The Inhibition of the Inducible Nitric Oxide Synthase Enhances the DPSC Mineralization under LPS-Induced Inflammation
Abstract
1. Introduction
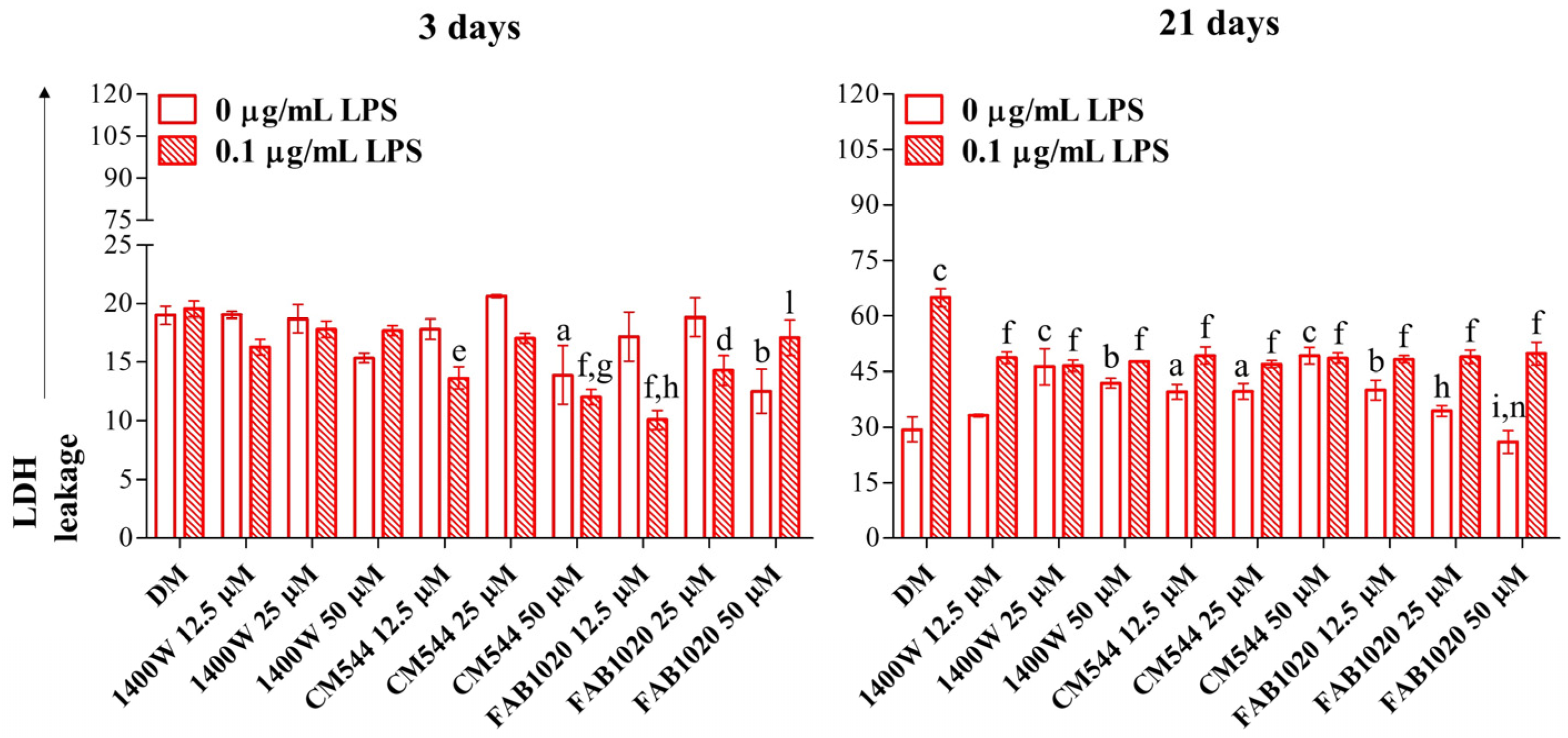
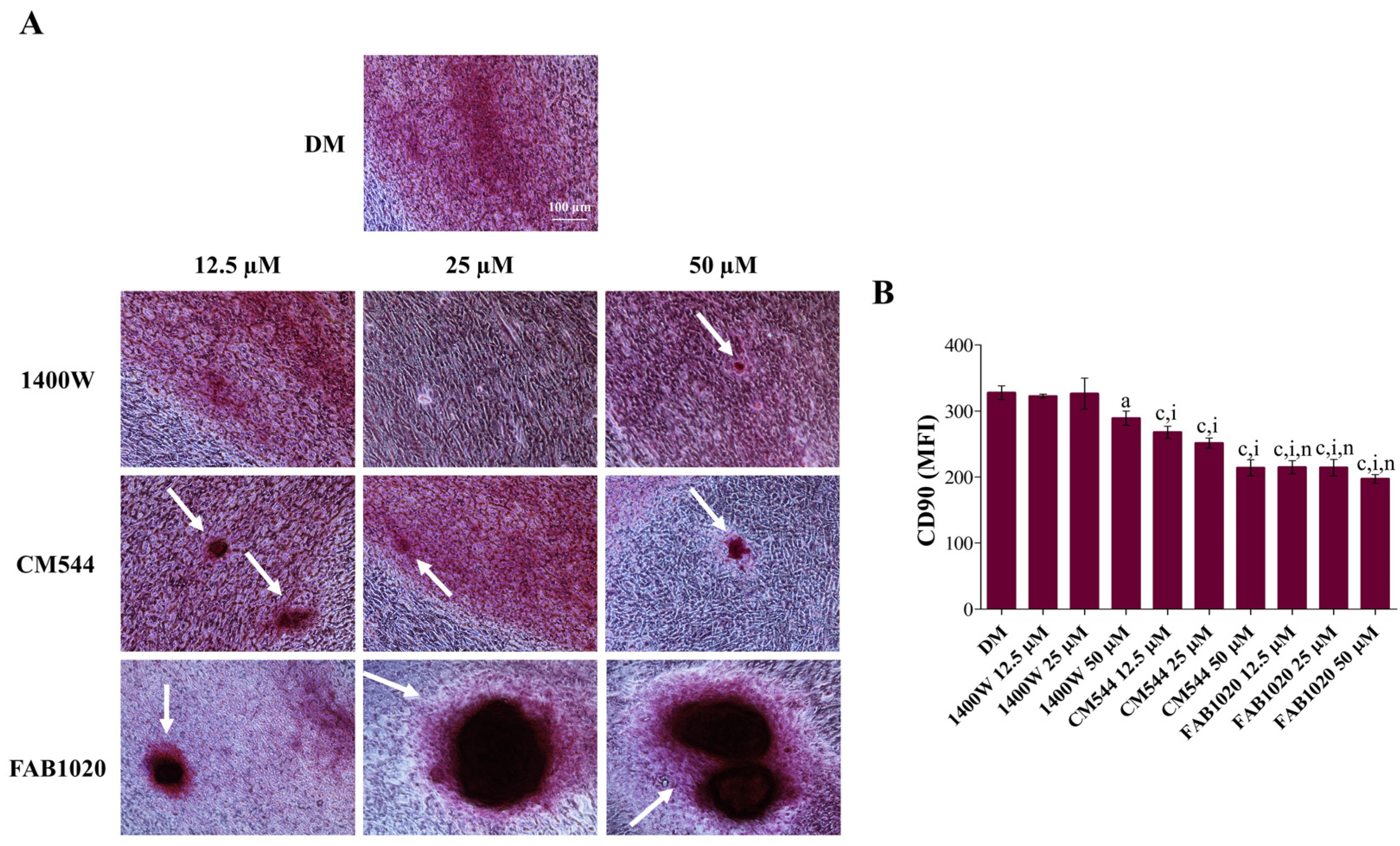
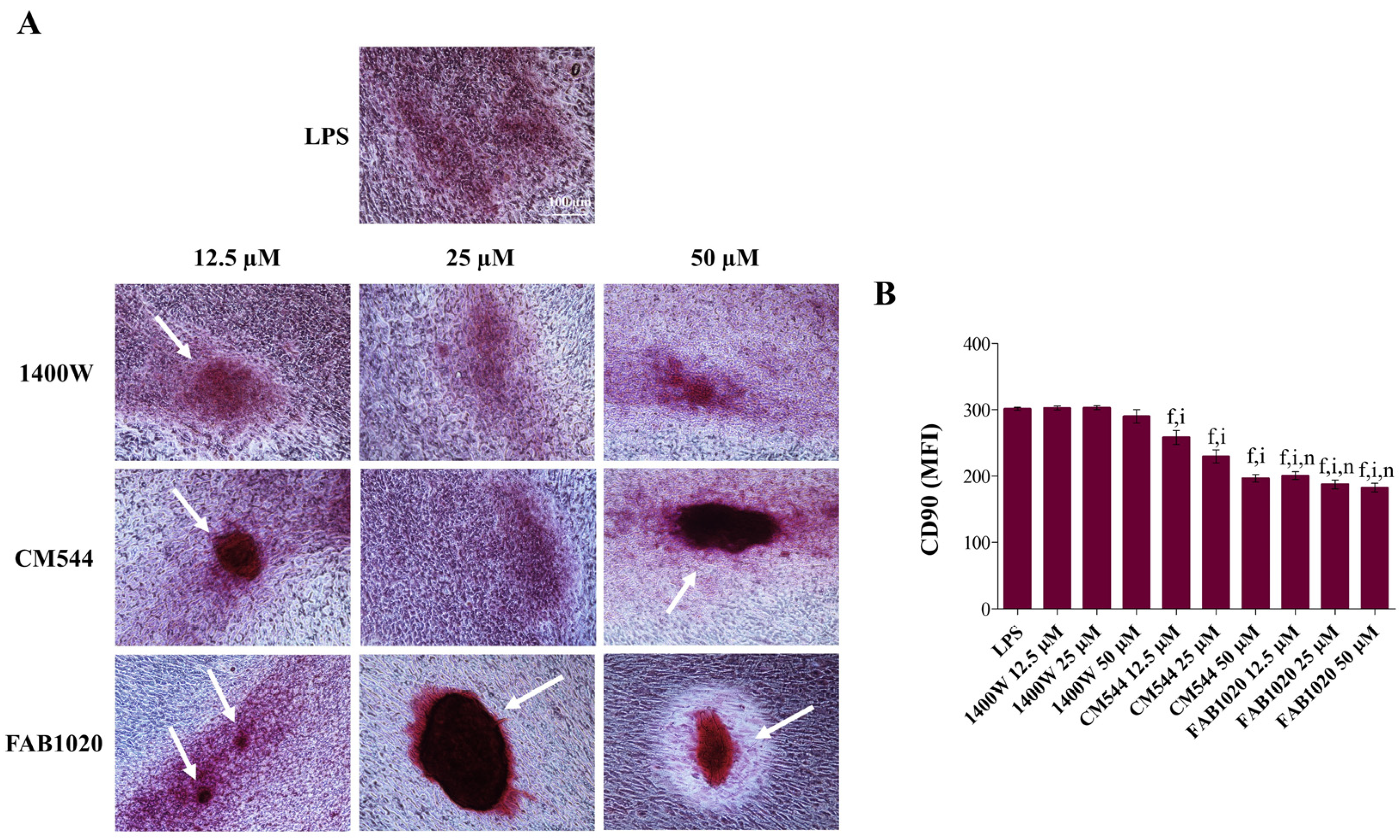
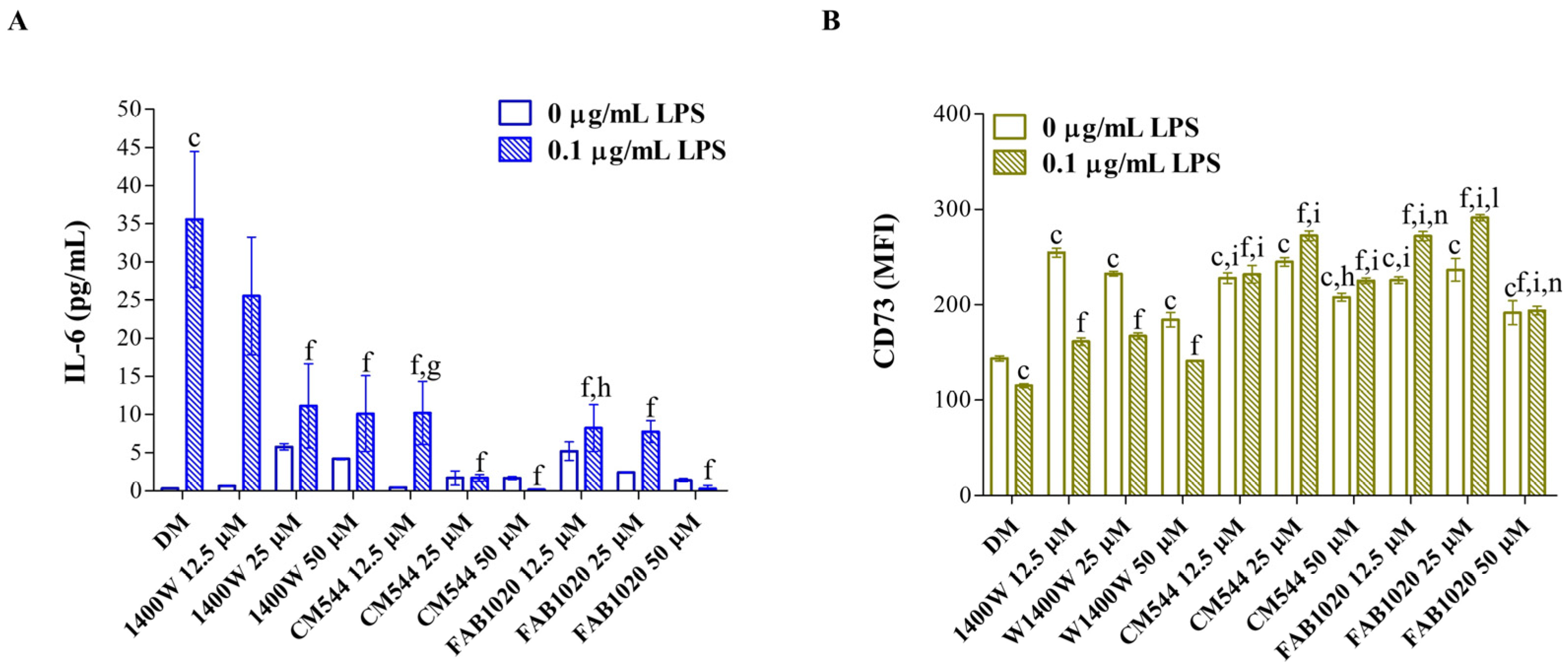
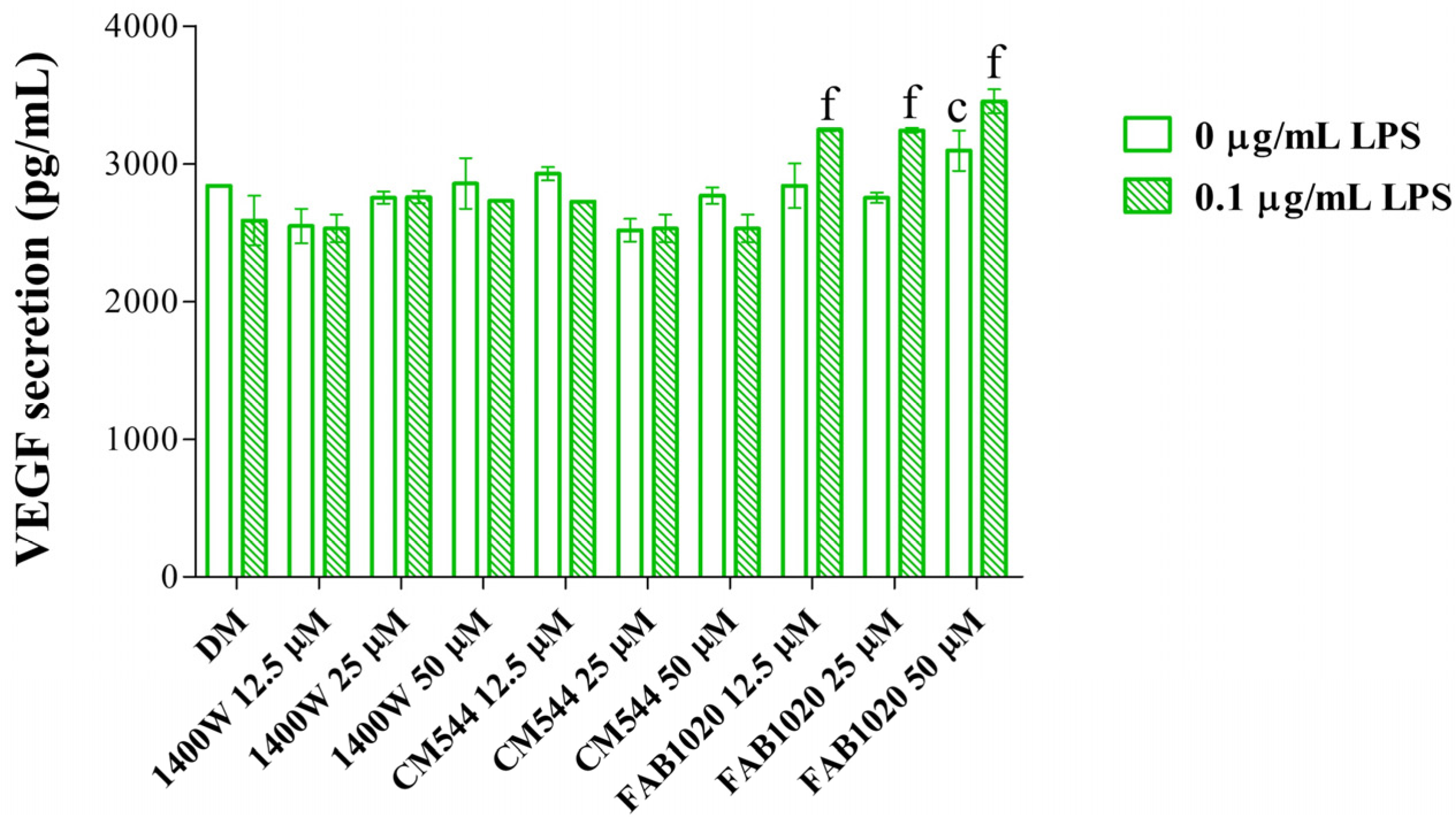
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of iNOS Inhibitors
3.2. Cell Culture
3.3. Establishment of Pro-Inflammatory Conditions and Cell Exposures to Inhibitors
3.4. Lactate Dehydrogenase (LDH) Release
3.5. Interleukin-6 Release
3.6. Immunophenotype In Vitro by Flow Cytometry
3.7. Extracellular Matrix Mineralization (Alizarin Red Staining)
3.8. ELISA Analysis of VEGF Secretion
3.9. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundberg, O.J.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853. [Google Scholar] [CrossRef] [PubMed]
- Mir, J.M.; Maurya, R.C. A gentle introduction to gasotransmitters with special reference to nitric oxide: Biological and chemical implications. Rev. Inorg. Chem. 2018, 38, 193–220. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed]
