[68Ga]Ga-DATA5m-LM4, a PET Radiotracer in the Diagnosis of SST2R-Positive Tumors: Preclinical and First Clinical Results
Abstract
1. Introduction
2. Results
2.1. Ligands and Radioligands
2.2. In Vitro Studies
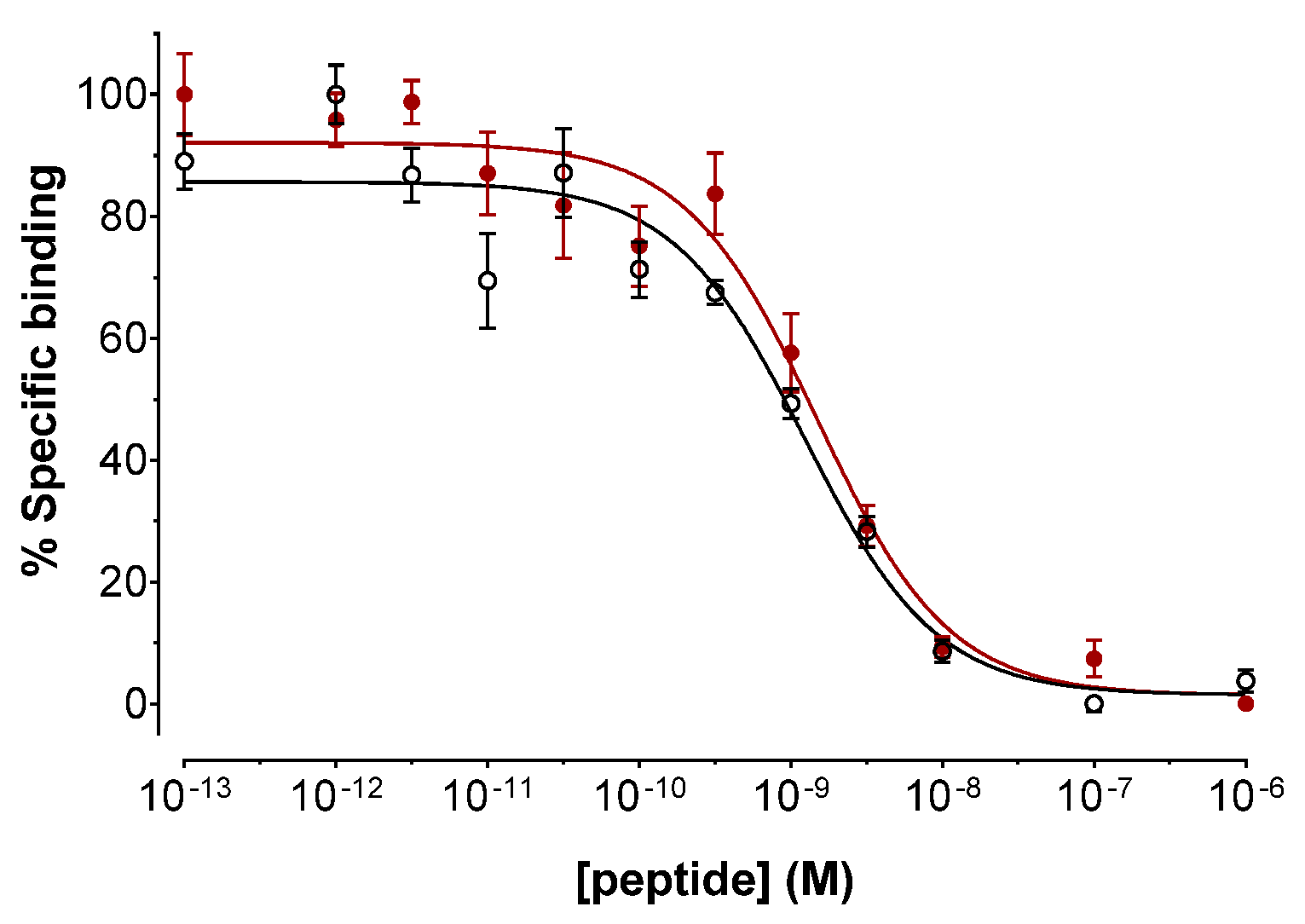
2.2.1. Binding Affinities for the Human SST2R
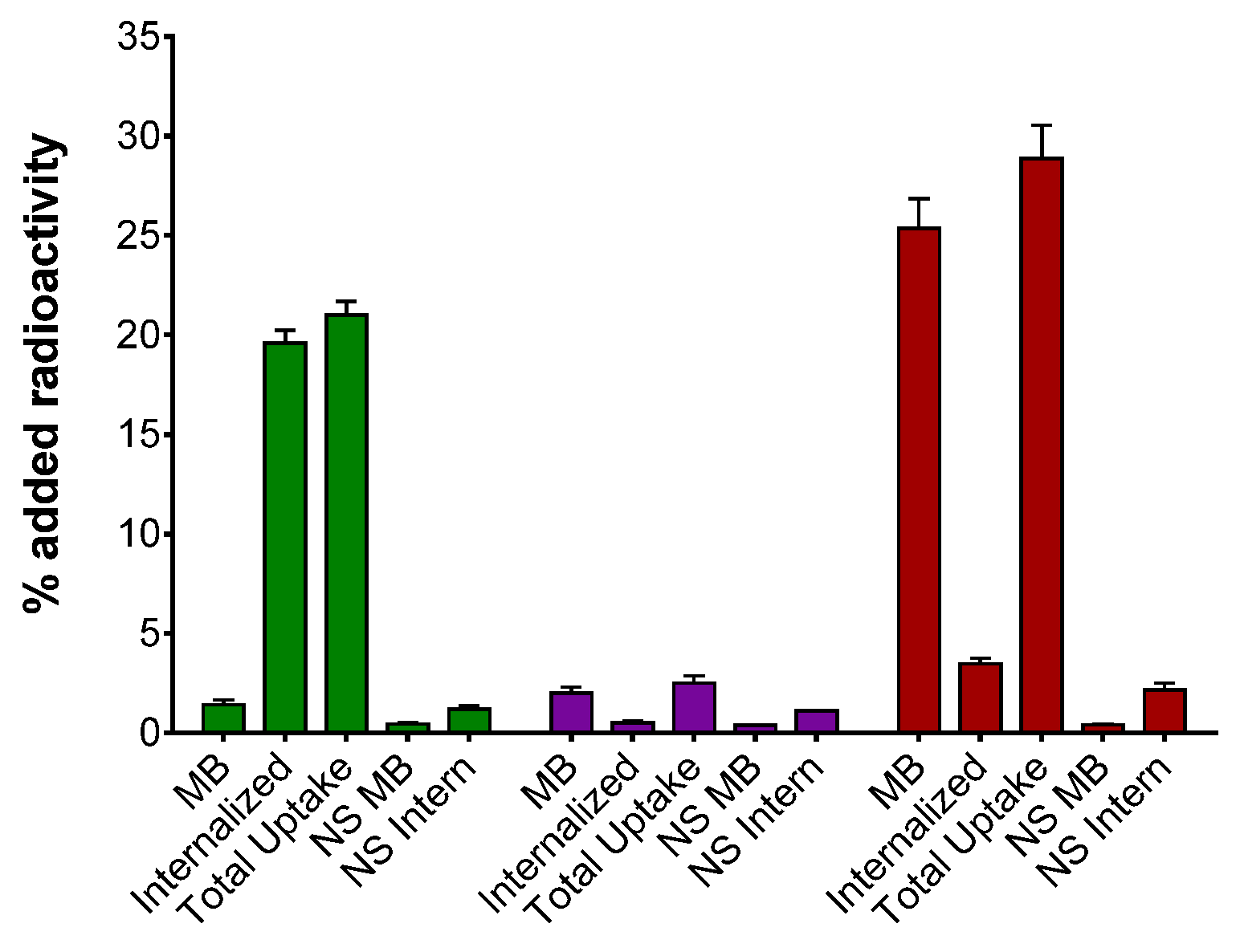
2.2.2. Uptake/Internalization in HEK293-SST2R Cells
2.3. Animal Studies
2.3.1. In Vivo Metabolic Stability of [67Ga]Ga-DATA5m-LM4
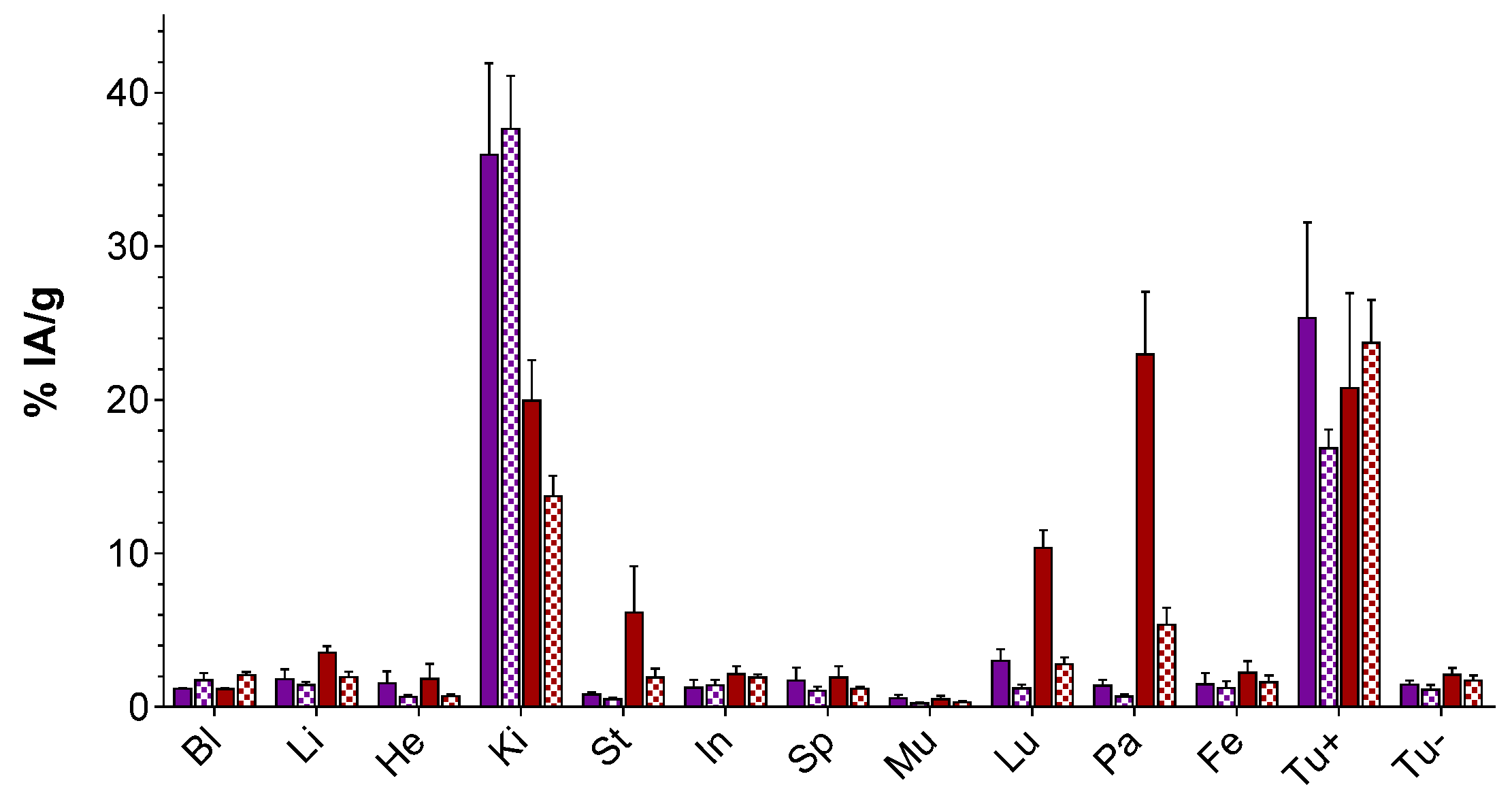
2.3.2. Comparison of [67Ga]Ga-DATA5m-LM4 vs. [67Ga]Ga-DOTA-LM3 in Tumor-Bearing Mice
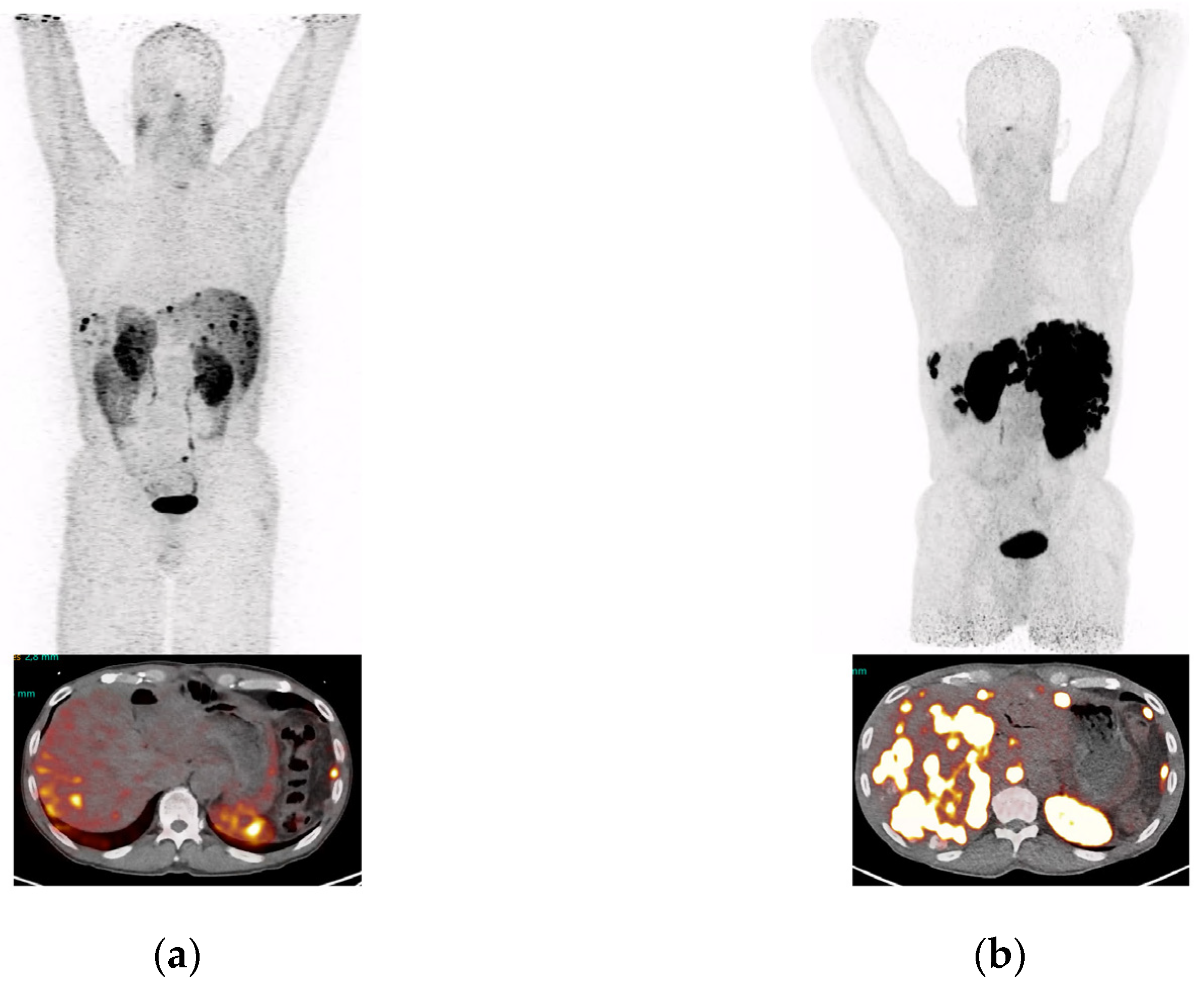
2.4. PET/CT of a NET Patient
3. Discussion
4. Materials and Methods
4.1. Chemicals, Ligands and Radionuclides
4.1.1. Radiolabeling
4.1.2. Radiochemical Analysis
4.2. Cell Studies
4.2.1. Cell Culture
4.2.2. Competition Binding Assays in HEK293-SST2R Cell Membranes
4.2.3. Radioligand Uptake/Internalization in HEK293-SST2R Cells
4.3. Animal Studies
4.3.1. Stability Studies
4.3.2. Biodistribution in SCID Mice Bearing Twin HEK293-SST2R and wtHEK293 Tumors
4.3.3. Statistical Analysis
4.4. Patient Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reubi, J.C. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology 2004, 80 (Suppl. 1), 51–56. [Google Scholar] [CrossRef] [PubMed]
- van Essen, M.; Sundin, A.; Krenning, E.P.; Kwekkeboom, D.J. Neuroendocrine tumours: The role of imaging for diagnosis and therapy. Nat. Rev. Endocrinol. 