Comparative Seeds Storage Transcriptome Analysis of Astronium fraxinifolium Schott, a Threatened Tree Species from Brazil
Abstract
1. Introduction
2. Results
2.1. Effects on Physiological Indexes during A. fraxinifolium Seed Treatment
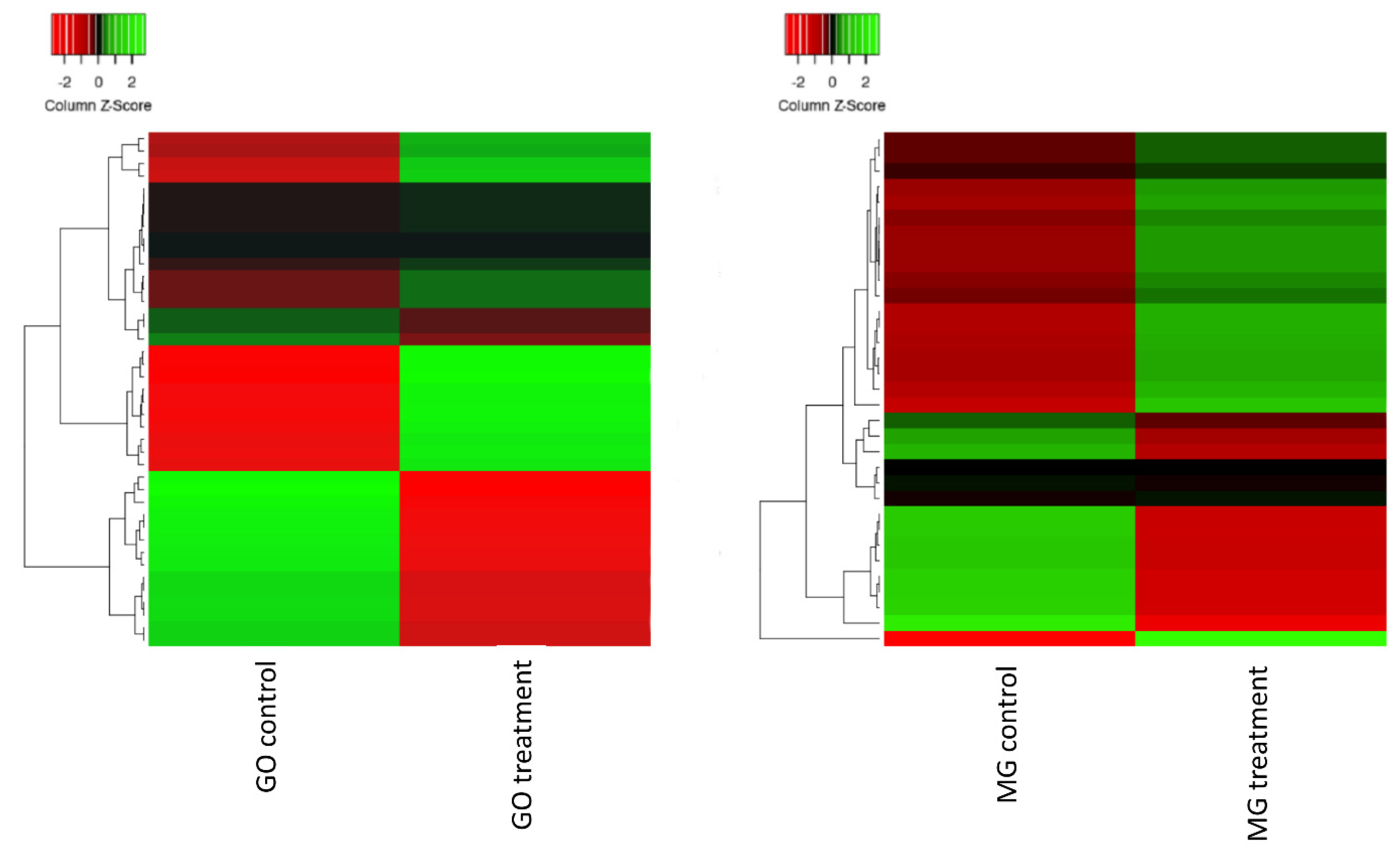
2.2. Sequencing Transcriptome Profile
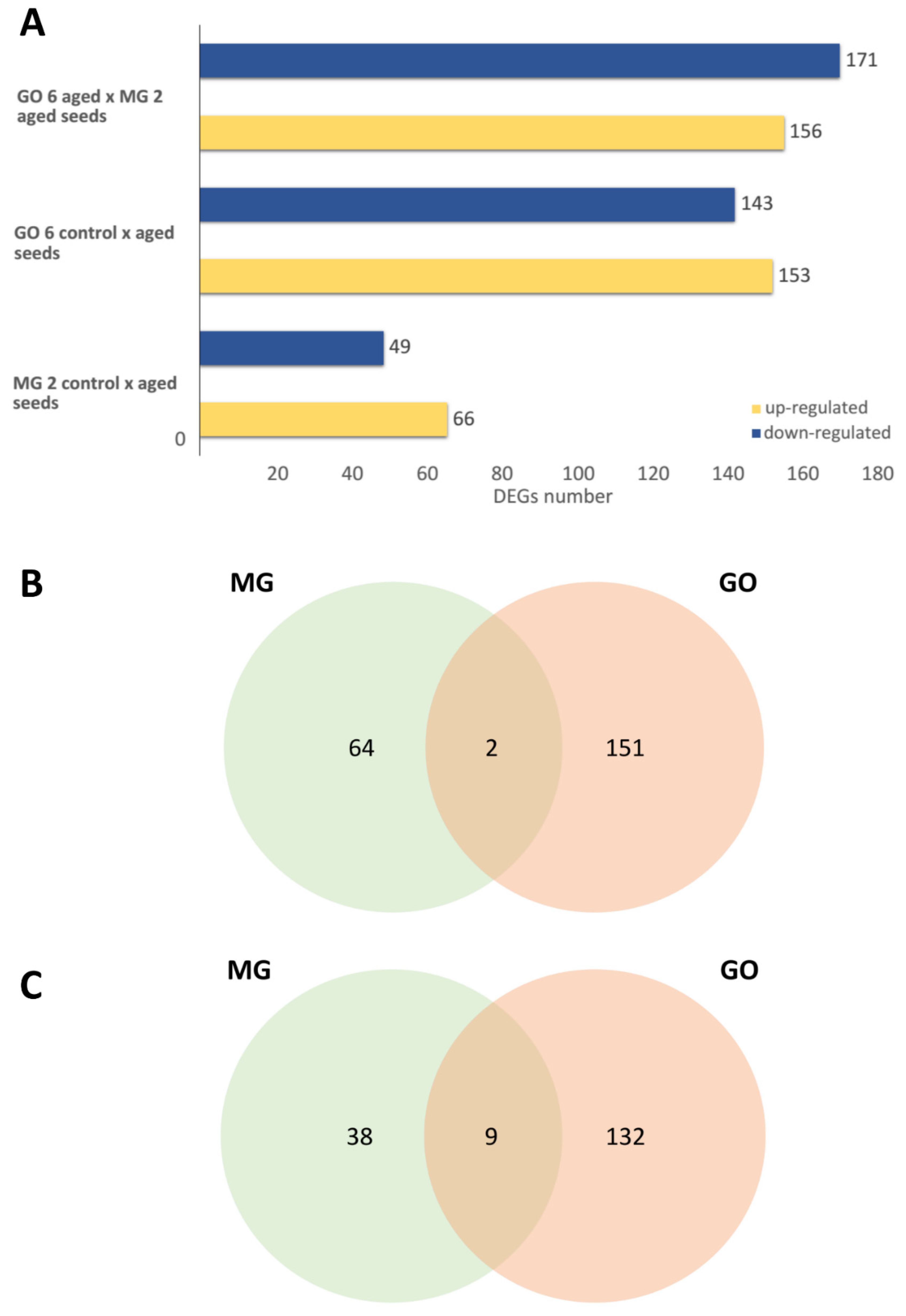
2.3. Comparison of Seed Longevity and Identification of Differentially Expressed Genes
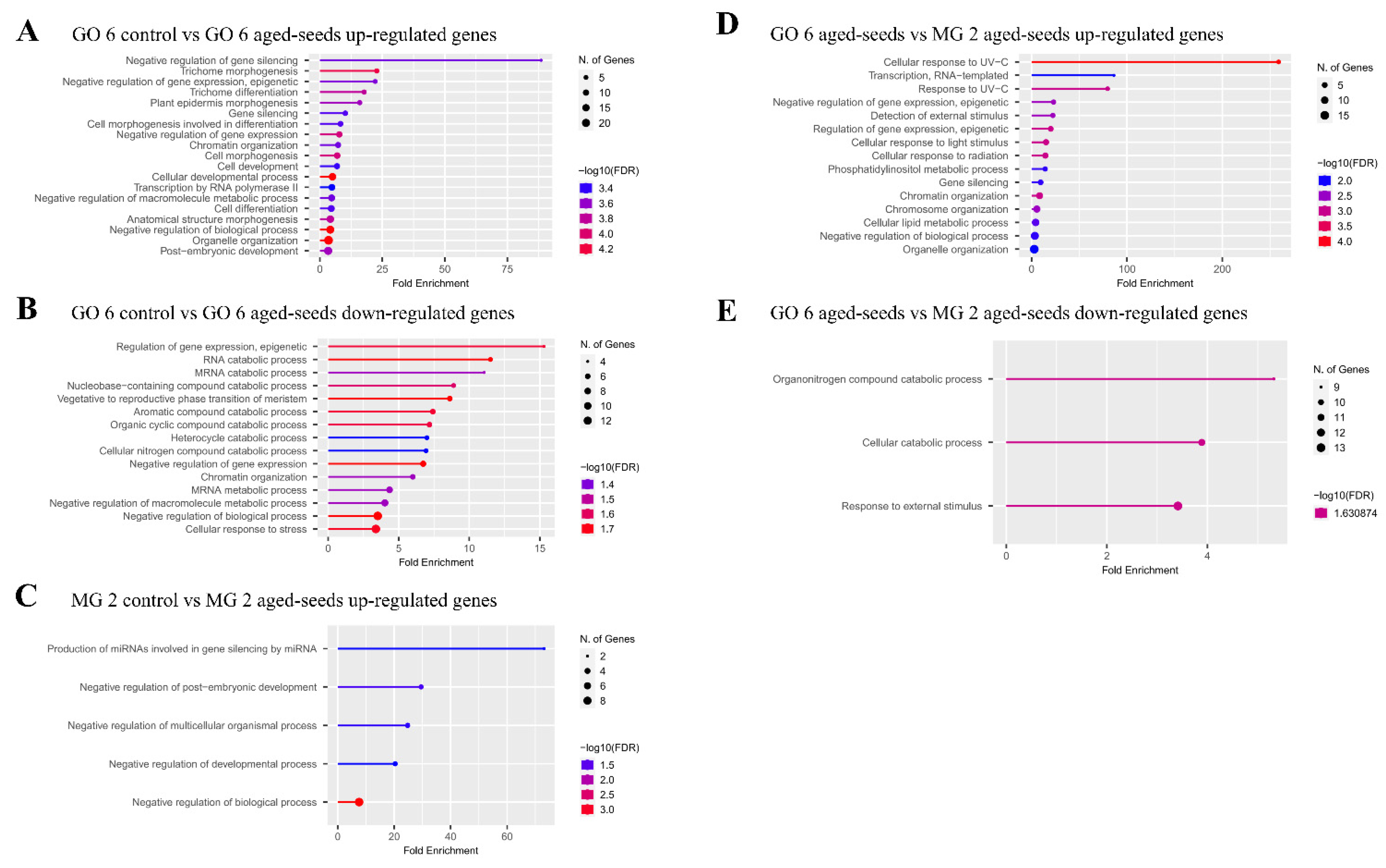
2.4. Identification of Transcription Factors and Related Transcription-Mediated Complex
3. Discussion
4. Materials and Methods
4.1. Sampling
4.2. Artificial Aging Treatment
4.3. Library Construction and Transcriptome Sequencing
4.4. De Novo Assembly, Functional Annotation and Differential Gene Expression
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The Ecophysiology of Seed Persistence: A Mechanistic View of the Journey to Germination or Demise: The Ecophysiology of Seed Persistence. Biol. Rev. 2015, 90, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Kijowska-Oberc, J.; Staszak, A.M.; Ratajczak, E. Climate change affects seed aging? Initiation mechanism and consequences of loss of forest tree seed viability. Trees 2021, 35, 1099–1108. [Google Scholar] [CrossRef]
- Walters, C.; Pence, V.C. The Unique Role of Seed Banking and Cryobiotechnologies in Plant Conservation. Plants People Planet 2021, 3, 83–91. [Google Scholar] [CrossRef]
- Donà, M.; Balestrazzi, A.; Mondoni, A.; Rossi, G.; Ventura, L.; Buttafava, A.; Macovei, A.; Sabatini, M.E.; Valassi, A.; Carbonera, D. DNA Profiling, Telomere Analysis and Antioxidant Properties as Tools for Monitoring Ex Situ Seed Longevity. Ann. Bot. 2013, 111, 987–998. [Google Scholar] [CrossRef]
- Faiad, M.G.R.; Goedert, C.O.; Wetzel, M.M.V.S.; Silva, D.S.; Pereira Neto, L. Banco de Germoplasma de Sementes da Embrapa; (Embrapa Recursos Genéticos e Biotecnologia. Documentos 71); Embrapa: Brasilia, Brazil, 2001. [Google Scholar]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the Fate of MRNA in Aging Seeds: Protection, Destruction, or Slow Decay? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.B.; Hill, L.M.; Walters, C. The Kinetics of Ageing in Dry-Stored Seeds: A Comparison of Viability Loss and RNA Degradation in Unique Legacy Seed Collections. Ann. Bot. 2019, 123, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Cheng, S.; Chen, Z.; Nie, G.; Xu, F.; Zhang, J.; Zhou, M.; Zhang, W.; Liao, Y.; Ye, J. Comparative Transcriptome Analysis Revealing the Potential Mechanism of Seed Germination Stimulated by Exogenous Gibberellin in Fraxinus hupehensis. BMC Plant Biol. 2019, 19, 199. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Li, X.; Peng, Y.; Zhang, H.; Hey, S.; Wang, G.; Wang, J.; Gu, R. Comparative Analysis of the Accelerated Aged Seed Transcriptome Profiles of Two Maize Chromosome Segment Substitution Lines. PLoS ONE 2019, 14, e0216977. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Walters, C. Understanding the Mechanisms and Kinetics of Seed Aging. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Surki, A.A.; de Vos, R.C.H.; Kodde, J. Seed Storage at Elevated Partial Pressure of Oxygen, a Fast Method for Analysing Seed Ageing under Dry Conditions. Ann. Bot. 2012, 110, 1149–1159. [Google Scholar] [CrossRef]
- Niñoles, R.; Planes, D.; Arjona, P.; Ruiz-Pastor, C.; Chazarra, R.; Renard, J.; Bueso, E.; Forment, J.; Serrano, R.; Kranner, I.; et al. Comparative analysis of wild-type accessions reveals novel determinants of Arabidopsis seed longevity. Plant Cell Environ. 2022, 45, 2708–2728. [Google Scholar] [CrossRef] [PubMed]
- Delouche, J.C. Germinação, deterioração e vigor de sementes. Seed News 2002, 24–31. [Google Scholar]
- Mcdonald, M.B. Seed Deterioration: Physiology, Repair and Assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Hu, D.; Ma, G.; Wang, Q.; Yao, J.; Wang, Y.; Pritchard, H.W.; Wang, X. Spatial and Temporal Nature of Reactive Oxygen Species Production and Programmed Cell Death in Elm ( Ulmus pumila L.) Seeds during Controlled Deterioration: Reactive Oxygen Species Production and Programmed Cell Death in Elm ( Ulmus pumila L.) Seeds. Plant Cell Environ. 2012, 35, 2045–2059. [Google Scholar] [CrossRef]
- Corbineau, F. Markers of Seed Quality: From Present to Future. Seed Sci. Res. 2012, 22, S61–S68. [Google Scholar] [CrossRef]
- Leprince, O.; Buitink, J. Desiccation Tolerance: From Genomics to the Field. Plant Sci. 2010, 179, 554–564. [Google Scholar] [CrossRef]
