Structural and Biophysical Characterization of Stable Alpha-Synuclein Oligomers
Abstract
1. Introduction
2. Results
2.1. Preparation of Stable α-Syn Oligomers
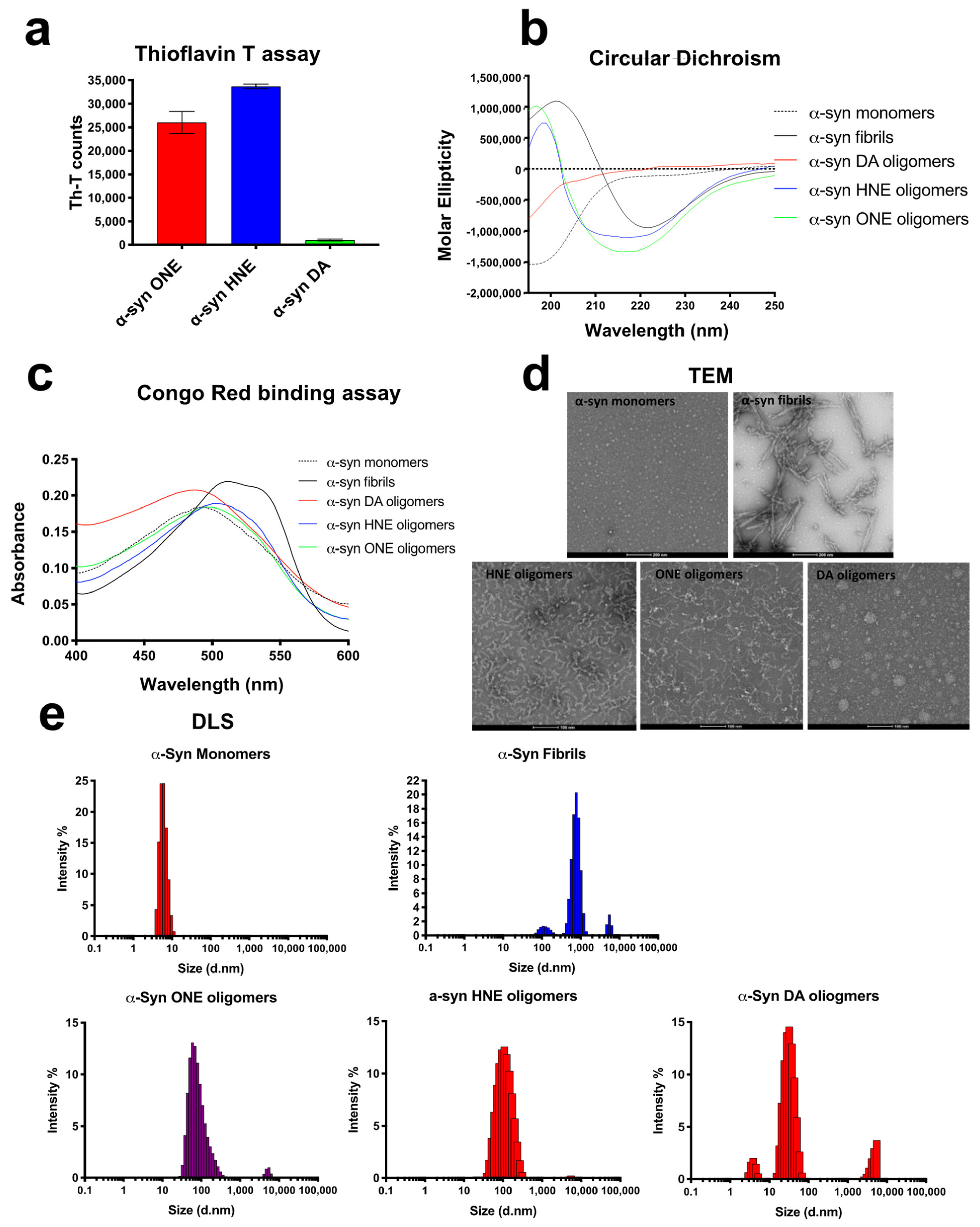
2.2. ONE-, HNE- and DA- Oligomers Display Size Differences and Greatly Differ in Their Oligomer Conformation
2.3. ONE-, HNE-, and DA-Oligomers Display Size Differences and Greatly Differ in Their Oligomer Conformation
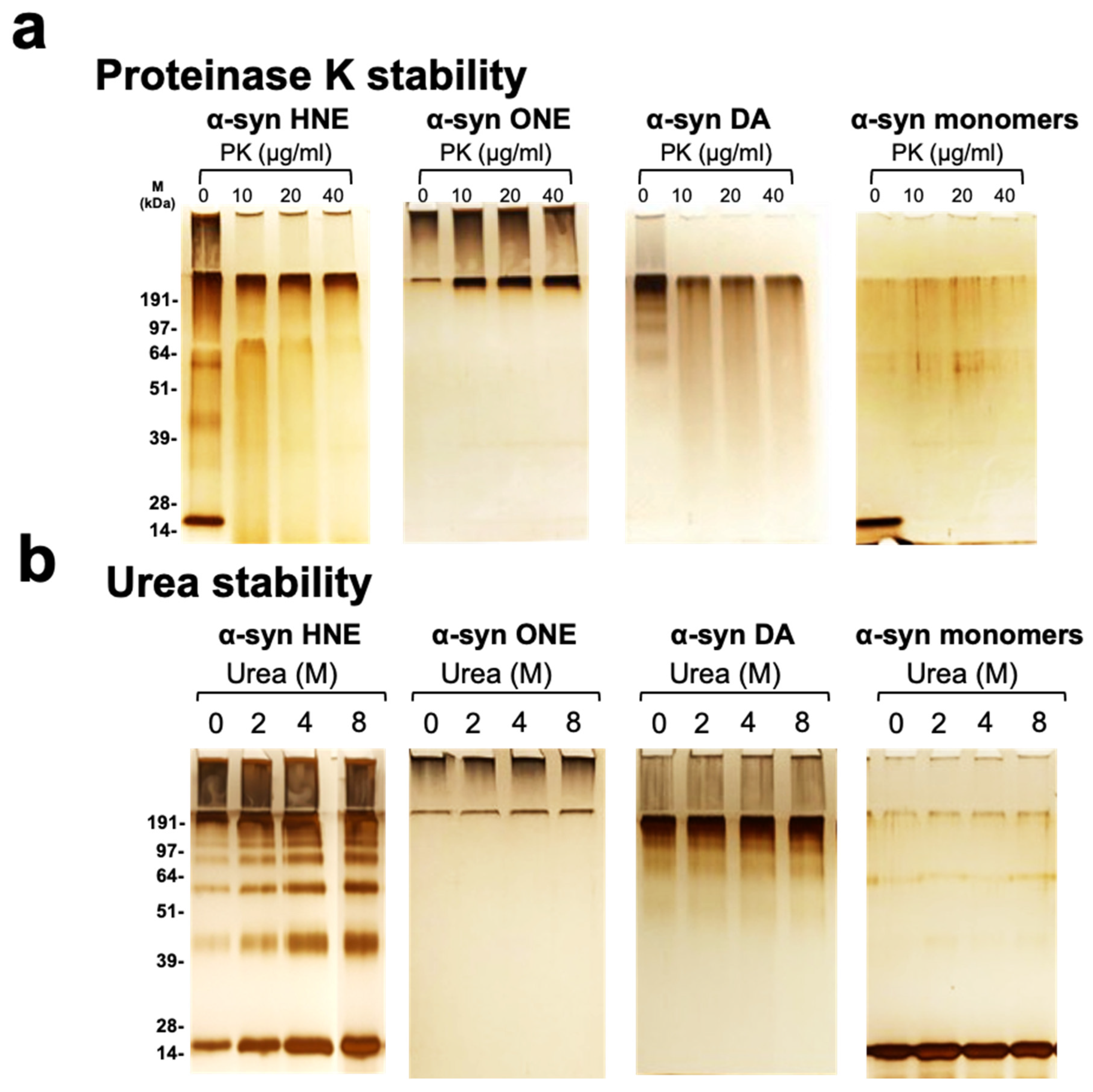
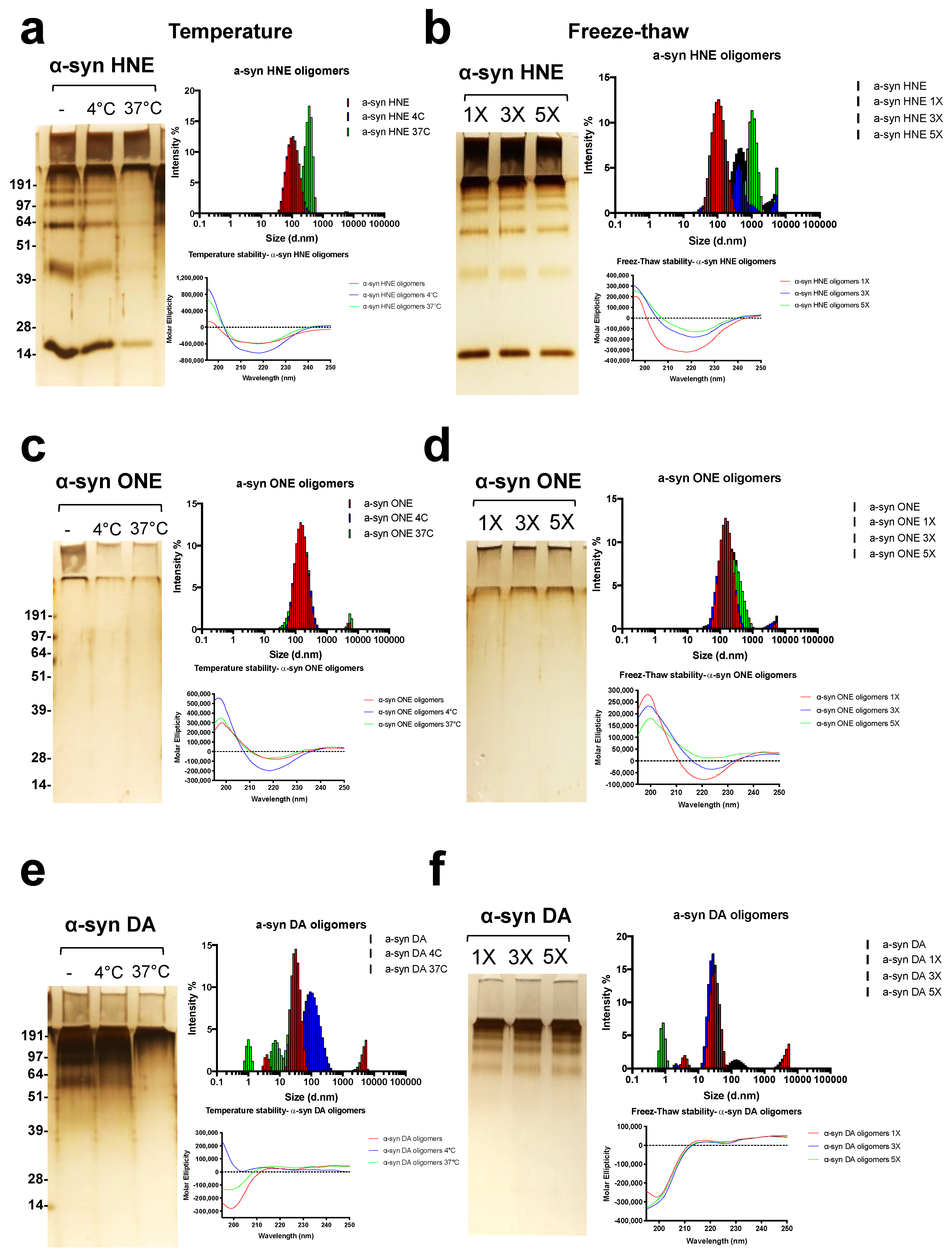
2.4. Stability
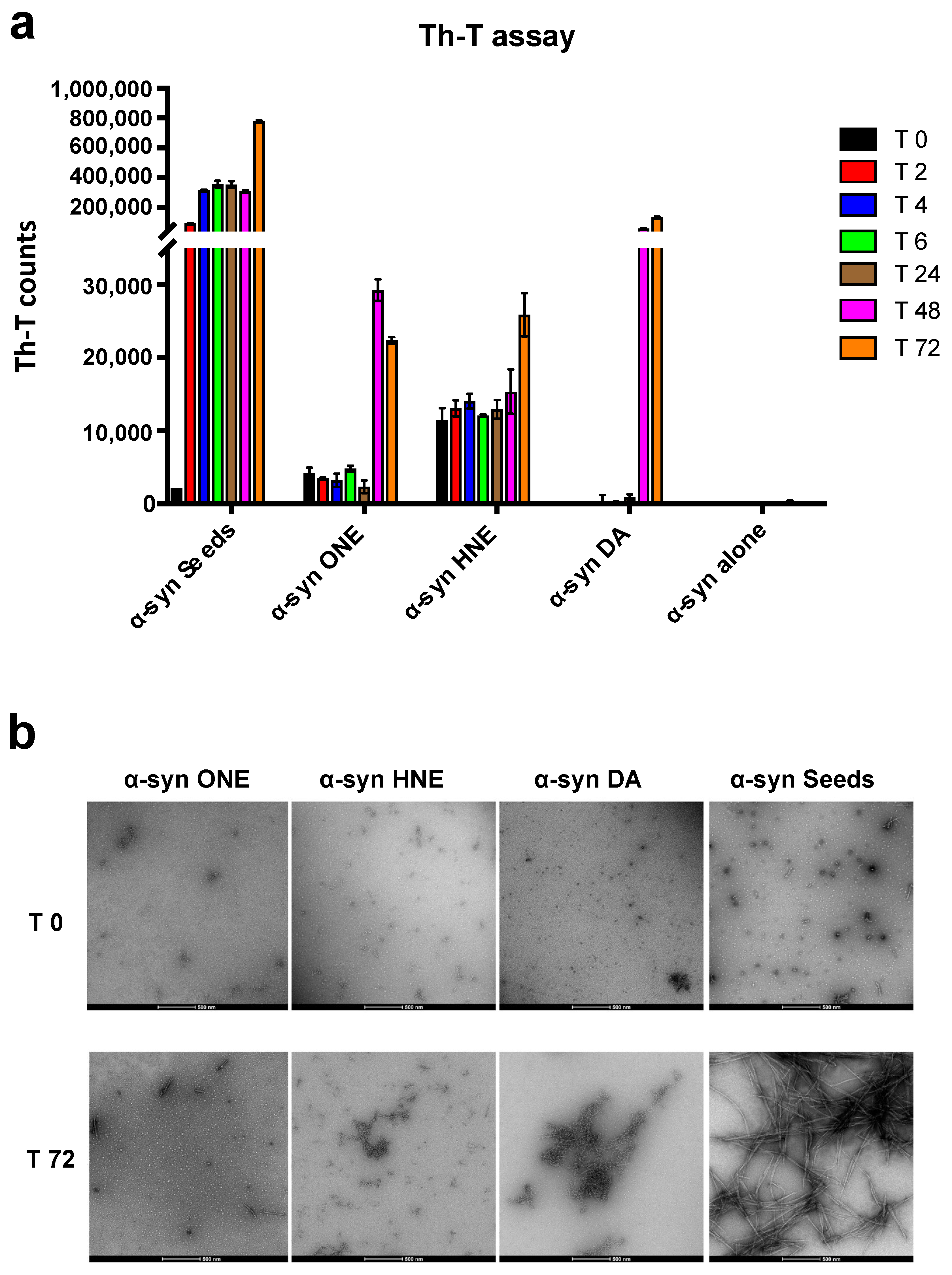
2.5. ONE and DA-Oligomers Can Seed the Aggregation of α-Syn Monomers at a Late Stage in an In Vitro Fibrillation Assay
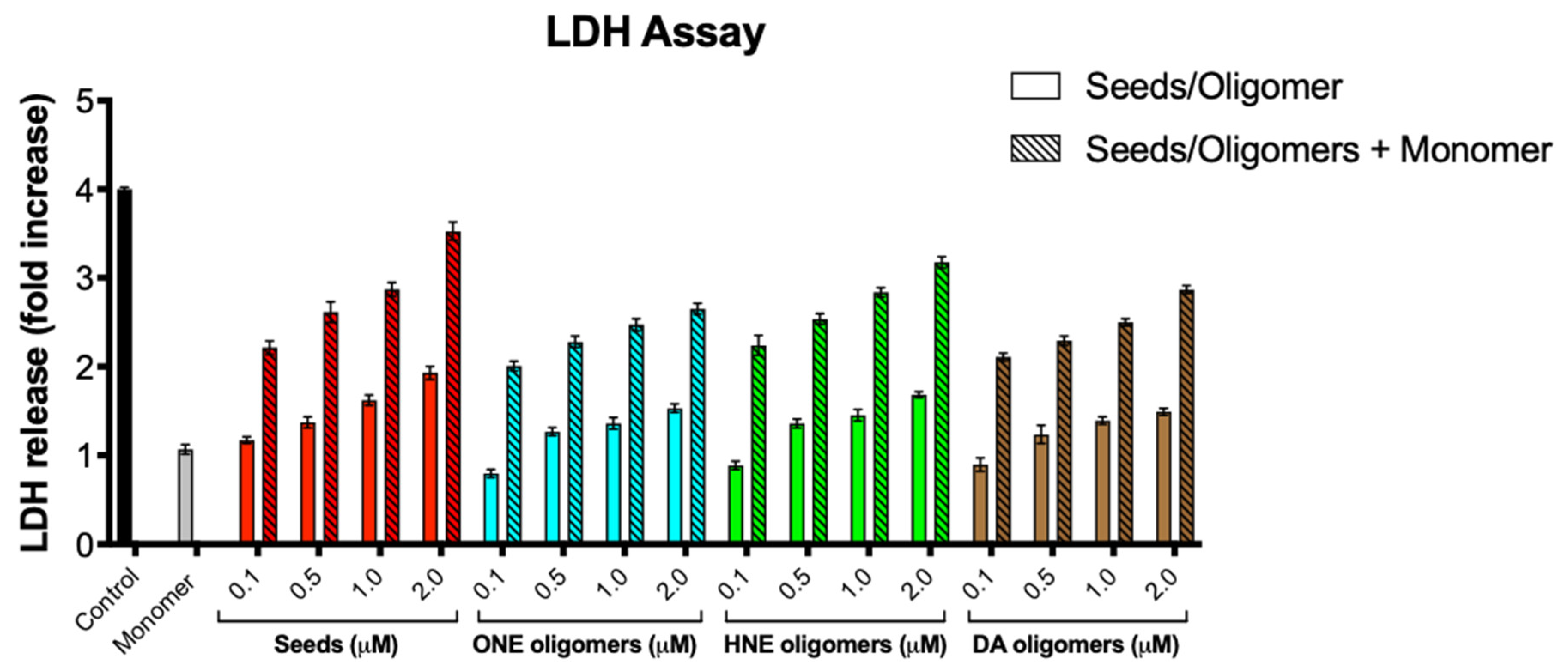
2.6. Toxicity of α-Syn Oligomers
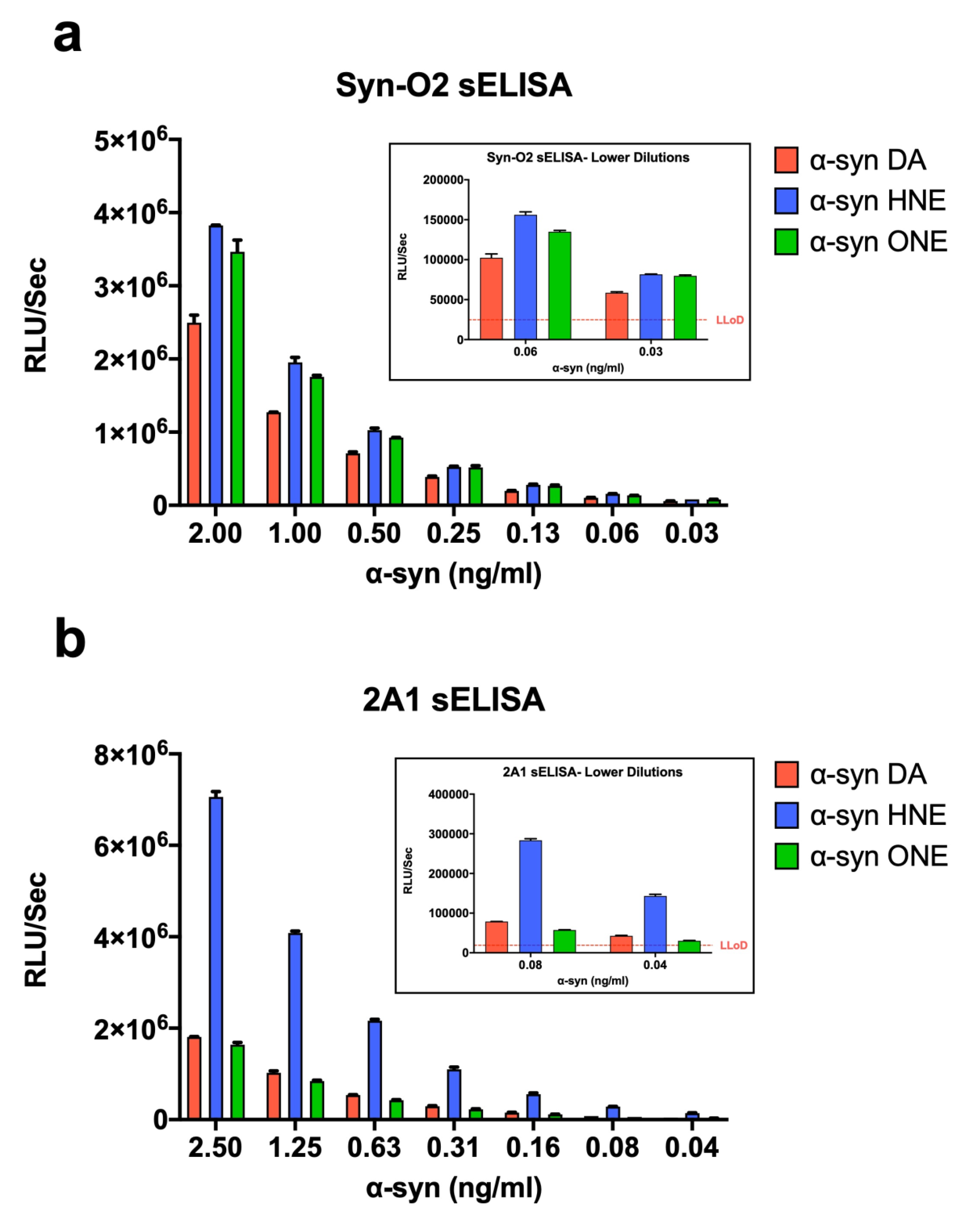
2.7. α-Syn Oligomers as Calibrators in ELISA
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of α-Syn
4.2. Generation of 4-Oxo-2-Nonenal (ONE)-, 4-Hydroxy-2-Nonenal (HNE)-, and Dopamine (DA)-Oligomers
4.3. Silver Staining
4.4. Western Blotting
4.5. Slot Blot Assay
4.6. In-Vitro Seeding Assay
4.7. LDH Activity Assay
4.8. TEM
4.9. Circular Dichroism (CD)
4.10. Congo Red
4.11. Dynamic Light Scattering (DLS)
4.12. Stability Test
4.13. Oligomers as Calibrators in ELISA
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCann, H.; Stevens, C.H.; Cartwright, H.; Halliday, G.M. α-Synucleinopathy Phenotypes. Parkinsonism Relat. Disord. 2014, 20 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, B.A.; Volpicelli-Daley, L.A. Initiation and Propagation of α-Synuclein Aggregation in the Nervous System. Mol. Neurodegener. 2020, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Volles, M.J.; Lansbury, P.T. Zeroing in on the Pathogenic Form of Alpha-Synuclein and Its Mechanism of Neurotoxicity in Parkinson’s Disease. Biochemistry 2003, 42, 7871–7878. [Google Scholar] [CrossRef] [PubMed]
- Abeliovich, A.; Schmitz, Y.; Fariñas, I.; Choi-Lundberg, D.; Ho, W.H.; Castillo, P.E.; Shinsky, N.; Verdugo, J.M.; Armanini, M.; Ryan, A.; et al. Mice Lacking Alpha-Synuclein Display Functional Deficits in the Nigrostriatal Dopamine System. Neuron 2000, 25, 239–252. [Google Scholar] [CrossRef]
- Nussbaum, R.L.; Polymeropoulos, M.H. Genetics of Parkinson’s Disease. Hum. Mol. Genet. 1997, 6, 1687–1691. [Google Scholar] [CrossRef]
- Lee, V.M.-Y.; Trojanowski, J.Q. Mechanisms of Parkinson’s Disease Linked to Pathological Alpha-Synuclein: New Targets for Drug Discovery. Neuron 2006, 52, 33–38. [Google Scholar] [CrossRef]
- Paxinou, E.; Chen, Q.; Weisse, M.; Giasson, B.I.; Norris, E.H.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.; Ischiropoulos, H. Induction of Alpha-Synuclein Aggregation by Intracellular Nitrative Insult. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 8053–8061. [Google Scholar] [CrossRef]
