Self-Diffusion in Confined Water: A Comparison between the Dynamics of Supercooled Water in Hydrophobic Carbon Nanotubes and Hydrophilic Porous Silica
Abstract
1. Introduction
2. Bulk Water
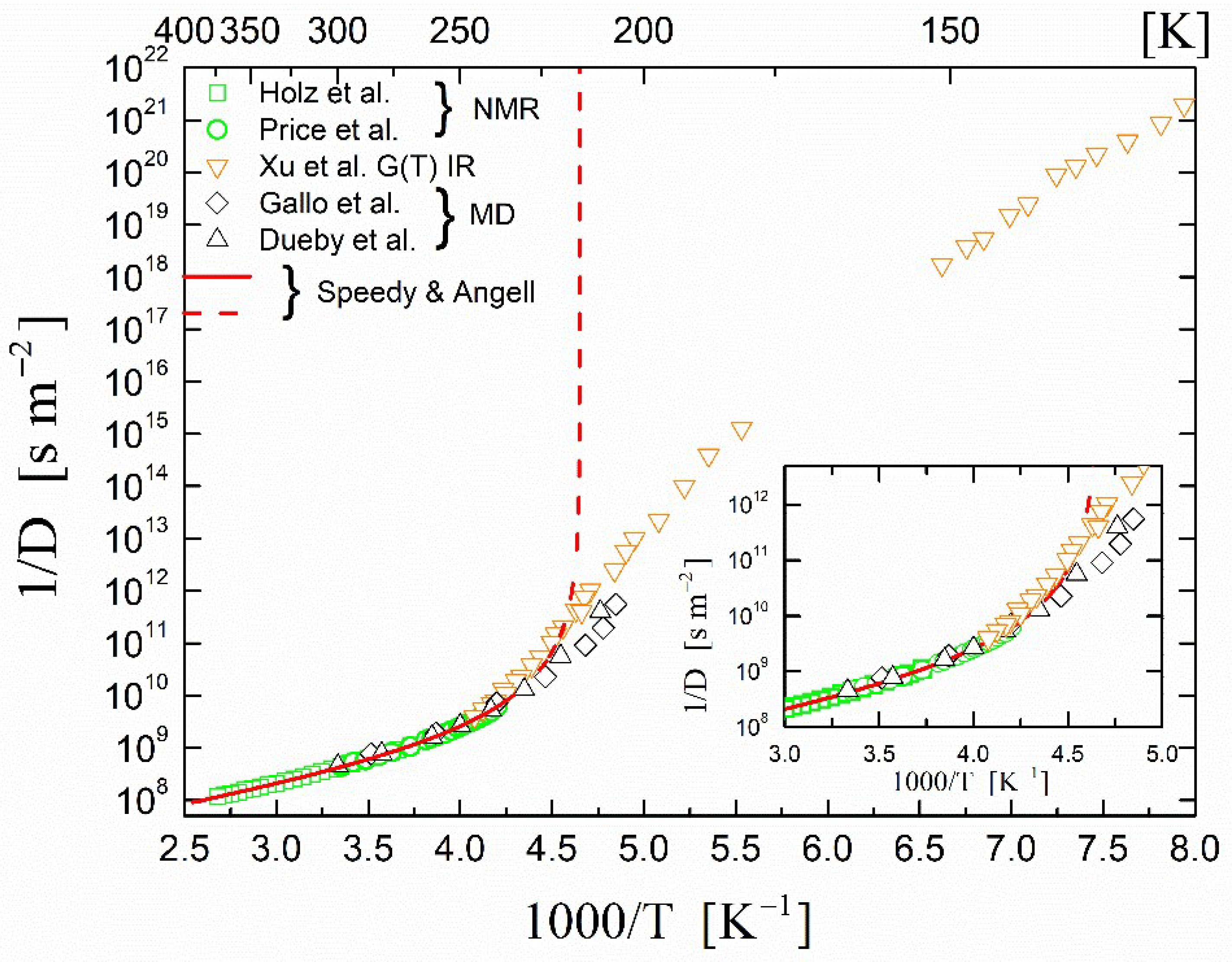
2.1. Self-Diffusion Coefficient
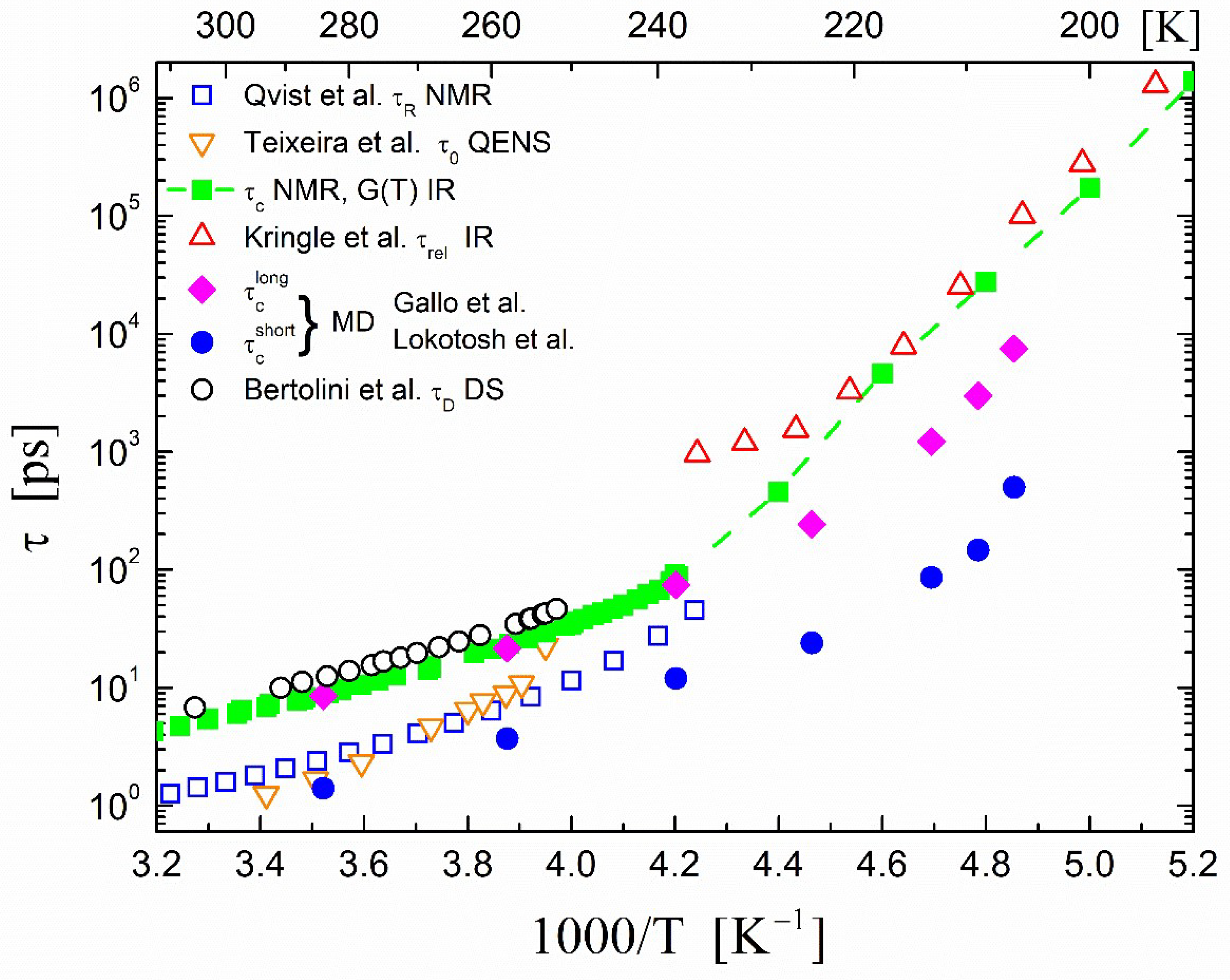
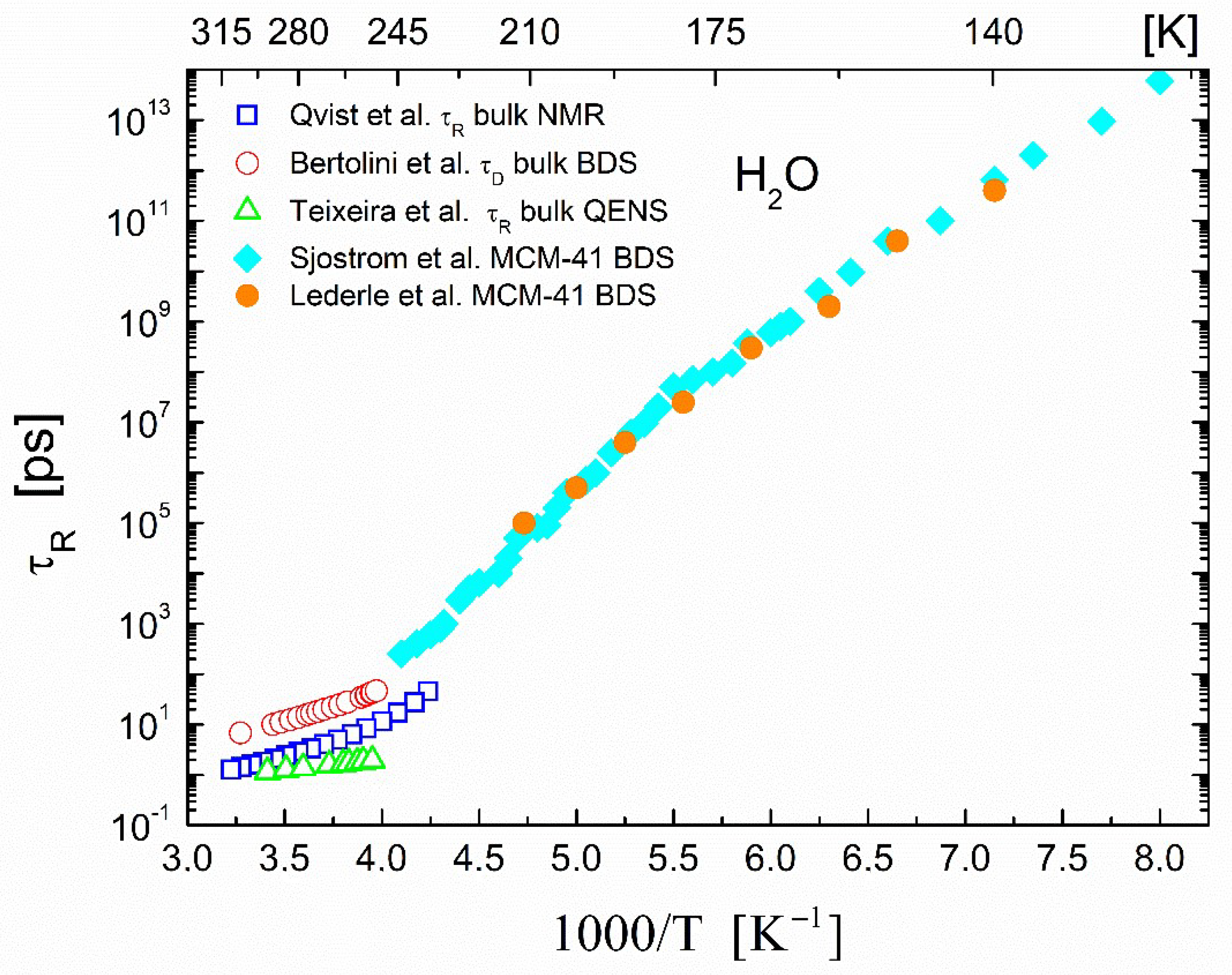
2.2. Correlation Times
3. Confined Water
3.1. Self-Diffusion Coefficients
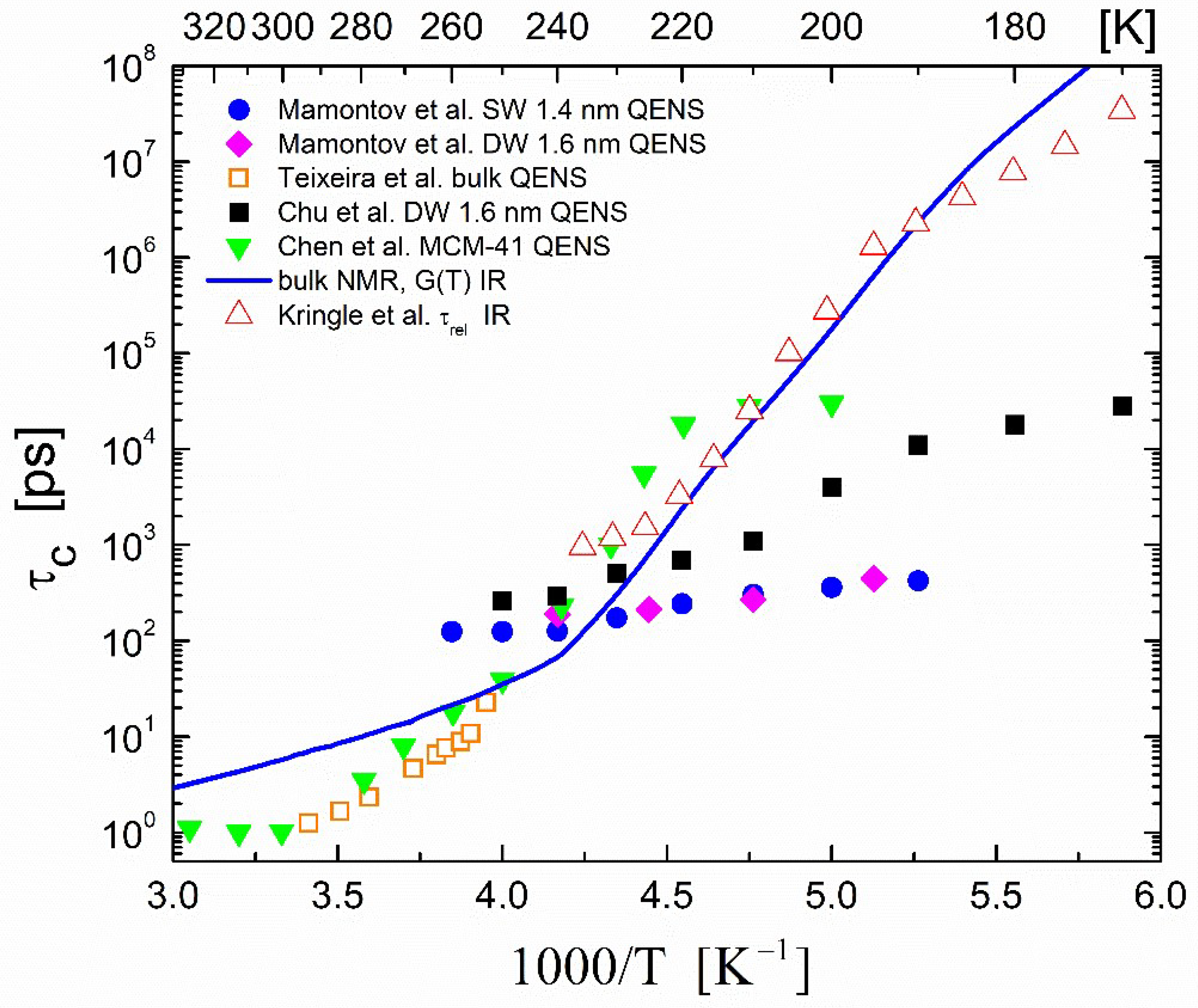
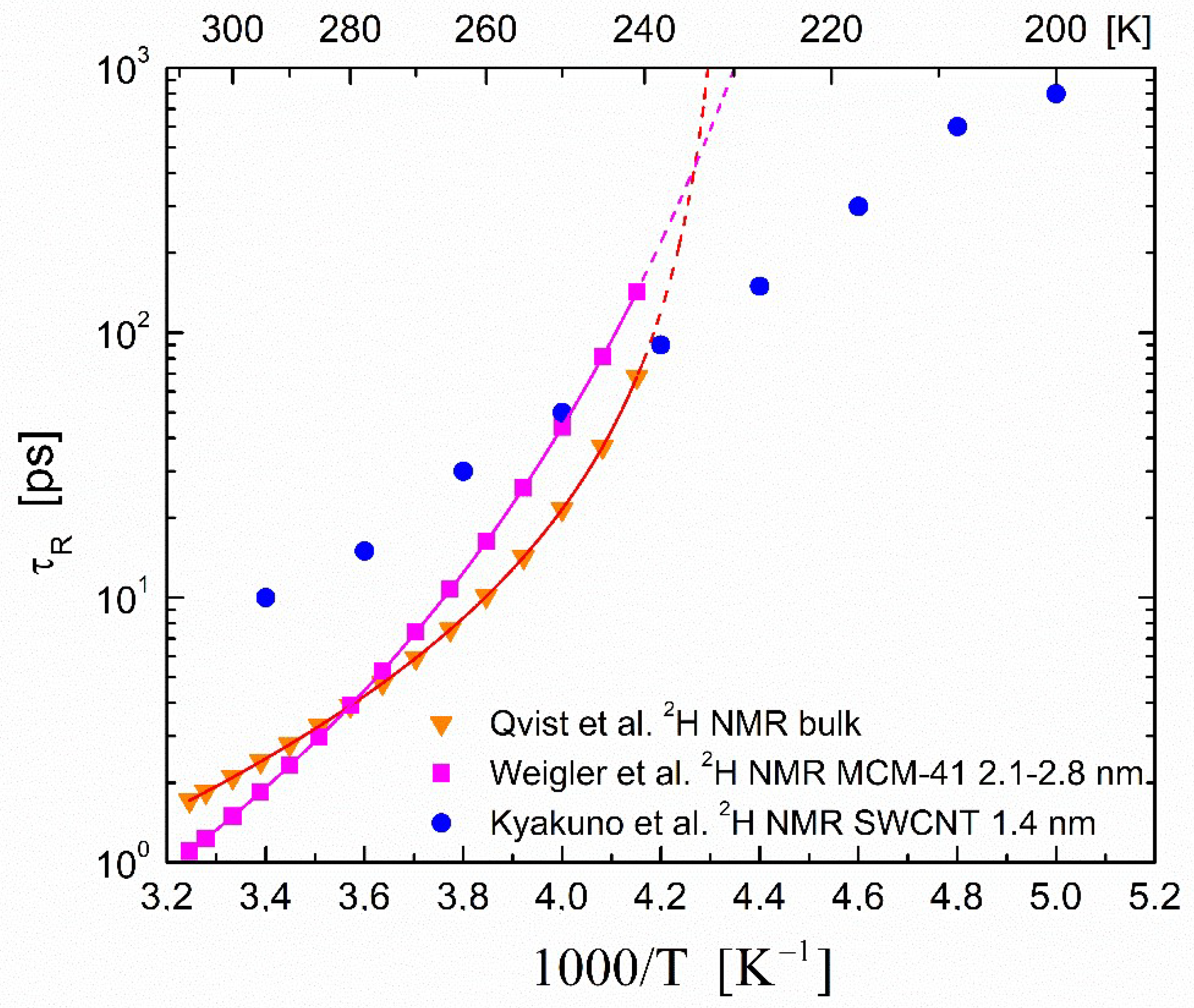
3.2. Correlation Times
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Speedy, R.J.; Angell, C.A. Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at −45 °C. J. Chem. Phys. 1976, 65, 851–858. [Google Scholar] [CrossRef]
- Lang, E.W.; Lüdemann, H.-D. Anomalies of Liquid Water. Angew. Chem. Int. Ed. 1982, 21, 315–329. [Google Scholar] [CrossRef]
- Gallo, P.; Amann-Winkel, K.; Angell, C.A.; Anisimov, M.A.; Caupin, F.; Chakravarty, C.; Lascaris, E.; Loerting, T.; Panagiotopoulos, A.Z.; Russo, J.; et al. Water: A Tale of Two Liquids. Chem. Rev. 2016, 116, 7463–7500. [Google Scholar] [CrossRef]
- Shi, R.; Tanaka, H. The anomalies and criticality of liquid water. Proc. Natl. Acad. Sci. USA 2020, 117, 26591–26599. [Google Scholar] [CrossRef] [PubMed]
- Debenedetti, P.G. Supercooled and glassy water. J. Phys. Condens. Matter 2003, 15, R1669–R1726. [Google Scholar] [CrossRef]
- Mishima, O.; Stanley, H.E. The relationship between liquid, supercooled and glassy water. Nature 1998, 396, 329–335. [Google Scholar] [CrossRef]
- Caupin, F. Escaping the no man’s land: Recent experiments on metastable liquid water. J. Non-Cryst. Solids 2015, 407, 441–448. [Google Scholar] [CrossRef]
- Cerveny, S.; Mallamace, F.; Swenson, J.; Vogel, M.; Xu, L. Confined Water as Model of Supercooled Water. Chem. Rev. 2016, 116, 7608–7625. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Bachler, J.; Bove, L.E.; Böhmer, R.; Camisasca, G.; Coronas, L.E.; Corti, H.R.; Ribeiro, I.D.A.; de Koning, M.; Franzese, G.; et al. Advances in the study of supercooled water. Eur. Phys. J. E 2021, 44, 143. [Google Scholar] [CrossRef]
- Striolo, A. Nano-Confined Water. In Nanomaterials: Design and Simulation; Balbuena, P.B., Seminario, J.M., Eds.; Theoretical and Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; pp. 245–274. ISBN 978-0-444-52826-1. [Google Scholar]
- Vogel, M. NMR studies on simple liquids in confinement. Eur. Phys. J. Spec. Top. 2010, 189, 47–64. [Google Scholar] [CrossRef]
- Stanley, H.; Buldyrev, S.; Kumar, P.; Mallamace, F.; Mazza, M.; Stokely, K.; Xu, L.; Franzese, G. Water in nanoconfined and biological environments. J. Non-Cryst. Solids 2011, 357, 629–640. [Google Scholar] [CrossRef]
