The Human Milk Microbiota Produces Potential Therapeutic Biomolecules and Shapes the Intestinal Microbiota of Infants
Abstract
1. Introduction
2. Results
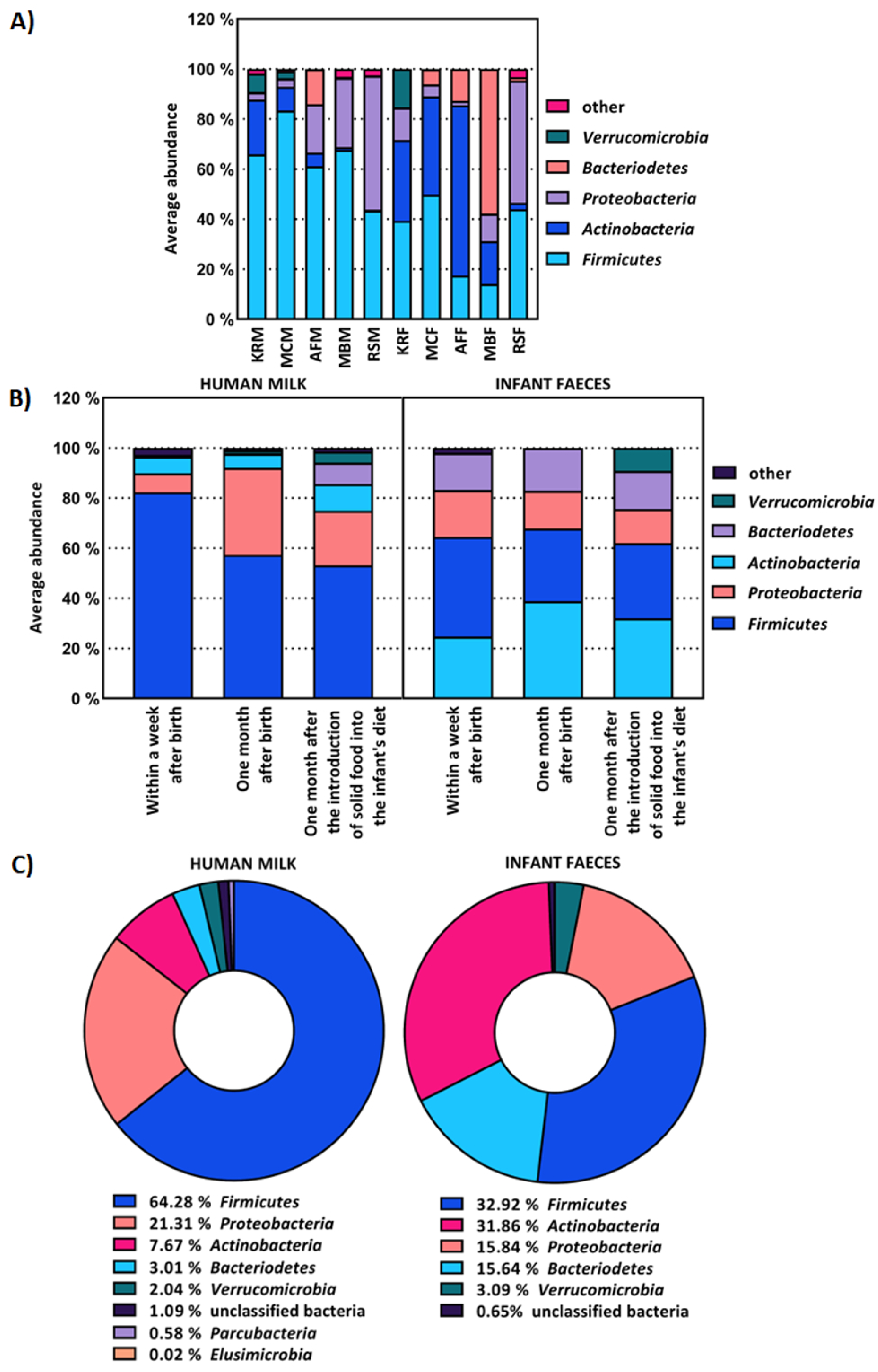
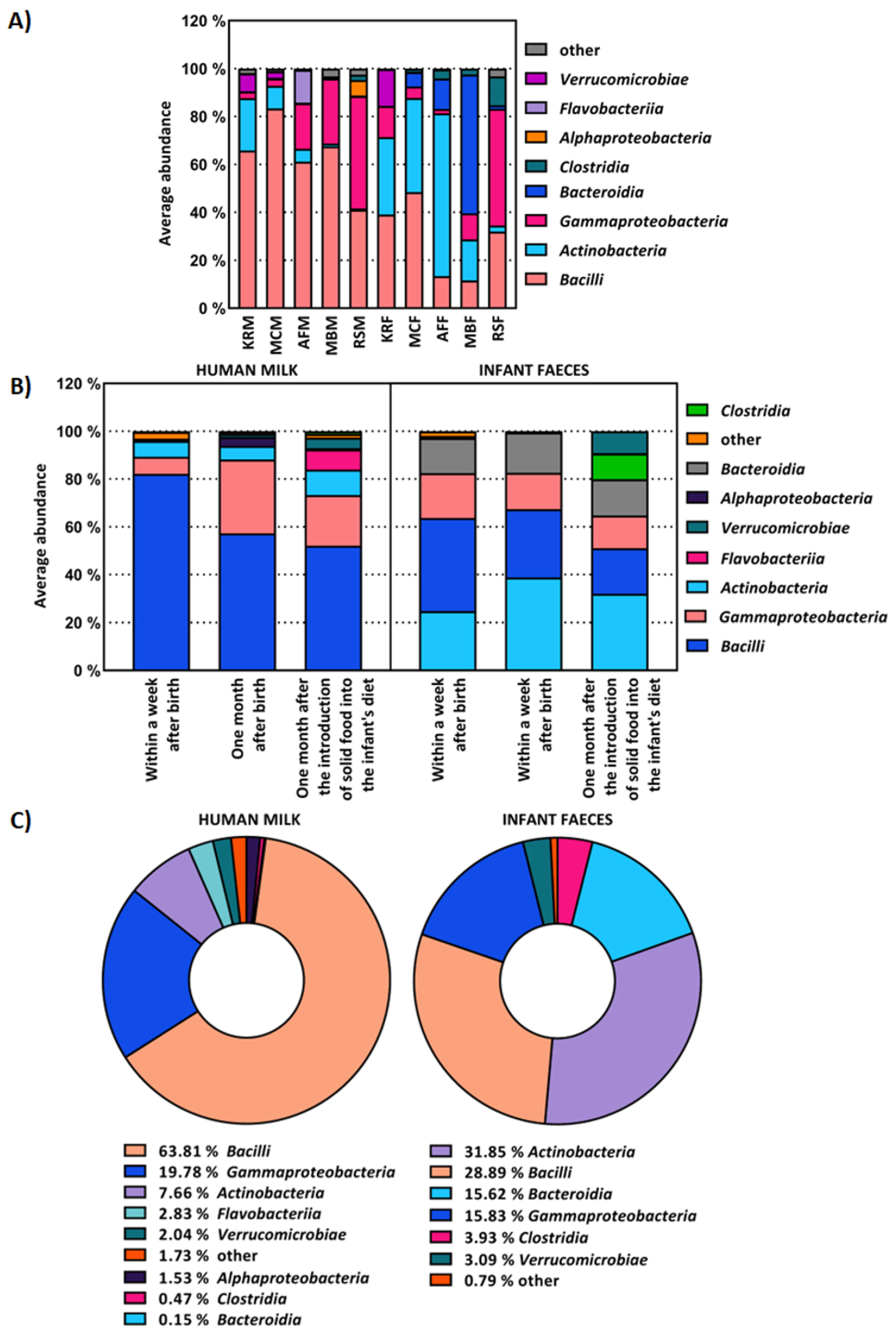
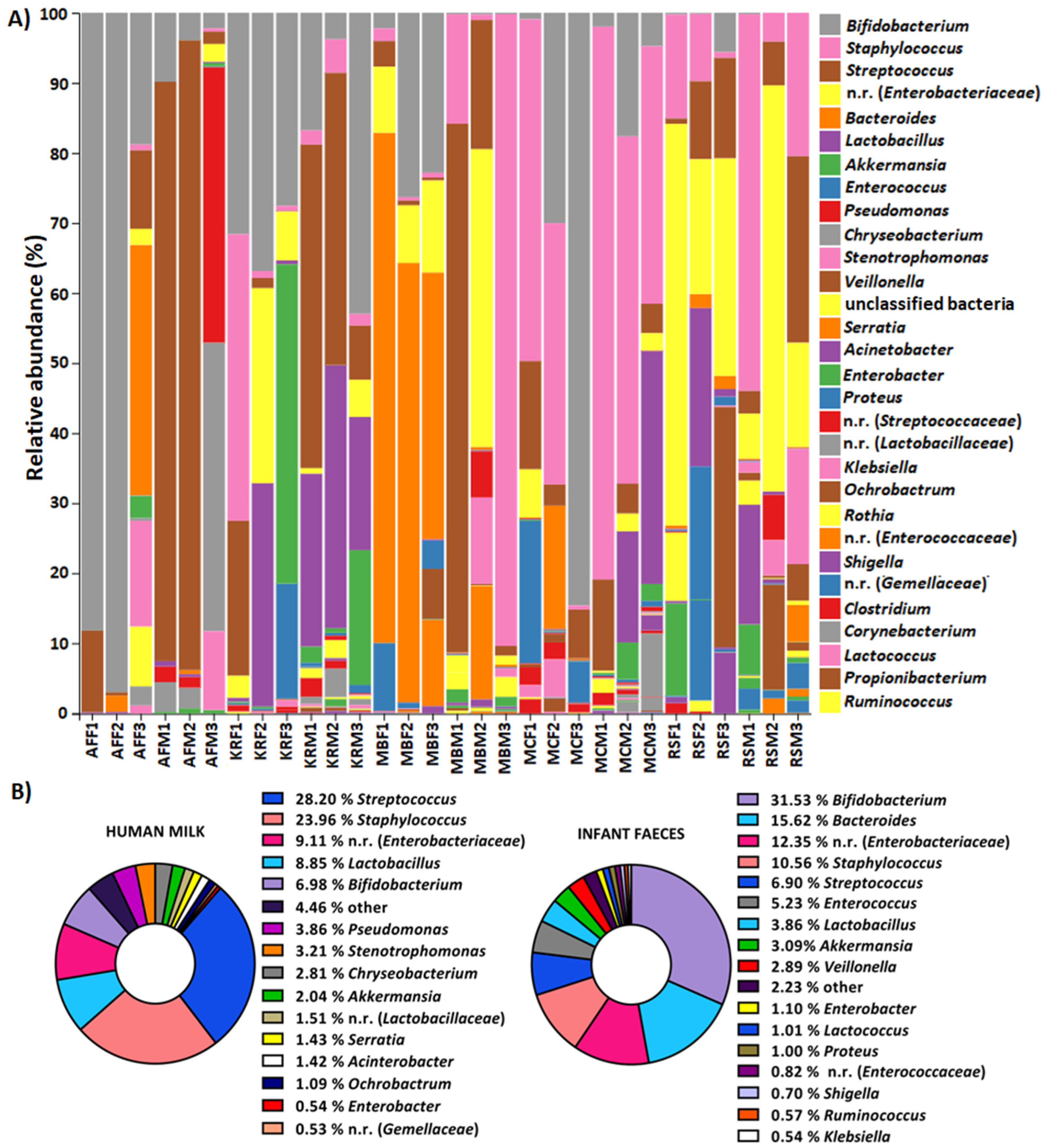
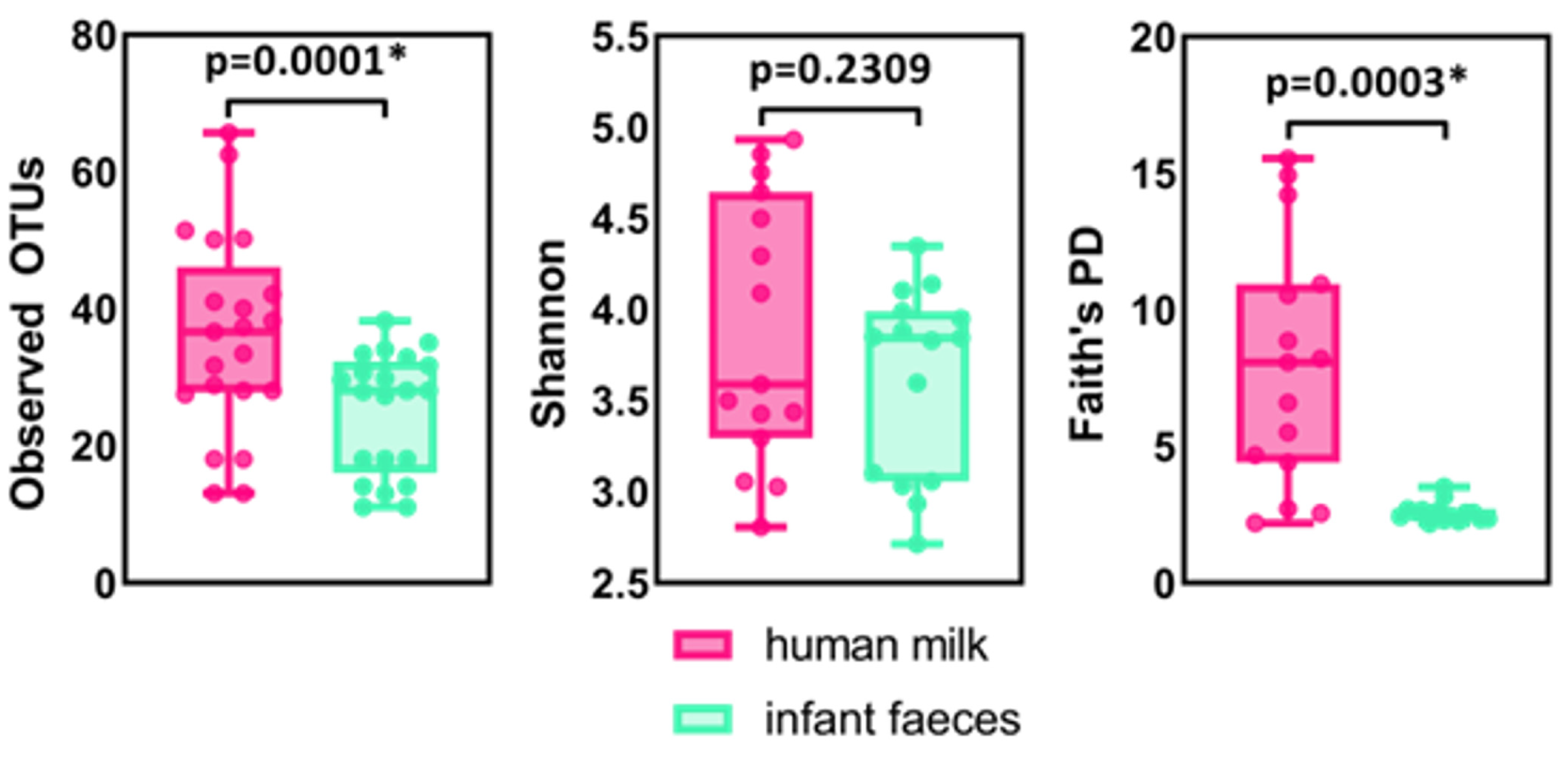
2.1. The Composition of Human Milk and Infant Faecal Microbiome
2.2. Synthesis of Potential Biomolecules by LAB Strains Isolated from the Human Milk Microbiota
3. Discussion
4. Materials and Methods
4.1. Collection of Human Milk and Infant Faecal Samples
4.2. Bacterial Strains
4.3. Bacterial Isolation, DNA Extraction and RAPD-PCR-Based Fingerprinting
4.4. LAB Selection and 16S rRNA Identification
4.5. WGS Identification of LAB Producers and Detection of Potential Therapeutic Biomolecules
4.5.1. Detection of S-Layer Proteins by SDS-PAGE and LC/MS Methods
4.5.2. Detection of Plantaricins by PCR and Agar Spot-Test
4.5.3. Detection of Exopolysaccharides by 1H NMR Method
4.5.4. Whole Genome Sequencing (WGS)