- Smith, W.W.; Margolis, R.L.; Li, X.; Troncoso, J.C.; Lee, M.K.; Dawson, V.L.; Dawson, T.M.; Iwatsubo, T.; Ross, C.A. Alpha-Synuclein Phosphorylation Enhances Eosinophilic Cytoplasmic Inclusion Formation in SH-SY5Y Cells. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5544–5552. [Google Scholar] [CrossRef]
- McFarlane, D.; Dybdal, N.; Donaldson, M.T.; Miller, L.; Cribb, A.E. Nitration and Increased Alpha-Synuclein Expression Associated with Dopaminergic Neurodegeneration in Equine Pituitary Pars Intermedia Dysfunction. J. Neuroendocrinol. 2005, 17, 73–80. [Google Scholar] [CrossRef]
- Conway, K.A.; Lee, S.J.; Rochet, J.C.; Ding, T.T.; Williamson, R.E.; Lansbury, P.T. Acceleration of Oligomerization, Not Fibrillization, Is a Shared Property of Both Alpha-Synuclein Mutations Linked to Early-Onset Parkinson’s Disease: Implications for Pathogenesis and Therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 571–576. [Google Scholar] [CrossRef]
- Caughey, B.; Lansbury, P.T. Protofibrils, Pores, Fibrils, and Neurodegeneration: Separating the Responsible Protein Aggregates from the Innocent Bystanders. Annu. Rev. Neurosci. 2003, 26, 267–298. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Jappelli, R.; Maji, S.K.; Desplats, P.A.; Boyer, L.; Aigner, S.; Hetzer, C.; Loher, T.; Vilar, M.; Campioni, S.; et al. In Vivo Demonstration That Alpha-Synuclein Oligomers Are Toxic. Proc. Natl. Acad. Sci. USA 2011, 108, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Angot, E.; Bergström, A.-L.; Steiner, J.A.; Pieri, L.; Paul, G.; Outeiro, T.F.; Melki, R.; Kallunki, P.; Fog, K.; et al. α-Synuclein Propagates from Mouse Brain to Grafted Dopaminergic Neurons and Seeds Aggregation in Cultured Human Cells. J. Clin. Investig. 2011, 121, 715–725. [Google Scholar] [CrossRef]
- Freundt, E.C.; Maynard, N.; Clancy, E.K.; Roy, S.; Bousset, L.; Sourigues, Y.; Covert, M.; Melki, R.; Kirkegaard, K.; Brahic, M. Neuron-to-Neuron Transmission of α-Synuclein Fibrils through Axonal Transport. Ann. Neurol. 2012, 72, 517–524. [Google Scholar] [CrossRef]
- Lee, H.-J.; Bae, E.-J.; Lee, S.-J. Extracellular α--Synuclein-a Novel and Crucial Factor in Lewy Body Diseases. Nat. Rev. Neurol. 2014, 10, 92–98. [Google Scholar] [CrossRef]
- de Oliveira, G.A.P.; Silva, J.L. Alpha-Synuclein Stepwise Aggregation Reveals Features of an Early Onset Mutation in Parkinson’s Disease. Commun. Biol. 2019, 2, 374. [Google Scholar] [CrossRef]
