are related to cholesterol content of bull spermatozoa. Asian Pac. J. Reprod. 2013, 2, 126–131. [Google Scholar] [CrossRef]
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E.; Mohey-Elsaeed, O. Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. Int. J. Vet. Sci. 2018, 6, S49–S56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, B.Y.; Prasad, J.K.; Ghosh, S.K.; Lone, S.A.; Kumar, A.; Mustapha, A.R.; Din, O.; Kumar, A. Effect of various levels of dissolved oxygen on reactive oxygen species and cryocapacitation-like changes in bull sperm. Reprod. Domest. Anim. 2018, 53, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ngou, A.A.; Ghosh, S.K.; Prasad, J.K.; Katiyar, R.; Kumar, A.; Rautela, R.; Bisla, A.; Srivastava, N.; Kumar, A. Exploring the role of E. coli derived enzyme, Oxyrase, as an oxagen scavenger to improve the cryotolerance of spermatozoa of Sahiwal bull. Cryobiology 2020, 97, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Benko, F.; Ďuračka, M.; Baňas, Š.; Lukáč, N.; Tvrdá, E. Biological Relevance of Free Radicals in the Process of Physiological Capacitation and Cryocapacitation. Oxygen 2021, 2, 164–176. [Google Scholar] [CrossRef]
- Gogoi, J.; Mondal, M.; Pal, P.; Upadhyay, V.R.; Kuri, P.; Mukherjee, I.; Tripura, S. Potential role of Catsper and proton ion channels in bull fertility. J. Pharm. Innov. 2022, 11, 313–321. [Google Scholar]
- Sun, X.; Zhu, Y.; Wang, L.; Liu, H.; Ling, Y.; Li, Z.; Sun, L. The Catsper channel and its role in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahban, R.; Nef, S. CatSper: The complex main gate of calcium entry in mammalian spermatozoa. Mol. Cell. Endrocrinol. 2020, 518, 110951. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Rahender, S. Catsper channel, sperm function and male fertility. Reprod. Biomed. Online 2015, 30, 28–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bystroff, C. Intramembranal disulfide cross-liking elucidates the super-quaternary structure of mammalian CatSpers. Reprod. Biol. 2018, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Vyklicka, L.; Lishko, P.V. Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell Biol. 2020, 63, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.C.P.; Pinto, N.A.; Torres, N.I.; Gonzálvez-Cota, A.L.; Luque, G.M.; Balestrini, P.A.; Romanowski, A.; Krapf, D.; Santi, C.M.; Trevino, C.L.; et al. CFTR/ENaC-dependent regulation of membrane potential during human sperm capacitation is initiated by bicarbonate uptake through NBC. J. Biol. Chem. 2018, 293, P9924–P9936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demarco, I.A.; Espinosa, F.; Edwards, J.; Sosnik, J.; De La Vega-Beltrán, J.L.; Hockensmith, J.W.; Kopf, G.S.; Darzson, A.; Visconti, P.E. Involvement of a Na+/HCO cotransporter in mouse sperm capacitation. J. Biol. Chem. 2003, 278, 7001–7009. [Google Scholar] [CrossRef] [Green Version]
- Rotman, T.; Etkovitz, N.; Spiegel, A.; Rubinstein, S.; Breitbart, H. Protein kinase A and protein kinase Cα/PPP1CC2 play opposing roles in the regulation of phosphatidylinositol 3-kinase activation in bovine sperm. Reproduction 2010, 140, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefiévre, L.; Jha, K.N.; de Lamirande, E.; Visconti, P.E.; Gagnoni, C. Activation of Protein Kinase A During Human Sperm Capacitation and Acrosome Reaction. J. Androl. 2013, 23, 709–716. [Google Scholar]
- Benko, F.; Mohammadi-Sangcheshmeh, A.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Physiological versus cryo-induced capacitation of bovine spermatozoa, part 1: Structural, functional, and oxidative similarities and differences. PLoS ONE 2022, 17, e0276683. [Google Scholar] [CrossRef]