2014, 10, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.; Krenning, E.P. Clinical history of the theranostic radionuclide approach to neuroendocrine tumors and other types of cancer: Historical review based on an interview of Eric P. Krenning by Rachel Levine. J. Nucl. Med. 2017, 58 (Suppl. 2), 3S–9S. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Carreras, C. Peptides and receptors in image-guided therapy: Theranostics for neuroendocrine neoplasms. Semin. Nucl. Med. 2012, 42, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R. Theranostics: From molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy-the Bad Berka experience. Theranostics 2012, 2, 437–447. [Google Scholar] [CrossRef]
- Decristoforo, C.; Maina, T.; Nock, B.; Gabriel, M.; Cordopatis, P.; Moncayo, R. 99mTc-Demotate 1: First data in tumour patients-results of a pilot/phase I study. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1211–1219. [Google Scholar] [CrossRef]
- de Jong, M.; Breeman, W.A.; Bakker, W.H.; Kooij, P.P.; Bernard, B.F.; Hofland, L.J.; Visser, T.J.; Srinivasan, A.; Schmidt, M.A.; Erion, J.L.; et al. Comparison of 111In-labeled somatostatin analogues for tumor scintigraphy and radionuclide therapy. Cancer Res. 1998, 58, 437–441. [Google Scholar]
- de Jong, M.; Breeman, W.A.; Kwekkeboom, D.J.; Valkema, R.; Krenning, E.P. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc. Chem. Res. 2009, 42, 873–880. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Zhang, J.; Kulkarni, H.R.; Baum, R.P. 225Ac-DOTATOC-targeted somatostatin receptor alpha-therapy in a patient with metastatic neuroendocrine tumor of the thymus, refractory to beta-radiation. Clin. Nucl. Med. 