A Changing Light Environment Induces Significant Lateral CO2 Diffusion within Maize Leaves
Abstract
:1. Introduction
2. Results
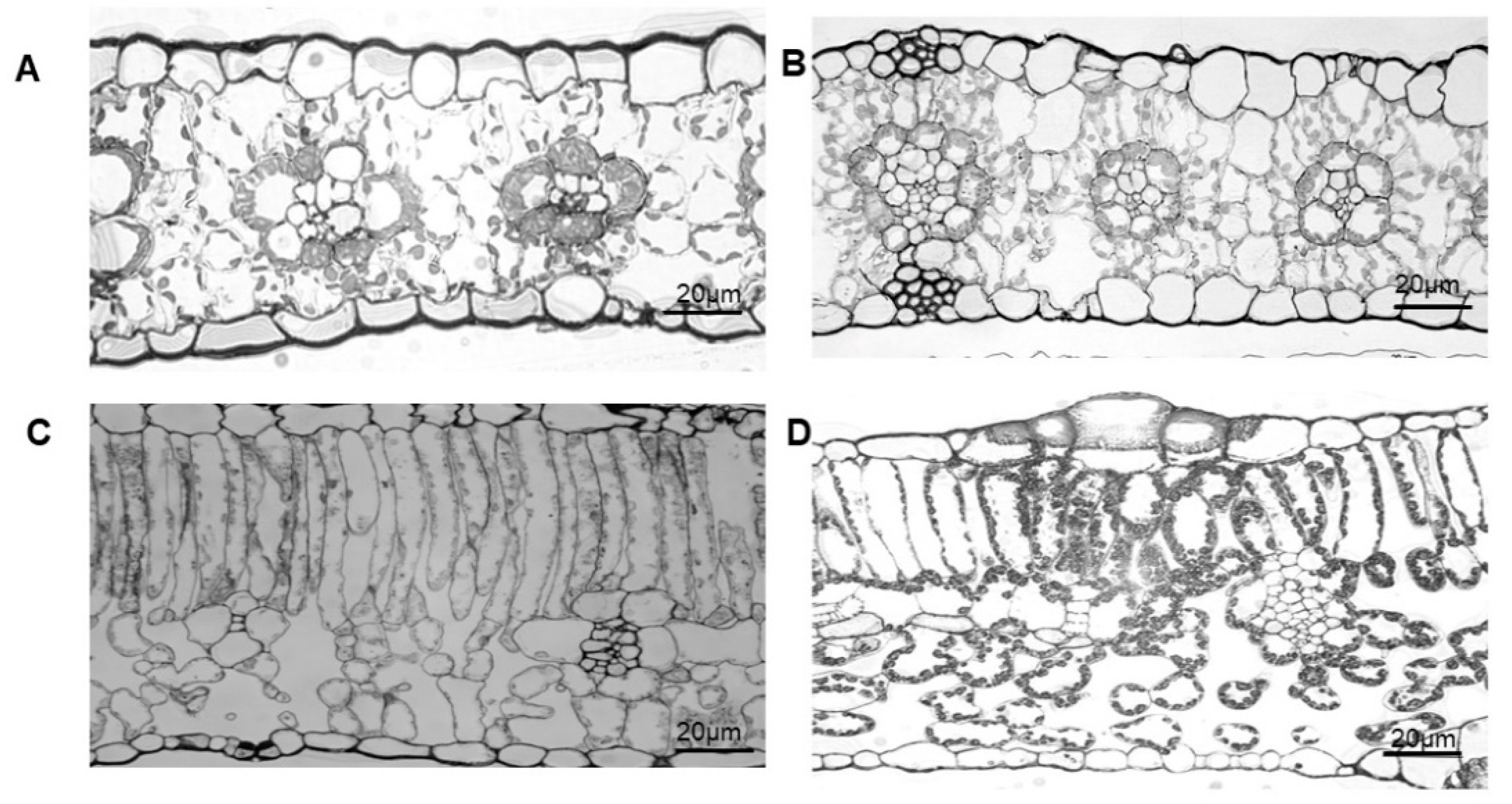
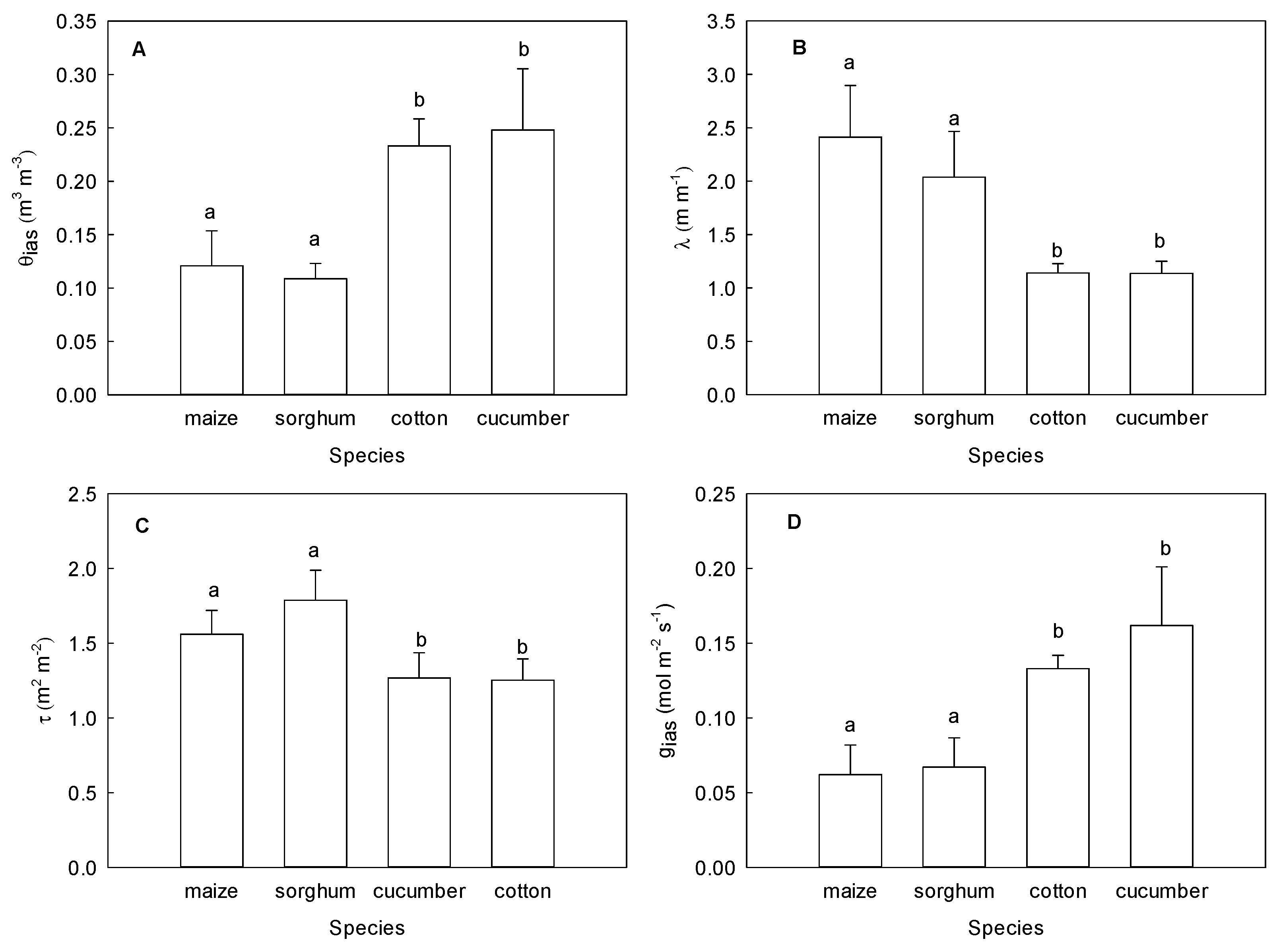
2.1. Differences in the Leaf Structure of Various Plant Species
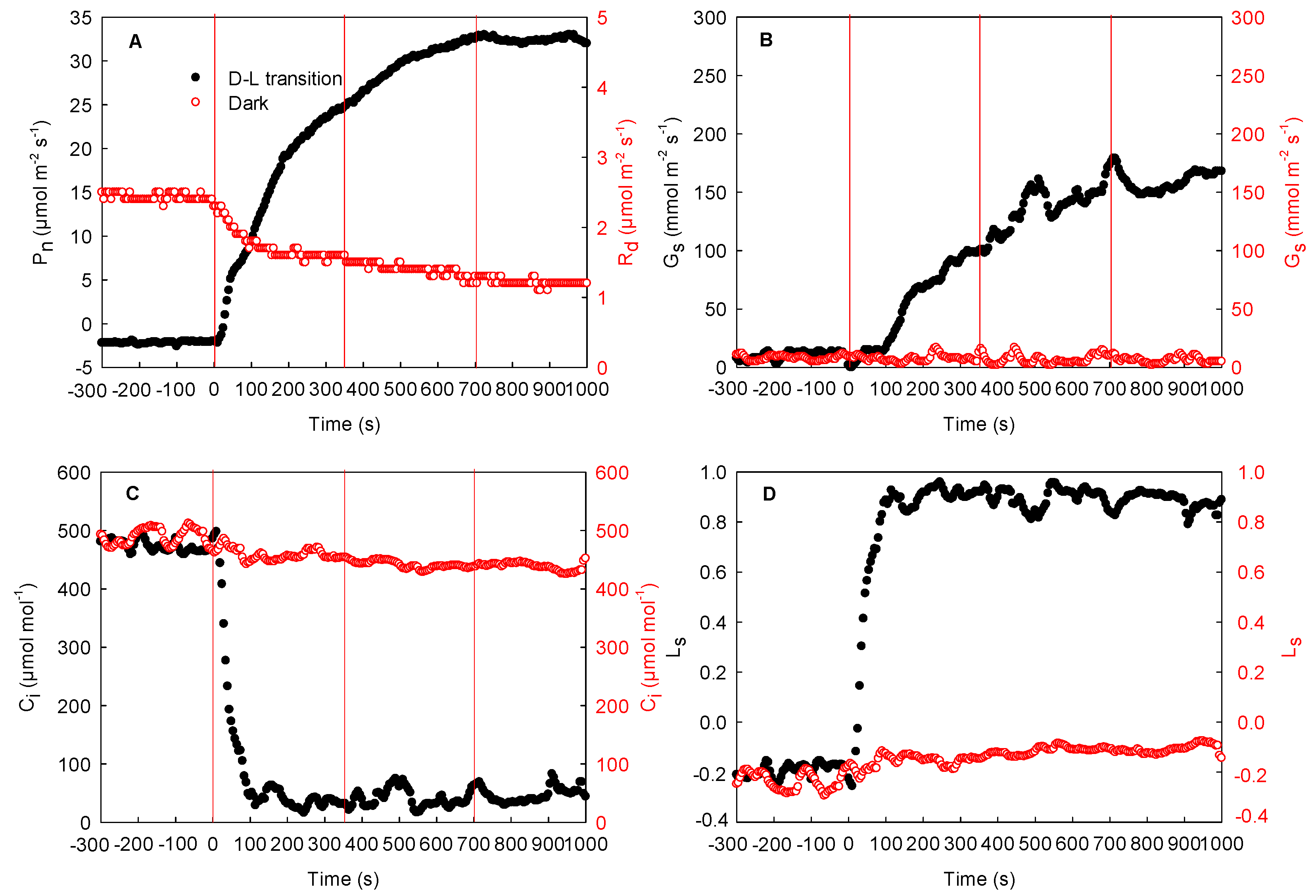