- Richert, R. Dynamics of Nanoconfined Supercooled Liquids. Annu. Rev. Phys. Chem. 2011, 62, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J. Recent experimental aspects of the structure and dynamics of liquid and supercooled water. Mol. Phys. 2012, 110, 249–258. [Google Scholar] [CrossRef]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Chopra, N.; Andrews, R.; Hinds, B.J. Enhanced flow in carbon nanotubes. Nature 2005, 438, 44. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast Mass Transport Through Sub-2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Whitby, M.; Quirke, N. Fluid flow in carbon nanotubes and nanopipes. Nat. Nanotechnol. 2007, 2, 87–94. [Google Scholar] [CrossRef]
- Joseph, S.; Aluru, N.R. Why Are Carbon Nanotubes Fast Transporters of Water? Nano Lett. 2008, 8, 452–458. [Google Scholar] [CrossRef]
- Kubo, R.; Toda, M.; Hashitsume, N. Statistical Physics. 2: Nonequilibrium Statistical Mechanics; Springer Series in Solid State Sciences; Springer: Berlin/Heidelberg, Germany, 1985; ISBN 978-3-540-11461-1. [Google Scholar]
- Hansen, J.-P.; McDonald, I.R. Theory of Simple Liquids, 2nd ed.; Academic Press: London, UK, 1986; ISBN 978-0-12-323852-8. [Google Scholar]
- Williams, G. Time-correlation functions and molecular motion. Chem. Soc. Rev. 1978, 7, 89–131. [Google Scholar] [CrossRef]
- Bagchi, B. Molecular Relaxation in Liquids; Oxford University Press: New York, NY, USA, 2012; ISBN 978-0-19-986332-7. [Google Scholar]
- Richards, M. Nuclear Magnetic Resonance in Adsorbed Helium. In Phase Transitions in Surface Films; NATO Advanced Study Institutes Series; Dash, J.G., Ruvalds, J., Eds.; Springer: Boston, MA, USA, 1980; Volume 51, pp. 165–192. ISBN 978-1-4613-3059-2. [Google Scholar]
- Zeidler, M.D. A Comparative Study of Quasielastic Neutron Scattering and NMR Relaxation in Liquid Acetonitrile. Ber. Der Bunsenges. Für Phys. Chem. 1971, 75, 769–776. [Google Scholar] [CrossRef]
- Lascombe, J. (Ed.) Molecular Motions in Liquids: Proceedings of the 24th Annual Meeting of the Société de Chimie Physique Paris-Orsay, 2–6 July 1972; Springer: Dordrecht, The Netherlands, 1974; ISBN 978-94-010-2179-1. [Google Scholar]
- Jobic, H.; Schmidt, W.; Krause, C.B.; Kärger, J. PFG NMR and QENS diffusion study of n-alkane homologues in MFI-type zeolites. Microporous Mesoporous Mater. 2006, 90, 299–306. [Google Scholar] [CrossRef]
- Chandler, D. Hydrophobicity: Two faces of water. Nature 2002, 417, 491. [Google Scholar] [CrossRef] [PubMed]
- Coe, M.K.; Evans, R.; Wilding, N.B. Density Depletion and Enhanced Fluctuations in Water near Hydrophobic Solutes: Identifying the Underlying Physics. Phys. Rev. Lett. 2022, 128, 045501. [Google Scholar] [CrossRef]
- Mallamace, F.; Mallamace, D.; Chen, S.-H.; Lanzafame, P.; Papanikolaou, G. Hydrophilic and Hydrophobic Effects on the Structure and Themodynamic Properties of Confined Water: Water in Solutions. Int. J. Mol. Sci. 2021, 22, 7547. [Google Scholar] [CrossRef] [PubMed]
- Chathoth, S.M.; Mamontov, E.; Kolesnikov, A.I.; Gogotsi, Y.; Wesolowski, D.J. Quasielastic neutron scattering study of water confined in carbon nanopores. Eur. Lett. 2011, 95, 56001. [Google Scholar] [CrossRef]