- Powell, A.A.; Matthews, S. Seed Aging/Repair Hypothesis Leads to New Testing Methods. Seed Technol. 2012, 34, 15–25. [Google Scholar]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-Wide Characterization of Seed Aging in Arabidopsis: A Comparison between Artificial and Natural Aging Protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef]
- Tetreault, H.; Fleming, M.; Hill, L.; Dorr, E.; Yeater, K.; Richards, C.; Walters, C. A Power Analysis for Detecting Aging of Dry-stored Soybean Seeds: Germination versus RNA Integrity Assessments. Crop Sci. 2022, csc2.20821. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Tang, Y.; Jiang, L.; He, W.; Lin, Q.; Yu, F.; Wang, L. Transcriptome-Wide Characterization of Seed Aging in Rice: Identification of Specific Long-Lived MRNAs for Seed Longevity. Front. Plant Sci. 2022, 13, 857390. [Google Scholar] [CrossRef]
- Chen, H.; Osuna, D.; Colville, L.; Lorenzo, O.; Graeber, K.; Küster, H.; Leubner-Metzger, G.; Kranner, I. Transcriptome-Wide Mapping of Pea Seed Ageing Reveals a Pivotal Role for Genes Related to Oxidative Stress and Programmed Cell Death. PLoS ONE 2013, 8, e78471. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Ma, X.; Jia, S.; Li, M.; Mao, P. Transcriptomic Analysis Reveals the Changes of Energy Production and AsA-GSH Cycle in Oat Embryos during Seed Ageing. Plant Physiol. Biochem. 2020, 153, 40–52. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Árvores Do Brasil Central: Espécies da Região Geoeconômica de Brasília/IBGE, Diretoria de Geociências; IBGE, DGC: Rio de Janeiro, Brazil, 2002; Volume 3, ISBN 85-240-0889-X. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas Do Brasil; 6; Instituto Plantarum de Estudos da Flora: Sao Paulo, Brazil, 2014; ISBN 85-86714-51-8. [Google Scholar]
- Salomão, A.N.; SILVA, J.A. Reserva Genética Florestal Tamanduá; Embrapa Recursos Genéticos e Biotecnologia: Brasilia, Brazil, 2006. [Google Scholar]
- Aguiar, A.V.D.; Bortolozo, F.R.; Moraes, M.L.T.; Sá, M.E. Determination of Genetic Parameters in a Astronium fraxinifolium Population by Seed Physiological Characteristics. Sci. For. 2001, 60, 88–97. [Google Scholar]
- IBAMA—Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis Lista Oficial de Flora Ameaçada de Extinção. Instrução Normativa; n. 6 2008.