2.2. Effect of Local Illumination on the Respiration Rate of Adjacent Region in the Same Leaves
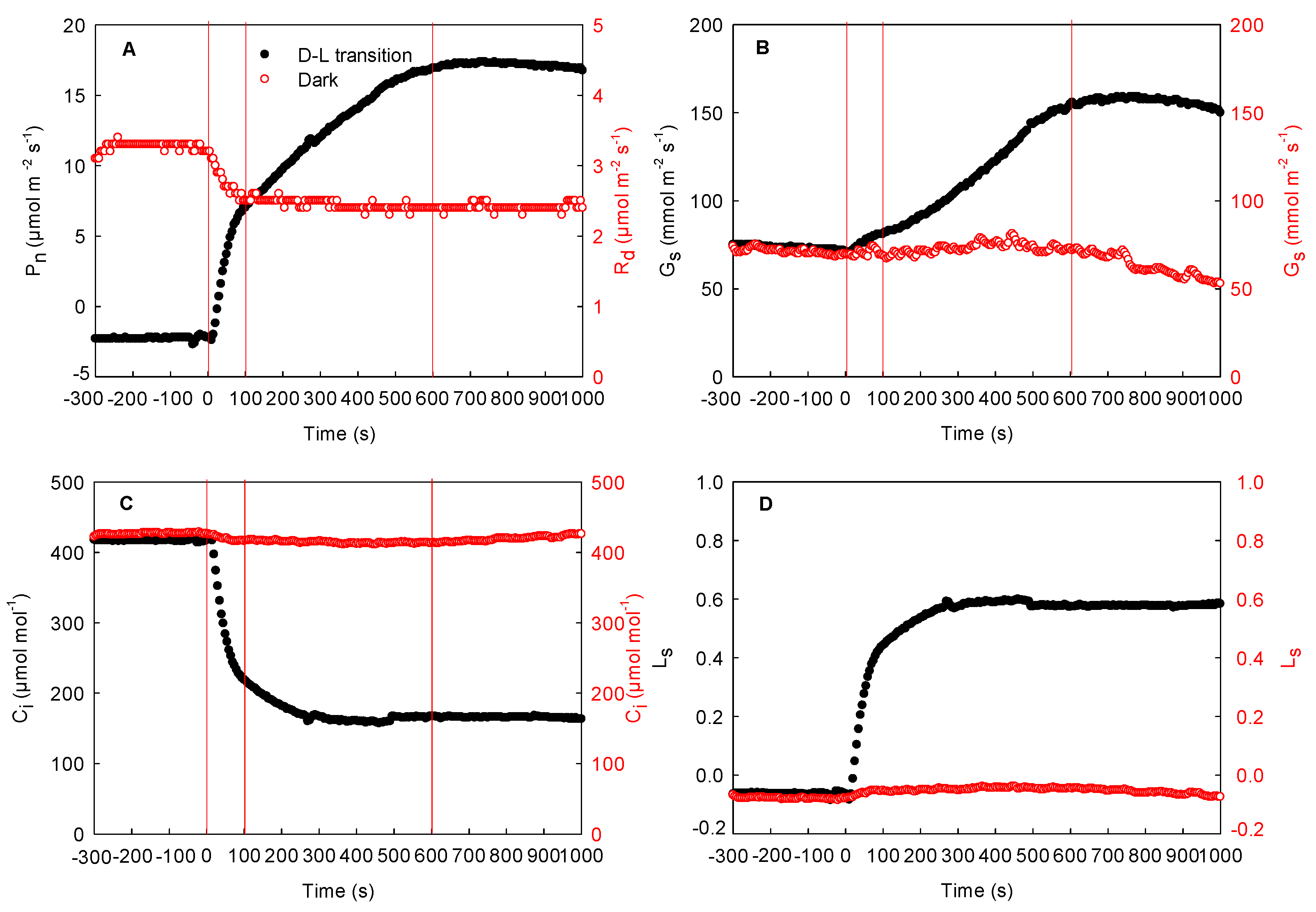
2.3. Effect of Local Illumination on the Photosynthetic Rate of the Adjacent Region in the Same Leaves
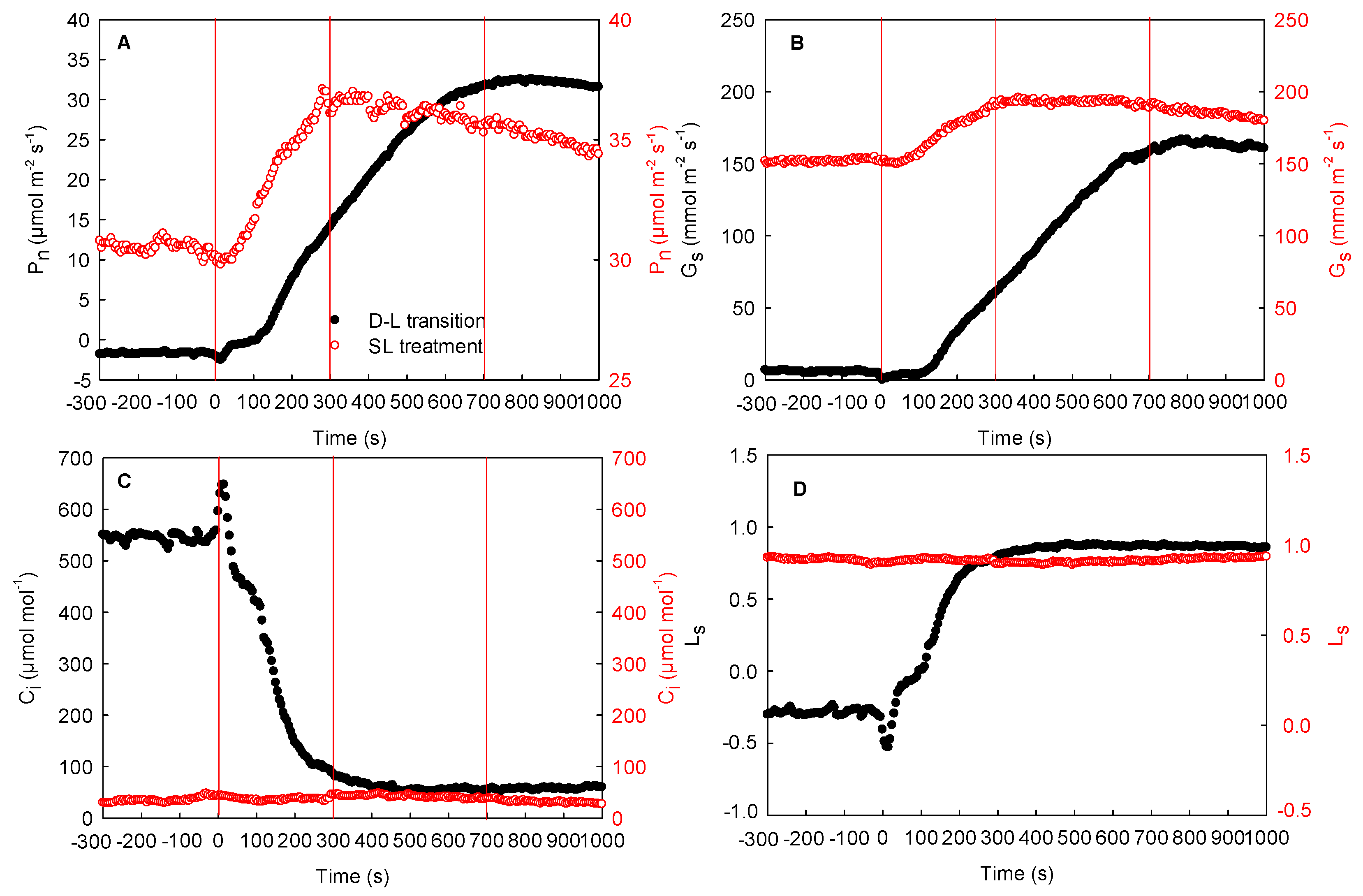
2.4. Effects of the Changing Light Environment on the Respiration and Photosynthetic Rate of the Same Maize Leaves in the Field