- De Lamirande, E.; Leclerc, P.; Gagnon, C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol. Hum. Reprod. 1997, 3, 175–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostek, A.; Janta, A.; Majewska, A.; Ciereszko, A. Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins. Int. J. Mol. Sci. 2021, 22, 7903. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.T.; van der Horst, G.; Mortimer, D. The future of computer-aided sperm analysis. Asian J. Androl. 2015, 17, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Itach, S.B.; Finklestein, M.; Etkovitz, N.; Breitbart, H. Hyper-activated motility in sperm capacitation is mediated by phospholipase D-dependent actin polymerization. Dev. Biol. 2012, 362, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitbart, H.; Finkelstein, M. Regulation of Sperm Capacitation and the Acrosome Reaction by PIP 2 and Actin Modulation. Asian J. Androl. 2015, 17, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Wiser, A.; Sachar, S.; Ghetler, Y.; Shulman, A.; Breitbart, H. Assessment of sperm hyperactivated motility and acrosome reaction can discriminate the use of spermatozoa for conventional in vitro fertilisation or intracytoplasmic sperm injection: Preliminary results. Andrologia 2014, 46, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2021, 106904, in press. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Liu, F.; Pan, Y.; Miao, L.; Zhu, Q.; Tan, S. The Feasibility of Antioxidants Avoiding Oxidative Damages from Reactive Oxygen Species in Cryopreservation. Front. Chem. 2021, 9, 648684. [Google Scholar] [CrossRef]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Sperm Membrane Behaviour during Cooling and Cryopreservation. Reprod. Domest. Anim. 2015, 50, 20–26. [Google Scholar] [CrossRef]
- Holt, W.V.; Medrano, A. Assessment of boar sperm function in relation to freezing and storage. J. Reprod. Infertil. 1997, 52, 213–222. [Google Scholar] [CrossRef]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar]
- De Leeuw, F.E.; Chen, H.C.; Colenbrander, B.; Verkleij, A.J. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 1990, 27, 171–183. [Google Scholar] [CrossRef]
- Holt, W.V.; North, R.D. Partially irreversible cold-induced lipid phase transitions in mammalian sperm plasma membrane domains: Freeze-fracture study. J. Exp. Zool. 1984, 230, 473–483. [Google Scholar] [CrossRef]
- Gadella, B.M.; Harrison, R.A. Capacitation induces cyclic adenosine 3′,5′-monophosphate-dependent, but apoptosis-unrelated, exposure of aminophospholipids at the apical head plasma membrane of boar sperm cells. Biol. Reprod. 2022, 67, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Breininger, E.; Cetica, P.D.; Beconi, M.T. Capacitation inducers act through diverse intracellular mechanisms in cryopreserved bovine sperm. Theriogenology 2010, 74, 1036–1049. [Google Scholar] [CrossRef] [PubMed]
- Vadnais, M.L.; Roberts, K.P. Effects of seminal plasma on cooling-induced capacitative changes in boar sperm. J. Androl. 2007, 28, 416–422. [Google Scholar] [CrossRef]
- Vadnais, M.L.; Althouse, G.C. Characterization of capacitation, cryoinjury, and the role of seminal plasma in porcine sperm. Theriogenology 2011, 76, 1508–1516. [Google Scholar] [CrossRef]
- Parker, N.A.; Bailey, T.L.; Bowen, J.M.; Ley, W.B.; Purswell, B.J.; Dascanio, J.J. In Vitro and Xenogenous Capacitation-like Changes of Fresh, Cooled, and Cryopreserved Stallion Sperm as Assessed By a Chlortetracycline Stain. J. Androl. 2000, 21, 45–52. [Google Scholar]
- Pérez, L.J.; Valcárcel, A.; de las Heras, M.A.; Moses, D.; Baldassarre, H. Evidence that frozen/thawed ram spermatozoa show accelerated capacitation in vitro as assessed by chlortetracycline assay. Theriogenology 1996, 46, 131–140. [Google Scholar] [CrossRef]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Young, S.; Krenz, H.; Tüttelmann, F.; Röpke, A.; Krallmann, C.; Kliesch, S.; Zeng, X.H.; Brenker, C.; Strünker, T. The Ca2+ channel CatSper is not activated by cAMP/PKA signaling but directly affected by chemicals used to probe the action of cAMP and PKA. J. Biol. Chem. 2020, 295, 13181–13193. [Google Scholar] [CrossRef] [PubMed]