- Inglis, P.W.; Ciampi, A.Y.; Salomão, A.N.; Costa, T.D.S.A.; Azevedo, V.C.R. Expression of Stress-Related Genes in Zebrawood (Astronium fraxinifolium, Anacardiaceae) Seedlings Following Germination in Microgravity. Genet. Mol. Biol. 2014, 37, 81–92. [Google Scholar] [CrossRef]
- Fu, Y.-B.; Ahmed, Z.; Diederichsen, A. Towards a Better Monitoring of Seed Ageing under Ex Situ Seed Conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef]
- Cornacini, M.R.; Alcantara, M.A.M.; Silva, J.R.D.; Corrêa, A.J.M.; Cambuim, J.; Manoel, R.; Alves, P.F.; Rossini, B.C.; Aguiar, A.V.D.; Moraes, M.L.T.; et al. Florescimento em teste de procedência e progênies de Astronium fraxinifolium schott (anacardiaceae) em três eventos reprodutivos. In A Produção do Conhecimento na Engenharia Florestal; Atena Editora: Ponta Grossa, Brazil, 2020; pp. 82–91. ISBN 9786557065006. [Google Scholar]
- Zhao, L.; Wang, S.; Fu, Y.-B.; Wang, H. Arabidopsis Seed Stored MRNAs Are Degraded Constantly over Aging Time, as Revealed by New Quantification Methods. Front. Plant Sci. 2020, 10, 1764. [Google Scholar] [CrossRef]
- Kijak, H.; Ratajczak, E. What Do We Know About the Genetic Basis of Seed Desiccation Tolerance and Longevity? Int. J. Mol. Sci. 2020, 21, 3612. [Google Scholar] [CrossRef]
- Saighani, K.; Kondo, D.; Sano, N.; Murata, K.; Yamada, T.; Kanekatsu, M. Correlation between Seed Longevity and RNA Integrity in the Embryos of Rice Seeds. Plant Biotechnol. 2021, 38, 277–283. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef]
- Ooms, J.; Leon-Kloosterziel, K.M.; Bartels, D.; Koornneef, M.; Karssen, C.M. Acquisition of Desiccation Tolerance and Longevity in Seeds of Arabidopsis thaliana (A Comparative Study Using Abscisic Acid-Insensitive Abi3 Mutants). Plant Physiol. 1993, 102, 1185–1191. [Google Scholar] [CrossRef]
- Sachetto-Martins, G.; Franco, L.O.; de Oliveira, D.E. Plant Glycine-Rich Proteins: A Family or Just Proteins with a Common Motif? Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2000, 1492, 1–14. [Google Scholar] [CrossRef]
- Aneeta; Sanan-Mishra, N.; Tuteja, N.; Kumar Sopory, S. Salinity- and ABA-Induced up-Regulation and Light-Mediated Modulation of MRNA Encoding Glycine-Rich RNA-Binding Protein from Sorghum Bicolor. Biochem. Biophys. Res. Commun. 2002, 296, 1063–1068. [Google Scholar] [CrossRef]
- Czolpinska, M.; Rurek, M. Plant Glycine-Rich Proteins in Stress Response: An Emerging, Still Prospective Story. Front. Plant Sci. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC Transporters. Arab. Book 2011, 9, e0153. [Google Scholar] [CrossRef]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L.; Martinoia, E.; Lee, Y. Abscisic Acid Transporters Cooperate to Control Seed Germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic Acid Signaling in Seeds and Seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, J.; Gao, X.; Tong, J.; Xiao, L.; Li, W.; Zhang, H. The Arabidopsis AP2/ERF Transcription Factor RAP2.6 Participates in ABA, Salt and Osmotic Stress Responses. Gene 2010, 457, 1–12. [Google Scholar] [CrossRef]
- Ali, M.A.; Abbas, A.; Kreil, D.P.; Bohlmann, H. Overexpression of the Transcription Factor RAP2.6 Leads to Enhanced Callose Deposition in Syncytia and Enhanced Resistance against the Beet Cyst Nematode Heterodera Schachtiiin Arabidopsis Roots. BMC Plant Biol. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ye, J.; Guo, X.; Chang, H.; Yuan, C.; Wang, Y.; Hu, S.; Liu, X.; Li, X. Arabidopsis Casein Kinase 1-like 2 Involved in Abscisic Acid Signal Transduction Pathways. J. Plant Interact. 2014, 9, 19–25. [Google Scholar] [CrossRef]
- Kriechbaumer, V.; Maneta-Peyret, L.; Fouillen, L.; Botchway, S.W.; Upson, J.; Hughes, L.; Richardson, J.; Kittelmann, M.; Moreau, P.; Hawes, C. The Odd One out: Arabidopsis Reticulon 20 Does Not Bend ER Membranes but Has a Role in Lipid Regulation. Sci. Rep. 2018, 8, 2310. [Google Scholar] [CrossRef]
- Wang, H.; Nagegowda, D.A.; Rawat, R.; Bouvier-Navé, P.; Guo, D.; Bach, T.J.; Chye, M.-L. Overexpression of Brassica juncea Wild-Type and Mutant HMG-CoA Synthase 1 in Arabidopsis up-Regulates Genes in Sterol Biosynthesis and Enhances Sterol Production and Stress Tolerance: HMGS-OEs Overaccumulate Sterols. Plant Biotechnol. J. 2012, 10, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fan, J.; Zhou, C.; Xu, C. Sterols Are Required for the Coordinated Assembly of Lipid Droplets in Developing Seeds. Nat. Commun. 2021, 12, 5598. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H. Multifaceted Roles of FHY3 and FAR1 in Light Signaling and Beyond. Trends Plant Sci. 2015, 20, 453–461. [Google Scholar] [CrossRef]
- Siddiqui, H.; Khan, S.; Rhodes, B.M.; Devlin, P.F. FHY3 and FAR1 Act Downstream of Light Stable Phytochromes. Front. Plant Sci. 2016, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Dapena, P.; Castaño, R.; Almoguera, C.; Jordano, J. Improved Resistance to Controlled Deterioration in Transgenic Seeds. Plant Physiol. 2006, 142, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Rosnoblet, C.; Aubry, C.; Leprince, O.; Vu, B.L.; Rogniaux, H.; Buitink, J. The Regulatory Gamma Subunit SNF4b of the Sucrose Non-Fermenting-Related Kinase Complex Is Involved in Longevity and Stachyose Accumulation during Maturation of Medicago Truncatula Seeds: MtSNF4b Is Involved in Seed Longevity and Stachyose Content. Plant J. 2007, 51, 47–59. [Google Scholar] [CrossRef]