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of In-flammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Birke, M.; Gallorini, M.; Petzel, C.; Bolay, C.; Waha, C.; Hiller, K.; Buchalla, W. HEMA-induced oxidative stress inhibits NF-κB nuclear translocation and TNF release from LTA- and LPS-stimulated immunocompetent cells. Dent Mater. 2021, 37, 175–190. [Google Scholar] [CrossRef]
- Di Nardo Di Maio, F.; Lohinai, Z.; D’Arcangelo, C.; De Fazio, P.E.; Speranza, L.; De Lutiis, M.; Patruno, A.; Grilli, A.; Felaco, M. Nitric oxide synthase in healthy and inflamed human dental pulp. J. Dent. Res. 2004, 83, 312–316. [Google Scholar] [CrossRef]
- Parwani, S.R.; Parwani, R.N. Nitric oxide and inflammatory periodontal disease. Gen Dent. 2015, 63, 34–40. [Google Scholar]
- Rothermund, K.; Calabrese, T.C.; Syed-Picard, F.N. Differential Effects of Escherichia coli—Versus Porphyromonas gingivalis—Derived Lipopolysaccharides on Dental Pulp Stem Cell Differentiation in Scaffold-free Engineered Tissues. J. Endod. 2022, 48, 1378–1386.e2. [Google Scholar] [CrossRef]
- Gallorini, M.; Widbiller, M.; Bolay, C.; Carradori, S.; Buchalla, W.; Cataldi, A.; Schweikl, H. Relevance of Cellular Redox Ho-meostasis for Vital Functions of Human Dental Pulp Cells. Antioxidants 2022, 11, 23. [Google Scholar] [CrossRef]