2021, 46, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Nunez, R. Targeted alpha-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic sstr-expressing neuroendocrine tumors: First-in-humans dose-escalation clinical trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Macke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Cescato, R.; Erchegyi, J.; Waser, B.; Piccand, V.; Maecke, H.R.; Rivier, J.E.; Reubi, J.C. Design and in vitro characterization of highly sst2-selective somatostatin antagonists suitable for radiotargeting. J. Med. Chem. 2008, 51, 4030–4037. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Nicolàs, G.P.; Wild, D. Somatostatin receptor antagonists for imaging and therapy. J. Nucl. Med. 2017, 58 (Suppl. 2), 61S–66S. [Google Scholar] [CrossRef]
- Wild, D.; Fani, M.; Béhé, M.; Brink, I.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; Weber, W.A. First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J. Nucl. Med. 2011, 52, 1412–1417. [Google Scholar] [CrossRef]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Fischer, R.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: A pilot study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef]
- Nicolàs, G.P.; Wild, D.; Fani, M. Reply: Advantages and limits of targeted radionuclide therapy with somatostatin antagonists. J. Nucl. Med. 2018, 59, 547–548. [Google Scholar] [CrossRef]
- Cescato, R.; Waser, B.; Fani, M.; Reubi, J.C. Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J. Nucl. Med. 2011, 52, 1886–1890. [Google Scholar] [CrossRef]