3. Discussion
3.1. Lateral CO2 Diffusion Supports Photosynthesis in Maize Leaves
3.2. Mechanism of Lateral CO2 Diffusion inside the Maize Leaves
3.3. The Significance of Lateral CO2 Diffusion within the Leaf
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Determination of Anatomical Structure and Leaf Porosity
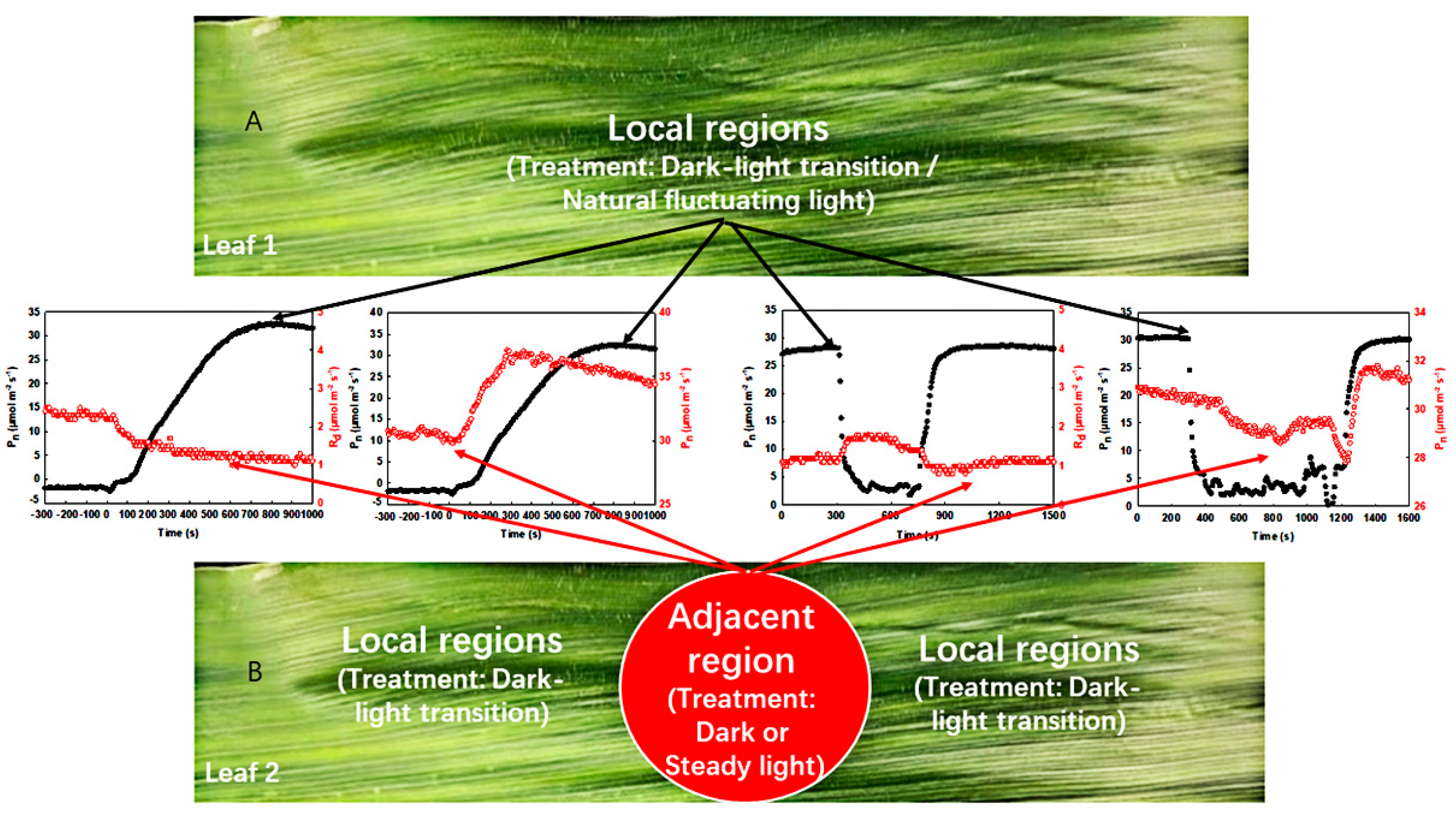
4.3. Determination of Gas Exchange under a Controlled Light Environment
- (1)
- First, the measurement of the photosynthetic induction under the dark–light transition in the local regions was carried out. In this process, the light intensity in the local regions was maintained in darkness (0 μmol−2 m−2 s–1). After stabilization of the respiration rate in the local regions, the Light-Emitting Diode (LED) of the leaf chamber in the local regions was then turned on (light intensity: 1600 μmol m−2 s−1). During this process, the photosynthetic induction under the dark–light transition was recorded every 5 s (Figure 3A).
