Combined Metabolomic and Quantitative RT-PCR Analyses Revealed the Synthetic Differences of 2-Acetyl-1-pyrroline in Aromatic and Non-Aromatic Vegetable Soybeans
Abstract
:1. Introduction
2. Results
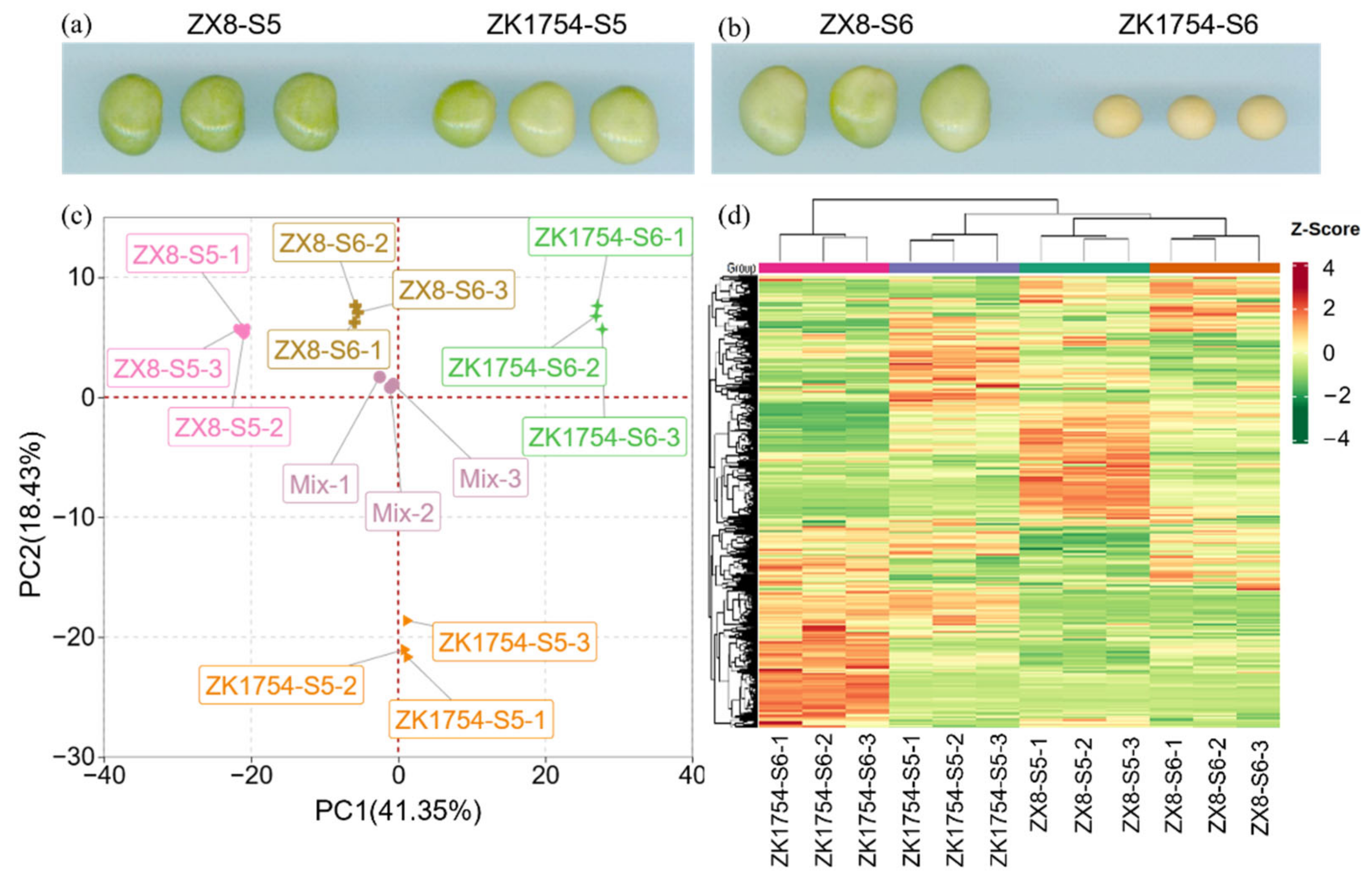
2.1. Metabolic Profiling of ZX8 and ZK1754 Grain at Two Developmental Stages
2.2. Differential Accumulation Metabolites (DAMs) in ZX8 and ZK1754 Soybean Grains