- Danzer, K.M.; Haasen, D.; Karow, A.R.; Moussaud, S.; Habeck, M.; Giese, A.; Kretzschmar, H.; Hengerer, B.; Kostka, M. Different Species of Alpha-Synuclein Oligomers Induce Calcium Influx and Seeding. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 9220–9232. [Google Scholar] [CrossRef] [PubMed]
- Pieri, L.; Madiona, K.; Melki, R. Structural and Functional Properties of Prefibrillar α-Synuclein Oligomers. Sci. Rep. 2016, 6, 24526. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hsu, L.J.; Xia, Y.; Takeda, A.; Sisk, A.; Sundsmo, M.; Masliah, E. Oxidative Stress Induces Amyloid-like Aggregate Formation of NACP/Alpha-Synuclein in Vitro. Neuroreport 1999, 10, 717–721. [Google Scholar] [CrossRef]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative Stress in Brain Aging, Neurodegenerative and Vascular Diseases: An Overview. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2005, 827, 65–75. [Google Scholar] [CrossRef]
- Cappai, R.; Leck, S.-L.; Tew, D.J.; Williamson, N.A.; Smith, D.P.; Galatis, D.; Sharples, R.A.; Curtain, C.C.; Ali, F.E.; Cherny, R.A.; et al. Dopamine Promotes Alpha-Synuclein Aggregation into SDS-Resistant Soluble Oligomers via a Distinct Folding Pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1377–1379. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Mishizen, A.J.; Giasson, B.I.; Lynch, D.R.; Thomas, S.A.; Nakashima, A.; Nagatsu, T.; Ota, A.; Ischiropoulos, H. Cytosolic Catechols Inhibit Alpha-Synuclein Aggregation and Facilitate the Formation of Intracellular Soluble Oligomeric Intermediates. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 10068–10078. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Baek, S.M.; Ho, D.-H.; Suk, J.-E.; Cho, E.-D.; Lee, S.-J. Dopamine Promotes Formation and Secretion of Non-Fibrillar Alpha-Synuclein Oligomers. Exp. Mol. Med. 2011, 43, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Cappai, R.; Barnham, K.J.; Pham, C.L.L. Modulation of Alpha-Synuclein Aggregation by Dopamine: A Review. Neurochem. Res. 2009, 34, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.H.; Giasson, B.I.; Hodara, R.; Xu, S.; Trojanowski, J.Q.; Ischiropoulos, H.; Lee, V.M.-Y. Reversible Inhibition of Alpha-Synuclein Fibrillization by Dopaminochrome-Mediated Conformational Alterations. J. Biol. Chem. 2005, 280, 21212–21219. [Google Scholar] [CrossRef]
- Maguire-Zeiss, K.A.; Short, D.W.; Federoff, H.J. Synuclein, Dopamine and Oxidative Stress: Co-Conspirators in Parkinson’s Disease? Brain Res. Mol. Brain Res. 2005, 134, 18–23. [Google Scholar] [CrossRef]
- Burke, W.J.; Li, S.W.; Williams, E.A.; Nonneman, R.; Zahm, D.S. 3,4-Dihydroxyphenylacetaldehyde Is the Toxic Dopamine Metabolite in Vivo: Implications for Parkinson’s Disease Pathogenesis. Brain Res. 2003, 989, 205–213. [Google Scholar] [CrossRef]