- (2)
- Secondly, the measurement of respiration in darkness and photosynthetic rate under steady light intensity in the adjacent region was carried out: The light intensity in the adjacent region was controlled to 0 or 1600 μmol m−2 s−1 via an LED of the leaf chamber, while the local regions were maintained in darkness. After a stable respiration rate or a steady-state photosynthetic rate in the adjacent region was achieved, the local regions were subjected to a light intensity of 1600 μmol m−2 s−1 controlled by an LED, and the changes in respiration rate or photosynthetic rate in the adjacent region under steady light intensity were recorded every 5 s (Figure 3B).
4.4. Determination of Gas Exchange in the Field
- (1)
- To investigate the effects of natural fluctuating light on photosynthesis in the local regions, the measurements in the local regions were carried out in the field with a gas exchange system (removal of LED light source) (Figure 3A).
- (2)
- To study the effects of natural fluctuating light on respiration or photosynthetic rate in the adjacent region, the measurements in the adjacent region were performed with a gas exchange system (with LED light source). The light intensity of the leaf chamber (adjacent region) was controlled to 0 or 1600 µmol m−2 s−1, and the respiration rate or photosynthetic rate in steady light intensity was determined, respectively (Figure 3B).
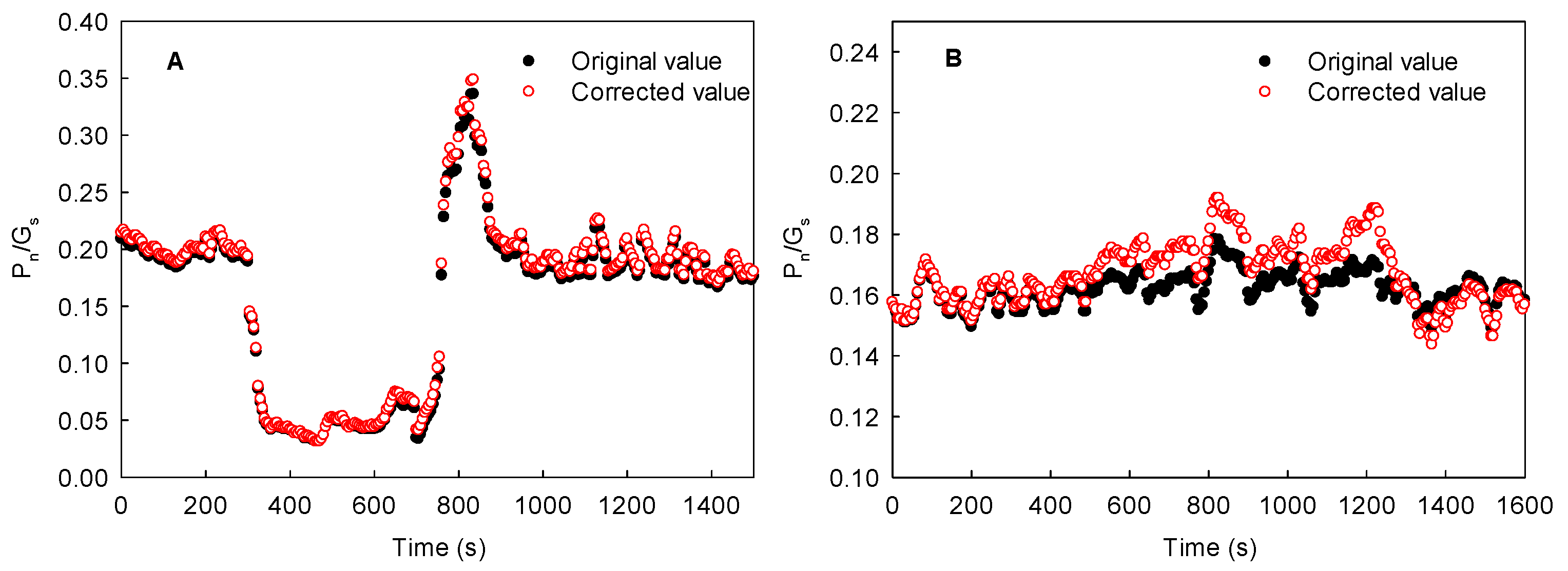
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.R. Mesophyll conductance: Walls, membranes and spatial complexity. New Phytol. 2020, 229, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Berghuijs, H.N.C.; Yin, X.Y.; Ho, Q.T.; Retta, M.A.; Nicolai, B.M.; Struik, P.C. Using a reaction-diffusion model to estimate day respiration and reassimilation of (photo)respired CO2 in leaves. New Phytol. 2019, 223, 619–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Zhang, Z.C.; Huang, G.J.; Li, Y. Leaf photosynthesis and its temperature response are different between growth stages and N supplies in rice plants. Int. J. Mol. Sci. 2022, 23, 3885. [Google Scholar] [CrossRef]
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. Major transitions in the evolution of early land plants: A bryological perspective. Ann. Bot. 2012, 109, 851–871. [Google Scholar] [CrossRef] [PubMed]
- Earles, J.M.; Theroux-Rancourt, G.; Roddy, A.B.; Gilbert, M.E.; McElrone, A.J.; Brodersen, C.R. Beyond porosity: 3D leaf intercellular airspace traits that impact mesophyll conductance. Plant Physiol. 2018, 178, 148–162. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.A.M.; Chitwood, D.H.; Azevedo, A.A.; Araújo, W.L.; Ribeiro, D.M.; Peres, L.E.P.; Martins, S.C.U.; Zsögön, A. Bundle sheath extensions affect leaf structural and physiological plasticity in response to irradiance. Plant Cell Environ. 2019, 42, 1575–1589. [Google Scholar] [CrossRef] [Green Version]
- Parkhurst, D.F. Diffusion of CO2 and other gases inside leaves. New Phytol. 1994, 126, 449–479. [Google Scholar] [CrossRef]
- Jahnke, S.; Krewitt, M. Atmospheric CO2 concentration may directly affect leaf respiration measurement in tobacco, but not respiration itself. Plant Cell Environ. 2002, 25, 641–651. [Google Scholar] [CrossRef]
- Pieruschka, R.; Schurr, U.; Jahnke, S. Lateral gas diffusion inside leaves. J. Exp. Bot. 2005, 56, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieruschka, R.; Chavarria-Krauser, A.; Schurr, U.; Jahnke, S. Photosynthesis in lightfleck areas of homobaric and heterobaric leaves. J. Exp. Bot. 2010, 61, 1031–1039. [Google Scholar] [CrossRef] [Green Version]