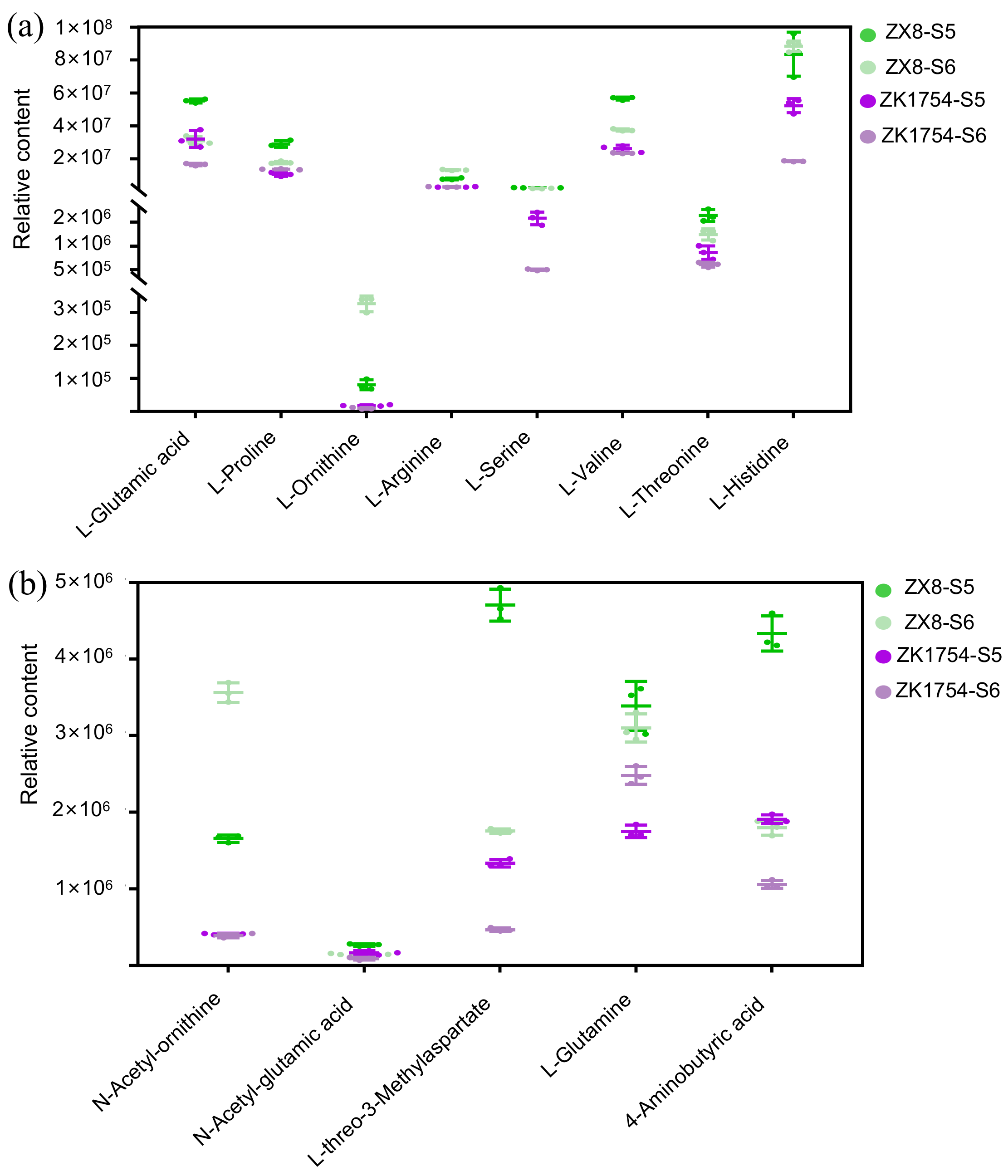
2.3. Special DAMs in Aromatic and Non-Aromatic Soybeans
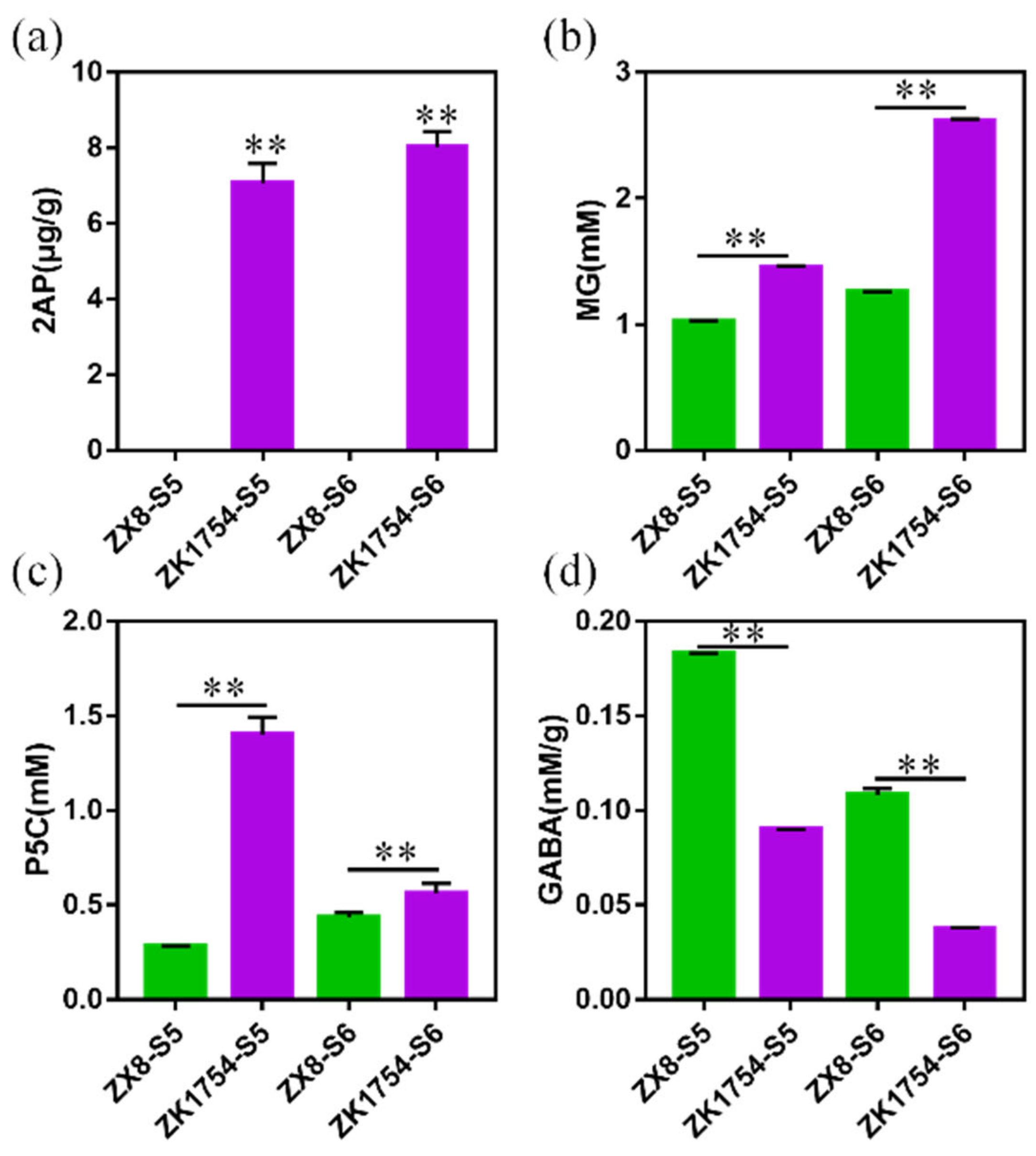
2.4. Quantification of the 2AP Synthesis-Related Metabolites
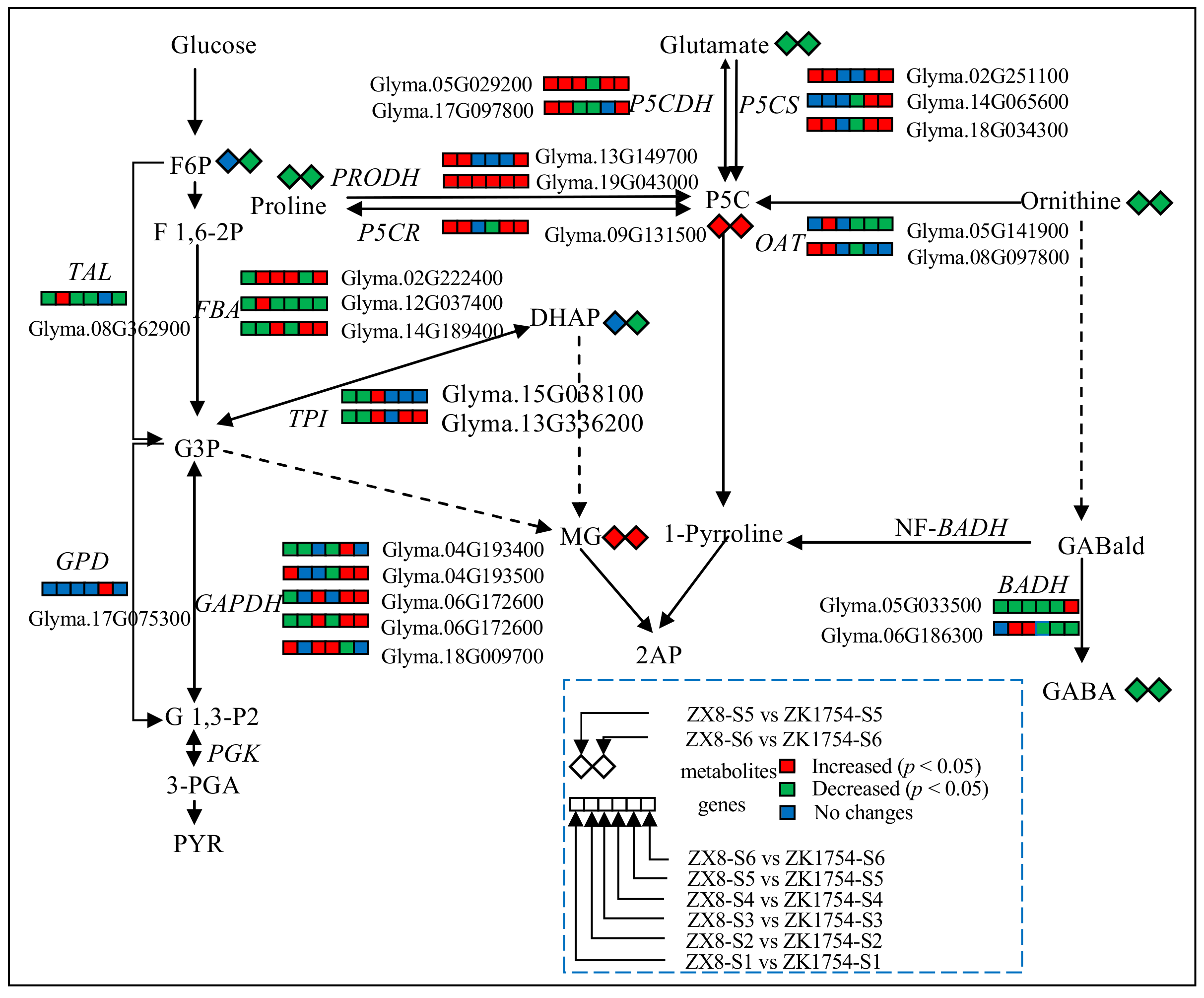
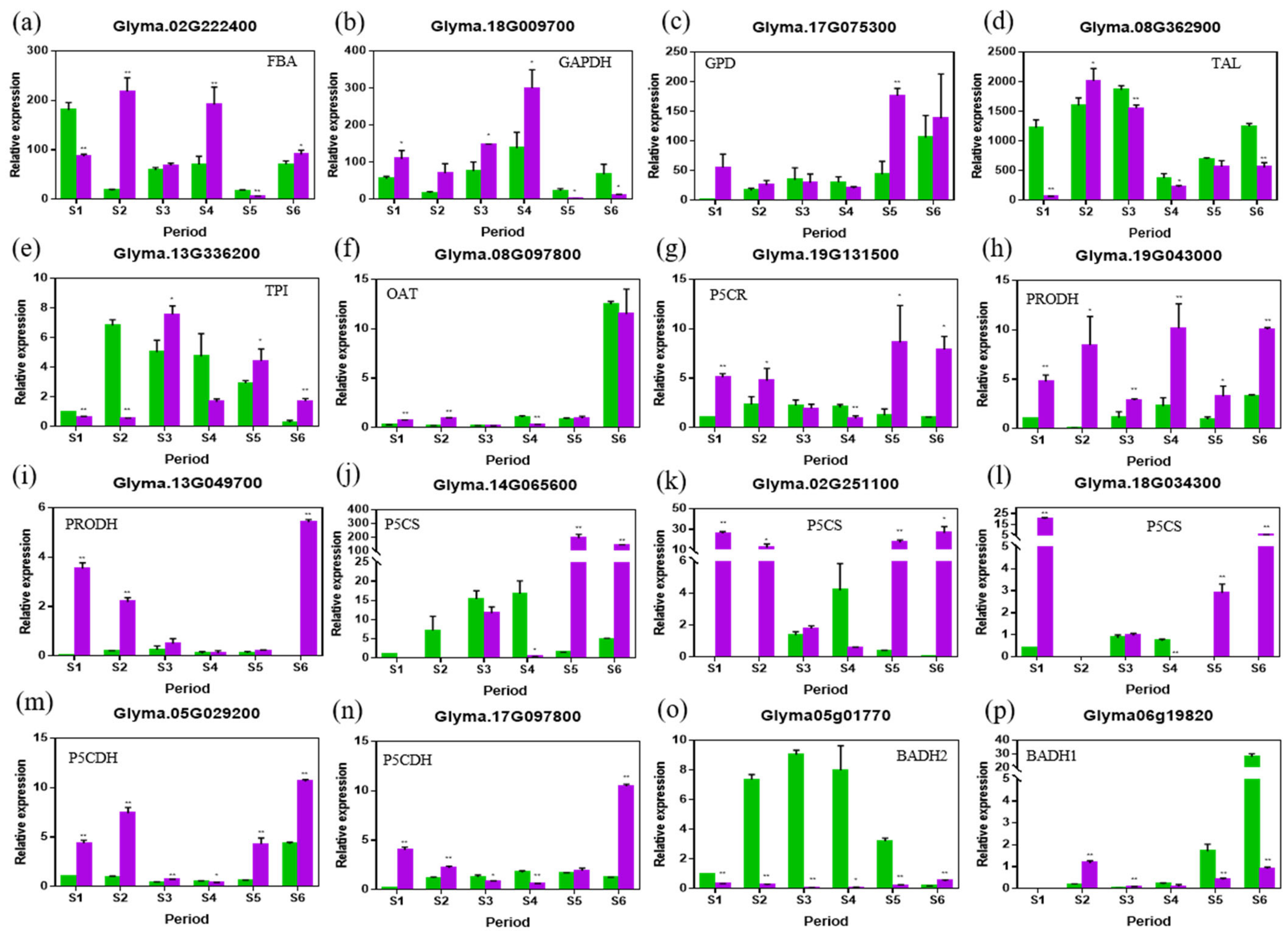
2.5. RT-PCR Analysis of the Genes in the 2AP Synthesis Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Extraction Procedure
4.3. Chromatography and Mass Spectrometry Acquisition Conditions
4.4. Data Analysis
4.5. Content Determination
4.6. RT-qPCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coward, L.; Barnes, N.C.; Setchell, K.; Barnes, S. Genistein, daidzein, and theirbeta-glycoside conjugates: Antitumor isoflavones in soybean food from American and Asian diets. J. Agric. Food Chem. 1993, 41, 1961–1967. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [Green Version]
- Statistics Department, Ministry of Agriculture, Forestry and Fisheries. Statistics on Production and Shipment of Vegetables Heisei 19; Statistics Department, Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2009; ISBN 4-541-03621-5.