- Diallo, S.O.; Vlcek, L.; Mamontov, E.; Keum, J.K.; Chen, J.; Hayes, J.J.S.; Chialvo, A.A. Translational diffusion of water inside hydrophobic carbon micropores studied by neutron spectroscopy and molecular dynamics simulation. Phys. Rev. E 2015, 91, 022124. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Nomura, K.-I.; Kalia, R.K.; Nakano, A.; Vashishta, P. Structure and dynamics of water confined in nanoporous carbon. Phys. Rev. Mater. 2018, 2, 115605. [Google Scholar] [CrossRef]
- Cerveny, S.; Barroso-Bujans, F.; Alegría, A.; Colmenero, J. Dynamics of Water Intercalated in Graphite Oxide. J. Phys. Chem. C 2010, 114, 2604–2612. [Google Scholar] [CrossRef]
- Chaikin, P.M.; Lubensky, T.C. Principles of Condensed Matter Physics, 1st ed.; Cambridge University Press: Cambridge, UK, 1995; ISBN 978-0-521-43224-5. [Google Scholar]
- Boon, J.-P.; Yip, S. Molecular Hydrodynamics; Dover Publications: New York, NY, USA, 1991; ISBN 978-0-486-66949-6. [Google Scholar]
- March, N.H.; Tosi, M.P. Introduction to Liquid State Physics; World Scientific: River Edge, NJ, USA, 2002; ISBN 978-981-02-4639-6. [Google Scholar]
- Kärger, J.; Valiullin, R.; Vasenkov, S. Molecular dynamics under confinement to one dimension: Options of measurement and accessible information. New J. Phys. 2005, 7, 15. [Google Scholar] [CrossRef]
- Weingärtner, H. Self Diffusion in Liquid Water. A Reassessment. Z. Für Phys. Chem. 1982, 132, 129–149. [Google Scholar] [CrossRef]
- Pruppacher, H.R. Self-Diffusion Coefficient of Supercooled Water. J. Chem. Phys. 1972, 56, 101–107. [Google Scholar] [CrossRef]
- Price, W.S. NMR Studies of Translational Motion; Cambridge Molecular Science; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780521806961. [Google Scholar]
- Evans, R. The interpretation of small molecule diffusion coefficients: Quantitative use of diffusion-ordered NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2020, 117, 33–69. [Google Scholar] [CrossRef]
- Cowan, B.; Fardis, M. Direct measurement of spin diffusion from spin relaxation times in solidHe3. Phys. Rev. B 1991, 44, 4304–4313. [Google Scholar] [CrossRef]
- Holz, M.; Heil, S.R.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]
- Price, W.S.; Ide, A.H.; Arata, Y. Self-Diffusion of Supercooled Water to 238 K Using PGSE NMR Diffusion Measurements. J. Phys. Chem. A 1999, 103, 448–450. [Google Scholar] [CrossRef]
- Xu, Y.; Petrik, N.G.; Smith, R.S.; Kay, B.D.; Kimmel, G.A. Growth rate of crystalline ice and the diffusivity of supercooled water from 126 to 262 K. Proc. Natl. Acad. Sci. USA 2016, 113, 14921–14925. [Google Scholar] [CrossRef]
- Gallo, P.; Sciortino, F.; Tartaglia, P.; Chen, S.-H. Slow Dynamics of Water Molecules in Supercooled States. Phys. Rev. Lett. 1996, 76, 2730–2733. [Google Scholar] [CrossRef]
- Dueby, S.; Dubey, V.; Daschakraborty, S. Decoupling of Translational Diffusion from the Viscosity of Supercooled Water: Role of Translational Jump Diffusion. J. Phys. Chem. B 2019, 123, 7178–7189. [Google Scholar] [CrossRef] [PubMed]
- Stirnemann, G.; Laage, D. Communication: On the origin of the non-Arrhenius behavior in water reorientation dynamics. J. Chem. Phys. 2012, 137, 031101. [Google Scholar] [CrossRef] [PubMed]