- Mansi, R.; Fani, M. Design and development of the theranostic pair 177Lu-OPS201/68Ga-OPS202 for targeting somatostatin receptor expressing tumors. J. Labelled Comp. Radiopharm. 2019, 62, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Braun, F.; Waser, B.; Beetschen, K.; Cescato, R.; Erchegyi, J.; Rivier, J.E.; Weber, W.A.; Maecke, H.R.; Reubi, J.C. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J. Nucl. Med. 2012, 53, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.A.; de Blois, E.; Sze Chan, H.; Konijnenberg, M.; Kwekkeboom, D.J.; Krenning, E.P. 68Ga-labeled DOTA-peptides and 68Ga-labeled radiopharmaceuticals for positron emission tomography: Current status of research, clinical applications, and future perspectives. Semin. Nucl. Med. 2011, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Breeman, W.A.; Klette, I.; Gottschaldt, M.; Odparlik, A.; Baehre, M.; Tworowska, I.; Schultz, M.K. Radiolabeling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat. Protoc. 2016, 11, 1057–1066. [Google Scholar] [CrossRef]
- Seemann, J.; Waldron, B.P.; Roesch, F.; Parker, D. Approaching ‘kit-type’ labelling with 68Ga: The DATA chelators. ChemMedChem 2015, 10, 1019–1026. [Google Scholar] [CrossRef]
- Spang, P.; Herrmann, C.; Roesch, F. Bifunctional gallium-68 chelators: Past, present, and future. Semin. Nucl. Med. 2016, 46, 373–394. [Google Scholar] [CrossRef]