- Conway, K.A.; Rochet, J.C.; Bieganski, R.M.; Lansbury, P.T. Kinetic Stabilization of the Alpha-Synuclein Protofibril by a Dopamine-Alpha-Synuclein Adduct. Science 2001, 294, 1346–1349. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and Biochemistry of 4-Hydroxynonenal, Malonaldehyde and Related Aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Lee, S.H.; Blair, I.A. Characterization of 4-Oxo-2-Nonenal as a Novel Product of Lipid Peroxidation. Chem. Res. Toxicol. 2000, 13, 698–702. [Google Scholar] [CrossRef]
- Qin, Z.; Hu, D.; Han, S.; Reaney, S.H.; Di Monte, D.A.; Fink, A.L. Effect of 4-Hydroxy-2-Nonenal Modification on Alpha-Synuclein Aggregation. J. Biol. Chem. 2007, 282, 5862–5870. [Google Scholar] [CrossRef] [PubMed]
- Näsström, T.; Wahlberg, T.; Karlsson, M.; Nikolajeff, F.; Lannfelt, L.; Ingelsson, M.; Bergström, J. The Lipid Peroxidation Metabolite 4-Oxo-2-Nonenal Cross-Links Alpha-Synuclein Causing Rapid Formation of Stable Oligomers. Biochem. Biophys. Res. Commun. 2009, 378, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Hattori, N.; Uchida, K.; Tanaka, M.; Stadtman, E.R.; Mizuno, Y. Immunohistochemical Detection of 4-Hydroxynonenal Protein Adducts in Parkinson Disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. α-Synuclein Occurs Physiologically as a Helically Folded Tetramer That Resists Aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Lee, V.M.-Y. Addition of Exogenous α-Synuclein Preformed Fibrils to Primary Neuronal Cultures to Seed Recruitment of Endogenous α-Synuclein to Lewy Body and Lewy Neurite-like Aggregates. Nat. Protoc. 2014, 9, 2135–2146. [Google Scholar] [CrossRef]
- Luk, K.C.; Song, C.; O’Brien, P.; Stieber, A.; Branch, J.R.; Brunden, K.R.; Trojanowski, J.Q.; Lee, V.M.-Y. Exogenous Alpha-Synuclein Fibrils Seed the Formation of Lewy Body-like Intracellular Inclusions in Cultured Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20051–20056. [Google Scholar] [CrossRef]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Abul Khair, S.B.; Kazim, A.S.; Minhas, S.T.; Al-Tel, T.H.; Al-Hayani, A.A.; Haque, M.E.; Eliezer, D.; et al. Structure Activity Relationship of Phenolic Acid Inhibitors of α-Synuclein Fibril Formation and Toxicity. Front. Aging Neurosci. 2014, 6, 197. [Google Scholar] [CrossRef]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Menon, S.A.; Abul Khair, S.B.; Lu, J.-H.; Safieh-Garabedian, B.; Al-Hayani, A.A.; Eliezer, D.; Li, M.; et al. Ginsenoside Rb1 Inhibits Fibrillation and Toxicity of Alpha-Synuclein and Disaggregates Preformed Fibrils. Neurobiol. Dis. 2015, 74, 89–101. [Google Scholar] [CrossRef]
- Majbour, N.K.; Vaikath, N.N.; van Dijk, K.D.; Ardah, M.T.; Varghese, S.; Vesterager, L.B.; Montezinho, L.P.; Poole, S.; Safieh-Garabedian, B.; Tokuda, T.; et al. Oligomeric and Phosphorylated Alpha-Synuclein as Potential CSF Biomarkers for Parkinson’s Disease. Mol. Neurodegener. 2016, 11, 7. [Google Scholar] [CrossRef]
- El-Agnaf, O.M.A.; Salem, S.A.; Paleologou, K.E.; Curran, M.D.; Gibson, M.J.; Court, J.A.; Schlossmacher, M.G.; Allsop, D. Detection of Oligomeric Forms of Alpha-Synuclein Protein in Human Plasma as a Potential Biomarker for Parkinson’s Disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 419–425. [Google Scholar] [CrossRef]
- Tokuda, T.; Qureshi, M.M.; Ardah, M.T.; Varghese, S.; Shehab, S.A.S.; Kasai, T.; Ishigami, N.; Tamaoka, A.; Nakagawa, M.; El-Agnaf, O.M.A. Detection of Elevated Levels of α-Synuclein Oligomers in CSF from Patients with Parkinson Disease. Neurology 2010, 75, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Cheon, S.-M.; Bae, H.-R.; Kim, S.-H.; Kim, J.W. Elevated Levels of α-Synuclein Oligomer in the Cerebrospinal Fluid of Drug-Naïve Patients with Parkinson’s Disease. J. Clin. Neurol. 2011, 7, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Paleologou, K.E.; Kragh, C.L.; Mann, D.M.A.; Salem, S.A.; Al-Shami, R.; Allsop, D.; Hassan, A.H.; Jensen, P.H.; El-Agnaf, O.M.A. Detection of Elevated Levels of Soluble Alpha-Synuclein Oligomers in Post-Mortem Brain Extracts from Patients with Dementia with Lewy Bodies. Brain J. Neurol. 2009, 132 Pt 4, 1093–1101. [Google Scholar] [CrossRef]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural Basis of Membrane Disruption and Cellular Toxicity by α-Synuclein Oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal Lipid Peroxidation in Substantia Nigra Is Increased in Parkinson’s Disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Uchida, K. 4-Hydroxy-2-Nonenal: A Product and Mediator of Oxidative Stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Rindgen, D.; Nakajima, M.; Wehrli, S.; Xu, K.; Blair, I.A. Covalent Modifications to 2’-Deoxyguanosine by 4-Oxo-2-Nonenal, a Novel Product of Lipid Peroxidation. Chem. Res. Toxicol. 1999, 12, 1195–1204. [Google Scholar] [CrossRef]
- Benedetti, A.; Comporti, M.; Esterbauer, H. Identification of 4-Hydroxynonenal as a Cytotoxic Product Originating from the Peroxidation of Liver Microsomal Lipids. Biochim. Biophys. Acta 1980, 620, 281–296. [Google Scholar] [CrossRef]
- Stewart, B.J.; Doorn, J.A.; Petersen, D.R. Residue-Specific Adduction of Tubulin by 4-Hydroxynonenal and 4-Oxononenal Causes Cross-Linking and Inhibits Polymerization. Chem. Res. Toxicol. 2007, 20, 1111–1119. [Google Scholar] [CrossRef]