- Hundertmark, M.; Buitink, J.; Leprince, O.; Hincha, D.K. The Reduction of Seed-Specific Dehydrins Reduces Seed Longevity in Arabidopsis thaliana. Seed Sci. Res. 2011, 21, 165–173. [Google Scholar] [CrossRef]
- Righetti, K.; Vu, J.L.; Pelletier, S.; Vu, B.L.; Glaab, E.; Lalanne, D.; Pasha, A.; Patel, R.V.; Provart, N.J.; Verdier, J.; et al. Inference of Longevity-Related Genes from a Robust Coexpression Network of Seed Maturation Identifies Regulators Linking Seed Storability to Biotic Defense-Related Pathways. Plant Cell 2015, 27, 2692–2708. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Oya, T. Tolerância à Seca Em Plantas: Mecanismos Fisiológicos e Moleculares. Biotecnologia Cienc. Desenvolv. 2001, 23, 12–18. [Google Scholar]
- Renard, J.; Niñoles, R.; Martínez-Almonacid, I.; Gayubas, B.; Mateos-Fernández, R.; Bissoli, G.; Bueso, E.; Serrano, R.; Gadea, J. Identification of Novel Seed Longevity Genes Related to Oxidative Stress and Seed Coat by Genome-wide Association Studies and Reverse Genetics. Plant Cell Environ. 2020, 43, 2523–2539. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chu, P.; Zhou, Y.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.T.; Jiang, L.; Wu, K.; Huang, S. Overexpression of AtOGG1, a DNA Glycosylase/AP Lyase, Enhances Seed Longevity and Abiotic Stress Tolerance in Arabidopsis. J. Exp. Bot. 2012, 63, 4107–4121. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Active Oxygen Species and Antioxidants in Seed Biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative Signaling in Seed Germination and Dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Birtić, S.; Anderson, K.M.; Pritchard, H.W. Glutathione Half-Cell Reduction Potential: A Universal Stress Marker and Modulator of Programmed Cell Death? Free. Radic. Biol. Med. 2006, 40, 2155–2165. [Google Scholar] [CrossRef]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E Is Essential for Seed Longevity and for Preventing Lipid Peroxidation during Germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef]
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the Testa on Seed Dormancy, Germination, and Longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed Longevity: Survival and Maintenance of High Germination Ability of Dry Seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Criqui, M.C.; de Almeida Engler, J.; Camasses, A.; Capron, A.; Parmentier, Y.; Inzé, D.; Genschik, P. Molecular Characterization of Plant Ubiquitin-Conjugating Enzymes Belonging to the UbcP4/E2-C/UBCx/UbcH10 Gene Family. Plant Physiol. 2002, 130, 1230–1240. [Google Scholar] [CrossRef]
- Bueso, E.; Ibañez, C.; Sayas, E.; Muñoz-Bertomeu, J.; Gonzalez-Guzmán, M.; Rodriguez, P.L.; Serrano, R. A Forward Genetic Approach in Arabidopsis thaliana Identifies a RING-Type Ubiquitin Ligase as a Novel Determinant of Seed Longevity. Plant Sci. 2014, 215–216, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, A.; Goslin, K.; Graciet, E. Ubiquitylation in Plants: Signaling Hub for the Integration of Environmental Signals. J. Exp. Bot. 2018, 69, 4511–4527. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, X.; Qi, X.; Fu, X.; Ghimire, S.; Ma, R.; Li, S.; Zhang, N.; Si, H. The Ubiquitin Conjugating Enzyme: An Important Ubiquitin Transfer Platform in Ubiquitin-Proteasome System. Int. J. Mol. Sci. 2020, 21, 2894. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, C.; Kim, D.Y.; Huang, Y.; Chatt, E.; He, P.; Vierstra, R.D.; Shan, L. Ubiquitylome Analysis Reveals a Central Role for the Ubiquitin-Proteasome System in Plant Innate Immunity. Plant Physiol. 2021, 185, 1943–1965. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.H.; Osborne, D.J. Function of DNA Synthesis and DNA Repair in the Survival of Embryos during Early Germination and in Dormancy. Seed Sci. Res. 1993, 3, 43–53. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A Plant DNA Ligase Is an Important Determinant of Seed Longevity: Characterization of Arabidopsis DNA Ligase 6. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Footitt, S.; Bray, C.M.; Finch-Savage, W.E.; West, C.E. DNA Damage Checkpoint Kinase ATM Regulates Germination and Maintains Genome Stability in Seeds. Proc. Natl. Acad. Sci. USA 2016, 113, 9647–9652. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Bray, C.M.; West, C.E. Seeds and the Art of Genome Maintenance. Front. Plant Sci. 2019, 10, 706. [Google Scholar] [CrossRef]
- Antonova, S.V.; Boeren, J.; Timmers, H.T.M.; Snel, B. Epigenetics and Transcription Regulation during Eukaryotic Diversification: The Saga of TFIID. Genes Dev. 2019, 33, 888–902. [Google Scholar] [CrossRef]
- Kornberg, R.D. Mediator and the Mechanism of Transcriptional Activation. Trends Biochem. Sci. 2005, 30, 235–239. [Google Scholar] [CrossRef]
- Conaway, R.C.; Conaway, J.W. Function and Regulation of the Mediator Complex. Curr. Opin. Genet. Dev. 2011, 21, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Thakur, J.K. Importance of Mediator Complex in the Regulation and Integration of Diverse Signaling Pathways in Plants. Front. Plant Sci. 2015, 6, 757. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.; Luo, H.; Foerster, A.M.; AbuQamar, S.; Du, H.-N.; Briggs, S.D.; Scheid, O.M.; Mengiste, T. HISTONE MONOUBIQUITINATION1 Interacts with a Subunit of the Mediator Complex and Regulates Defense against Necrotrophic Fungal Pathogens in Arabidopsis. Plant Cell 2009, 21, 1000–1019. [Google Scholar] [CrossRef]