- Sáez, P.L.; Bravo, L.A.; Cavieres, L.A.; Vallejos, V.; Sanhueza, C.; Font-Carrascosa, M.; Gil-Pelegrín, E.; Peguero-Pina, J.J.; Galmés, J. Photosynthetic limitations in two Antarctic vascular plants: Importance of leaf anatomical traits and Rubisco kinetic parameters. J. Exp. Bot. 2017, 68, 2871–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syvertsen, J.P.; Lloyd, J.; McConchie, C.; Kriedemann, P.E.; Farquhar, G.D. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell Environ. 1995, 18, 149–157. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Reichstein, M. Controls on the emission of plant volatiles through stomata: Differential sensitivity of emission rates to stomatal closure explained. J. Geophys. Res. 2003, 108, 4208. [Google Scholar] [CrossRef]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Ribas-Carbó, M.; Tosens, T.; Vislap, V.; Niinemets, Ü. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Harwood, R.; Théroux-Rancourt, G.; Barbour, M.M. Understanding airspace in leaves: 3D anatomy and directional tortuosity. Plant Cell Environ. 2021, 44, 2455–2465. [Google Scholar] [CrossRef]
- Lawson, T.; Morison, J. Visualising patterns of CO2 diffusion in leaves. New Phytol. 2006, 169, 637–640. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Lawson, T. Does lateral gas diffusion in leaves matter? Plant Cell Environ. 2007, 30, 1072–1085. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Lawson, T.; Cornic, G. Lateral CO2 diffusion inside dicotyledonous leaves can be substantial: Quantification in different light intensities. Plant Physiol. 2007, 145, 680–690. [Google Scholar] [CrossRef]
- Long, S.P.; Farage, P.K.; Bolha´r-Nordenkampf, H.R.; Rohrhofer, U. Separating the contribution of the upper and the lower mesophyll to photosynthesis in Zea mays L. leaves. Planta 1989, 177, 207–216. [Google Scholar] [CrossRef]
- Graham, E.A.; Mulkey, S.S.; Kitajima, K.; Phillips, N.G.; Wright, S.J. Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc. Natl. Acad. Sci. USA 2003, 100, 572–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, W.; Lindner, S.; Dubbert, M.; Otieno, D.; Ko, J.H.; Muraoka, H.; Werner, C.; Tenhunen, J. Supplement understanding of the relative importance of biophysical factors in determination of photosynthetic capacity and photosynthetic productivity in rice ecosystems. Agric. Forest Meteorol. 2017, 232, 550–565. [Google Scholar] [CrossRef]
- Pieruschka, R.; Schurr, U.; Jensen, M.; Wolff, W.F.; Jahnke, S. Lateral diffusion of CO2 from shaded to illuminated leaf parts affects photosynthesis inside homobaric leaves. New Phytol. 2006, 169, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Pieruschka, R.; Chavarria-Krauser, A.; Cloos, K.; Scharr, H.; Schurr, U.; Jahnke, S. Photosynthesis can be enhanced by lateral CO2 diffusion inside leaves over distances of several millimeters. New Phytol. 2008, 178, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Morison, J.I.L.; Gallouet, E.; Lawson, T.; Cornic, G.; Herbin, R.; Baker, N.R. Lateral diffusion of CO2 in leaves is not sufficient to support photosynthesis. Plant Physiol. 2005, 139, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Hatch, M.D.; Burnell, J.N. Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiol. 1990, 93, 825–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badger, M.R.; Price, G.D. The role of carbonic anhydrase in photosynthesis. Annu. Rev. Plant Phys. 1994, 45, 369–392. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Fernández, J.A.; Rubio, L.; Pérez, L.; Terés, J.; Barceló, J. Transport and use of bicarbonate in plants: Current knowledge and challenges ahead. Int. J. Mol. Sci. 2018, 19, 1352. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Liang, J.J.; Zhou, Y.; Tian, T.; Zhang, B.L.; Duanmu, D.Q. Molecular characterization of carbonic anhydrase genes in Lotus japonicus and their potential roles in symbiotic nitrogen fixation. Int. J. Mol. Sci. 2021, 22, 7766. [Google Scholar] [CrossRef]
- Chatterjee, J.; Coe, R.A.; Acebron, K.; Thakur, V.; Yennamalli, R.M.; Danila, F.; Lin, H.C.; Balahadia, C.P.; Bagunu, E.; Padhmapriya, P.O.S.; et al. A low CO2-responsive mutant of Setaria viridis reveals that reduced carbonic anhydrase limits C4 photosynthesis. J. Exp. Bot. 2021, 72, 3122–3136. [Google Scholar] [CrossRef] [PubMed]
- Poincelot, R.P. Intracellular distribution of carbonic anhydrase in spinach leaves. Biochim. Et Biophys. Acta (BBA)-Enzymol. 1972, 258, 637–642. [Google Scholar] [CrossRef]
- Friso, G.; Majeran, W.; Huang, M.; Sun, Q.; van Wijk, K.J. Reconstruction of metabolic pathways, protein expression, and homeostasis machineries across maize bundle sheath and mesophyll chloroplasts: Large-scale quantitative proteomics using the first maize genome assembly. Plant Physiol. 2010, 152, 1219–1250. [Google Scholar] [CrossRef] [Green Version]
- Bräutigam, A.; Mullick, T.; Schliesky, S.; Weber, A.P.M. Critical assessment of assembly strategies for non-model species mRNA-Seq data and application of next-generation sequencing to the comparison of C3 and C4 species. J. Exp. Bot. 2011, 62, 3093–3102. [Google Scholar] [CrossRef] [Green Version]
- Studer, A.J.; Gandin, A.; Kolbe, A.R.; Wang, L.; Cousins, A.B.; Brutnell, T.P. A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a ca1ca2 double mutant in maize. Plant Physiol. 2014, 165, 608–617. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Chen, H.M.; Jiang, C.D.; Shi, L. Quantification of leaf anatomical structure and its application in a C4 plant, sorghum. Chin. Bull. Bot. 2014, 49, 173–182. (In Chinese) [Google Scholar]
- Jiang, C.D.; Wang, X.; Gao, H.Y.; Shi, L.; Chow, W.S. Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-light tolerance in sorghum. Plant Physiol. 2011, 155, 1416–1424. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-Y.; Zou, Q.-Q.; Ji, W.-T.; Wang, Y.-W.; Zhang, W.-F.; Jiang, C.-D. A Changing Light Environment Induces Significant Lateral CO2 Diffusion within Maize Leaves. Int. J. Mol. Sci. 2022, 23, 14530. https://doi.org/10.3390/ijms232314530
Wu H-Y, Zou Q-Q, Ji W-T, Wang Y-W, Zhang W-F, Jiang C-D. A Changing Light Environment Induces Significant Lateral CO2 Diffusion within Maize Leaves. International Journal of Molecular Sciences. 2022; 23(23):14530. https://doi.org/10.3390/ijms232314530
Chicago/Turabian StyleWu, Han-Yu, Qing-Qing Zou, Wen-Tao Ji, Ying-Wei Wang, Wang-Feng Zhang, and Chuang-Dao Jiang. 2022. "A Changing Light Environment Induces Significant Lateral CO2 Diffusion within Maize Leaves" International Journal of Molecular Sciences 23, no. 23: 14530. https://doi.org/10.3390/ijms232314530
APA StyleWu, H.-Y., Zou, Q.-Q., Ji, W.-T., Wang, Y.-W., Zhang, W.-F., & Jiang, C.-D. (2022). A Changing Light Environment Induces Significant Lateral CO2 Diffusion within Maize Leaves. International Journal of Molecular Sciences, 23(23), 14530. https://doi.org/10.3390/ijms232314530