- An, S. Nitric Oxide in Dental Pulp Tissue: From Molecular Understanding to Clinical Application in Regenerative Endodontic Procedures. Tissue Eng. Part B Rev. 2020, 26, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho, J.E.M.; Aguiar, M.T.; Machado, L.C.; Carlos, A.C.A.M.; Nunes Alves, A.P.N.; Mesquita, K.C.; de Miranda Candeiro, G.T.; de Barros Silva, P.G. Intense orthodontic force induces the three dental pulp nitric oxide synthase isoforms and leads to orofacial discomfort in rats. Orthod. Craniofacial Res. 2022, 25, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Ziche, M. Constitutive and Inducible Nitric Oxide Synthase: Role in Angiogenesis. Antioxidants Redox Signal. 2002, 4, 817–823. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2019, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Maccallini, C.; Di Matteo, M.; Gallorini, M.; Montagnani, M.; Graziani, V.; Ammazzalorso, A.; Amoia, P.; De Filippis, B.; Di Silvestre, S.; Fantacuzzi, M.; et al. Discovery of N-{3-[(ethanimidoylamino)methyl]benzyl}-l-prolinamide dihydrochloride: A new potent and selective inhibitor of the inducible nitric oxide synthase as a promising agent for the therapy of malignant glioma. Eur. J. Med. Chem. 2018, 152, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Maccallini, C.; Arias, F.; Gallorini, M.; Amoia, P.; Ammazzalorso, A.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Cataldi, A.; Camacho, M.E.; et al. Antiglioma Activity of Aryl and Amido-Aryl Acetamidine Derivatives Targeting iNOS: Synthesis and Biological Evaluation. ACS Med. Chem. Lett. 2020, 11, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Maccallini, C.; Ammazzalorso, A.; Amoia, P.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Cataldi, A.; Amoroso, R. The Selective Acetamidine-Based iNOS Inhibitor CM544 Reduces Glioma Cell Proliferation by Enhancing PARP-1 Cleavage In Vitro. Int. J. Mol. Sci. 2019, 20, 495. [Google Scholar] [CrossRef]
- Gallorini, M.; Rapino, M.; Schweikl, H.; Cataldi, A.; Amoroso, R.; Maccallini, C. Selective Inhibitors of the Inducible Nitric Oxide Synthase as Modulators of Cell Responses in LPS-Stimulated Human Monocytes. Molecules 2021, 26, 4419. [Google Scholar] [CrossRef]