- Pasrija, R.; Thakur, J.K. Tissue Specific Expression Profile of Mediator Genes in Arabidopsis. Plant Signal. Behav. 2013, 8, e23983. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, Z.; Chen, S.; Beachy, R.N. RF2b, a Rice BZIP Transcription Activator, Interacts with RF2a and Is Involved in Symptom Development of Rice Tungro Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 687–692. [Google Scholar] [CrossRef]
- Alonso, R.; Oñate-Sánchez, L.; Weltmeier, F.; Ehlert, A.; Diaz, I.; Dietrich, K.; Vicente-Carbajosa, J.; Dröge-Laser, W. A Pivotal Role of the Basic Leucine Zipper Transcription Factor BZIP53 in the Regulation of Arabidopsis Seed Maturation Gene Expression Based on Heterodimerization and Protein Complex Formation. Plant Cell 2009, 21, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, L.; Wan, L.; Huai, D.; Kang, Y.; Shi, L.; Jiang, H.; Lei, Y.; Liao, B. Genome-Wide Systematic Characterization of BZIP Transcription Factors and Their Expression Profiles during Seed Development and in Response to Salt Stress in Peanut. BMC Genom. 2019, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Qian, J.; Liu, X.; Xu, H.; Zhang, G.; Ren, J.; Wang, L.; Zhang, L.; Yu, H. Comparative Transcriptome Analysis Revealed Candidate Genes Potentially Related to Desiccation Sensitivity of Recalcitrant Quercus variabilis Seeds. Front. Plant Sci. 2021, 12, 717563. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Yao, X.; Wang, K.; Sheng, Y.; Lv, L. De Novo Transcriptome Assembly of the Cotyledon of Camellia oleifera for Discovery of Genes Regulating Seed Germination. BMC Plant Biol. 2022, 22, 265. [Google Scholar] [CrossRef]
- Vlachonasios, K.E.; Thomashow, M.F.; Triezenberg, S.J. Disruption Mutations of ADA2b and GCN5 Transcriptional Adaptor Genes Dramatically Affect Arabidopsis Growth, Development, and Gene Expression. Plant Cell 2003, 15, 626–638. [Google Scholar] [CrossRef]
- Vlachonasios, K.E.; Kaldis, A.; Nikoloudi, A.; Tsementzi, D. The Role of Transcriptional Coactivator ADA2b in Arabidopsis Abiotic Stress Responses. Plant Signal. Behav. 2011, 6, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Wilson, I.W.; Yang, J.; Buerstenbinder, K.; Llewellyn, D.; Dennis, E.S.; Sauter, M.; Dolferus, R. Arabidopsis RAP2.2: An Ethylene Response Transcription Factor That Is Important for Hypoxia Survival. Plant Physiol. 2010, 153, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.V.; Iyer, S.; Amerhauser, C.; Lehmann, M.; van Dongen, J.T.; Geigenberger, P. Oxygen Sensing via the Ethylene Response Transcription Factor RAP2.12 Affects Plant Metabolism and Performance under Both Normoxia and Hypoxia. Plant Physiol. 2016, 172, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Giuntoli, B.; Licausi, F.; van Veen, H.; Perata, P. Functional Balancing of the Hypoxia Regulators RAP2.12 and HRA1 Takes Place in Vivo in Arabidopsis Thaliana Plants. Front. Plant Sci. 2017, 8, 591. [Google Scholar] [CrossRef]
- Choi, Y.; Gehring, M.; Johnson, L.; Hannon, M.; Harada, J.J.; Goldberg, R.B.; Jacobsen, S.E.; Fischer, R.L. DEMETER, a DNA Glycosylase Domain Protein, Is Required for Endosperm Gene Imprinting and Seed Viability in Arabidopsis. Cell 2002, 110, 33–42. [Google Scholar] [CrossRef]
- Park, J.-S.; Frost, J.M.; Park, K.; Ohr, H.; Park, G.T.; Kim, S.; Eom, H.; Lee, I.; Brooks, J.S.; Fischer, R.L.; et al. Control of DEMETER DNA Demethylase Gene Transcription in Male and Female Gamete Companion Cells in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 2078–2083. [Google Scholar] [CrossRef]
- Zhang, C.; Hung, Y.-H.; Rim, H.J.; Zhang, D.; Frost, J.M.; Shin, H.; Jang, H.; Liu, F.; Xiao, W.; Iyer, L.M.; et al. The Catalytic Core of DEMETER Guides Active DNA Demethylation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 17563–17571. [Google Scholar] [CrossRef]
- Galon, Y.; Snir, O.; Fromm, H. How Calmodulin Binding Transcription Activators (CAMTAs) Mediate Auxin Responses. Plant Signal. Behav. 2010, 5, 1311–1314. [Google Scholar] [CrossRef][Green Version]
- Shkolnik, D.; Finkler, A.; Pasmanik-Chor, M.; Fromm, H. Calmodulin-Binding Transcription Activator 6: A Key Regulator of Na+ Homeostasis during Germination. Plant Physiol. 2019, 180, 1101–1118. [Google Scholar] [CrossRef]
- Ali, E.; Raza, M.A.; Cai, M.; Hussain, N.; Shahzad, A.N.; Hussain, M.; Ali, M.; Bukhari, S.A.H.; Sun, P. Calmodulin-Binding Transcription Activator (CAMTA) Genes Family: Genome-Wide Survey and Phylogenetic Analysis in Flax (Linum usitatissimum). PLoS ONE 2020, 15, e0236454. [Google Scholar] [CrossRef]
- Misra, A.; McKnight, T.D.; Mandadi, K.K. Bromodomain Proteins GTE9 and GTE11 Are Essential for Specific BT2-Mediated Sugar and ABA Responses in Arabidopsis Thaliana. Plant Mol. Biol. 2018, 96, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Azhakanandam, S.; Franks, R.G. SEUSS and SEUSS-LIKE Transcriptional Adaptors Regulate Floral and Embryonic Development in Arabidopsis. Plant Physiol. 2010, 152, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Zhang, X.; You, Y.; Lin, L.; Zhou, W.; Li, C. SEUSS Integrates Transcriptional and Epigenetic Control of Root Stem Cell Organizer Specification. EMBO J. 2020, 39, e105047. [Google Scholar] [CrossRef] [PubMed]