- Balbach, M.; Hamzeh, H.; Jikeli, J.F.; Brenker, C.; Schiffer, C.; Hansen, J.N.; Neugebauer, P.; Trötschel, C.; Jovine, L.; Han, L.; et al. Molecular Mechanism Underlying the Action of Zona-pellucida Glycoproteins on Mouse Sperm. Front. Cell Dev. Biol. 2020, 8, 572735. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ren, D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod. Biol. Endocrinol. 2009, 7, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumigama, S.; Mansell, S.; Miller, M.; Lishko, P.V.; Cherr, G.N.; Meyers, S.A.; Tollner, T. Progesterone Accelerates the Completion of Sperm Capacitation and Activates CatSper Channel in Spermatozoa from the Rhesus Macaque. Biol. Reprod. 2015, 93, 130. [Google Scholar] [CrossRef]
- Avenarius, M.R.; Hildebrand, M.S.; Zhang, Y.; Meyer, N.C.; Smith, L.L.H.; Kahrizi, K.; Najmabadi, H.; Smith, R.J.H. Human Male Infertility Caused by Mutations in the CATSPER1 Channel Protein. Am. J. Hum. Genet. 2009, 84, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Avidan, N.; Tamary, H.; Dgany, O.; Cattan, D.; Pariente, A.; Thulliez, M.; Borot, N.; Moati, L.; Barthelme, A.; Shalmon, L.; et al. CATSPER2, a Human Autosomal Nonsyndromic Male Infertility Gene. Eur. J. Hum. Genet. 2003, 11, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.F.; Syritsyna, O.; Fellous, M.; Serres, C.; Mannowetz, N.; Kirichok, Y.; Lishko, P.V. Disruption of the Principal, Progesterone-Activated Sperm Ca2+ Channel in a CatSper2-Deficient Infertile Patient. Proc. Natl. Acad. Sci. USA 2013, 110, 6823–6828. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.; Wolff, C.A.; Suarez, S.S. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fertil. Dev. 2009, 21, 345–350. [Google Scholar] [CrossRef]
- Chung, J.J.; Miki, K.; Kim, D.; Shim, S.H.; Shi, H.F.; Hwang, J.Y.; Cai, X.; Iseri, Y.; Zhuang, X.; Clapham, D.E. CatSperζ regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. eLife 2017, 6, e23082. [Google Scholar] [CrossRef] [PubMed]
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petyim, S.; Choavaratana, R.; Kunathikom, S. Freezing effect on post-thawed sperm characteristics especially sperm DNA integrity comparing between liquid nitrogen vapour and computerized program freezer. Siriraj Med. J. 2017, 59, 298–302. [Google Scholar]
- Alshawa, E.; Laqqan, M.; Montenarh, M.; Hammadeh, M.E. Influence of cryopreservation on the CATSPER2 and TEKT2 expression levels and protein levels in human spermatozoa. Toxicol. Rep. 2019, 6, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Ramió-Lluch, L.; Bucci, D.; Fernández-Novell, J.M.; Peña, A.; Rodríguez-Gil, J.E. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology 2011, 76, 1450–1464. [Google Scholar] [CrossRef]
- Harrison, R.A.; White, I.G. Glycolytic enzymes in the spermatozoa and cytoplasmic droplets of bull, boar and ram, and their leakage after shock. J. Reprod. Fertil. 1972, 30, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.G.; Hu, S.; Han, C.; Zhu, Q.C.; Yan, G.J.; Hu, J.H. Association of heat shock protein 90 with motility of post-thawed sperm in bulls. Cryobiology 2015, 70, 164–169. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Hong, J.-Y.; Yan, G.-J.; Wang, Y.-F.; Li, Q.-W.; Hu, J.-H. Association of heat shock protein 70 with motility of frozen-thawed sperm in bulls. Czech J. Anim. Sci. 2015, 60, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Xu, Y.; Tang, M.; Fang, J.; Sun, H.; Sun, Y.; Gu, M.; Liu, Z.; Zhang, Z.; et al. Proteomic characteristics of human sperm cryopreservation. Proteomics 2014, 14, 298–310. [Google Scholar] [CrossRef]