- Arikit, S.; Yoshihashi, T.; Wanchana, S.; Uyen, T.T.; Huong, N.T.T.; Wongpornchai, S.; Vanavichit, A. Deficiency in the amino aldehyde dehydrogenase encoded by GmAMADH2, the homologue of rice Os2AP, enhances 2-acetyl-1-pyrroline biosynthesis in soybeans (Glycine max L.). Plant Biotech. J. 2010, 9, 75–87. [Google Scholar] [CrossRef]
- Wu, M.-L.; Chou, K.-L.; Wu, C.-R.; Chen, J.-K.; Huang, T.-C. Characterization and the possible formation mechanism of 2-acetyl-1-pyrroline in aromatic vegetable soybean (Glycine max L.). J. Food Sci. 2009, 74, 192–197. [Google Scholar] [CrossRef]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [CrossRef]
- Huang, T.C.; Teng, C.S.; Chang, J.L.; Chuang, H.S.; Ho, C.T.; Wu, M.L. biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J. Agric. Food Chem. 2008, 56, 7399–7404. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O.; Turnbaugh, J.G. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma volatile analyses and 2AP characterization at various developmental stages in basmati and non-basmati scented rice (Oryza sativa L.) cultivars. Rice 2016, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Routray, W.; Rayaguru, K. 2-Acetyl-1-pyrroline: A key aroma component of aromatic rice and other food products. Food Rev. Int. 2018, 34, 539–565. [Google Scholar] [CrossRef]
- Fushimi, T.; Masuda, R. 2-Acetyl-1-pyrroline concentration of the vegetable soybean. In Proceedings of the 2nd International Vegetable Soybean Conference, Tacoma, WA, USA, 10–12 August 2001; Washington State University: Pullman, WA, USA, 2001; Volume 39. [Google Scholar]
- Chen, S.; Yang, Y.; Shi, W.; Ji, Q.; He, F.; Zhang, Z.; Cheng, Z.; Liu, X.; Xu, M. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef]
- Ahn, S.N.; Bollich, C.N.; Tanksley, S.D. RFLP tagging of a gene for aroma in rice. Theor. Appl. Genet. 1992, 84, 825–828. [Google Scholar] [CrossRef]
- Lorieux, M.; Petrov, M.; Huang, N.; Guiderdoni, E.; Ghesquire, A. Aroma in rice: Genetic analysis of a quantitative trait. Theor. Appl. Genet. 1996, 93, 1145–1151. [Google Scholar] [CrossRef]
- Wanchana, S. Identification of Genes Controlling Grain Aroma and Amylose Content for Positional Cloning and Marker-Assisted Selection Program in Rice (Oryza sativa L.). Ph.D. Thesis, Kasetsart University, Bangkok, Thailand, 2005. [Google Scholar]
- Bradbury, L.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.; Waters, D. The gene for fragrance in rice. Plant Biotechnol. J. 2005, 3, 363–370. [Google Scholar] [CrossRef]
- Shi, W.; Yi, Y.; Chen, S.; Xu, M. Discovery of a new fragrance allele and the development of functional markers for the breeding of fragrant rice varieties. Mol. Breed. 2008, 22, 185–192. [Google Scholar] [CrossRef]
- Khandagale, K.S.; Chavhan, R.; Nadaf, A.B. RNAi-mediated down regulation of BADH2 gene for expression of 2-acetyl-1-pyrroline in non-scented Indica rice IR-64 (Oryza sativa L.). 3 Biotechnol. 2020, 10, 145. [Google Scholar] [CrossRef]
- Niu, X.; Tang, W.; Huang, W.; Ren, G.; Wang, Q.; Luo, D.; Xiao, Y.; Yang, S.; Wang, F.; Lu, B.R.; et al. RNAi-directed downregulation of OsBADH2 results in aroma (2-acetyl-1-pyrroline) production in rice (Oryza sativa L.). BMC Plant Biol. 2008, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Abdelrahman, M.; Li, J.; Wang, F.; Ji, Z.; Qi, H.; Wang, C.; Zhao, K. CRISPR/Cas9 induces exon skipping that facilitates development of fragrant rice. Plant Biotechnol. J. 2021, 19, 642–644. [Google Scholar] [CrossRef]
