FaesAP3_1 Regulates the FaesELF3 Gene Involved in Filament-Length Determination of Long-Homostyle Fagopyrum esculentum
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of FaesELF3 and FaesELF3 Promoter (pFaesELF3) from Long-Homostyle Common Buckwheat
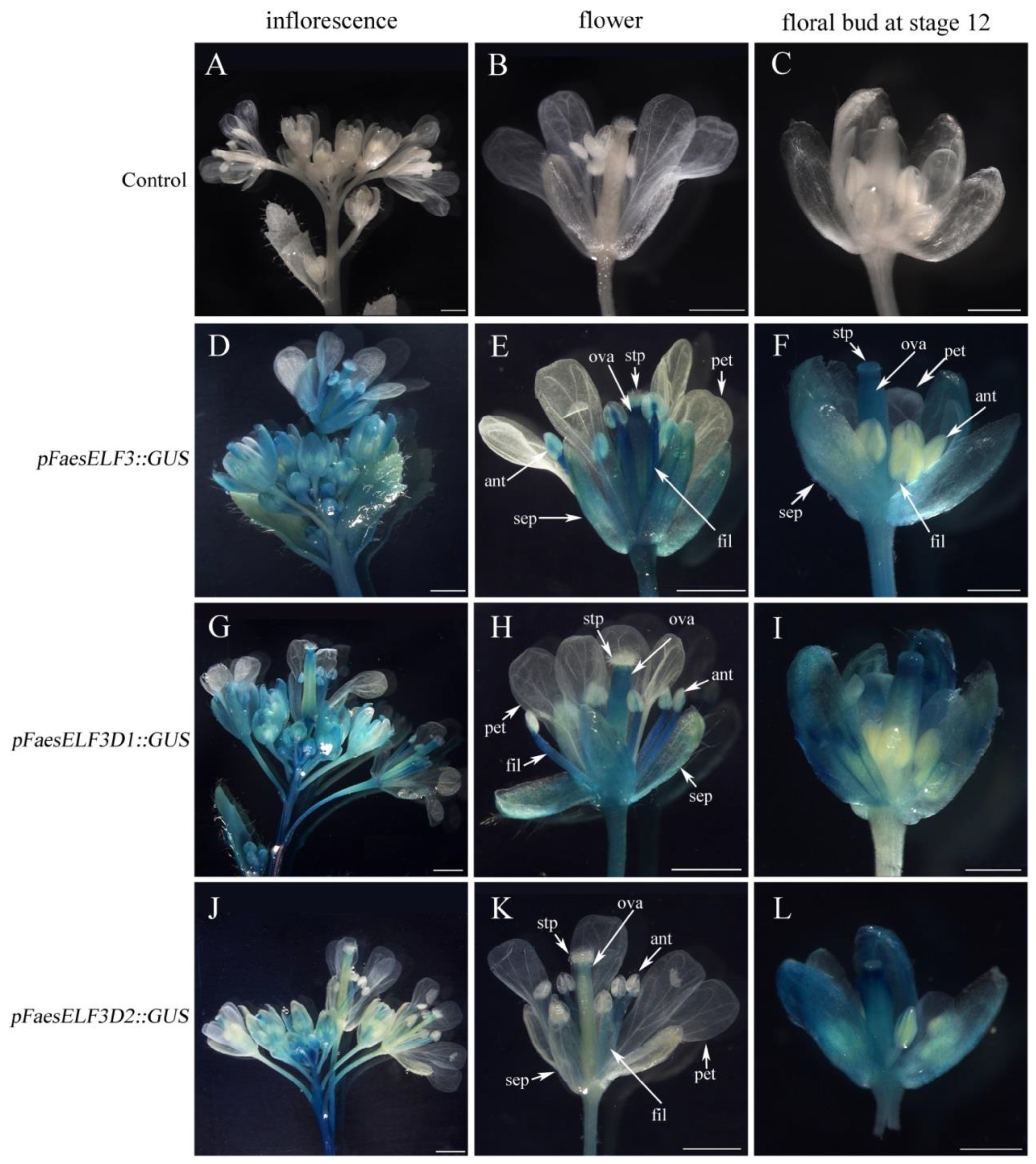
2.2. Deletion Analysis of the pFaesELF3 in Transgenic Arabidopsis
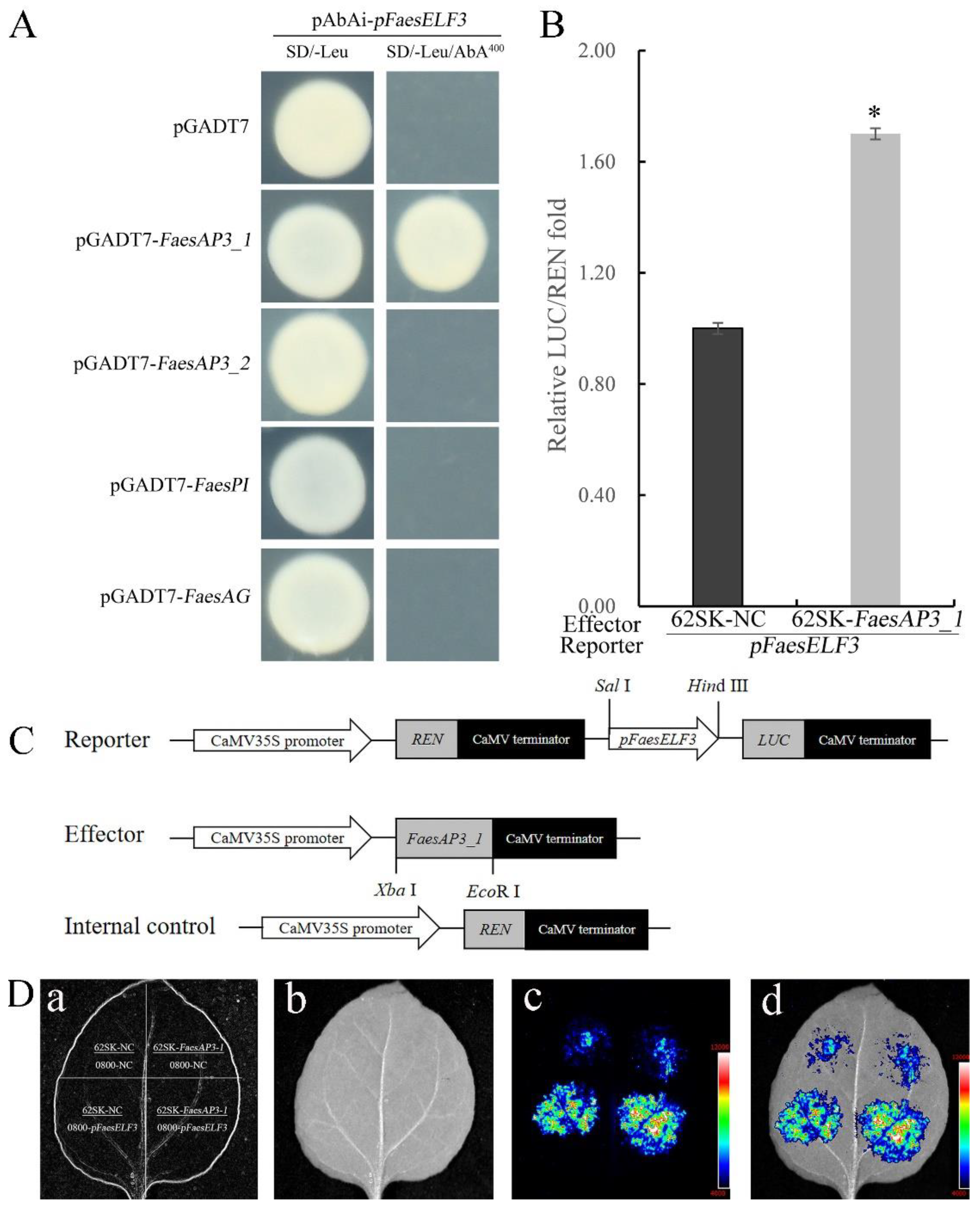
2.3. B-Class MADS-Box Transcription Factor FaesAP3_1 to Target FaesELF3 for Stamen Development
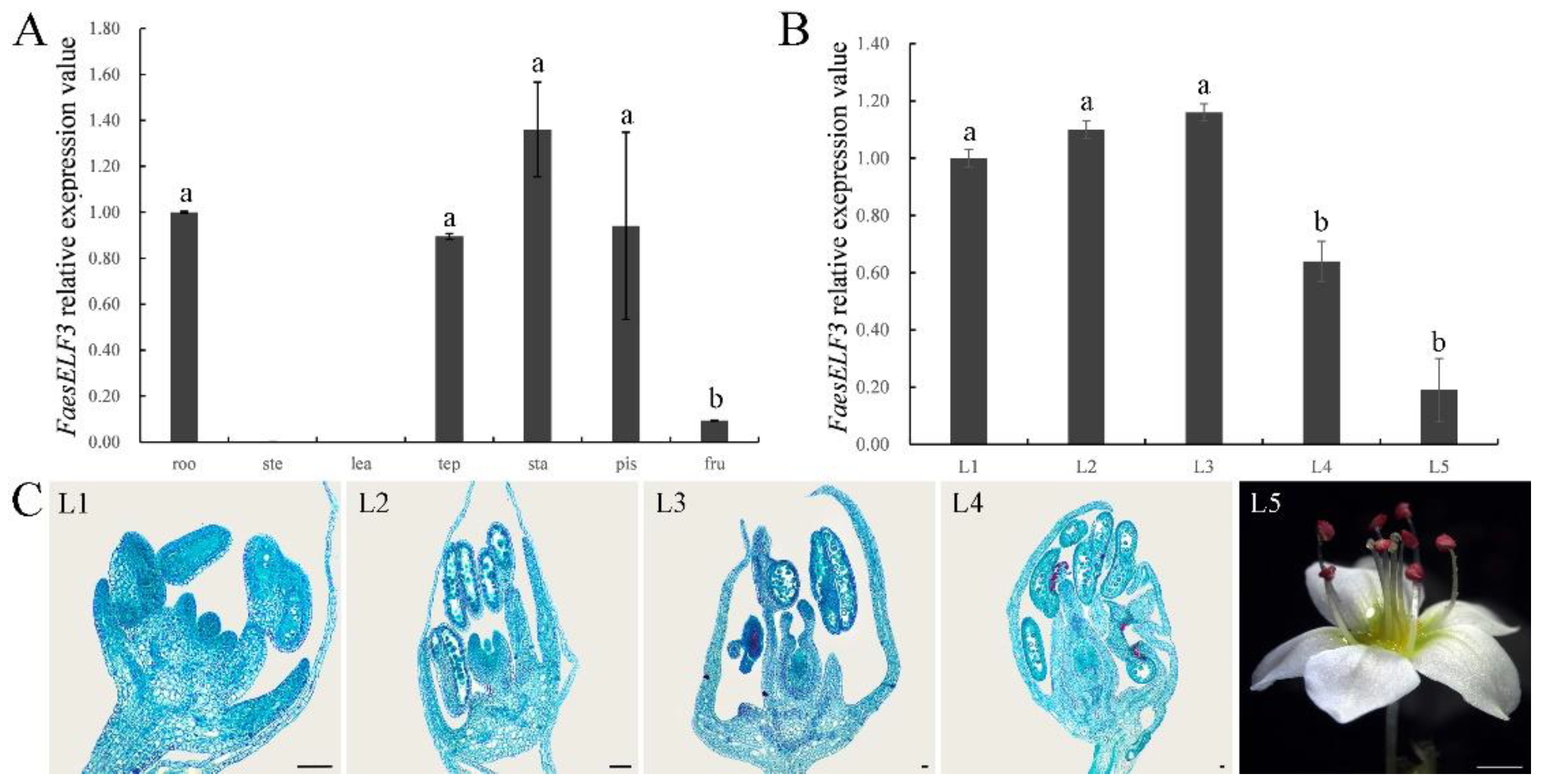
2.4. Expression Analysis of FaesELF3 in LH Flower F. Esculentum
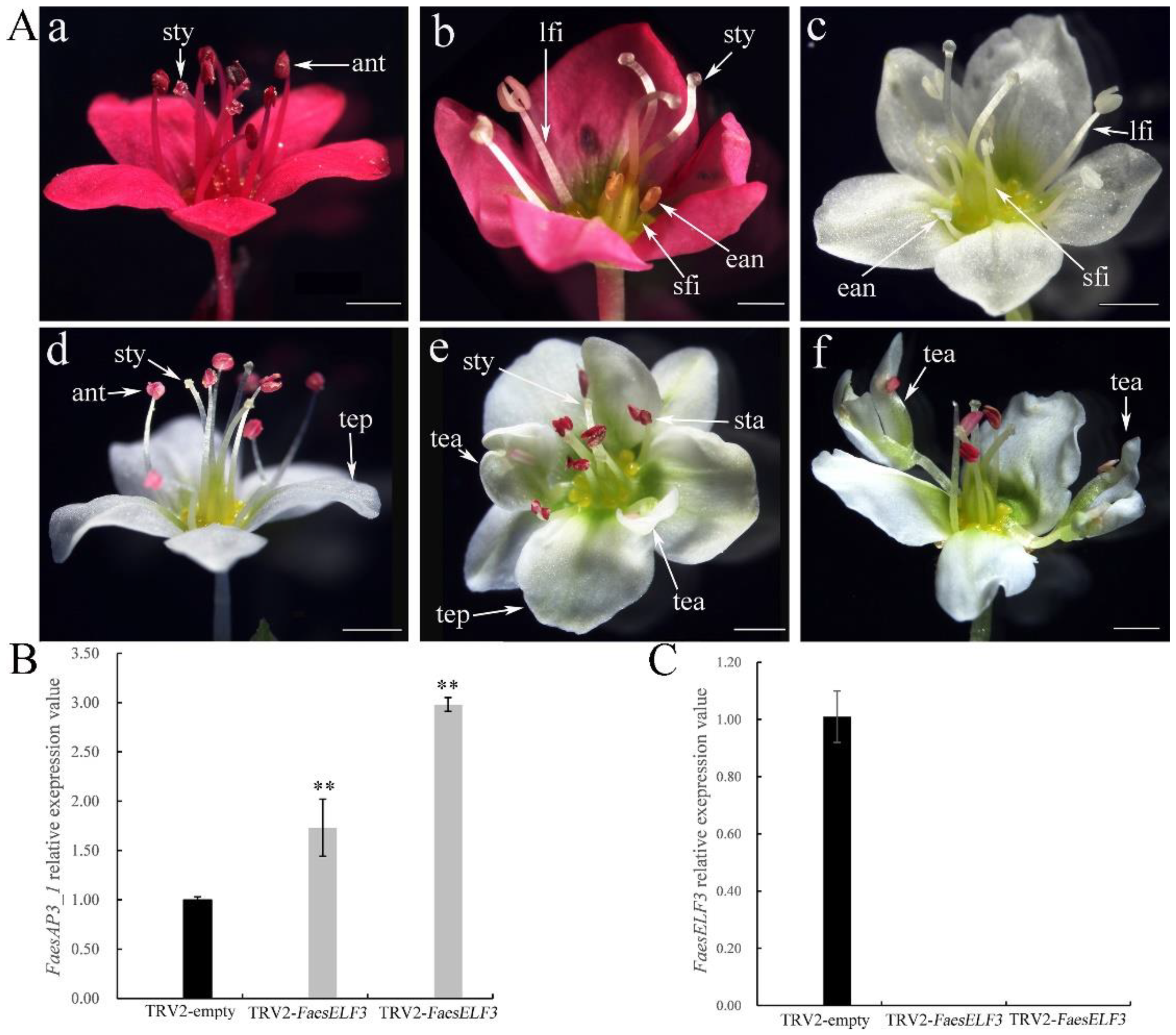
2.5. Characterization of FaesAP3_1 and FaesELF3-Silenced Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Isolation and Characterization of FaesELF3 and FaesELF3 Promoter (pFaesELF3) from Long-Homostyle Common Buckwheat
4.3. Characterization of pFaesELF3 Activity from the 5′ Deleted Promoter Fragments in Transgenic Arabidopsis
4.4. Yeast One-Hybrid Assay
4.5. Dual-Luciferase Reporter Assay
4.6. Cytomorphological Observation and Expression Analysis of FaesELF3
4.7. VIGS Assay in Long-Homostyle Buckwheat
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Chen, X.; Zeng, L.; Ma, Z.; Liu, Z. Characterization of PISTILLATA-like Genes and Their Promoters from the Distyly Fagopyrum esculentum. Plants 2022, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Yasui, Y. Buckwheat Heteromorphic Self-Incompatibility: Genetics, Genomics and Application to Breeding. Breed. Sci. 2020, 70, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, J.; Wang, X.; Liu, Z. Isolation and Characterization of APETALA3 Orthologs and Promoters from the Distylous Fagopyrum esculentum. Plants 2021, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Yasui, Y. Gene flow signature in the S-allele region of cultivated buckwheat. BMC Plant Biol. 2019, 19, 125. [Google Scholar] [CrossRef]
- Matsui, K.; Mizuno, N.; Ueno, M.; Takeshima, R.; Yasui, Y. Development of co-dominant markers linked to a hemizygous region that is related to the self-compatibility locus (S) in buckwheat (Fagopyrum esculentum). Breed. Sci. 2020, 70, 112–117. [Google Scholar] [CrossRef]
- Kappel, C.; Huu, C.N.; Lenhard, M. A short story gets longer: Recent insights into the molecular basis of heterostyly. J. Exp. Bot. 2017, 68, 5719–5730. [Google Scholar] [CrossRef]
- Anwer, M.U.; Davis, A.; Davis, S.J.; Quint, M. Photoperiod sensing of the circadian clock is controlled by EARLY FLOWERING 3 and GIGANTEA. Plant J. 2020, 101, 1397–1410. [Google Scholar] [CrossRef]
- Zhu, Z.; Quint, M.; Anwer, M.U. Arabidopsis EARLY FLOWERING 3 controls temperature responsiveness of the circadian clock independently of the evening complex. J. Exp. Bot. 2022, 73, 1049–1061. [Google Scholar] [CrossRef]
- Bu, T.; Lu, S.; Wang, K.; Dong, L.; Li, S.; Xie, Q.; Xu, X.; Cheng, Q.; Chen, L.; Fang, C.; et al. A critical role of the soybean evening complex in the control of photoperiod sensitivity and adaptation. Proc. Natl. Acad. Sci. USA 2021, 118, e2010241118. [Google Scholar] [CrossRef]