- Pereira Neto, L.G.; Sartori, M.M.P.; Toorop, P.E.; Silva, E.A.A. Seed Longevity Differs in Astronium fraxinifolium Schott from Two Geographic Regions in Brazil. Agraria 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved Equations for the Prediction of Seed Longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Ministério da Agricultura. Brasil Regras Para Análise de Sementes, 1st ed.; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2009; ISBN 978-85-99851-70-8. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De Novo Transcript Sequence Reconstruction from RNA-Seq Using the Trinity Platform for Reference Generation and Analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol 2010, 11, R106. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S. Blast2GO: A Comprehensive Suite for Functional Analysis in Plant Genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
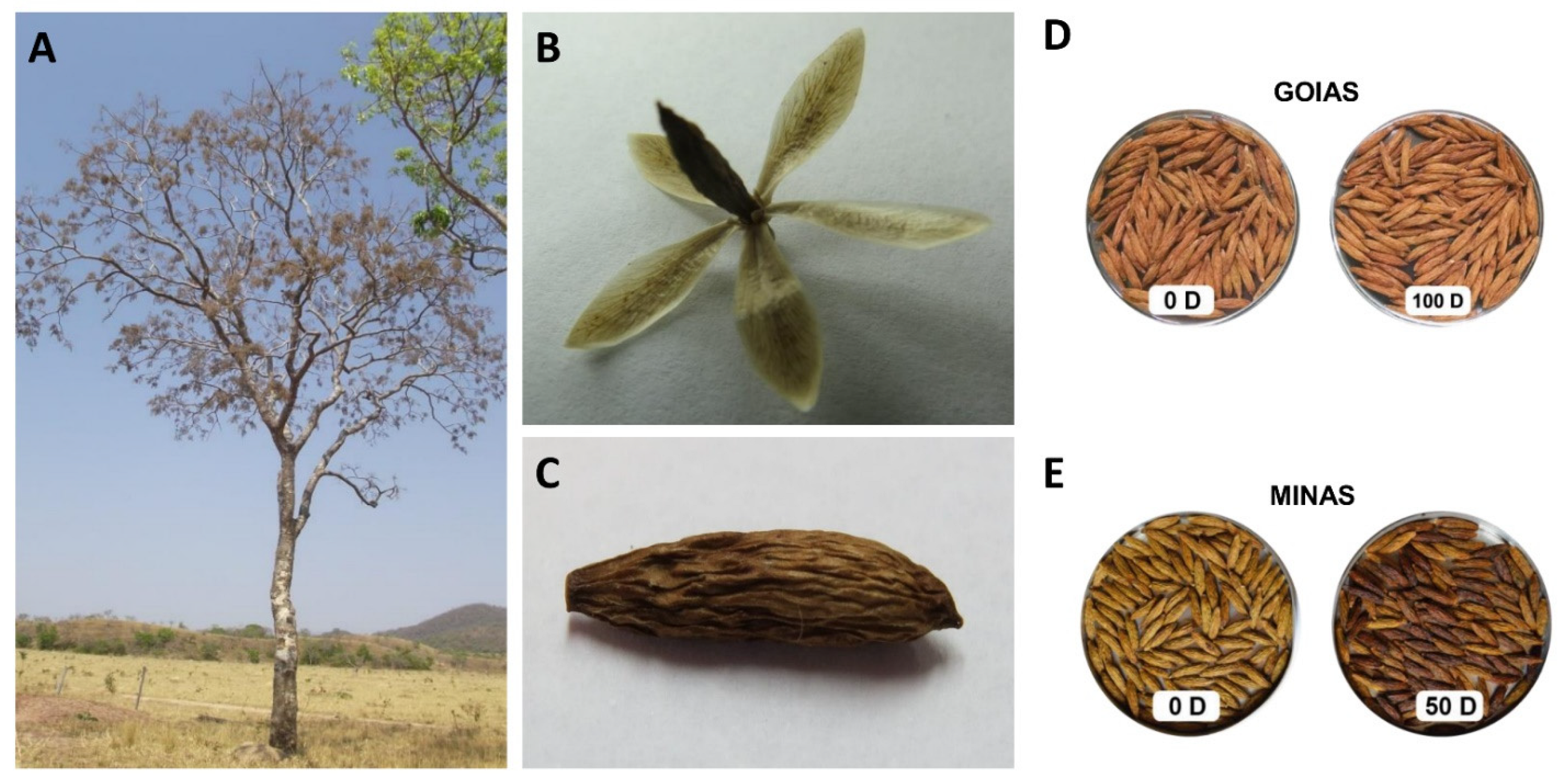

| Relative Humidity | ||||
|---|---|---|---|---|
| 60% | 65% | 70% | ||
| GOIAS | Sigma | 17.65 | 10.97 | 4.82 |
| P50 | 69.50 | 35.30 | 24.50 | |
| MINAS | Sigma | 9.91 | 5.74 | 3.17 |
| P50 | 26.90 | 15.60 | 8.80 | |
| Germination (%) | ||||
| 0 days | 8 days | 18 days | 21 days | |
| GOIAS | 98 | 97 | 96 | 97 |
| MINAS | 93 | 90 | 92 | 81 |
| Treatment | Total Number of Reads | Number of Trimmed Reads | Mapped Reads | % Mapped Reads | Reads not Mapped | % Reads not Mapped |
|---|---|---|---|---|---|---|
| GOIAS control × aged seeds | 73,058,144 | 69,236,758 | 59,600,544 | 86.08 | 9,636,214 | 13.91 |
| MINAS control × aged seeds | 128,874,380 | 121,011,132 | 104,046,698 | 85.98 | 16,964,434 | 14.01 |
| GOIAS aged × MINAS aged seeds | 103,382,264 | 97,804,592 | 85,003,636 | 86.91 | 12,800,956 | 13.08 |
| GOIAS control × MINAS control | 95,814,580 | 90,062,502 | 76,652,780 | 85.11 | 13,409,722 | 14.88 |
| ID | Gene Ontology | Gene Function | |
|---|---|---|---|
| GOIAS up-regulated genes (GOIAS control vs. induced aged seeds) | c20908_g1_i5 | ZINC | PHD finger protein ING1 (Protein INHIBITOR OF GROWTH 1) |
| c26185_g2_i3 | TRANSP. MAGNESIUM | Probable magnesium transporter NIPA6 | |
| c26616_g1_i3 | ASPARTIC | Aspartic proteinase 39 | |
| c27188_g2_i2 | COMP. MEMBRANE | AP3-complex subunit beta-A | |
| c27812_g1_i1 | MICROTUBULE | 65-kDa microtubule-associated protein 6 | |
| c28091_g1_i6 | HELICASE | Pre-mRNA-splicing factor ATP-dependent RNA helicase DEAH7 | |
| c29015_g2_i10 | TRANSCRIPTION | Protein ALWAYS EARLY 3 | |
| c29097_g1_i10 | KINASE | Serine/threonine-protein kinase PBL34 | |
| c29650_g1_i7 | MEMBRANE COMPONENT | DUF21 domain-containing protein At4g14240 | |
| c30095_g2_i20 | AMINOTRANSFERASE | Branched-chain-amino-acid aminotransferase-like protein 2 | |
| c30277_g1_i5 | HELICASE | Probable helicase MAGATAMA 3 | |
| c30355_g2_i6 | DNA POLYMERASE | DNA-directed RNA polymerase III subunit 1 | |
| c30426_g3_i2 | CoA LIGASE | 4-coumarate-CoA ligase-like 9 | |
| c30654_g1_i9 | KINASE | C-type lectin receptor-like tyrosine-protein kinase At1g52310 | |
| c30712_g3_i1 | SUGAR TRANSPORT | Probable sugar phosphate/phosphate translocator At1g06470 | |
| c30834_g1_i8 | NAP1 | Protein NAP1 | |
| c31008_g2_i9 | TPS1 | Alpha,alpha-trehalose-phosphate synthase | |
| c31494_g2_i12 | KINASE | Probable leucine-rich repeat receptor-like protein kinase At5g49770 | |
| MINAS up-regulated genes (MINAS control vs. induced aged seeds) | c12237_g1_i2 | CARBOHYDRATE TRANSPORT | Probable sugar phosphate/phosphate translocator At3g14410 |
| c18835_g1_i2 | NUTRIENT RESERVE | Vicilin-like seed storage protein At2g28490 | |
| c20908_g1_i3 | ZINC TRANSPORT | PHD finger protein ING1 | |
| c21711_g1_i3 | FATTY ACID OXIDATION | Protein HOTHEAD | |
| c22798_g1_i3 | XILAN CATABOLISM | Beta-D-xylosidase 4 | |
| c24339_g1_i1 | LIPID TRANSPORT | Late embryogen esis abundant protein D-29 | |
| c25778_g1_i1 | POLYAMINE TRANSPORT | Probable polyamine transporter At3g19553 | |
| c27644_g1_i4 | TRANSCRIPTION | Transcription factor GTE10 | |
| c27917_g1_i3 | STARCH BIOSYNTHESIS | Granule-bound starch synthase 1, chloroplastic | |
| c28093_g1_i3 | HELICASE | ATP-dependent helicase BRM | |
| c28543_g1_i4 | UBIQUITIN PROTEIN | Protein FIZZY-RELATED 2 | |
| c28665_g1_i2 | TOXIC SUBSTANCES CATABOLISM | Glutathione S-transferase U17 | |
| c29854_g1_i7 | NUCLEAR ORGANIZATION | Protein CROWDED NUCLEI 1 | |
| c30020_g1_i5 | ZINC TRANSPORT | Putative zinc transporter At3g08650 | |
| c30925_g1_i2 | STARCH BIOSYNTHESIS | 4-alpha-glucanotransferase DPE2 | |
| c30946_g1_i1 | UBIQUITIN PROTEIN | Prob. ubiquitin-conjugating enzyme E2 24 | |
| c30965_g1_i3 | TRANSCRIPTION | Two-component response regulator-like APRR5 | |
| c31176_g2_i7 | DNA POLYMERASE II | DNA polymerase epsilon catalytic subunit A | |
| GOIAS down-regulated genes (GOIAS control vs. induced aged seeds) | c20908_g1_i3 | ZINC | PHD finger protein ING1 |
| c21777_g1_i4 | ZINC | Pentatricopeptide repeat-containing protein At1g19720 | |
| c23560_g1_i3 | KINASE | Probable inactive leucine-rich repeat receptor-like protein kinase At1g66830 | |
| c23779_g4_i5 | TRANSCRIPTION | Protein FAR1-RELATED SEQUENCE 5 | |
| c24185_g1_i5 | LYSINE | 4-hydroxy-tetrahydrodipicolinate synthase, chloroplastic | |
| c26084_g1_i5 | KINASE | Casein kinase I isoform delta-like | |
| c26241_g6_i5 | ACID NUCLEIC POLY(A) | Polyadenylate-binding protein RBP47B | |
| c26944_g1_i1 | ATP | Feruloyl esterase A | |
| c27020_g1_i4 | ABA | Glycine-rich domain-containing protein 1 | |