- Vanavichit, A.; Tragoonrung, S.; Toojinda, T.; Wanchana, S.; Kamolsukyunyong, W. Transgenic Rice Plants with Reduced Expression of Os2AP and Elevated Levels of 2-acetyl-1-pyrroline. U.S. Patent 7,319,181, 15 January 2008. [Google Scholar]
- Amarawathi, Y.; Singh, R.; Singh, A.K.; Singh, V.P.; Mohapatra, T.; Sharma, T.R.; Singh, N.K. Mapping of quantitative trait loci for basmati quality traits in rice (Oryza sativa L.). Mol. Breed. 2007, 21, 49–65. [Google Scholar] [CrossRef]
- Juwattanasomran, R.; Somta, P.; Chankaew, S.; Shimizu, T.; Wongpornchai, S.; Kaga, A.; Srinives, P. A SNP in GmBADH2 gene associates with fragrance in vegetable soybean variety “kaori” and SNAP marker development for the fragrance. Theor. Appl. Genet. 2011, 122, 533–541. [Google Scholar] [CrossRef]
- Qian, L.; Jin, H.; Yang, Q.; Zhu, L.; Yu, X.; Fu, X.; Zhao, M.; Yuan, F. A sequence variation in GmBADH2 enhances soybean aroma and is a functional marker for improving soybean flavor. Int. J. Mol. Sci. 2022, 23, 4116. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, T.; Zheng, A.; He, L.; Lai, R.; Liu, J.; Xing, P.; Tang, X. Exogenous proline induces regulation in 2-acetyl-1-pyrroline (2-AP) biosynthesis and quality characters in fragrant rice (Oryza sativa L.). Sci. Rep. 2020, 10, 13971. [Google Scholar] [CrossRef]
- Sharma, S.; Mustafiz, A.; Singla-Pareek, S.L.; Shankar Srivastava, P.; Sopory, S.K. Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signal. Behav. 2012, 7, 1337–1345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Wang, N.N.; Zhang, Y.H.; Feng, Q.Z.; Yang, C.W.; Liu, B. Exogenous proline induces regulation in 2-acetyl-1-pyrroline (2-AP) biosynthesis and quality characters in fragrant rice. Genet. Mol. Res. 2013, 12, 1269–1277. [Google Scholar] [CrossRef]
- Yun, D.Y.; Kang, Y.G.; Kim, M.; Kim, D.; Hong, Y.S. Metabotyping of different soybean genotypes and distinct metabolism in their seeds and leaves. Food Chem. 2020, 330, 127198–127208. [Google Scholar] [CrossRef]
- Ho, H.M.; Leung, L.K.; Chan, F.L.; Huang, Y.; Chen, Z.Y. Soy leaf lowers the ratio of non-HDL to HDL cholesterol in hamsters. J. Agric. Food Chem. 2003, 51, 4554–4558. [Google Scholar] [CrossRef]
- Kwak, Y.; Ju, J. Glycine max Merr. Leaf extract possesses anti-oxidant properties, decreases inflammatory mediator production in murine macrophages, and inhibits growth, migration, and adhesion in human cancer cells. Food Sci. Biotechnol. 2017, 26, 245–253. [Google Scholar] [CrossRef]
- Ryu, R.; Jeong, T.-S.; Kim, Y.J.; Choi, J.-Y.; Cho, S.-J.; Kwon, E.-Y.; Jung, U.J.; Ji, H.-S.; Shin, D.-H.; Choi, M.-S. Beneficial effects of pterocarpan-high soybean leaf extract on metabolic syndrome in overweight and obese korean subjects: Randomized controlled Trial. Nutrients 2016, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Deng, B.; Tian, S.; Guo, M.; Liu, H.; Zhao, X. Metabolic and transcriptomic analyses reveal different metabolite biosynthesis profiles between leaf buds and mature leaves in Ziziphus jujuba mill. Food Chem. 2021, 347, 129005–129015. [Google Scholar] [CrossRef]