- Huang, H.; Gehan, M.A.; Huss, S.E.; Alvarez, S.; Lizarraga, C.; Gruebbling, E.L.; Gierer, J.; Naldrett, M.J.; Bindbeutel, R.K.; Evans, B.S.; et al. Cross-species complementation reveals conserved functions for EARLY FLOWERING 3 between monocots and dicots. Plant Direct 2017, 1, e00018. [Google Scholar] [CrossRef]
- Ning, Y.; Shi, X.; Wang, R.; Fan, J.; Park, C.H.; Zhang, C.; Zhang, T.; Ouyang, X.; Li, S.; Wang, G.L. OsELF3-2, an Ortholog of Arabidopsis ELF3, Interacts with the E3 Ligase APIP6 and Negatively Regulates Immunity against Magnaporthe oryzae in Rice. Mol. Plant 2015, 8, 1679–1682. [Google Scholar] [CrossRef]
- Itoh, H.; Tanaka, Y.; Izawa, T. Genetic Relationship Between Phytochromes and OsELF3-1 Reveals the Mode of Regulation for the Suppression of Phytochrome Signaling in Rice. Plant Cell Physiol. 2019, 60, 549–561. [Google Scholar] [CrossRef]
- De Folter, S.; Angenent, G.C. Trans meets cis in MADS science. Trends Plant Sci. 2006, 11, 224–231. [Google Scholar] [CrossRef]
- Melzer, R.; Kaufmann, K.; Theiben, G. Missing Links: DNA-Binding and Target Gene Specificity of Floral Homeotic Proteins. Adv. Bot. Res. 2006, 44, 209–236. [Google Scholar] [CrossRef]
- Schilling, S.; Pan, S.; Kennedy, A.; Melzer, R. MADS-box genes and crop domestication: The jack of all traits. J. Exp. Bot. 2018, 69, 1447–1469. [Google Scholar] [CrossRef]
- Fang, Z.W.; Li, X.P.; Li, X.F.; Liu, Z.X. FaesPI, a Fagopyrum esculentum PISTILLATA Ortholog, Is Involved Only in Stamen Development. J. Plant Biol. 2015, 58, 102–109. [Google Scholar] [CrossRef]