4.6. Microbiome Analysis with QIIME 2™—Determination of Taxonomy and α- and β-Diversity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventura, M.; O’Flaherty, S.; Claesson, M.J.; Turroni, F.; Klaenhammer, T.R.; van Sinderen, D.; O’Toole, P.W. Genome-scale analyses of health-promoting bacteria: Probiogenomics. Nat. Rev. Microbiol. 2009, 7, 61–71. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.; Marchesi, J.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Douillard, F.P.; de Vos, W.M. Biotechnology of health-promoting bacteria. Biotechnol. Adv. 2019, 37, 107369. [Google Scholar] [CrossRef]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 26. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, E.; Carasi, P.; Mobili, P.; Serradell, M.A.; Gómez-Zavaglia, A. Role of S-layer proteins in bacteria. World J. Microbiol. Biotechnol. 2015, 31, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Hynönen, U.; Palva, A. Lactobacillus surface layer proteins: Structure, function and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef]
- Klotz, C.; Goh, Y.J.; O’Flaherty, S.; Barrangou, R. S-layer associated proteins contribute to the adhesive and immunomodulatory properties of Lactobacillus acidophilus NCFM. BMC Microbiol. 2020, 20, 248. [Google Scholar] [CrossRef]
- Rodrigues-Oliveira, T.; Souza, A.A.; Kruger, R.; Schuster, B.; Maria de Freitas, S.; Kyaw, C.M. Environmental factors influence the Haloferax volcanii S-layer protein structure. PLoS ONE 2019, 14, e0216863. [Google Scholar] [CrossRef]
- Butorac, K.; Banić, M.; Novak, J.; Leboš Pavunc, A.; Uroić, K.; Durgo, K.; Oršolić, N.; Kukolj, M.; Radović, S.; Scalabrin, S.; et al. The functional capacity of plantaricin-producing Lactobacillus plantarum SF9C and S-layer-carrying Lactobacillus brevis SF9B to withstand gastrointestinal transit. Microb. Cell Fact. 2020, 19, 106. [Google Scholar] [CrossRef]
- Butorac, K.; Novak, J.; Bellich, B.; Terán, L.C.; Banić, M.; Leboš Pavunc, A.; Zjalić, S.; Cescutti, P.; Šušković, J.; Kos, B. Lyophilized alginate-based microspheres containing Lactobacillus fermentum D12, an exopolysaccharides producer, contribute to the strain’s functionality in vitro. Microb. Cell Fact. 2021, 20, 85. [Google Scholar] [CrossRef]