- Qvist, J.; Schober, H.; Halle, B. Structural dynamics of supercooled water from quasielastic neutron scattering and molecular simulations. J. Chem. Phys. 2011, 134, 144508. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, R.; Delmelle, M.; Cannistraro, S. Role of hydrogen-bond cooperativity and free-volume fluctuations in the non-Arrhenius behavior of water self-diffusion: A continuity-of-states model. Phys. Rev. E 1994, 49, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Russo, J.; Tanaka, H. Origin of the emergent fragile-to-strong transition in supercooled water. Proc. Natl. Acad. Sci. USA 2018, 115, 9444–9449. [Google Scholar] [CrossRef]
- Muthachikavil, A.V.; Kontogeorgis, G.M.; Liang, X.; Lei, Q.; Peng, B. Structural characteristics of low-density environments in liquid water. Phys. Rev. E 2022, 105, 034604. [Google Scholar] [CrossRef] [PubMed]
- Reif, F. Fundamentals of Statistical and Thermal Physics; McGraw-Hill Series in Fundamentals of Physics; International ed., [Nachdr.]; MacGraw-Hill: Auckland, New Zealand; Hamburg, Germany, 2008; ISBN 978-0-07-085615-8. [Google Scholar]
- Zwanzig, R. Time-Correlation Functions and Transport Coefficients in Statistical Mechanics. Annu. Rev. Phys. Chem. 1965, 16, 67–102. [Google Scholar] [CrossRef]
- Cowan, B. Nuclear Magnetic Resonance and Relaxation, 1st ed.; Cambridge University Press: Cambridge, UK, 1997; ISBN 978-0-521-30393-4. [Google Scholar]
- Hindman, J.C.; Svirmickas, A.; Wood, M. Relaxation processes in water. A study of the proton spin-lattice relaxation time. J. Chem. Phys. 1973, 59, 1517–1522. [Google Scholar] [CrossRef]
- Hertz, H. Chapter 5 Microdynamic behaviour of liquids as studied by NMR relaxation times. Prog. Nucl. Magn. Reson. Spectrosc. 1967, 3, 159–230. [Google Scholar] [CrossRef]
- Kringle, L.; Thornley, W.A.; Kay, B.D.; Kimmel, G.A. Structural relaxation and crystallization in supercooled water from 170 to 260 K. Proc. Natl. Acad. Sci. USA 2021, 118, e2022884118. [Google Scholar] [CrossRef]
- Lokotosh, T.V.; Magazù, S.; Maisano, G.; Malomuzh, N.P. Nature of self-diffusion and viscosity in supercooled liquid water. Phys. Rev. E 2000, 62, 3572–3580. [Google Scholar] [CrossRef]
- Teixeira, J.; Bellissent-Funel, M.-C.; Chen, S.H.; Dianoux, A.J. Experimental determination of the nature of diffusive motions of water molecules at low temperatures. Phys. Rev. A 1985, 31, 1913–1917. [Google Scholar] [CrossRef]
- Gillen, K.T.; Douglass, D.C.; Hoch, M.J.R. Self-Diffusion in Liquid Water to −31 °C. J. Chem. Phys. 1972, 57, 5117–5119. [Google Scholar] [CrossRef]
- Joseph, S.; Aluru, N.R. Pumping of Confined Water in Carbon Nanotubes by Rotation-Translation Coupling. Phys. Rev. Lett. 2008, 101, 064502. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, D.; Cassettari, M.; Salvetti, G. The dielectric relaxation time of supercooled water. J. Chem. Phys. 1982, 76, 3285–3290. [Google Scholar] [CrossRef]
- Lang, E.W.; Lüdemann, H.-D. High Pressure O-17 Longitudinal Relaxation Time Studies in Supercooled H2O and D2O. Ber. Der Bunsenges. Für Phys. Chem. 1981, 85, 603–611. [Google Scholar] [CrossRef]