- Seemann, J.; Waldron, B.; Parker, D.; Roesch, F. DATATOC: A novel conjugate for kit-type 68Ga labelling of TOC at ambient temperature. EJNMMI Radiopharm. Chem. 2017, 1, 4. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Nagel, J.; Sinnes, J.P.; Roesch, F.; Maina, T. Novel bifunctional data chelator for quick access to site-directed PET Ga-68-radiotracers: Preclinical proof-of-principle with [Tyr3]octreotide. Dalton Trans. 2017, 46, 14584–14590. [Google Scholar] [CrossRef]
- Yadav, D.; Ballal, S.; Yadav, M.P.; Tripathi, M.; Roesch, F.; Bal, C. Evaluation of [68Ga]Ga-DATA-TOC for imaging of neuroendocrine tumours: Comparison with [68Ga]Ga-DOTA-NOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 860–869. [Google Scholar] [CrossRef]
- Sinnes, J.P.; Nagel, J.; Waldron, B.P.; Maina, T.; Nock, B.A.; Bergmann, R.K.; Ullrich, M.; Pietzsch, J.; Bachmann, M.; Baum, R.P.; et al. Instant kit preparation of 68Ga-radiopharmaceuticals via the hybrid chelator DATA: Clinical translation of [68Ga]Ga-DATA-TOC. EJNMMI Res. 2019, 9, 48. [Google Scholar] [CrossRef]
- Baum, R.P.; Zhang, J.; Schuchardt, C.; Müller, D.; Macke, H. First-in-humans study of the SSTR antagonist 177Lu-DOTA-LM3 for peptide receptor radionuclide therapy in patients with metastatic neuroendocrine neoplasms: Dosimetry, safety, and efficacy. J. Nucl. Med. 2021, 62, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.A.; Perez-Tilve, D.; Liu, F.; Gelfanov, V.; DiMarchi, R.D.; Mayer, J.P. Pyridyl-alanine as a hydrophilic, aromatic element in peptide structural optimization. J. Med. Chem. 2016, 59, 8061–8067. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Cescato, R.; Waser, B.; Tatsi, A.; Kaloudi, A.; Krenning, E.P.; de Jong, M.; Nock, B.A.; Reubi, J.C. [111In-DOTA]LTT-SS28, a first pansomatostatin radioligand for in vivo targeting of somatostatin receptor-positive tumors. J. Med. Chem. 2014, 57, 6564–6571. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To serve and protect”: Enzyme inhibitors as radiopeptide escorts promote tumor targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanellopoulos, P.; Nock, B.A.; Greifenstein, L.; Baum, R.P.; Roesch, F.; Maina, T. [68Ga]Ga-DATA5m-LM4, a PET Radiotracer in the Diagnosis of SST2R-Positive Tumors: Preclinical and First Clinical Results. Int. J. Mol. Sci. 2022, 23, 14590. https://doi.org/10.3390/ijms232314590
Kanellopoulos P, Nock BA, Greifenstein L, Baum RP, Roesch F, Maina T. [68Ga]Ga-DATA5m-LM4, a PET Radiotracer in the Diagnosis of SST2R-Positive Tumors: Preclinical and First Clinical Results. International Journal of Molecular Sciences. 2022; 23(23):14590. https://doi.org/10.3390/ijms232314590
Chicago/Turabian StyleKanellopoulos, Panagiotis, Berthold A. Nock, Lukas Greifenstein, Richard P. Baum, Frank Roesch, and Theodosia Maina. 2022. "[68Ga]Ga-DATA5m-LM4, a PET Radiotracer in the Diagnosis of SST2R-Positive Tumors: Preclinical and First Clinical Results" International Journal of Molecular Sciences 23, no. 23: 14590. https://doi.org/10.3390/ijms232314590
APA StyleKanellopoulos, P., Nock, B. A., Greifenstein, L., Baum, R. P., Roesch, F., & Maina, T. (2022). [68Ga]Ga-DATA5m-LM4, a PET Radiotracer in the Diagnosis of SST2R-Positive Tumors: Preclinical and First Clinical Results. International Journal of Molecular Sciences, 23(23), 14590. https://doi.org/10.3390/ijms232314590