| c27495_g7_i6 | TRANSCRIPTION | Protein RTF1 homolog | |
| c27942_g1_i1 | ACID NUCLEIC CONNECTION | Putative G3BP-like protein | |
| c28093_g1_i5 | HELICASE | ATP-dependent helicase BRM | |
| c28984_g1_i3 | STEROL SYNTHESIS | 3beta-hydroxysteroid dehydrogenase/decarboxylase isoform 1 | |
| c29201_g1_i2 | ETHYLENE | Ethylene-responsive transcription factor RAP2-12 | |
| c29563_g1_i8 | STARCH | Phosphoglucan phosphatase LSF1, chloroplastic | |
| c29870_g1_i3 | ATP | ABC transporter C family member 4 | |
| c29888_g6_i1 | ATP | Endoribonuclease Dicer homolog 1 | |
| c30277_g1_i3 | HELICASE | Probable helicase MAGATAMA 3 | |
| c30555_g2_i7 | RNA POLYMERASE II | Mediator of RNA polymerase II transcription subunit 23 | |
| c30654_g1_i3 | KINASE | C-type lectin receptor-like tyrosine-protein kinase At1g52310 | |
| c30709_g2_i2 | ACID NUCLEIC CONNECTION | Polyribonucleotide nucleotidyltransferase 1, chloroplastic | |
| c31008_g2_i6 | TPS1 | Alpha,alpha-trehalose-phosphate synthase | |
| c31157_g1_i3 | UBIQUITIN PROTEIN | BTB/POZ domain-containing protein At1g04390 | |
| MINAS down-regulated genes (MINAS control vs. induced aged seeds) | c12237_g1_i3 | CARBOHYDRATE TRANSPORT | Prob. sugar phosphate/phosphate translocator At3g14410 |
| c20908_g1_i5 | RNA TRANSCRIPTION | PHD finger protein ING1 (Protein INHIBITOR OF GROWTH 1) | |
| c22798_g1_i2 | XYLAN CATABOLISM | Beta-D-xylosidase 4 | |
| c25158_g2_i2 | MICROTUBULES | Tubulin alpha chain | |
| c26737_g1_i2 | UBIQUITIN PROTEIN | E3 ubiquitin-protein ligase At4g11680 | |
| c27190_g4_i4 | UBIQUITIN PROTEIN | Protein pleiotropic regulatory locus 1 | |
| c28984_g1_i3 | STEROL BIOSYNTHESIS | 3beta-hydroxysteroid dehydrogenase/decarboxylase isoform 1 | |
| c29111_g2_i10 | RNA POLYMERASE | Transcription initiation factor TFIID sub. 2 | |
| c29114_g2_i8 | AMINO ACID TRANSPORT | Cationic amino acid transporter 9, chloroplastic | |
| c29438_g1_i2 | ROOT GROWTH | Boron transporter 1 | |
| c29489_g1_i2 | RNA TRANSCRIPTION | Protein FAR1-RELATED SEQUENCE 5 | |
| c30224_g2_i4 | CYTOSOL | F-box protein At1g78280 | |
| c30545_g1_i5 | CARBOHYDRATE CATABOLISM | Beta-galactosidase 5 (Lactase 5) | |
| c30986_g1_i2 | DNA POLYMERASE | DNA polymerase zeta catalytic subunit | |
| c31176_g2_i8 | DNA POLYMERASE | DNA polymerase epsilon catalytic sub. A |
| GOIAS Control vs. GOIAS Aged-Seeds | ||
|---|---|---|
| id | sprot_Top_BLASTX_hit | Description |
| c30555_g2_i9 | MED23_ARATH | Mediator of RNA polymerase II transcription subunit 23 |
| c29094_g5_i2 | CMTA4_ARATH | Calmodulin-binding transcription activator 4 |
| c29111_g2_i6 | TAF2_ARATH | Transcription initiation factor TFIID subunit 2 |
| c22254_g1_i5 | RF2B_ORYSJ | Transcription factor RF2b |
| c29294_g1_i2 | MED12_ARATH | Mediator of RNA polymerase II transcription subunit 12 |
| c27419_g2_i1 | TAD2B_ARATH | Transcriptional adapter ADA2b |
| c24172_g1_i1 | UNE10_ARATH | Transcription factor UNE10 |
| c31090_g2_i9 | MD37D_ARATH | Probable mediator of RNA polymerase II transcription subunit 37c |
| c29201_g1_i2 | RA212_ARATH | Ethylene-responsive transcription factor RAP2-12 |
| c30555_g2_i7 | MED23_ARATH | Mediator of RNA polymerase II transcription subunit 23 |
| c29246_g1_i1 | DME_ARATH | Transcriptional activator DEMETER |
| c24653_g1_i4 | KELP_ARATH | RNA polymerase II transcriptional coactivator KELP |
| c26529_g2_i3 | MD33A_ARATH | Mediator of RNA polymerase II transcription subunit 33A |
| MINAS Control vs. MINAS Aged-Seeds | ||
| id | sprot_Top_BLASTX_hit | Description |
| c27644_g1_i4 | GTE10_ARATH | Transcription factor GTE10 |
| c29111_g2_i10 | TAF2_ARATH | Transcription initiation factor TFIID subunit 2 |
| GOIAS Aged-Seeds vs. MINAS Aged-Seeds | ||
| id | sprot_Top_BLASTP_hit | Description |
| c29111_g2_i10 | TAF2_ARATH | Transcription initiation factor TFIID subunit 2 |
| c29413_g3_i6 | PUR_ARATH | Transcription factor Pur-alpha 1 |
| c30283_g2_i3 | SEUSS_ARATH | Transcriptional corepressor SEUSS |
| c30495_g3_i3 | UNE12_ARATH | Transcription factor UNE12 |
| c30555_g2_i6 | MED23_ARATH | Mediator of RNA polymerase II transcription subunit 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira Neto, L.G.; Rossini, B.C.; Marino, C.L.; Toorop, P.E.; Silva, E.A.A. Comparative Seeds Storage Transcriptome Analysis of Astronium fraxinifolium Schott, a Threatened Tree Species from Brazil. Int. J. Mol. Sci. 2022, 23, 13852. https://doi.org/10.3390/ijms232213852
Pereira Neto LG, Rossini BC, Marino CL, Toorop PE, Silva EAA. Comparative Seeds Storage Transcriptome Analysis of Astronium fraxinifolium Schott, a Threatened Tree Species from Brazil. International Journal of Molecular Sciences. 2022; 23(22):13852. https://doi.org/10.3390/ijms232213852
Chicago/Turabian StylePereira Neto, Leonel Gonçalves, Bruno Cesar Rossini, Celso Luis Marino, Peter E. Toorop, and Edvaldo Aparecido Amaral Silva. 2022. "Comparative Seeds Storage Transcriptome Analysis of Astronium fraxinifolium Schott, a Threatened Tree Species from Brazil" International Journal of Molecular Sciences 23, no. 22: 13852. https://doi.org/10.3390/ijms232213852
APA StylePereira Neto, L. G., Rossini, B. C., Marino, C. L., Toorop, P. E., & Silva, E. A. A. (2022). Comparative Seeds Storage Transcriptome Analysis of Astronium fraxinifolium Schott, a Threatened Tree Species from Brazil. International Journal of Molecular Sciences, 23(22), 13852. https://doi.org/10.3390/ijms232213852