- Grottelli, S.; Amoroso, R.; Macchioni, L.; D’Onofrio, F.; Fettucciari, K.; Bellezza, I.; Maccallini, C. Acetamidine-Based iNOS Inhibitors as Molecular Tools to Counteract Inflammation in BV2 Microglial Cells. Molecules 2020, 25, 2646. [Google Scholar] [CrossRef]
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp Stem Cell–Mediated Functional Pulp Regeneration. J. Dent. Res. 2018, 98, 27–35. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Di Carlo, R.; Pilato, S.; Ricci, A.; Schweikl, H.; Cataldi, A.; Fontana, A.; Zara, S. Liposomes embedded with differentiating factors as a new strategy for enhancing DPSC osteogenic commitment. Eur. Cells Mater. 2021, 41, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Mei, Y.-F.; Atsuta, I.; Danjo, A.; Yamaza, H.; Hama, S.; Nishida, K.; Tang, R.; Kyumoto-Nakamura, Y.; Uehara, N.; et al. Exogenous nitric oxide stimulates the odontogenic differentiation of rat dental pulp stem cells. Sci. Rep. 2018, 8, 3419. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Qin, A.; Chen, S.; Wang, P.; Huang, X.; Zhang, Y.; Liang, L.; Du, L.-R.; Lai, D.-H.; Ding, L.; Yu, X.; et al. Knockout of NOS2 Promotes Adipogenic Differentiation of Rat MSCs by Enhancing Activation of JAK/STAT3 Signaling. Front. Cell Dev. Biol. 2021, 9, 638518. [Google Scholar] [CrossRef]