- Wakte, K.V.; Kad, T.D.; Zanan, R.L.; Nadaf, A.B. Mechanism of 2-acetyl-1-pyrroline biosynthesis in Bassia latifolia Roxb. flowers. Physiol. Mol. Biol. Plants 2011, 17, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, L.M.T.; Gillies, S.A.; Brushett, D.J.; Waters, D.L.E.; Henry, R.J. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 2008, 68, 439–449. [Google Scholar] [CrossRef]
- Chen, M.; Wei, X.; Shao, G.; Tang, S.; Luo, J.; Hu, P. Fragrance of the rice grain achieved via artificial microRNA-induced down-regulation of OsBADH2. Plant Breed. 2012, 131, 584–590. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Waters, D.L.E.; Henry, R.J. The effect of salt on betaine aldehyde dehydrogenase transcript levels and 2-acetyl-1-pyrroline concentration in fragrant and non-fragrant rice (Oryza sativa). Plant Sci. 2008, 175, 539–546. [Google Scholar] [CrossRef]
- He, Q.; Yu, J.; Kim, T.S.; Cho, Y.H.; Lee, Y.S.; Park, Y.J. Resequencing Reveals Different Domestication Rate for BADH1 and BADH2 in Rice (Oryza sativa). PLoS ONE 2015, 10, e134801. [Google Scholar] [CrossRef]
- Bradbury, L.M.T.; Henry, R.J.; Jin, Q.; Reinke, R.F.; Waters, D.L.E. A perfect marker for fragrance genotyping in rice. Mol. Breed. 2005, 16, 279–283. [Google Scholar] [CrossRef]
- Huang, T.C.; Huang, Y.W.; Hung, H.J.; Ho, C.T.; Wu, M.L. Δ1-Pyrroline-5-carboxylic acid formed by proline dehydrogenase from the Bacillus subtilis ssp. natto expressed in Escherichia coli as a precursor for 2-acetyl-1-pyrroline. J. Agric. Food Chem. 2007, 55, 5097–5102. [Google Scholar] [CrossRef]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9–18. [Google Scholar] [CrossRef]
- Zhao, D.W.; Pu, X.-Y.; Zeng, Y.W.; Li, B.X.; Juan, D.U.; Yang, S.M. Determination of the γ-aminobutyric acid in barley. J. Triticeae Crops 2009, 29, 69–72. [Google Scholar]
| Class | ZX8-S5 vs. ZK1754-S5 | ZX8-S6 vs. ZK1754-S6 | ZX8-S5 vs. ZX8-S6 | ZK1754-S5 vs. ZK1754-S6 | ||||
|---|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | Up | Down | |
| Flavonoids | 37 | 5 | 33 | 9 | 3 | 6 | 9 | 10 |
| Lipids | 0 | 32 | 35 | 11 | 1 | 38 | 34 | 14 |
| Amino acids and derivatives | 4 | 21 | 4 | 29 | 10 | 6 | 12 | 19 |
| Saccharides and Alcohols | 17 | 2 | 8 | 13 | 14 | 3 | 8 | 16 |
| Organic acids | 7 | 7 | 7 | 11 | 3 | 6 | 6 | 11 |
| Nucleotides and derivatives | 8 | 5 | 19 | 10 | 2 | 12 | 14 | 12 |
| Phenolic acids | 6 | 4 | 11 | 9 | 1 | 9 | 5 | 8 |
| Alkaloids | 2 | 8 | 2 | 7 | 0 | 5 | 2 | 3 |
| Vitamin | 3 | 0 | 1 | 4 | 4 | 0 | 2 | 7 |
| Others | 1 | 1 | 8 | 0 | 1 | 0 | 2 | 0 |
| Total | 86 | 85 | 128 | 103 | 40 | 85 | 94 | 102 |
| Metabolites | ZX8-S5 | ZK1754-S5 | ZX8-S6 | ZK1754-S6 | ZX8-S5 | ZX8-S6 | ZK1754-S5 | ZK1754-S6 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Content | Relative Content | Relative Content | Relative Content | |||||||||
| L-glutamic acid | 55,170,000 | 31,964,333 | * | 30,996,333 | 16,549,000 | ** | 55,170,000 | 30,996,333 | * | 31,964,333 | 16,549,000 | ** |
| L-proline | 29,087,667 | 10,533,167 | ** | 17,831,333 | 13,705,000 | ** | 29,087,667 | 17,831,333 | ** | 10,533,167 | 13,705,000 | ** |
| L-ornithine | 80,612 | 17,967 | * | 326,013 | 9045 | ** | 80,612 | 326,013 | ** | 17,967 | 9045 | * |
| L-arginine | 7,828,300 | 2,859,900 | ** | 13,208,000 | 2,840,367 | ** | 7,828,300 | 13,208,000 | * | 2,859,900 | 2,840,367 | |
| γ-Aminobutyric acid | 4,331,533 | 1,907,767 | ** | 1,795,967 | 1,059,767 | ** | 4,331,533 | 1,795,967 | ** | 1,907,767 | 1,059,767 | ** |
| 4-Hydroxy- proline | 223,263 | 243,827 | 168,980 | 202,517 | 223,263 | 168,980 | * | 243,827 | 202,517 | |||