- Lankhorst, D.; Schriever, J.; Leyte, J.C. Determination of the Rotational Correlation Time of Water by Proton NMR Relaxation in H217O and Some Related Results. Ber. Der Bunsenges. Für Phys. Chem. 1982, 86, 215–221. [Google Scholar] [CrossRef]
- Qvist, J.; Mattea, C.; Sunde, E.P.; Halle, B. Rotational dynamics in supercooled water from nuclear spin relaxation and molecular simulations. J. Chem. Phys. 2012, 136, 204505. [Google Scholar] [CrossRef] [PubMed]
- Brodin, A. Depolarized light scattering study of glycerol. Eur. Phys. J. B 2005, 44, 3–14. [Google Scholar] [CrossRef]
- Bloembergen, N. (Ed.) Encounters in Magnetic Resonances: Selected Papers of Nicolaas Bloembergen; World Scientific Series in 20th Century Physics; World Scientific: Singapore, 1996; ISBN 978-981-02-2590-2. [Google Scholar]
- Poole, C.P.; Farach, H.A. Theory of Magnetic Resonance, 2nd ed.; Wiley: New York, NY, USA, 1987; ISBN 978-0-471-81530-6. [Google Scholar]
- Laage, D.; Hynes, J.T. A Molecular Jump Mechanism of Water Reorientation. Science 2006, 311, 832–835. [Google Scholar] [CrossRef]
- Kyakuno, H.; Yahiro, H.; Inami, Y.; Fukuoka, T.; Matsuda, K.; Miyata, Y.; Yanagi, K.; Maniwa, Y.; Kataura, H.; Saito, T.; et al. Confined water inside single-walled carbon nanotubes: Global phase diagram and effect of finite length. J. Chem. Phys. 2011, 134, 244501. [Google Scholar] [CrossRef]
- Mamontov, E.; Burnham, C.J.; Chen, S.-H.; Moravsky, A.P.; Loong, C.-K.; De Souza, N.R.; Kolesnikov, A. Dynamics of water confined in single- and double-wall carbon nanotubes. J. Chem. Phys. 2006, 124, 194703. [Google Scholar] [CrossRef]
- Gkoura, L.; Diamantopoulos, G.; Fardis, M.; Homouz, D.; Alhassan, S.; Beazi-Katsioti, M.; Karagianni, M.; Anastasiou, A.; Romanos, G.; Hassan, J.; et al. The peculiar size and temperature dependence of water diffusion in carbon nanotubes studied with 2D NMR diffusion–relaxation D–T2eff spectroscopy. Biomicrofluidics 2020, 14, 034114. [Google Scholar] [CrossRef]
- Chen, S.-H.; Mallamace, F.; Mou, C.-Y.; Broccio, M.; Corsaro, C.; Faraone, A.; Liu, L. The violation of the Stokes–Einstein relation in supercooled water. Proc. Natl. Acad. Sci. USA 2006, 103, 12974–12978. [Google Scholar] [CrossRef] [PubMed]
- Weigler, M.; Winter, E.; Kresse, B.; Brodrecht, M.; Buntkowsky, G.; Vogel, M. Static field gradient NMR studies of water diffusion in mesoporous silica. Phys. Chem. Chem. Phys. 2020, 22, 13989–13998. [Google Scholar] [CrossRef] [PubMed]
- Kyakuno, H.; Fukasawa, M.; Ichimura, R.; Matsuda, K.; Nakai, Y.; Miyata, Y.; Saito, T.; Maniwa, Y. Diameter-dependent hydrophobicity in carbon nanotubes. J. Chem. Phys. 2016, 145, 064514. [Google Scholar] [CrossRef]
- Ciesla, U.; Schüth, F. Ordered mesoporous materials. Microporous Mesoporous Mater. 1999, 27, 131–149. [Google Scholar] [CrossRef]
- Briganti, G.; Rogati, G.; Parmentier, A.; Maccarini, M.; De Luca, F. Neutron scattering observation of quasi-free rotations of water confined in carbon nanotubes. Sci. Rep. 2017, 7, 45021. [Google Scholar] [CrossRef] [PubMed]