- Philipp, D.; Suhr, L.; Wahlers, T.; Choi, Y.-H.; Paunel-Görgülü, A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res. Ther. 2018, 9, 286. [Google Scholar] [CrossRef]
- Takedachi, M.; Oohara, H.; Smith, B.J.; Iyama, M.; Kobashi, M.; Maeda, K.; Long, C.L.; Humphrey, M.B.; Stoecker, B.J.; Toyosawa, S.; et al. CD73-generated adenosine promotes osteoblast differentiation. J. Cell. Physiol. 2011, 227, 2622–2631. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef] [PubMed]
- Rapino, M.; Di Valerio, V.; Zara, S.; Gallorini, M.; Marconi, G.D.; Sancilio, S.; Marsich, E.; Ghinassi, B.; di Giacomo, V.; Cataldi, A. Chit-lac-coated Thermosets Enhance Osteogenesis and Angiogenesis in a Co-culture of Dental Pulp Stem Cells and Endothelial Cells. Nanomaterials 2019, 9, 928. [Google Scholar] [CrossRef] [PubMed]
- Janebodin, K.; Chavanachat, R.; Hays, A.; Gil, M.R. Silencing VEGFR-2 Hampers Odontoblastic Differentiation of Dental Pulp Stem Cells. Front. Cell Dev. Biol. 2021, 9, 665886. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L. Nitric Oxide and Angiogenesis. J. Neuro-Oncology 2000, 50, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Saura, M.; Tarin, C.; Zaragoza, C. Recent insights into the implication of nitric oxide in osteoblast differentiation and prolif-eration during bone development. Sci. World J. 2010, 10, 624–632. [Google Scholar] [CrossRef]
- Gallorini, M.; Zara, S.; Ricci, A.; Mangano, F.G.; Cataldi, A.; Mangano, C. The Open Cell Form of 3D-Printed Titanium Im-proves Osteconductive Properties and Adhesion Behavior of Dental Pulp Stem Cells. Materials 2021, 14, 5308. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Krifka, S.; Widbiller, M.; Schröder, A.; Brochhausen, C.; Cataldi, A.; Hiller, K.-A.; Buchalla, W.; Schweikl, H. Distinguished properties of cells isolated from the dentin-pulp interface. Ann. Anat. 2020, 234, 151628. [Google Scholar] [CrossRef] [PubMed]
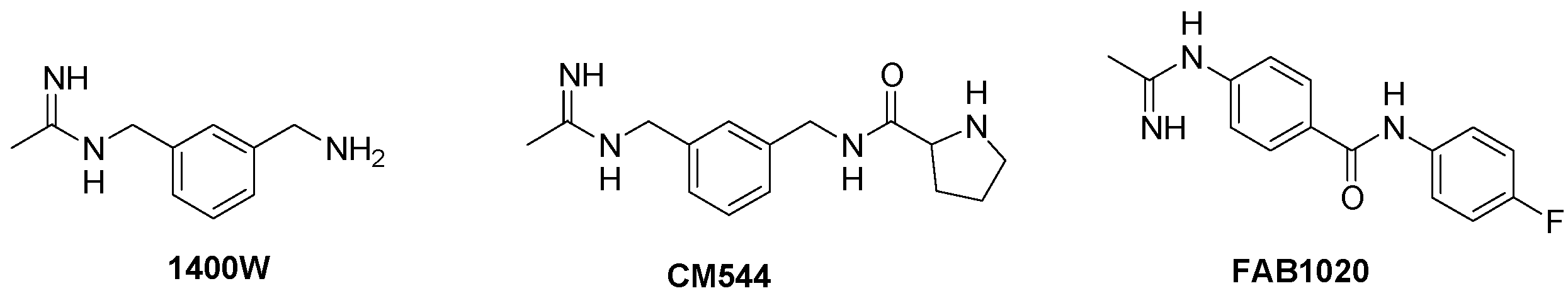
| Cpd | iNOS IC50 (nM) | eNOS/iNOS Selectivity | logP * | TPSA * |
|---|---|---|---|---|
| 1400W | 81 | 3800 | 1.06 | 61.90 |
| CM544 | 56 | 4569 | 1.31 | 68.22 |