- Novak, J.; Leboš Pavunc, A.; Butorac, K.; Banić, M.; Čuljak, N.; Rak, H.; Blažević, M.; Iveljić, A.-M.; Šušković, J.; Kos, B. Caseinolytic proteases of Lactobacillus and Lactococcus strains isolated from fermented dairy products. Mljekarstvo 2022, 72, 11–21. [Google Scholar] [CrossRef]
- Novak, J.; Butorac, K.; Leboš Pavunc, A.; Banić, M.; Butorac, A.; Lepur, A.; Oršolić, N.; Tonković, K.; Bendelja, K.; Čuljak, N.; et al. A Lactic Acid Bacteria Consortium Impacted the Content of Casein-Derived Biopeptides in Dried Fresh Cheese. Molecules 2022, 27, 160. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Quorum sensing circuits in the communicating mechanisms of bacteria and its implication in the biosynthesis of bacteriocins by lactic acid bacteria: A review. Probiotics Antimicrob. Proteins 2020, 12, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M.T. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef]
- Casto-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Rojas, M.F.; Reinheimer, J.A.; Binetti, A.G. Lactobacillus fermentum: Could EPS production ability be responsible for functional properties? Food Microbiol. 2020, 90, 103465. [Google Scholar] [CrossRef]
- Kozak, K.; Charbonneau, D.; Sanozky-Dawes, R.; Klaenhammer, T. Characterization of bacterial isolates from the microbiota of mothers’ breast milk and their infants. Gut Microbes 2015, 6, 341–351. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Rutten, N.B.M.M.; Boekhorst, J.; Saulnier, D.M.; Kortman, G.A.M.; Contractor, N.; Kullen, M.; Floris, E.; Harmsen, H.J.M.; Vlieger, A.M.; et al. Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures. Sci. Rep. 2017, 7, 8327. [Google Scholar] [CrossRef]
- Lyons, K.E.; Shea, C.A.O.; Grimaud, G.; Ryan, C.A.; Dempsey, E.; Kelly, A.L.; Ross, R.P.; Stanton, C. The human milk microbiome aligns with lactation stage and not birth mode. Sci. Rep. 2022, 12, 5598. [Google Scholar] [CrossRef]
- Biagi, E.; Quercia, S.; Aceti, A.; Beghetti, I.; Rampelli, S.; Turroni, S.; Faldella, G.; Candela, M.; Brigidi, P.; Corvaglia, L. The bacterial ecosystem of mother’s milk and infant’s mouth and gut. Front. