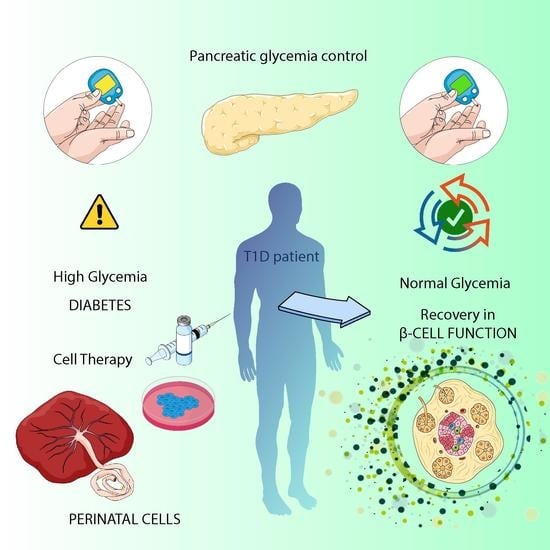
Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation
Abstract
1. Introduction
2. Perinatal Cells
2.1. Amniotic Epithelial Cells (AECs)
2.1.1. Differentiating Potential of AECs
2.1.2. Immunomodulatory Capacity of AECs
2.2. Wharton’s Jelly Mesenchymal Stem/Stromal Cells (WJ-MSCs)
2.2.1. Differentiating Potential of WJ-MSCs
2.2.2. Immunomodulatory Capacity of WJ-MSCs
2.2.3. Co-Culture Strategies Based on WJ-MSCs
2.3. Other Sources of Perinatal Cells
2.3.1. Amniotic Fluid Stem Cells (AFSCs)
2.3.2. Amniotic Membrane Mesenchymal Stem Cells (AM-MSCs)
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchetti, P.; Bugliani, M.; De Tata, V.; Suleiman, M.; Marselli, L. Pancreatic Beta Cell Identity in Humans and the Role of Type 2 Diabetes. Front. Cell Dev. Biol. 2017, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Chapter 1: Epidemiology of Type 1 Diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Desai, S.; Deshmukh, A. Mapping of Type 1 Diabetes Mellitus. Curr. Diabetes Rev. 2020, 16, 438–441. [Google Scholar] [CrossRef]
- Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. Clinical Islet Transplantation: Is the Future Finally Now? Curr. Opin. Organ Transpl. 2018, 23, 428–439. [Google Scholar] [CrossRef]
- Skyler, J.S. Hope vs Hype: Where Are We in Type 1 Diabetes? Diabetologija 2018, 61, 509–516. [Google Scholar] [CrossRef]
- Sneddon, J.B.; Tang, Q.; Stock, P.; Bluestone, J.A.; Roy, S.; Desai, T.; Hebrok, M. Stem Cell Therapies for Treating Diabetes: Progress and Remaining Challenges. Cell Stem Cell 2018, 22, 810–823. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Pokrywczynska, M.; Ricordi, C. Clinical Pancreatic Islet Transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 Diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.G.; Millman, J.R. Applications of IPSC-Derived Beta Cells from Patients with Diabetes. Cell Rep. Med. 2021, 2, 100238. [Google Scholar] [CrossRef]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic Endoderm Derived from Human Embryonic Stem Cells Generates Glucose-Responsive Insulin-Secreting Cells In Vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Cito, M.; Pellegrini, S.; Piemonti, L.; Sordi, V. The Potential and Challenges of Alternative Sources of β Cells for the Cure of Type 1 Diabetes. Endocr. Connect. 2018, 7, R114–R125. [Google Scholar] [CrossRef]
- Suman, S.; Domingues, A.; Ratajczak, J.; Ratajczak, M.Z. Potential Clinical Applications of Stem Cells in Regenerative Medicine. Adv. Exp. Med. Biol. 2019, 1201, 1–22. [Google Scholar] [CrossRef]
- Hyun, I. The Bioethics of Stem Cell Research and Therapy. J. Clin. Investig. 2010, 120, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bhonde, R.R.; Sheshadri, P.; Sharma, S.; Kumar, A. Making Surrogate β-Cells from Mesenchymal Stromal Cells: Perspectives and Future Endeavors. Int. J. Biochem. Cell Biol. 2014, 46, 90–102. [Google Scholar] [CrossRef]
- Murray, H.E.; Zafar, A.; Qureshi, K.M.; Paget, M.B.; Bailey, C.J.; Downing, R. The Potential Role of Multifunctional Human Amniotic Epithelial Cells in Pancreatic Islet Transplantation. J. Tissue Eng. Regen. Med. 2021, 15, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.-J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise Review: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Silini, A.R.; Masserdotti, A.; Papait, A.; Parolini, O. Shaping the Future of Perinatal Cells: Lessons from the Past and Interpretations of the Present. Front. Bioeng. Biotechnol. 2019, 7, 75. [Google Scholar] [CrossRef]
- Okere, B.; Alviano, F.; Costa, R.; Quaglino, D.; Ricci, F.; Dominici, M.; Paolucci, P.; Bonsi, L.; Iughetti, L. In Vitro Differentiation of Human Amniotic Epithelial Cells into Insulin-Producing 3D Spheroids. Int. J. Immunopathol. Pharm. 2015, 28, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.R.; Di Pietro, R.; Lang-Olip, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaite, V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature. Front. Bioeng. Biotechnol. 2020, 8, 1438. [Google Scholar] [CrossRef]
- Cross, J.C. Formation of the Placenta and Extraembryonic Membranes. Ann. N. Y. Acad. Sci. 1998, 857, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bourne, G.L. The Microscopic Anatomy of the Human Amnion and Chorion. Am. J. Obstet. Gynecol. 1960, 79, 1070–1073. [Google Scholar] [CrossRef]
- Alviano, F.; Fossati, V.; Marchionni, C.; Arpinati, M.; Bonsi, L.; Franchina, M.; Lanzoni, G.; Cantoni, S.; Cavallini, C.; Bianchi, F.; et al. Term Amniotic Membrane Is a High Throughput Source for Multipotent Mesenchymal Stem Cells with the Ability to Differentiate into Endothelial Cells In Vitro. BMC Dev. Biol. 2007, 7, 11. [Google Scholar] [CrossRef]
- Stefańska, K.; Ożegowska, K.; Hutchings, G.; Popis, M.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Pieńkowski, W.; Gutaj, P.; Shibli, J.A.; et al. Human Wharton’s Jelly—Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. J. Clin. Med. 2020, 9, 1102. [Google Scholar] [CrossRef]
- McElreavey, K.D.; Irvine, A.I.; Ennis, K.T.; McLean, W.H. Isolation, Culture and Characterisation of Fibroblast-like Cells Derived from the Wharton’s Jelly Portion of Human Umbilical Cord. Biochem. Soc. Trans. 1991, 19, 29S. [Google Scholar] [CrossRef]
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The Immunomodulatory Properties of Amniotic Cells: The Two Sides of the Coin. Cell Transpl. 2018, 27, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Motedayyen, H.; Esmaeil, N.; Tajik, N.; Khadem, F.; Ghotloo, S.; Khani, B.; Rezaei, A. Method and Key Points for Isolation of Human Amniotic Epithelial Cells with High Yield, Viability and Purity. BMC Res. Notes 2017, 10, 552. [Google Scholar] [CrossRef]
- Trosan, P.; Smeringaiova, I.; Brejchova, K.; Bednar, J.; Benada, O.; Kofronova, O.; Jirsova, K. The Enzymatic De-Epithelialization Technique Determines Denuded Amniotic Membrane Integrity and Viability of Harvested Epithelial Cells. PLoS ONE 2018, 13, e0194820. [Google Scholar] [CrossRef]
- Centurione, L.; Passaretta, F.; Centurione, M.A.; De Munari, S.; Vertua, E.; Silini, A.; Liberati, M.; Parolini, O.; Di Pietro, R. Mapping of the Human Placenta. Cell Transpl. 2018, 27, 12–22. [Google Scholar] [CrossRef]
- Fatimah, S.S.; Tan, G.C.; Chua, K.H.; Tan, A.E.; Hayati, A.R. Effects of Epidermal Growth Factor on the Proliferation and Cell Cycle Regulation of Cultured Human Amnion Epithelial Cells. J. Biosci. Bioeng. 2012, 114, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in Culture Expanded Human Amniotic Epithelial Cells: Implications for Potential Therapeutic Applications. PLoS ONE 2011, 6, e26136. [Google Scholar] [CrossRef]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem Cell Characteristics of Amniotic Epithelial Cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Koike, N.; Sugimoto, J.; Okabe, M.; Arai, K.; Nogami, M.; Okudera, H.; Yoshida, T. Distribution of Amniotic Stem Cells in Human Term Amnion Membrane. Microscopy 2022, 71, 66–76. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Mosaffa, N.; Nikoo, S.; Bozorgmehr, M.; Ghods, R.; Kazemnejad, S.; Rezania, S.; Keshavarzi, B.; Arefi, S.; Ramezani-Tehrani, F.; et al. Isolation and Partial Characterization of Human Amniotic Epithelial Cells: The Effect of Trypsin. Avicenna J. Med. Biotechnol. 2014, 6, 10. [Google Scholar]
- Miki, T. Stem Cell Characteristics and the Therapeutic Potential of Amniotic Epithelial Cells. Am. J. Reprod. Immunol. 2018, 80, e13003. [Google Scholar] [CrossRef]
- Jiawen, S.; Jianjun, Z.; Jiewen, D.; Dedong, Y.; Hongbo, Y.; Jun, S.; Xudong, W.; Shen, S.G.F.; Lihe, G. Osteogenic Differentiation of Human Amniotic Epithelial Cells and Its Application in Alveolar Defect Restoration. Steam Cells Transl. Med. 2014, 3, 1504–1513. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, D. Application of Human Amniotic Epithelial Cells in Regenerative Medicine: A Systematic Review. Stem Cell Res. Ther. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, F.; Gramignoli, R.; Dorko, K.; Miki, T.; Ranade, A.R.; Paola Serra, M.; Doratiotto, S.; Sini, M.; Sharma, S.; Mitamura, K.; et al. Hepatic Differentiation of Amniotic Epithelial Cells. Hepatology 2011, 53, 1719–1729. [Google Scholar] [CrossRef]
- Yao, M.; Chen, J.; Yang, X.-X.; Zhang, X.-L.; Ji, Q.-S.; Zhou, Q.; Xu, J.-T. Differentiation of Human Amniotic Epithelial Cells into Corneal Epithelial-like Cells in Vitro. Int. J. Ophthalmol. 2013, 6, 564–572. [Google Scholar] [CrossRef]
- Niknejad, H.; Peirovi, H.; Ahmadiani, A.; Ghanavi, J.; Jorjani, M. Differentiation Factors That Influence Neuronal Markers Expression in Vitro from Human Amniotic Epithelial Cells. Eur. Cell Mater. 2010, 19, 22–29. [Google Scholar] [CrossRef]
- Bhandari, D.R.; Seo, K.-W.; Sun, B.; Seo, M.-S.; Kim, H.-S.; Seo, Y.-J.; Marcin, J.; Forraz, N.; Roy, H.L.; Larry, D.; et al. The Simplest Method for in Vitro β-Cell Production from Human Adult Stem Cells. Differentiation 2011, 82, 144–152. [Google Scholar] [CrossRef]
- Wei, J.P.; Zhang, T.S.; Kawa, S.; Aizawa, T.; Ota, M.; Akaike, T.; Kato, K.; Konishi, I.; Nikaido, T. Human Amnion-Isolated Cells Normalize Blood Glucose in Streptozotocin-Induced Diabetic Mice. Cell Transpl. 2003, 12, 545–552. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, Q.; Liu, T.; Guo, L. Human Amnion Epithelial Cells Can Be Induced to Differentiate into Functional Insulin-Producing Cells. Acta Biochim. Biophys. Sin. 2008, 40, 830–839. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Qin, J.; Chang, M.; Wang, S.; Li, Y.; Pei, X.; Wang, Y. Enhanced Differentiation of Human Pluripotent Stem Cells into Pancreatic Endocrine Cells in 3D Culture by Inhibition of Focal Adhesion Kinase. Stem Cell Res. Ther. 2020, 11, 488. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.G.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E.E. Production of Pancreatic Hormone–Expressing Endocrine Cells from Human Embryonic Stem Cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef]
- Luo, Y.; Cheng, Y.-W.; Yu, C.-Y.; Liu, R.-M.; Zhao, Y.-J.; Chen, D.-X.; Zhong, J.-J.; Xiao, J.-H. Effects of Hyaluronic Acid on Differentiation of Human Amniotic Epithelial Cells and Cell-Replacement Therapy in Type 1 Diabetic Mice. Exp. Cell Res. 2019, 384, 111642. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Lebreton, F.; Lavallard, V.; Bellofatto, K.; Bonnet, R.; Wassmer, C.H.; Perez, L.; Kalandadze, V.; Follenzi, A.; Boulvain, M.; Kerr-Conte, J.; et al. Insulin-Producing Organoids Engineered from Islet and Amniotic Epithelial Cells to Treat Diabetes. Nat. Commun. 2019, 10, 4491. [Google Scholar] [CrossRef]
- Lebreton, F.; Bellofatto, K.; Wassmer, C.H.; Perez, L.; Lavallard, V.; Parnaud, G.; Cottet-Dumoulin, D.; Kerr-Conte, J.; Pattou, F.; Bosco, D.; et al. Shielding Islets with Human Amniotic Epithelial Cells Enhances Islet Engraftment and Revascularization in a Murine Diabetes Model. Am. J. Transplant. 2020, 20, 1551–1561. [Google Scholar] [CrossRef]
- Wassmer, C.-H.; Lebreton, F.; Bellofatto, K.; Perez, L.; Cottet-Dumoulin, D.; Andres, A.; Bosco, D.; Berney, T.; Othenin-Girard, V.; Martinez De Tejada, B.; et al. Bio-Engineering of Pre-Vascularized Islet Organoids for the Treatment of Type 1 Diabetes. Transpl. Int. 2022, 35, 10214. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Parolini, O.; Caruso, M. Review: Preclinical Studies on Placenta-Derived Cells and Amniotic Membrane: An Update. Placenta 2011, 32 (Suppl. S2), S186–S195. [Google Scholar] [CrossRef]
- Wassmer, C.-H.; Lebreton, F.; Bellofatto, K.; Bosco, D.; Berney, T.; Berishvili, E. Generation of Insulin-Secreting Organoids: A Step toward Engineering and Transplanting the Bioartificial Pancreas. Transpl Int. 2020, 33, 1577–1588. [Google Scholar] [CrossRef]
- Moodley, Y.; Ilancheran, S.; Samuel, C.; Vaghjiani, V.; Atienza, D.; Williams, E.D.; Jenkin, G.; Wallace, E.; Trounson, A.; Manuelpillai, U. Human Amnion Epithelial Cell Transplantation Abrogates Lung Fibrosis and Augments Repair. Am. J. Respir. Crit. Care Med. 2010, 182, 643–651. [Google Scholar] [CrossRef]
- Ilancheran, S.; Michalska, A.; Peh, G.; Wallace, E.M.; Pera, M.; Manuelpillai, U. Stem Cells Derived from Human Fetal Membranes Display Multilineage Differentiation Potential. Biol. Reprod. 2007, 77, 577–588. [Google Scholar] [CrossRef]
- Morandi, F.; Marimpietri, D.; Görgens, A.; Gallo, A.; Srinivasan, R.C.; El-Andaloussi, S.; Gramignoli, R. Human Amnion Epithelial Cells Impair T Cell Proliferation: The Role of HLA-G and HLA-E Molecules. Cells 2020, 9, 2123. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.; McMichael, A.J. Functions of Nonclassical MHC and Non-MHC-Encoded Class I Molecules. Curr. Opin. Immunol. 1999, 11, 100–108. [Google Scholar] [CrossRef]
- Rizzo, R.; Campioni, D.; Stignani, M.; Melchiorri, L.; Bagnara, G.P.; Bonsi, L.; Alviano, F.; Lanzoni, G.; Moretti, S.; Cuneo, A.; et al. A Functional Role for Soluble HLA-G Antigens in Immune Modulation Mediated by Mesenchymal Stromal Cells. Cytotherapy 2008, 10, 364–375. [Google Scholar] [CrossRef]
- Zhu, D.; Tan, J.; Maleken, A.S.; Muljadi, R.; Chan, S.T.; Lau, S.N.; Elgass, K.; Leaw, B.; Mockler, J.; Chambers, D.; et al. Human Amnion Cells Reverse Acute and Chronic Pulmonary Damage in Experimental Neonatal Lung Injury. Stem Cell Res Ther. 2017, 8, 257. [Google Scholar] [CrossRef]
- Li, H.; Niederkorn, J.Y.; Neelam, S.; Mayhew, E.; Word, R.A.; McCulley, J.P.; Alizadeh, H. Immunosuppressive Factors Secreted by Human Amniotic Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 900–907. [Google Scholar] [CrossRef]
- Tan, J.L.; Chan, S.T.; Lo, C.Y.; Deane, J.A.; McDonald, C.A.; Bernard, C.C.; Wallace, E.M.; Lim, R. Amnion Cell-Mediated Immune Modulation Following Bleomycin Challenge: Controlling the Regulatory T Cell Response. Stem Cell Res. Ther. 2015, 6, 8. [Google Scholar] [CrossRef]
- Fathi, I.; Miki, T. Human Amniotic Epithelial Cells Secretome: Components, Bioactivity, and Challenges. Front. Med. 2022, 8. [Google Scholar] [CrossRef]
- Tan, J.L.; Lau, S.N.; Leaw, B.; Nguyen, H.P.T.; Salamonsen, L.A.; Saad, M.I.; Chan, S.T.; Zhu, D.; Krause, M.; Kim, C.; et al. Amnion Epithelial Cell-Derived Exosomes Restrict Lung Injury and Enhance Endogenous Lung Repair. Stem Cells Transl. Med. 2018, 7, 180–196. [Google Scholar] [CrossRef]
- Cargnoni, A.; Farigu, S.; Cotti Piccinelli, E.; Bonassi Signoroni, P.; Romele, P.; Vanosi, G.; Toschi, I.; Cesari, V.; Barros Sant’Anna, L.; Magatti, M.; et al. Effect of Human Amniotic Epithelial Cells on Pro-Fibrogenic Resident Hepatic Cells in a Rat Model of Liver Fibrosis. J. Cell Mol. Med. 2018, 22, 1202–1213. [Google Scholar] [CrossRef]
- Song, Y.-S.; Joo, H.-W.; Park, I.-H.; Shen, G.-Y.; Lee, Y.; Shin, J.H.; Kim, H.; Shin, I.-S.; Kim, K.-S. Transplanted Human Amniotic Epithelial Cells Secrete Paracrine Proangiogenic Cytokines in Rat Model of Myocardial Infarctio. Cell Transpl. 2015, 24, 2055–2064. [Google Scholar] [CrossRef]
- Vosdoganes, P.; Wallace, E.M.; Chan, S.T.; Acharya, R.; Moss, T.J.M.; Lim, R. Human Amnion Epithelial Cells Repair Established Lung Injury. 2013. Available online: https://journals.sagepub.com/doi/10.3727/096368912X657657 (accessed on 25 September 2022).
- Hodge, A.; Andrewartha, N.; Lourensz, D.; Strauss, R.; Correia, J.; Goonetilleke, M.; Yeoh, G.; Lim, R.; Sievert, W. Human Amnion Epithelial Cells Produce Soluble Factors That Enhance Liver Repair by Reducing Fibrosis While Maintaining Regeneration in a Model of Chronic Liver Injury. 2020. Available online: https://journals.sagepub.com/doi/10.1177/0963689720950221 (accessed on 25 September 2022).
- Jirsova, K.; Jones, G.L.A. Amniotic Membrane in Ophthalmology: Properties, Preparation, Storage and Indications for Grafting—A Review. Cell Tissue Bank 2017, 18, 193–204. [Google Scholar] [CrossRef]
- Castillo-Melendez, M.; Yawno, T.; Jenkin, G.; Miller, S. Stem Cell Therapy to Protect and Repair the Developing Brain: A Review of Mechanisms of Action of Cord Blood and Amnion Epithelial Derived Cells. Front. Neurosci. 2013, 7, 194. [Google Scholar] [CrossRef]
- Podestà, M.A.; Remuzzi, G.; Casiraghi, F. Mesenchymal Stromal Cells for Transplant Tolerance. Front. Immunol. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Jun, Y.; Kang, A.R.; Lee, J.S.; Park, S.-J.; Lee, D.Y.; Moon, S.-H.; Lee, S.-H. Microchip-Based Engineering of Super-Pancreatic Islets Supported by Adipose-Derived Stem Cells. Biomaterials 2014, 35, 4815–4826. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.M.; Oliver, R.J.; Paget, M.B.; Murray, H.E.; Bailey, C.J.; Downing, R. Human Amniotic Epithelial Cells Induce Localized Cell-Mediated Immune Privilege in Vitro: Implications for Pancreatic Islet Transplantation. Cell Transpl. 2011, 20, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Lee, J.; Yesmin, S.; Paget, M.B.; Bailey, C.J.; Murray, H.E.; Downing, R. Rotational Culture and Integration with Amniotic Stem Cells Reduce Porcine Islet Immunoreactivity in Vitro and Slow Xeno-Rejection in a Murine Model of Islet Transplantation. Xenotransplantation 2019, 26, e12508. [Google Scholar] [CrossRef]
- Cui, W.; Khan, K.M.; Ma, X.; Chen, G.; Desai, C.S. Human Amniotic Epithelial Cells and Human Amniotic Membrane as a Vehicle for Islet Cell Transplantation. Transpl. Proc. 2020, 52, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, F.; Hanna, R.; Wassmer, C.H.; Bellofatto, K.; Perez, L.; Othenin-Girard, V.; de Tejada, B.M.; Cohen, M.; Berishvili, E. Mechanisms of Immunomodulation and Cytoprotection Conferred to Pancreatic Islet by Human Amniotic Epithelial Cells. Stem Cell Rev. Rep. 2022, 18, 346–359. [Google Scholar] [CrossRef]
- Kamal, M.M.; Kassem, D.H. Therapeutic Potential of Wharton’s Jelly Mesenchymal Stem Cells for Diabetes: Achievements and Challenges. Front. Cell Dev. Biol. 2020, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Fong, C.-Y.; Gauthaman, K. Taking Stem Cells to the Clinic: Major Challenges. J. Cell Biochem. Suppl. 2008, 105, 1352–1360. [Google Scholar] [CrossRef]
- Bai, C.; Gao, Y.; Li, Q.; Feng, Y.; Yu, Y.; Meng, G.; Zhang, M.; Guan, W. Differentiation of Chicken Umbilical Cord Mesenchymal Stem Cells into Beta-like Pancreatic Islet Cells. Artif. Cells Nanomed. Biotechnol. 2015, 43, 106–111. [Google Scholar] [CrossRef]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells 2008, 26, 2865–2874. [Google Scholar] [CrossRef]
- El Omar, R.; Beroud, J.; Stoltz, J.-F.; Menu, P.; Velot, E.; Decot, V. Umbilical Cord Mesenchymal Stem Cells: The New Gold Standard for Mesenchymal Stem Cell-Based Therapies? Tissue Eng. Part B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef]
- La Rocca, G.; Anzalone, R.; Corrao, S.; Magno, F.; Loria, T.; Lo Iacono, M.; Di Stefano, A.; Giannuzzi, P.; Marasà, L.; Cappello, F.; et al. Isolation and Characterization of Oct-4+/HLA-G+ Mesenchymal Stem Cells from Human Umbilical Cord Matrix: Differentiation Potential and Detection of New Markers. Histochem. Cell Biol. 2009, 131, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, R.; Lo Iacono, M.; Loria, T.; Di Stefano, A.; Giannuzzi, P.; Farina, F.; La Rocca, G. Wharton’s Jelly Mesenchymal Stem Cells as Candidates for Beta Cells Regeneration: Extending the Differentiative and Immunomodulatory Benefits of Adult Mesenchymal Stem Cells for the Treatment of Type 1 Diabetes. Stem Cell Rev. Rep. 2011, 7, 342–363. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, Y.; Liu, J. Mesenchymal Stem Cells: An Excellent Candidate for the Treatment of Diabetes Mellitus. Int. J. Endocrinol. 2021, 2021, 9938658. [Google Scholar] [CrossRef]
- Johnson, C.L.; Soeder, Y.; Dahlke, M.H. Concise Review: Mesenchymal Stromal Cell-Based Approaches for the Treatment of Acute Respiratory Distress and Sepsis Syndromes. Stem Cells Transl. Med. 2017, 6, 1141–1151. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Zhou, J.; Deng, X.; He, W.; Liu, X.; Li, Y.; Zhong, N.; Sang, L. Current Status of Cell-Based Therapies for COVID-19: Evidence From Mesenchymal Stromal Cells in Sepsis and ARDS. Front. Immunol. 2021, 12, 738697. [Google Scholar] [CrossRef] [PubMed]
- Gorman, E.; Millar, J.; McAuley, D.; O’Kane, C. Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome (ARDS), Sepsis, and COVID-19 Infection: Optimizing the Therapeutic Potential. Expert Rev. Respir. Med. 2021, 15, 301–324. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Kouroupis, D.; Lanzoni, G.; Linetsky, E.; Messinger Cayetano, S.; Wishnek Metalonis, S.; Leñero, C.; Stone, L.D.; Ruiz, P.; Correa, D.; Ricordi, C. Umbilical Cord-Derived Mesenchymal Stem Cells Modulate TNF and Soluble TNF Receptor 2 (STNFR2) in COVID-19 ARDS Patients. Eur. Rev. Med. Pharm. Sci. 2021, 25, 4435–4438. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Gibellini, D.; Astori, G.; Bernardi, M.; Bozza, A.; Chieregato, K.; Elice, F.; Ugel, S.; Caligola, S.; de Sanctis, F.; et al. The Immune Modulatory Effects of Umbilical Cord-Derived Mesenchymal Stromal Cells in Severe COVID-19 Pneumonia. Stem Cell Res. Ther. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Cai, J.; Wu, Z.; Xu, X.; Liao, L.; Chen, J.; Huang, L.; Wu, W.; Luo, F.; Wu, C.; Pugliese, A.; et al. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 2016, 39, 149–157. [Google Scholar] [CrossRef]
- Hu, J.; Yu, X.; Wang, Z.; Wang, F.; Wang, L.; Gao, H.; Chen, Y.; Zhao, W.; Jia, Z.; Yan, S.; et al. Long Term Effects of the Implantation of Wharton’s Jelly-Derived Mesenchymal Stem Cells from the Umbilical Cord for Newly-Onset Type 1 Diabetes Mellitus. Endocr. J. 2013, 60, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Liang, H.; Tang, D.; Huang, M.; Zhao, J.; Yang, X.; Liu, Y.; Shu, L.; Wang, J.; et al. Efficacy of Mesenchymal Stem Cell Transplantation Therapy for Type 1 and Type 2 Diabetes Mellitus: A Meta-Analysis. Stem Cell Res. Ther. 2021, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shen, S.-M.; Ling, Q.; Wang, B.; Li, L.-R.; Zhang, W.; Qu, D.-D.; Bi, Y.; Zhu, D.-L. One Repeated Transplantation of Allogeneic Umbilical Cord Mesenchymal Stromal Cells in Type 1 Diabetes: An Open Parallel Controlled Clinical Study. Stem Cell Res. 2021, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Afzali, H.; Raoofi, A.; Nouri, M.; Naser, R.; Gholami, O.; Nasiry, D.; Mohammadnia, A.; Razzaghi, Z.; Alimohammadi, A.; et al. Topical Spray of Wharton’s Jelly Mesenchymal Stem Cells Derived from Umbilical Cord Accelerates Diabetic Wound Healing. J. Cosmet. Derm. 2022, 10, 5156–5167. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, R.F.; Hammad, L.N.; Kamal, M.M.; El Mesallamy, H.O. A Comparison of Wharton’s Jelly and Cord Blood as a Source of Mesenchymal Stem Cells for Diabetes Cell Therapy. Regen. Med. 2015, 10, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Kassem, D.H.; Kamal, M.M. Therapeutic Efficacy of Umbilical Cord-Derived Stem Cells for Diabetes Mellitus: A Meta-Analysis Study. Stem Cell Res. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Wang, H.-S.; Lin, G.-J.; Chou, S.-C.; Chu, T.-H.; Chuan, W.-T.; Lu, Y.-J.; Weng, Z.-C.; Su, C.-H.; Hsieh, P.-S.; et al. Undifferentiated Wharton’s Jelly Mesenchymal Stem Cell Transplantation Induces Insulin-Producing Cell Differentiation and Suppression of T-Cell-Mediated Autoimmunity in Nonobese Diabetic Mice. Cell Transpl. 2015, 24, 1555–1570. [Google Scholar] [CrossRef]
- Kassem, D.H.; Kamal, M.M.; El-Kholy, A.E.-L.G.; El-Mesallamy, H.O. Association of Expression Levels of Pluripotency/Stem Cell Markers with the Differentiation Outcome of Wharton’s Jelly Mesenchymal Stem Cells into Insulin Producing Cells. Biochimie 2016, 127, 187–195. [Google Scholar] [CrossRef]
- Kassem, D.H.; Kamal, M.M.; El-Kholy, A.E.-L.G.; El-Mesallamy, H.O. Exendin-4 Enhances the Differentiation of Wharton’s Jelly Mesenchymal Stem Cells into Insulin-Producing Cells through Activation of Various β-Cell Markers. Stem Cell Res. 2016, 7, 108. [Google Scholar] [CrossRef]
- Ranjbaran, H.; Abediankenari, S.; Khalilian, A.; Rahmani, Z.; Momeninezhad Amiri, M.; Hosseini Khah, Z. Differentiation of Wharton’s Jelly Derived Mesenchymal Stem Cells into Insulin Producing Cells. Int. J. Hematol Oncol. Stem Cell Res. 2018, 12, 220–229. [Google Scholar]
- Ren, H.; Sang, Y.; Zhang, F.; Liu, Z.; Qi, N.; Chen, Y. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016, 2016, 3516574. [Google Scholar] [CrossRef]
- Gao, L.R.; Zhang, N.K.; Zhang, Y.; Chen, Y.; Wang, L.; Zhu, Y.; Tang, H.H. Overexpression of Apelin in Wharton’ Jelly Mesenchymal Stem Cell Reverses Insulin Resistance and Promotes Pancreatic β Cell Proliferation in Type 2 Diabetic Rats. Stem Cell Res. 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Beikmohammadi, L.; Bandehpour, M.; Hashemi, S.M.; Kazemi, B. Generation of Insulin-Producing Hepatocyte-like Cells from Human Wharton’s Jelly Mesenchymal Stem Cells as an Alternative Source of Islet Cells. J. Cell Physiol. 2019, 234, 17326–17336. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Chen, T.-H.; Huang, B.-S.; Chen, P.-H.; Su, C.-H.; Shyu, J.-F.; Tsai, P.-J. Comparison between the Therapeutic Effects of Differentiated and Undifferentiated Wharton’s Jelly Mesenchymal Stem Cells in Rats with Streptozotocin-Induced Diabetes. World J. Stem Cells 2020, 12, 139–151. [Google Scholar] [CrossRef]
- Aierken, A.; Li, B.; Liu, P.; Cheng, X.; Kou, Z.; Tan, N.; Zhang, M.; Yu, S.; Shen, Q.; Du, X.; et al. Melatonin Treatment Improves Human Umbilical Cord Mesenchymal Stem Cell Therapy in a Mouse Model of Type II Diabetes Mellitus via the PI3K/AKT Signaling Pathway. Stem Cell Res. 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Azarbarz, N.; Khorsandi, L.; Nejaddehbashi, F.; Neisi, N.; Nejad, D.B. Decellularized Wharton’s Jelly Scaffold Enhances Differentiation of Mesenchymal Stem Cells to Insulin-Secreting Cells. Tissue Cell. 2022, 79, 101938. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Marrazzo, P.; Crupi, A.N.; Alviano, F.; Teodori, L.; Bonsi, L. Exploring the Roles of MSCs in Infections: Focus on Bacterial Diseases. J. Mol. Med. 2019, 97, 437–450. [Google Scholar] [CrossRef]
- LPS-Preconditioned Mesenchymal Stromal Cells Modify Macrophage Polarization for Resolution of Chronic Inflammation via Exosome-Shuttled Let-7b—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26386558/ (accessed on 25 September 2022).
- Kouroupis, D.; Correa, D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 621748. [Google Scholar] [CrossRef]
- Xie, A.W.; Zacharias, N.A.; Binder, B.Y.K.; Murphy, W.L. Controlled Aggregation Enhances Immunomodulatory Potential of Mesenchymal Stromal Cell Aggregates. Stem Cells Transl. Med. 2021, 10, 1184–1201. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of Human Mesenchymal Stromal Cells (MSCs) into 3D Spheroids Enhances Their Antiinflammatory Properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Sun, L.; Jeske, R.; Nkosi, D.; York, S.B.; Liu, Y.; Grant, S.C.; Meckes, D.G.; Li, Y. Engineering Extracellular Vesicles by Three-Dimensional Dynamic Culture of Human Mesenchymal Stem Cells. J. Extracell Vesicles 2022, 11, e12235. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, F.; Farsinejad, A.; Nematollahi-Mahani, S.A.; Eslaminejad, T.; Nematollahi-Mahani, S.N. Suspension Culture Alters Insulin Secretion in Induced Human Umbilical Cord Matrix-Derived Mesenchymal Cells. Cell J. 2016, 18, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Chandravanshi, B.; Datar, S.; Bhonde, R. Response of Chick B Islets to Insulin Secretagogues Is Comparable to Those of Human Islet Equivalents. JOP 2015, 16, 318–323. [Google Scholar] [CrossRef]
- Xiang, C.; Xie, Q.-P. Protection of Mouse Pancreatic Islet Function by Co-culture with Hypoxia Pre-treated Mesenchymal Stromal Cells. Mol. Med. Rep. 2018, 18, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, L.; Yang, L.; Wang, X.; Zhao, C.; Zhao, D. Protective Effect of Mesenchymal Stem Cells on Isolated Islets Survival and Against Hypoxia Associated With the HIF-1α/PFKFB3 Pathway. Cell Transpl. 2022, 31, 09636897211073127. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Ma, X.; Yang, C.; Chen, Z.; Rong, P.; Wu, M.; Jiang, J.; Tan, M.; Yi, S.; Wang, W. Human Mesenchymal-Stem-Cells-Derived Exosomes Are Important in Enhancing Porcine Islet Resistance to Hypoxia. Xenotransplantation 2018, 25, e12405. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Wang, Y.; Dong, Y.; Wang, F.-S.; Ding, Y.; Kang, Y.; Xu, X. Roles of the Co-culture of Human Umbilical Cord Wharton’s Jelly-derived Mesenchymal Stem Cells with Rat Pancreatic Cells in the Treatment of Rats with Diabetes Mellitus. Exp. Med. 2014, 8, 1389–1396. [Google Scholar] [CrossRef][Green Version]
- Dietrich, I.; Girdlestone, J.; Giele, H. Differential Cytokine Expression in Direct and Indirect Co-Culture of Islets and Mesenchymal Stromal Cells. Cytokine 2022, 150, 155779. [Google Scholar] [CrossRef]
- Kim, K.-S.; Choi, Y.K.; Kim, M.J.; Hwang, J.W.; Min, K.; Jung, S.Y.; Kim, S.-K.; Choi, Y.-S.; Cho, Y.-W. Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell. Diabetes Metab. J. 2021, 45, 260–269. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, X.; Cai, J.; Chen, J.; Huang, L.; Wu, W.; Pugliese, A.; Li, S.; Ricordi, C.; Tan, J. Prevention of Chronic Diabetic Complications in Type 1 Diabetes by Co-Transplantation of Umbilical Cord Mesenchymal Stromal Cells and Autologous Bone Marrow: A Pilot Randomized Controlled Open-Label Clinical Study with 8-Year Follow-Up. Cytotherapy 2022, 24, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Moschidou, D.; Mukherjee, S.; Blundell, M.P.; Jones, G.N.; Atala, A.J.; Thrasher, A.J.; Fisk, N.M.; de Coppi, P.; Guillot, P.V. Human Mid-Trimester Amniotic Fluid Stem Cells Cultured Under Embryonic Stem Cell Conditions with Valproic Acid Acquire Pluripotent Characteristics. Stem Cells Dev. 2013, 22, 444–458. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of Amniotic Stem Cell Lines with Potential for Therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Moorefield, E.C.; McKee, E.E.; Solchaga, L.; Orlando, G.; Yoo, J.J.; Walker, S.; Furth, M.E.; Bishop, C.E. Cloned, CD117 Selected Human Amniotic Fluid Stem Cells Are Capable of Modulating the Immune Response. PLoS ONE 2011, 6, e26535. [Google Scholar] [CrossRef]
- Luo, C.; Jia, W.; Wang, K.; Chi, F.; Gu, Y.; Yan, X.; Zou, G.; Duan, T.; Zhou, Q. Human Amniotic Fluid Stem Cells Suppress PBMC Proliferation through IDO and IL-10-Dependent Pathways. Curr. Stem Cell Res. 2014, 9, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Subhan, B.S.; Kwong, J.; Kuhn, J.F.; Monas, A.; Sharma, S.; Rabbani, P.S. Amniotic Fluid-Derived Multipotent Stromal Cells Drive Diabetic Wound Healing through Modulation of Macrophages. J. Transl. Med. 2021, 19, 16. [Google Scholar] [CrossRef]
- Sato, Y.; Ochiai, D.; Abe, Y.; Masuda, H.; Fukutake, M.; Ikenoue, S.; Kasuga, Y.; Shimoda, M.; Kanai, Y.; Tanaka, M. Prophylactic Therapy with Human Amniotic Fluid Stem Cells Improved Survival in a Rat Model of Lipopolysaccharide-Induced Neonatal Sepsis through Immunomodulation via Aggregates with Peritoneal Macrophages. Stem Cell Res. 2020, 11, 300. [Google Scholar] [CrossRef]
- Villani, V.; Milanesi, A.; Sedrakyan, S.; Da Sacco, S.; Angelow, S.; Conconi, M.T.; Di Liddo, R.; de Filippo, R.; Perin, L. Amniotic Fluid Stem Cells Prevent β-Cell Injury. Cytotherapy 2014, 16, 41–55. [Google Scholar] [CrossRef]
- Pianta, S.; Bonassi Signoroni, P.; Muradore, I.; Rodrigues, M.F.; Rossi, D.; Silini, A.; Parolini, O. Amniotic Membrane Mesenchymal Cells-Derived Factors Skew T Cell Polarization toward Treg and Downregulate Th1 and Th17 Cells Subsets. Stem Cell Rev. Rep. 2015, 11, 394–407. [Google Scholar] [CrossRef]
- Magaña-Guerrero, F.S.; Domínguez-López, A.; Martínez-Aboytes, P.; Buentello-Volante, B.; Garfias, Y. Human Amniotic Membrane Mesenchymal Stem Cells Inhibit Neutrophil Extracellular Traps through TSG-6. Sci. Rep. 2017, 7, 12426. [Google Scholar] [CrossRef]
- Magatti, M.; de Munari, S.; Vertua, E.; Nassauto, C.; Albertini, A.; Wengler, G.S.; Parolini, O. Amniotic Mesenchymal Tissue Cells Inhibit Dendritic Cell Differentiation of Peripheral Blood and Amnion Resident Monocytes. Cell Transpl. 2009, 18, 899–914. [Google Scholar] [CrossRef]
- Kharat, A.; Chandravanshi, B.; Gadre, S.; Patil, V.; Bhonde, R.; Dubhashi, A. IGF-1 and Somatocrinin Trigger Islet Differentiation in Human Amniotic Membrane Derived Mesenchymal Stem Cells. Life Sci. 2019, 216, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.S.; Sudhakar, M.; Nair, P.D.; Bhonde, R.R. Reversal of Experimental Diabetes in Mice by Transplantation of Neo-Islets Generated from Human Amnion-Derived Mesenchymal Stromal Cells Using Immuno-Isolatory Macrocapsules. Cytotherapy 2010, 12, 982–991. [Google Scholar] [CrossRef]
- Li, J.; Koike-Soko, C.; Sugimoto, J.; Yoshida, T.; Okabe, M.; Nikaido, T. Human Amnion-Derived Stem Cells Have Immunosuppressive Properties on NK Cells and Monocytes. Cell Transpl. 2015, 24, 2065–2076. [Google Scholar] [CrossRef]
- Kubo, M.; Sonoda, Y.; Muramatsu, R.; Usui, M. Immunogenicity of Human Amniotic Membrane in Experimental Xenotransplantation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1539–1546. [Google Scholar]
- Najar, M.; Fayyad-Kazan, M.; Meuleman, N.; Bron, D.; Fayyad-Kazan, H.; Lagneaux, L. Immunological Impact of Wharton’s Jelly Mesenchymal Stromal Cells and Natural Killer Cell Co-Culture. Mol. Cell Biochem. 2018, 447, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Al-Mrahleh, M.; Matar, S.; Jafar, H.; Wehaibi, S.; Aslam, N.; Awidi, A. Human Wharton’s Jelly-Derived Mesenchymal Stromal Cells Primed by Tumor Necrosis Factor-α and Interferon-γ Modulate the Innate and Adaptive Immune Cells of Type 1 Diabetic Patients. Front. Immunol. 2021, 12, 732549. [Google Scholar] [CrossRef]
- Maraldi, T.; Beretti, F.; Guida, M.; Zavatti, M.; de Pol, A. Role of Hepatocyte Growth Factor in the Immunomodulation Potential of Amniotic Fluid Stem Cells. Stem Cells Transl. Med. 2015, 4, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Full Article: Comparative Analysis of Human Mesenchymal Stem Cells from Fetal-Bone Marrow, Adipose Tissue, and Warton’s Jelly as Sources of Cell Immunomodulatory Therapy. Available online: https://www.tandfonline.com/doi/full/10.1080/21645515.2015.1030549 (accessed on 26 September 2022).
- Alipour, R.; Motedayyen, H.; Sereshki, N.; Rafiee, M.; Alsahebfosul, F.; Pourazar, A. Human Amniotic Epithelial Cells Affect the Functions of Neutrophils. Int. J. Stem Cells 2020, 13, 212–220. [Google Scholar] [CrossRef]
- Motedayyen, H.; Zarnani, A.-H.; Tajik, N.; Ghotloo, S.; Rezaei, A. Immunomodulatory Effects of Human Amniotic Epithelial Cells on Naive CD4+ T Cells from Women with Unexplained Recurrent Spontaneous Abortion. Placenta 2018, 71, 31–40. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Fan, X.; Li, L.; Xiao, Y.; Luo, P.; Liu, Y.; Wang, L.; Cui, Z.; He, F.; et al. Amniotic Epithelial Cells Accelerate Diabetic Wound Healing by Modulating Inflammation and Promoting Neovascularization. Stem Cells Int. 2018, 2018, 1082076. [Google Scholar] [CrossRef]
- Dymowska, M.; Aksamit, A.; Zielniok, K.; Kniotek, M.; Kaleta, B.; Roszczyk, A.; Zych, M.; Dabrowski, F.; Paczek, L.; Burdzinska, A. Interaction between Macrophages and Human Mesenchymal Stromal Cells Derived from Bone Marrow and Wharton’s Jelly—A Comparative Study. Pharmaceutics 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Manuelpillai, U.; Tchongue, J.; Lourensz, D.; Vaghjiani, V.; Samuel, C.S.; Liu, A.; Williams, E.D.; Sievert, W. Transplantation of Human Amnion Epithelial Cells Reduces Hepatic Fibrosis in Immunocompetent CCl4-Treated Mice. Cell Transpl. 2010, 19, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.J.; Inatomi, T.J.; Sotozono, C.J.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth Factor MRNA and Protein in Preserved Human Amniotic Membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- Alhomrani, M.; Correia, J.; Zavou, M.; Leaw, B.; Kuk, N.; Xu, R.; Saad, M.I.; Hodge, A.; Greening, D.W.; Lim, R.; et al. The Human Amnion Epithelial Cell Secretome Decreases Hepatic Fibrosis in Mice with Chronic Liver Fibrosis. Front. Pharm. 2017, 8, 748. [Google Scholar] [CrossRef]
- Thakur, G.; Bok, E.-Y.; Kim, S.-B.; Jo, C.-H.; Oh, S.-J.; Baek, J.-C.; Park, J.-E.; Kang, Y.-H.; Lee, S.-L.; Kumar, R.; et al. Scaffold-Free 3D Culturing Enhance Pluripotency, Immunomodulatory Factors, and Differentiation Potential of Wharton’s Jelly-Mesenchymal Stem Cells. Eur. J. Cell Biol. 2022, 101, 151245. [Google Scholar] [CrossRef]
- Joerger-Messerli, M.S.; Marx, C.; Oppliger, B.; Mueller, M.; Surbek, D.V.; Schoeberlein, A. Mesenchymal Stem Cells from Wharton’s Jelly and Amniotic Fluid. Best Pract. Res. Clin. Obs. Gynaecol. 2016, 31, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Koike, C.; Zhou, K.; Takeda, Y.; Fathy, M.; Okabe, M.; Yoshida, T.; Nakamura, Y.; Kato, Y.; Nikaido, T. Characterization of Amniotic Stem Cells. Cell. Reprogramm. 2014, 16, 298–305. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Ali, H.; AlKandari, S.; Atizado, V.L.; Akhter, N.; Al-Mulla, F.; Atari, M. Defined Three-Dimensional Culture Conditions Mediate Efficient Induction of Definitive Endoderm Lineage from Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cells. Stem Cell Res. 2016, 7, 165. [Google Scholar] [CrossRef]
- Mu, X.-P.; Ren, L.-Q.; Yan, H.-W.; Zhang, X.-M.; Xu, T.-M.; Wei, A.-H.; Jiang, J.-L. Enhanced Differentiation of Human Amniotic Fluid-Derived Stem Cells into Insulin-Producing Cells In Vitro. J. Diabetes Investig. 2017, 8, 34–43. [Google Scholar] [CrossRef]
- Cargnoni, A.; Papait, A.; Masserdotti, A.; Pasotti, A.; Stefani, F.R.; Silini, A.R.; Parolini, O. Extracellular Vesicles From Perinatal Cells for Anti-Inflammatory Therapy. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, C.-H.; Berishvili, E. Immunomodulatory Properties of Amniotic Membrane Derivatives and Their Potential in Regenerative Medicine. Curr. Diabetes Rep. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhong, C.; Yang, X.; Shu, F.; Xiao, S.; Gong, T.; Luo, P.; Li, L.; Chen, Z.; Zheng, Y.; et al. Exosomes Derived from Human Amniotic Epithelial Cells Accelerate Diabetic Wound Healing via PI3K-AKT-MTOR-Mediated Promotion in Angiogenesis and Fibroblast Function. Burn. Trauma 2020, 8, tkaa020. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Cargnoni, A.; Silini, A.R.; Parolini, O. Chapter 11—Epithelial and Mesenchymal Stromal Cells from the Amniotic Membrane: Both Potent Immunomodulators. In Perinatal Stem Cells; Atala, A., Cetrulo, K.J., Taghizadeh, R.R., Murphy, S.V., Cetrulo, C.L., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 147–155. ISBN 978-0-12-812015-6. [Google Scholar] [CrossRef]
- Vieira Paladino, F.; de Moraes Rodrigues, J.; da Silva, A.; Goldberg, A.C. The Immunomodulatory Potential of Wharton’s Jelly Mesenchymal Stem/Stromal Cells. Stem Cells Int. 2019, 2019, 3548917. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, X.; Ni, P.; Qiu, X.; Lin, X.; Wu, W.; Xie, L.; Lin, L.; Min, J.; Lai, X.; et al. Immunological Characteristics of Human Umbilical Cord Mesenchymal Stem Cells and the Therapeutic Effects of Their Transplantion on Hyperglycemia in Diabetic Rats. Int. J. Mol. Med. 2014, 33, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.M.; Li, H.; Kirkham, A.M.; Tieu, A.; Maganti, H.B.; Shorr, R.; Fergusson, D.A.; Lalu, M.M.; Elomazzen, H.; Allan, D.S. MSC-Derived Extracellular Vesicles to Heal Diabetic Wounds: A Systematic Review and Meta-Analysis of Preclinical Animal Studies. Stem Cell Rev. Rep. 2022, 18, 968–979. [Google Scholar] [CrossRef]
- Silini, A.R.; Papait, A.; Cargnoni, A.; Vertua, E.; Romele, P.; Bonassi Signoroni, P.; Magatti, M.; de Munari, S.; Masserdotti, A.; Pasotti, A.; et al. CM from Intact HAM: An Easily Obtained Product with Relevant Implications for Translation in Regenerative Medicine. Stem Cell Res. 2021, 12, 540. [Google Scholar] [CrossRef]
- Zia, S.; Cavallo, C.; Vigliotta, I.; Parisi, V.; Grigolo, B.; Buda, R.; Marrazzo, P.; Alviano, F.; Bonsi, L.; Zattoni, A.; et al. Effective Label-Free Sorting of Multipotent Mesenchymal Stem Cells from Clinical Bone Marrow Samples. Bioengineering 2022, 9, 49. [Google Scholar] [CrossRef]
- Zeynaloo, E.; Stone, L.D.; Dikici, E.; Ricordi, C.; Deo, S.K.; Bachas, L.G.; Daunert, S.; Lanzoni, G. Delivery of Therapeutic Agents and Cells to Pancreatic Islets: Towards a New Era in the Treatment of Diabetes. Mol. Asp. Med. 2022, 83, 101063. [Google Scholar] [CrossRef]
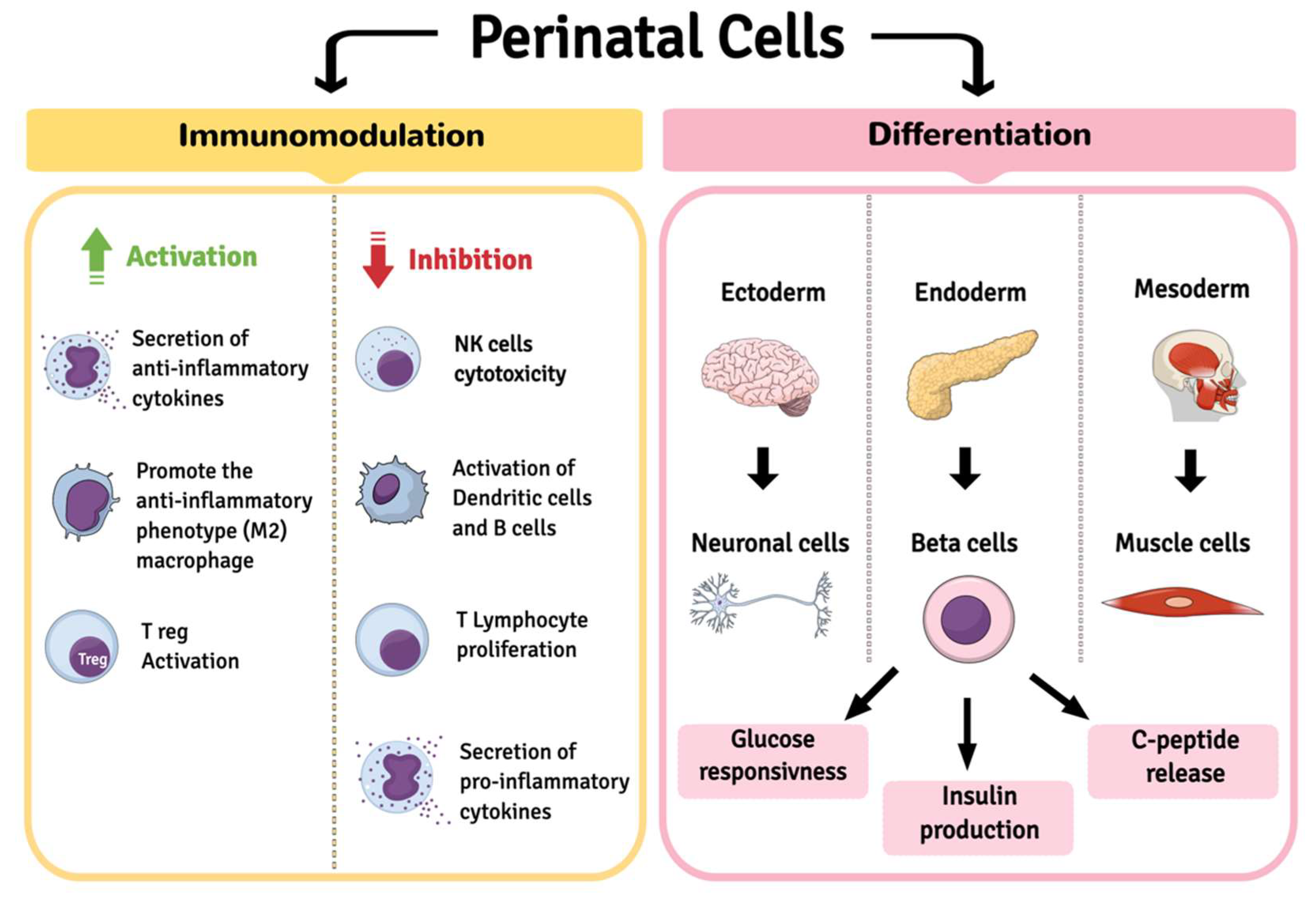
| Immunomodulation Activity | AESC | WJ-MSC | AFSC | AM-MSC |
|---|---|---|---|---|
| Inhibiting the cytotoxicity of natural killer cells | [137,138] | [139] | [133] | |
| Reducing of the activation of dendritic cells | [62] | [140] | [134] | |
| Reducing B cell activation | [62] | [141] | ||
| Anti-proliferative effect on activated PBMC | [21,74] | [142] | [128] | |
| [143,144] | [142] | |||
| Promoting the anti-inflammatory phenotype (M2) of macrophage populations | [145] | [146] | [129,130] | |
| Reducing T lymphocytes proliferation | [75,138] | [81,115,140] | [133] | [133] |
| In vivo allogenic transplantation | [37,51,75,147] | [84,85,99,112] | ||
| Increased expression of: TGF-β1, IL-6, TSG-6, PGE-2 | [148] | [112,140] | ||
| Secretion of EVs | [115,149] | [115] | ||
| Secretion of: IL-10, PGE2, hyaluronic acid | [112,140] | |||
| Expression of HLA Ib | [57,58,59] | [81] | ||
| Expression of migration inhibitor factor (MIF) | [62] | |||
| Differentiation capacity | AECs | WJ-MSCs | AFSCs | AM-MSCs |
| Expression of pluripotency markers: NANOG, OCT-4, SSEA-3, SSEA-4, TRA1-60, TRA1-80 | [34] | [150] | [151] | [152] |
| Pancreatic-endodermic lineage | [18,43,45] | [95,102,106,153] | [154] | [135,136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paris, F.; Pizzuti, V.; Marrazzo, P.; Pession, A.; Alviano, F.; Bonsi, L. Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation. Int. J. Mol. Sci. 2022, 23, 14597. https://doi.org/10.3390/ijms232314597
Paris F, Pizzuti V, Marrazzo P, Pession A, Alviano F, Bonsi L. Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation. International Journal of Molecular Sciences. 2022; 23(23):14597. https://doi.org/10.3390/ijms232314597
Chicago/Turabian StyleParis, Francesca, Valeria Pizzuti, Pasquale Marrazzo, Andrea Pession, Francesco Alviano, and Laura Bonsi. 2022. "Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation" International Journal of Molecular Sciences 23, no. 23: 14597. https://doi.org/10.3390/ijms232314597
APA StyleParis, F., Pizzuti, V., Marrazzo, P., Pession, A., Alviano, F., & Bonsi, L. (2022). Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation. International Journal of Molecular Sciences, 23(23), 14597. https://doi.org/10.3390/ijms232314597