- Apetri, M.M.; Maiti, N.C.; Zagorski, M.G.; Carey, P.R.; Anderson, V.E. Secondary Structure of Alpha-Synuclein Oligomers: Characterization by Raman and Atomic Force Microscopy. J. Mol. Biol. 2006, 355, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Celej, M.S.; Sarroukh, R.; Goormaghtigh, E.; Fidelio, G.D.; Ruysschaert, J.-M.; Raussens, V. Toxic Prefibrillar α-Synuclein Amyloid Oligomers Adopt a Distinctive Antiparallel β-Sheet Structure. Biochem. J. 2012, 443, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Emadi, S.; Kasturirangan, S.; Wang, M.S.; Schulz, P.; Sierks, M.R. Detecting Morphologically Distinct Oligomeric Forms of Alpha-Synuclein. J. Biol. Chem. 2009, 284, 11048–11058. [Google Scholar] [CrossRef] [PubMed]
- Gallea, J.I.; Celej, M.S. Structural Insights into Amyloid Oligomers of the Parkinson Disease-Related Protein α-Synuclein. J. Biol. Chem. 2014, 289, 26733–26742. [Google Scholar] [CrossRef] [PubMed]
- Gurry, T.; Ullman, O.; Fisher, C.K.; Perovic, I.; Pochapsky, T.; Stultz, C.M. The Dynamic Structure of α-Synuclein Multimers. J. Am. Chem. Soc. 2013, 135, 3865–3872. [Google Scholar] [CrossRef]
- Hong, D.-P.; Fink, A.L.; Uversky, V.N. Structural Characteristics of Alpha-Synuclein Oligomers Stabilized by the Flavonoid Baicalein. J. Mol. Biol. 2008, 383, 214–223. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Cho, M.-K.; Kumar, A.; Maier, E.; Siebenhaar, C.; Becker, S.; Fernandez, C.O.; Lashuel, H.A.; Benz, R.; Lange, A.; et al. Structural Properties of Pore-Forming Oligomers of Alpha-Synuclein. J. Am. Chem. Soc. 2009, 131, 17482–17489. [Google Scholar] [CrossRef]
- Rekas, A.; Knott, R.B.; Sokolova, A.; Barnham, K.J.; Perez, K.A.; Masters, C.L.; Drew, S.C.; Cappai, R.; Curtain, C.C.; Pham, C.L.L. The Structure of Dopamine Induced Alpha-Synuclein Oligomers. Eur. Biophys. J. EBJ 2010, 39, 1407–1419. [Google Scholar] [CrossRef]
- Mysling, S.; Betzer, C.; Jensen, P.H.; Jorgensen, T.J.D. Characterizing the Dynamics of α-Synuclein Oligomers Using Hydrogen/Deuterium Exchange Monitored by Mass Spectrometry. Biochemistry 2013, 52, 9097–9103. [Google Scholar] [CrossRef]
- Cremades, N.; Chen, S.W.; Dobson, C.M. Structural Characteristics of α-Synuclein Oligomers. Int. Rev. Cell Mol. Biol. 2017, 329, 79–143. [Google Scholar] [CrossRef]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Böckmann, A.; Meier, B.H.; et al. Structural and Functional Characterization of Two Alpha-Synuclein Strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef] [PubMed]
- Ruesink, H.; Reimer, L.; Gregersen, E.; Moeller, A.; Betzer, C.; Jensen, P.H. Stabilization of α-Synuclein Oligomers Using Formaldehyde. PLoS ONE 2019, 14, e0216764. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.L.; Brown, D.R. Seeking a Mechanism for the Toxicity of Oligomeric α-Synuclein. Biomolecules 2015, 5, 282–305. [Google Scholar] [CrossRef]
- Zhang, N.-Y.; Tang, Z.; Liu, C.-W. Alpha-Synuclein Protofibrils Inhibit 26 S Proteasome-Mediated Protein Degradation: Understanding the Cytotoxicity of Protein Protofibrils in Neurodegenerative Disease Pathogenesis. J. Biol. Chem. 2008, 283, 20288–20298. [Google Scholar] [CrossRef] [PubMed]
- Hinault, M.-P.; Cuendet, A.F.H.; Mattoo, R.U.H.; Mensi, M.; Dietler, G.; Lashuel, H.A.; Goloubinoff, P. Stable Alpha-Synuclein Oligomers Strongly Inhibit Chaperone Activity of the Hsp70 System by Weak Interactions with J-Domain Co-Chaperones. J. Biol. Chem. 2010, 285, 38173–38182. [Google Scholar] [CrossRef]
- Cremades, N.; Cohen, S.I.A.; Deas, E.; Abramov, A.Y.; Chen, A.Y.; Orte, A.; Sandal, M.; Clarke, R.W.; Dunne, P.; Aprile, F.A.; et al. Direct Observation of the Interconversion of Normal and Toxic Forms of α-Synuclein. Cell 2012, 149, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG Redirects Amyloidogenic Polypeptides into Unstructured, off-Pathway Oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Zhou, W.; Long, C.; Reaney, S.H.; Di Monte, D.A.; Fink, A.L.; Uversky, V.N. Methionine Oxidation Stabilizes Non-Toxic Oligomers of Alpha-Synuclein through Strengthening the Auto-Inhibitory Intra-Molecular Long-Range Interactions. Biochim. Biophys. Acta 2010, 1802, 322–330. [Google Scholar] [CrossRef]
- Lorenzen, N.; Lemminger, L.; Pedersen, J.N.; Nielsen, S.B.; Otzen, D.E. The N-Terminus of α-Synuclein Is Essential for Both Monomeric and Oligomeric Interactions with Membranes. FEBS Lett. 2014, 588, 497–502. [Google Scholar] [CrossRef]
- Galvin, J.E. Interaction of Alpha-Synuclein and Dopamine Metabolites in the Pathogenesis of Parkinson’s Disease: A Case for the Selective Vulnerability of the Substantia Nigra. Acta Neuropathol. 2006, 112, 115–126. [Google Scholar] [CrossRef]
- Bemporad, F.; Chiti, F. Protein Misfolded Oligomers: Experimental Approaches, Mechanism of Formation, and Structure-Toxicity Relationships. Chem. Biol. 2012, 19, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Clavaguera, F.; Tolnay, M. The Propagation of Prion-like Protein Inclusions in Neurodegenerative Diseases. Trends Neurosci. 2010, 33, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vicente, M.; Talloczy, Z.; Kaushik, S.; Massey, A.C.; Mazzulli, J.; Mosharov, E.V.; Hodara, R.; Fredenburg, R.; Wu, D.-C.; Follenzi, A.; et al. Dopamine-Modified Alpha-Synuclein Blocks Chaperone-Mediated Autophagy. J. Clin. Investig. 2008, 118, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Choi, M.