- Li, L.Y.; Fang, Z.W.; Li, X.P.; Liu, Z.X. Isolation and Characterization of the C-class MADS-box Gene from the Distylous Pseudo-cereal Fagopyrum esculentum. J. Plant Biol. 2017, 60, 189–198. [Google Scholar] [CrossRef]
- Wenkel, S.; Turck, F.; Singer, K.; Gissot, L.; Le Gourrierec, J.; Samach, A.; Coupland, G. CONSTANS and the CCAAT Box Binding Complex Share a Functionally Important Domain and Interact to Regulate Flowering of Arabidopsis. Plant Cell 2006, 18, 2971–2984. [Google Scholar] [CrossRef]
- Dinh, T.T.; Girke, T.; Liu, X.; Yant, L.; Schmid, M.; Chen, X. The Floral Homeotic Protein APETALA2 Recognizes and Acts through an AT-Rich Sequence Element. Development 2012, 139, 1978–1986. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Leonard, J.M.; Monteros, A.; Liu, P.P.; Nonogaki, H. A Novel Endo-β-Mannanase Gene in Tomato LeMAN5 Is Associated with Anther and Pollen Development. Plant Physiol. 2004, 134, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.J.; Bate, N.; Combe, J.; Sullivan, J.; Sweetman, J.; Swan, C.; Lonsdale, D.M.; Twell, D. Functional Analysis of cis-Regulatory Elements within the Promoter of the Tobacco Late Pollen Gene g10. Plant Mol. Biol. 2001, 45, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.; Nieto, C.; Avila, J.; Canas, L.; Diaz, I.; Paz-Ares, J. Dual DNA binding specificity of a petal epidermis-specific MYB transcription factor (MYB.Ph3) from Petunia hybrida. EMBO J. 1995, 14, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chung, H.J.; Thomas, T.L. Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J. 1997, 11, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Xie, Z.; Zou, X.; Casaretto, J.; Ho, T.D.; Shen, Q.J. A Rice WRKY Gene Encodes a Transcriptional Repressor of the Gibberellin Signaling Pathway in Aleurone Cells. Plant Physiol. 2004, 134, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Chiang, C.M.; Tseng, T.H.; Yu, S.M. Interaction between Rice MYBGA and the Gibberellin Response Element Controls Tissue-Specific Sugar Sensitivity of alpha-Amylase Genes. Plant Cell 2006, 18, 2326–2340. [Google Scholar] [CrossRef]
- Mena, M.; Cejudo, F.J.; Isabel-Lamoneda, I.; Carbonero, P. A Role for the DOF Transcription Factor BPBF in the Regulation of Gibberellin-Responsive Genes in Barley Aleurone. Plant Physiol. 2002, 130, 111–119. [Google Scholar] [CrossRef]
- Baumann, K.; De Paolis, A.; Costantino, P.; Gualberti, G. The DNA binding site of the Dof protein NtBBF1 is essential for tissue-specific and auxin-regulated expression of the rolB oncogene in plants. Plant Cell 1999, 11, 323–333. [Google Scholar] [CrossRef]
- Ross, E.J.; Stone, J.M.; Elowsky, C.G.; Arredondo-Peter, R.; Klucas, R.V.; Sarath, G. Activation of the Oryza sativa non-symbiotic haemoglobin-2 promoter by the cytokinin-regulated transcription factor, ARR1. J. Exp. Bot. 2004, 55, 1721–1731. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.H.; Fujii, H.; Zheng, X.; Zhu, J.K. A R2R3 Type MYB Transcription Factor Is Involved in the Cold Regulation of CBF Genes and in Acquired Freezing Tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef]
- Simpson, S.D.; Nakashima, K.; Narusaka, Y.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003, 33, 259–270. [Google Scholar] [CrossRef]