- Alexiadis, A.; Kassinos, S. Self-diffusivity, hydrogen bonding and density of different water models in carbon nanotubes. Mol. Simul. 2008, 34, 671–678. [Google Scholar] [CrossRef]
- Alexiadis, A.; Kassinos, S. Molecular Simulation of Water in Carbon Nanotubes. Chem. Rev. 2008, 108, 5014–5034. [Google Scholar] [CrossRef]
- Gordillo, M.C.; Marti, J. Hydrogen bond structure of liquid water confined in nanotubes. Chem. Phys. Lett. 2000, 329, 341–345. [Google Scholar] [CrossRef]
- Borg, M.K.; Lockerby, D.A.; Ritos, K.; Reese, J.M. Multiscale simulation of water flow through laboratory-scale nanotube membranes. J. Membr. Sci. 2018, 567, 115–126. [Google Scholar] [CrossRef]
- Striolo, A.; Chialvo, A.A.; Gubbins, K.E.; Cummings, P.T. Water in carbon nanotubes: Adsorption isotherms and thermodynamic properties from molecular simulation. J. Chem. Phys. 2005, 122, 234712. [Google Scholar] [CrossRef]
- Bordin, J.R.; Diehl, A.; Barbosa, M.C. Relation Between Flow Enhancement Factor and Structure for Core-Softened Fluids Inside Nanotubes. J. Phys. Chem. B 2013, 117, 7047–7056. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Garde, S.; Hummer, G. Osmotic water transport through carbon nanotube membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.-C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef]
- Hassan, J.; Diamantopoulos, G.; Gkoura, L.; Karagianni, M.; Alhassan, S.; Kumar, S.V.; Katsiotis, M.S.; Karagiannis, T.; Fardis, M.; Panopoulos, N.; et al. Ultrafast Stratified Diffusion of Water Inside Carbon Nanotubes; Direct Experimental Evidence with 2D D–T2 NMR Spectroscopy. J. Phys. Chem. C 2018, 122, 10600–10606. [Google Scholar] [CrossRef]
- De Marzio, M.; Camisasca, G.; Rovere, M.; Gallo, P. Fragile to strong crossover and Widom line in supercooled water: A comparative study. Front. Phys. 2018, 13, 136103. [Google Scholar] [CrossRef]
- Swenson, J. Comment on “Pressure Dependence of Fragile-to-Strong Transition and a Possible Second Critical Point in Supercooled Confined Water”. Phys. Rev. Lett. 2006, 97, 189801. [Google Scholar] [CrossRef]
- Hedstrom, J.; Swenson, J.; Bergman, R.; Jansson, H.; Kittaka, S. Does confined water exhibit a fragile-to-strong transition? Eur. Phys. J. Spec. Top. 2007, 141, 53–56. [Google Scholar] [CrossRef]
- Lederle, C.; Sattig, M.; Vogel, M. Effects of Partial Crystallization on the Dynamics of Water in Mesoporous Silica. J. Phys. Chem. C 2018, 122, 15427–15434. [Google Scholar] [CrossRef]
- Chu, X.-Q.; Kolesnikov, A.I.; Moravsky, A.P.; Garcia-Sakai, V.; Chen, S.-H. Observation of a dynamic crossover in water confined in double-wall carbon nanotubes. Phys. Rev. E 2007, 76, 021505. [Google Scholar] [CrossRef]
- Sjöström, J.; Swenson, J.; Bergman, R.; Kittaka, S. Investigating hydration dependence of dynamics of confined water: Monolayer, hydration water and Maxwell–Wagner processes. J. Chem. Phys. 2008, 128, 154503. [Google Scholar] [CrossRef]