- Yoon, S.J.; Rahman, M.S.; Kwon, W.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Proteomic identification of cryostress in epididymal spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.A.; Storey, B.T. Bicarbonate is essential for fertilization of mouse eggs: Mouse sperm require it to undergo the acrosome reaction. Biol. Reprod. 1986, 34, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.X.; Roldan, E.R. Bicarbonate/CO2 is not required for zona pellucida- or progesterone-induced acrosomal exocytosis of mouse spermatozoa but is essential for capacitation. Biol. Reprod. 1995, 52, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Boatman, D.E.; Robbins, R.S. Bicarbonate: Carbon-dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol. Reprod. 1991, 44, 806–813. [Google Scholar] [CrossRef]
- Jensen, L.J.; Schmitt, B.M.; Berger, U.V.; Nsumu, N.N.; Boron, W.F.; Hediger, M.A.; Brown, D.; Breton, S. Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol. Reprod. 1999, 60, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavarocchi, E.; Whitfield, M.; Saez, F.; Touré, A. Sperm Ion Transporters and Channels in Human Asthenozoospermia: Genetic Etiology, Lessons from Animal Models, and Clinical Perspectives. Int. J. Mol. Sci. 2022, 23, 3926. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Magnoux, E.; Royere, D.; Barthelemy, C.; Dacheux, J.L.; Gatti, J.L. Internal ph of human spermatozoa: Effect of ions, human follicular fluid and progesterone. Mol. Hum. Reprod. 1996, 2, 219–224. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, L.; Jiang, M.; Liu, B.; Xian, Y.; Liu, S.; Liu, X.; Zhao, W.; Li, F. Extend the Survival of Human Sperm In Vitro in Non-Freezing Conditions: Damage Mechanisms, Preservation Technologies, and Clinical Applications. Cells 2022, 11, 2845. [Google Scholar] [CrossRef]
- Dhumal, S.S.; Naik, P.; Dakshinamurthy, S.; Sullia, K. Semen ph and its correlation with motility and count—A study in subfertile men. JBRA Assist. Reprod. 2021, 25, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Stival, C.; Ritagliati, C.; Xu, X.; Gervasi, M.G.; Luque, G.M.; Baró Graf, C.; De la Vega-Beltrán, J.L.; Torres, N.; Darszon, A.; Krapf, D.; et al. Disruption of protein kinase A localization induces acrosomal exocytosis in capacitated mouse sperm. J. Biol. Chem. 2018, 293, 9435–9447. [Google Scholar] [CrossRef] [Green Version]
- Shabb, J.B. Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 2001, 101, 2381–2411. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Carmona, H.; Barón, L.; Kong, M.; Morales, P. Protein Kinase A (PRKA) Activity Is Regulated by the Proteasome at the Onset of Human Sperm Capacitation. Cells 2021, 10, 3501. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ Signaling in Mammalian Spermatozoa. Mol. Cell Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef]
- Nolan, M.A.; Babcock, D.F.; Wennemuth, G.; Brown, W.; Burton, K.A.; McKnight, G.S. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 13483–13488. [Google Scholar] [CrossRef] [Green Version]
- Zilli, L.; Schiavone, R.; Storelli, C.; Vilella, S. Molecular mechanisms determining sperm motility initiation in two sparids (Sparus aurata and Lithognathus mormyrus). Biol. Reprod. 2008, 79, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Fujinoki, M.; Okuno, M. K+-independent initiation of motility in chum salmon sperm treated with an organic alcohol, glycerol. J. Exp. Biol. 2005, 208, 4549–4556. [Google Scholar] [CrossRef] [Green Version]
- De Jonge, C.J.; Han, J.-L.; Lawrie, H.; Mack, S.R.; Zaneveld, L.J.D. Modulation of human sperm acrosome reaction by effectors of the adenylate cyclase/cAMP second messenger pathway. J. Exp. Zool. 1991, 258, 113–125. [Google Scholar] [CrossRef]