- Xue, G.P. Characterisation of the DNA-binding profile of barley HvCBF1 using an enzymatic method for rapid, quantitative and high-throughput analysis of the DNA-binding activity. Nucleic Acids Res. 2002, 30, e77. [Google Scholar] [CrossRef]
- Smyth, D.R.; Bowman, J.L.; Meyerowitz, E.M. Early flower development in Arabidopsis. Plant Cell 1990, 2, 755–767. [Google Scholar] [CrossRef]
- Fang, Z.W.; Qi, R.; Li, X.F.; Liu, Z.X. Ectopic expression of FaesAP3, a Fagopyrum esculentum (Polygonaceae) AP3 orthologous gene rescues stamen development in an Arabidopsis ap3 mutant. Gene 2014, 550, 200–206. [Google Scholar] [CrossRef]
- Wuest, S.E.; O’Maoileidigh, D.S.; Rae, L.; Kwasniewska, K.; Raganelli, A.; Hanczaryk, K.; Lohan, A.J.; Loftus, B.; Graciet, E.; Wellmer, F. Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc. Natl. Acad. Sci. USA 2012, 109, 13452–13457. [Google Scholar] [CrossRef]
- Wang, P.; Liao, H.; Zhang, W.; Yu, X.; Zhang, R.; Shan, H.; Duan, X.; Yao, X.; Kong, H. Flexibility in the structure of spiral flowers and its underlying mechanisms. Nat. Plants 2015, 2, 15188. [Google Scholar] [CrossRef]
- Deveaux, Y.; Conde, E.; Silva, N.; Manicacci, D.; Le Guilloux, M.; Brunaud, V.; Belcram, H.; Joets, J.; Soubigou-Taconnat, L.; Delannoy, E.; et al. Transcriptome Analysis Reveals Putative Target Genes of APETALA3-3 During Early Floral Development in Nigella damascena L. Front. Plant Sci. 2021, 12, 660803. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, Y.; Zhang, K.; Fang, Z. Ectopic Expression of a Fagopyrum esculentum APETALA1 Ortholog Only Rescues Sepal Development in Arabidopsis Ap1 Mutant. Int. J. Mol. Sci. 2019, 20, 2021. [Google Scholar] [CrossRef]
- Liu, Z.X.; Xiong, H.Y.; Li, L.Y.; Fei, Y.J. Functional Conservation of an AGAMOUS Orthologous Gene Controlling Reproductive Organ Development in the Gymnosperm Species Taxus chinensis var. mairei. J. Plant Biol. 2018, 61, 50–59. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Yang, Q.; Zeng, L.; Li, J.; Jiao, X.; Liu, Z. FaesAP3_1 Regulates the FaesELF3 Gene Involved in Filament-Length Determination of Long-Homostyle Fagopyrum esculentum. Int. J. Mol. Sci. 2022, 23, 14403. https://doi.org/10.3390/ijms232214403
Ma Z, Yang Q, Zeng L, Li J, Jiao X, Liu Z. FaesAP3_1 Regulates the FaesELF3 Gene Involved in Filament-Length Determination of Long-Homostyle Fagopyrum esculentum. International Journal of Molecular Sciences. 2022; 23(22):14403. https://doi.org/10.3390/ijms232214403
Chicago/Turabian StyleMa, Zhiyuan, Qingyu Yang, Lingtian Zeng, Jiayi Li, Xinyu Jiao, and Zhixiong Liu. 2022. "FaesAP3_1 Regulates the FaesELF3 Gene Involved in Filament-Length Determination of Long-Homostyle Fagopyrum esculentum" International Journal of Molecular Sciences 23, no. 22: 14403. https://doi.org/10.3390/ijms232214403
APA StyleMa, Z., Yang, Q., Zeng, L., Li, J., Jiao, X., & Liu, Z. (2022). FaesAP3_1 Regulates the FaesELF3 Gene Involved in Filament-Length Determination of Long-Homostyle Fagopyrum esculentum. International Journal of Molecular Sciences, 23(22), 14403. https://doi.org/10.3390/ijms232214403