- Kyakuno, H.; Matsuda, K.; Nakai, Y.; Ichimura, R.; Saito, T.; Miyata, Y.; Hata, K.; Maniwa, Y. Rotational dynamics and dynamical transition of water inside hydrophobic pores of carbon nanotubes. Sci. Rep. 2017, 7, 14834. [Google Scholar] [CrossRef] [PubMed]
- Weigler, M.; Brodrecht, M.; Buntkowsky, G.; Vogel, M. Reorientation of Deeply Cooled Water in Mesoporous Silica: NMR Studies of the Pore-Size Dependence. J. Phys. Chem. B 2019, 123, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Al Araimi, M.; Lutsyk, P.; Verbitsky, A.; Piryatinski, Y.; Shandura, M.; Rozhin, A. A dioxaborine cyanine dye as a photoluminescence probe for sensing carbon nanotubes. Beilstein J. Nanotechnol. 2016, 7, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Lutsyk, P.; Piryatinski, Y.; AlAraimi, M.; Arif, R.; Shandura, M.; Kachkovsky, O.; Verbitsky, A.; Rozhin, A. Emergence of Additional Visible-Range Photoluminescence Due to Aggregation of Cyanine Dye: Astraphloxin on Carbon Nanotubes Dispersed with Anionic Surfactant. J. Phys. Chem. C 2016, 120, 20378–20386. [Google Scholar] [CrossRef]
- Lutsyk, P.; Piryatinski, Y.; Shandura, M.; AlAraimi, M.; Tesa, M.; Arnaoutakis, G.E.; Melvin, A.A.; Kachkovsky, O.; Verbitsky, A.; Rozhin, A. Self-Assembly for Two Types of J-Aggregates: cis-Isomers of Dye on the Carbon Nanotube Surface and Free Aggregates of Dye trans-Isomers. J. Phys. Chem. C 2019, 123, 19903–19911. [Google Scholar] [CrossRef]
- Lee, S.H.; Rossky, P.J. A comparison of the structure and dynamics of liquid water at hydrophobic and hydrophilic surfaces—a molecular dynamics simulation study. J. Chem. Phys. 1994, 100, 3334–3345. [Google Scholar] [CrossRef]
- Kumar, P.; Buldyrev, S.V.; Starr, F.W.; Giovambattista, N.; Stanley, H.E. Thermodynamics, structure, and dynamics of water confined between hydrophobic plates. Phys. Rev. E 2005, 72, 051503. [Google Scholar] [CrossRef]
 ), dark grey triangles (
), dark grey triangles ( ), and stars (★) are the D values of H2O confined in SW 1.1 nm, MW 3.0 nm, and DW 3.5 nm, respectively, acquired via 2D D-T2 static field gradient NMR experiments (Gkoura et al. [74]). Inverted open triangles (▽) are the self-diffusion coefficients obtained from the pulsed field gradient NMR experiments of water confined in MCM-41 (Chen et al. [75]). Open circles (◯), open squares (□), and open upright triangles (△) are the self-diffusion coefficients obtained from static field gradient NMR experiments on water in open 2.8 nm, capped 2.8 nm, and capped 2.1 nm MCM-41 samples, respectively (Weigler et al. [76]).
), and stars (★) are the D values of H2O confined in SW 1.1 nm, MW 3.0 nm, and DW 3.5 nm, respectively, acquired via 2D D-T2 static field gradient NMR experiments (Gkoura et al. [74]). Inverted open triangles (▽) are the self-diffusion coefficients obtained from the pulsed field gradient NMR experiments of water confined in MCM-41 (Chen et al. [75]). Open circles (◯), open squares (□), and open upright triangles (△) are the self-diffusion coefficients obtained from static field gradient NMR experiments on water in open 2.8 nm, capped 2.8 nm, and capped 2.1 nm MCM-41 samples, respectively (Weigler et al. [76]).