- Visconti, P.E.; Johnson, L.R.; Oyaski, M.; Fornes, M.; Moss, S.B.; Gerton, G.L.; Kopf, G.S. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev. Biol. 1997, 192, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Lee-Estevez, M.; Herrera, L.; Díaz, R.; Beltrán, J.; Figueroa, E.; Dumorné, K.; Ulloa-Rodríguez, P.; Short, S.; Risopatrón, J.; Valdebenito, I.; et al. Effects of cryopreservation on cAMP-dependent protein kinase and AMP-activated protein kinase in Atlantic salmon (Salmo salar) spermatozoa: Relation with post-thaw motility. Anim. Reprod. Sci. 2019, 209, 106133. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.; Hu, C.; Hao, H.; Zhang, J.; Li, K.; Zhao, X.; Qin, T.; Zhao, K.; Zhu, H.; et al. Identification of differentially expressed proteins in fresh and frozen-thawed boar spermatozoa by iTRAQ-coupled 2D LC-MS/MS. Reproduction 2014, 147, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliciello, A.; Gottesman, M.E.; Avvedimento, E.V. The biological functions of A-kinase anchor proteins. J. Mol. Biol. 2001, 308, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Heydari, S.; Eidi, A.; Kouhkan, F.; Tvrda, E.; Mohammadi-Sangcheshmeh, A. Effects of increasing lipopolysaccharide concentrations on in vitro developmental competence of ovine oocytes. Anim. Reprod. 2020, 17, e20190125. [Google Scholar] [CrossRef]
- Ďuračka, M.; Husarčíková, K.; Jančov, M.; Galovičová, L.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Staphylococcus-Induced Bacteriospermia In Vitro: Consequences on the Bovine Spermatozoa Quality, Extracellular Calcium and Magnesium Content. Animals 2021, 11, 3309. [Google Scholar] [CrossRef]
- Zanganeh, Z.; Zhandi, M.; Zare-Shahneh, A.; Najafi, A.; Mahdi Nabi, M.; Mohammadi-Sangcheshmeh, A. Does rosemary aqueous extract improve buck semen cryopreservation? Small Rumin. Res. 2013, 114, 120–125. [Google Scholar] [CrossRef]
- Benko, F.; Geschwandtnerová, A.; Žiarovská, J.; Lukáč, N.; Tvrdá, E. Expression of HSP90 Gene in the Cryopreserved Bovine Spermatozoa. Sci. Pap. Anim. Sci. Biotechnol. 2021, 54, 73–77. [Google Scholar]





| Primer Name | Primer Sequences | Accession Number | Amplicon Size |
|---|---|---|---|
| Bov CATSPER1-F | TACTCTGACCCCAAACGCTT | XM_024987569.1 | 100 bp |
| Bov CATSPER1-R | GGCTGTCCAGGTAGATGAGG | ||
| Bov CATSPER2-F | CCTCAAGAGCATGACCTTCC | NM_001192477.1 | 103 bp |
| Bov CATSPER2-R | GCGAGTTGAACGGGTGTAAT | ||
| Bov GAPDH-F | GATGGTGAAGGTCGGAGTGAAC | NM_001034034.2 | 100 bp |
| Bov GAPDH-R | GTCATTGATGGCGACGATGT | ||
| Bov NBC-F | CAGCCATGACCCACAGGAAT | AF308160.1 | 146 bp |
| Bov NBC-R | AGTCTACCTCGCCAACAAGC | ||
| Bov PKA-F | GAAGCCCAAAGCCAGCTCTA | NM_174236.1 | 109 bp |
| Bov PKA-R | TTTGATTGAGTCCCAGGCCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benko, F.; Fialková, V.; Žiarovská, J.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A. Int. J. Mol. Sci. 2022, 23, 14646. https://doi.org/10.3390/ijms232314646
Benko F, Fialková V, Žiarovská J, Ďuračka M, Lukáč N, Tvrdá E. In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A. International Journal of Molecular Sciences. 2022; 23(23):14646. https://doi.org/10.3390/ijms232314646
Chicago/Turabian StyleBenko, Filip, Veronika Fialková, Jana Žiarovská, Michal Ďuračka, Norbert Lukáč, and Eva Tvrdá. 2022. "In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A" International Journal of Molecular Sciences 23, no. 23: 14646. https://doi.org/10.3390/ijms232314646
APA StyleBenko, F., Fialková, V., Žiarovská, J., Ďuračka, M., Lukáč, N., & Tvrdá, E. (2022). In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A. International Journal of Molecular Sciences, 23(23), 14646. https://doi.org/10.3390/ijms232314646