| 5-Aminovaleric acid | 217,463 | 194,113 | 183,020 | 156,257 | 217,463 | 183,020 | 194,113 | 156,257 | ||||
| N-Acetyl-ornithine | 1,656,333 | 410,120 | ** | 3,561,533 | 388,720 | ** | 1,656,333 | 3,561,533 | ** | 410,120 | 388,720 | |
| N-Acetyl-glutamic acid | 268,810 | 165,237 | * | 149,453 | 89,232 | ** | 268,810 | 149,453 | * | 165,237 | 89,232 | ** |
| L-threo-3-Methylaspartate | 4,702,967 | 1,334,233 | ** | 1,754,200 | 466,573 | ** | 4,702,967 | 1,754,200 | ** | 1,334,233 | 466,573 | ** |
| L-Glutamine | 3,387,033 | 1,750,967 | ** | 3,099,633 | 2,480,767 | * | 3,387,033 | 3,099,633 | 1,750,967 | 2,480,767 | * | |
| α-Ketoglutaric acid | 381,923 | 320,593 | 384,210 | 276,077 | * | 381,923 | 384,210 | 320,593 | 276,077 | |||
| Isocitric Acid | 9,293,833 | 9,384,800 | 10,395,433 | 11,808,667 | 9,293,833 | 10,395,433 | 9,384,800 | 11,808,667 | ||||
| Citric Acid | 31,133,667 | 32,903,333 | * | 36,366,667 | 34,530,333 | 9,293,833 | 36,366,667 | ** | 32,903,333 | 34,530,333 | * | |
| Succinic Acid | 43,160,333 | 39,516,000 | 29,892,000 | 7,596,200 | ** | 43,160,333 | 29,892,000 | * | 39,516,000 | 7,596,200 | ** | |
| Agmatine | 45,548 | 33,690 | * | 44,307 | 49,773 | 45,548 | 44,307 | 33,690 | 49,773 | * | ||
| Guanidinoacetate | 9798 | 8694 | 10,489 | 7968 | 9798 | 10,489 | 8694 | 7968 | ||||
| 4-Guanidinobutanal | 18,032,667 | 7,821,300 | * | 10,096,567 | 5,234,500 | ** | 18,032,667 | 10,096,567 | * | 7,821,300 | 5,234,500 | * |
| 4-Guanidinobutyric acid | 1,022,693 | 1,005,407 | 1,186,733 | 1,078,267 | 1,022,693 | 1,186,733 | 1,005,407 | 1,078,267 | ||||
| 4-Acetamidobutyric acid | 34,808 | 27,498 | 82,552 | 69,309 | 34,808 | 82,552 | 27,498 | 69,309 | ||||
| Dihydroxyacetone phosphate | 76,280 | 110,893 | 122,330 | 9 | ** | 76,280 | 122,330 | 110,893 | 9 | ** | ||
| D-Fructose 6-Phosphate | 5,871,767 | 6,243,100 | 4,351,700 | 18,964 | ** | 5,871,767 | 4,351,700 | 6,243,100 | 18,964 | ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Qian, L.; Chi, Z.; Jia, X.; Qi, F.; Yuan, F.; Liu, Z.; Zheng, Y. Combined Metabolomic and Quantitative RT-PCR Analyses Revealed the Synthetic Differences of 2-Acetyl-1-pyrroline in Aromatic and Non-Aromatic Vegetable Soybeans. Int. J. Mol. Sci. 2022, 23, 14529. https://doi.org/10.3390/ijms232314529
Zhao M, Qian L, Chi Z, Jia X, Qi F, Yuan F, Liu Z, Zheng Y. Combined Metabolomic and Quantitative RT-PCR Analyses Revealed the Synthetic Differences of 2-Acetyl-1-pyrroline in Aromatic and Non-Aromatic Vegetable Soybeans. International Journal of Molecular Sciences. 2022; 23(23):14529. https://doi.org/10.3390/ijms232314529
Chicago/Turabian StyleZhao, Man, Linlin Qian, Zhuoyu Chi, Xiaoli Jia, Fengjie Qi, Fengjie Yuan, Zhiqiang Liu, and Yuguo Zheng. 2022. "Combined Metabolomic and Quantitative RT-PCR Analyses Revealed the Synthetic Differences of 2-Acetyl-1-pyrroline in Aromatic and Non-Aromatic Vegetable Soybeans" International Journal of Molecular Sciences 23, no. 23: 14529. https://doi.org/10.3390/ijms232314529
APA StyleZhao, M., Qian, L., Chi, Z., Jia, X., Qi, F., Yuan, F., Liu, Z., & Zheng, Y. (2022). Combined Metabolomic and Quantitative RT-PCR Analyses Revealed the Synthetic Differences of 2-Acetyl-1-pyrroline in Aromatic and Non-Aromatic Vegetable Soybeans. International Journal of Molecular Sciences, 23(23), 14529. https://doi.org/10.3390/ijms232314529