 ), dark grey triangles (
), dark grey triangles ( ), and stars (★) are the D values of H2O confined in SW 1.1 nm, MW 3.0 nm, and DW 3.5 nm, respectively, acquired via 2D D-T2 static field gradient NMR experiments (Gkoura et al. [74]). Inverted open triangles (▽) are the self-diffusion coefficients obtained from the pulsed field gradient NMR experiments of water confined in MCM-41 (Chen et al. [75]). Open circles (◯), open squares (□), and open upright triangles (△) are the self-diffusion coefficients obtained from static field gradient NMR experiments on water in open 2.8 nm, capped 2.8 nm, and capped 2.1 nm MCM-41 samples, respectively (Weigler et al. [76]).
), and stars (★) are the D values of H2O confined in SW 1.1 nm, MW 3.0 nm, and DW 3.5 nm, respectively, acquired via 2D D-T2 static field gradient NMR experiments (Gkoura et al. [74]). Inverted open triangles (▽) are the self-diffusion coefficients obtained from the pulsed field gradient NMR experiments of water confined in MCM-41 (Chen et al. [75]). Open circles (◯), open squares (□), and open upright triangles (△) are the self-diffusion coefficients obtained from static field gradient NMR experiments on water in open 2.8 nm, capped 2.8 nm, and capped 2.1 nm MCM-41 samples, respectively (Weigler et al. [76]).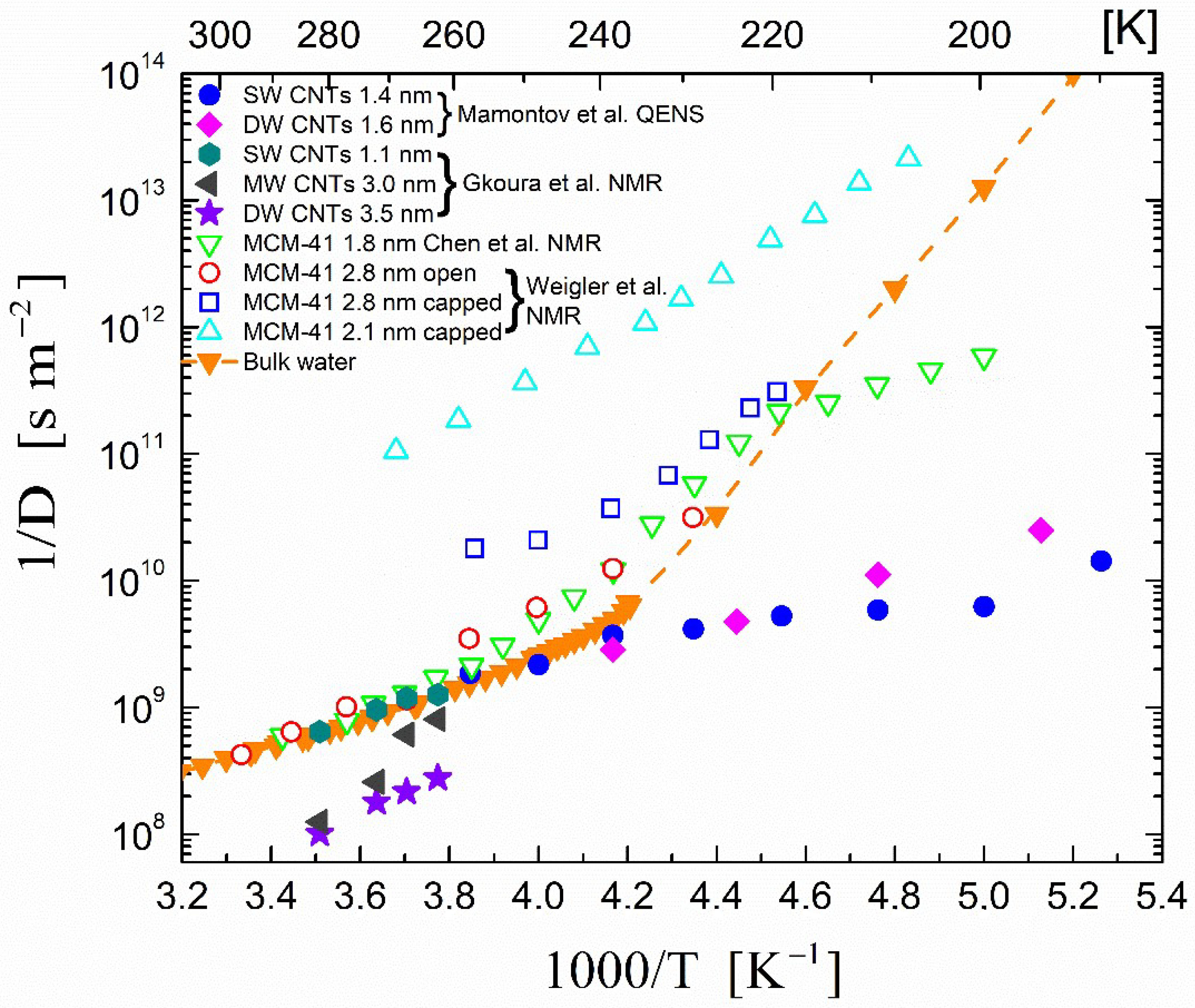
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fardis, M.; Karagianni, M.; Gkoura, L.; Papavassiliou, G. Self-Diffusion in Confined Water: A Comparison between the Dynamics of Supercooled Water in Hydrophobic Carbon Nanotubes and Hydrophilic Porous Silica. Int. J. Mol. Sci. 2022, 23, 14432. https://doi.org/10.3390/ijms232214432
Fardis M, Karagianni M, Gkoura L, Papavassiliou G. Self-Diffusion in Confined Water: A Comparison between the Dynamics of Supercooled Water in Hydrophobic Carbon Nanotubes and Hydrophilic Porous Silica. International Journal of Molecular Sciences. 2022; 23(22):14432. https://doi.org/10.3390/ijms232214432
Chicago/Turabian StyleFardis, Michael, Marina Karagianni, Lydia Gkoura, and George Papavassiliou. 2022. "Self-Diffusion in Confined Water: A Comparison between the Dynamics of Supercooled Water in Hydrophobic Carbon Nanotubes and Hydrophilic Porous Silica" International Journal of Molecular Sciences 23, no. 22: 14432. https://doi.org/10.3390/ijms232214432
APA StyleFardis, M., Karagianni, M., Gkoura, L., & Papavassiliou, G. (2022). Self-Diffusion in Confined Water: A Comparison between the Dynamics of Supercooled Water in Hydrophobic Carbon Nanotubes and Hydrophilic Porous Silica. International Journal of Molecular Sciences, 23(22), 14432. https://doi.org/10.3390/ijms232214432