| FAB1020 | 11 | >900 | 2.70 | 64.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldi, A.; Amoroso, R.; di Giacomo, V.; Zara, S.; Maccallini, C.; Gallorini, M. The Inhibition of the Inducible Nitric Oxide Synthase Enhances the DPSC Mineralization under LPS-Induced Inflammation. Int. J. Mol. Sci. 2022, 23, 14560. https://doi.org/10.3390/ijms232314560
Cataldi A, Amoroso R, di Giacomo V, Zara S, Maccallini C, Gallorini M. The Inhibition of the Inducible Nitric Oxide Synthase Enhances the DPSC Mineralization under LPS-Induced Inflammation. International Journal of Molecular Sciences. 2022; 23(23):14560. https://doi.org/10.3390/ijms232314560
Chicago/Turabian StyleCataldi, Amelia, Rosa Amoroso, Viviana di Giacomo, Susi Zara, Cristina Maccallini, and Marialucia Gallorini. 2022. "The Inhibition of the Inducible Nitric Oxide Synthase Enhances the DPSC Mineralization under LPS-Induced Inflammation" International Journal of Molecular Sciences 23, no. 23: 14560. https://doi.org/10.3390/ijms232314560
APA StyleCataldi, A., Amoroso, R., di Giacomo, V., Zara, S., Maccallini, C., & Gallorini, M. (2022). The Inhibition of the Inducible Nitric Oxide Synthase Enhances the DPSC Mineralization under LPS-Induced Inflammation. International Journal of Molecular Sciences, 23(23), 14560. https://doi.org/10.3390/ijms232314560