-G.; Kim, J.-Y.; Yang, Y.; Lai, Y.; Kweon, D.-H.; Lee, N.K.; Shin, Y.-K. Large α-Synuclein Oligomers Inhibit Neuronal SNARE-Mediated Vesicle Docking. Proc. Natl. Acad. Sci. USA 2013, 110, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, B.D.; Claessens, M.M.A.E.; Subramaniam, V. Lipid Bilayer Disruption by Oligomeric Alpha-Synuclein Depends on Bilayer Charge and Accessibility of the Hydrophobic Core. Biochim. Biophys. Acta 2009, 1788, 1271–1278. [Google Scholar] [CrossRef]
- Ding, T.T.; Lee, S.-J.; Rochet, J.-C.; Lansbury, P.T. Annular Alpha-Synuclein Protofibrils Are Produced When Spherical Protofibrils Are Incubated in Solution or Bound to Brain-Derived Membranes. Biochemistry 2002, 41, 10209–10217. [Google Scholar] [CrossRef]
- Herrera, F.E.; Chesi, A.; Paleologou, K.E.; Schmid, A.; Munoz, A.; Vendruscolo, M.; Gustincich, S.; Lashuel, H.A.; Carloni, P. Inhibition of Alpha-Synuclein Fibrillization by Dopamine Is Mediated by Interactions with Five C-Terminal Residues and with E83 in the NAC Region. PLoS ONE 2008, 3, e3394. [Google Scholar] [CrossRef]
- Vaikath, N.N.; Erskine, D.; Morris, C.M.; Majbour, N.K.; Vekrellis, K.; Li, J.-Y.; El-Agnaf, O.M.A. Heterogeneity in α-Synuclein Subtypes and Their Expression in Cortical Brain Tissue Lysates from Lewy Body Diseases and Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2019, 45, 597–608. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaikath, N.; Sudhakaran, I.; Abdi, I.; Gupta, V.; Majbour, N.; Ghanem, S.; Abdesselem, H.; Vekrellis, K.; El-Agnaf, O. Structural and Biophysical Characterization of Stable Alpha-Synuclein Oligomers. Int. J. Mol. Sci. 2022, 23, 14630. https://doi.org/10.3390/ijms232314630
Vaikath N, Sudhakaran I, Abdi I, Gupta V, Majbour N, Ghanem S, Abdesselem H, Vekrellis K, El-Agnaf O. Structural and Biophysical Characterization of Stable Alpha-Synuclein Oligomers. International Journal of Molecular Sciences. 2022; 23(23):14630. https://doi.org/10.3390/ijms232314630
Chicago/Turabian StyleVaikath, Nishant, Indulekha Sudhakaran, Ilham Abdi, Vijay Gupta, Nour Majbour, Simona Ghanem, Houari Abdesselem, Kostas Vekrellis, and Omar El-Agnaf. 2022. "Structural and Biophysical Characterization of Stable Alpha-Synuclein Oligomers" International Journal of Molecular Sciences 23, no. 23: 14630. https://doi.org/10.3390/ijms232314630
APA StyleVaikath, N., Sudhakaran, I., Abdi, I., Gupta, V., Majbour, N., Ghanem, S., Abdesselem, H., Vekrellis, K., & El-Agnaf, O. (2022). Structural and Biophysical Characterization of Stable Alpha-Synuclein Oligomers. International Journal of Molecular Sciences, 23(23), 14630. https://doi.org/10.3390/ijms232314630




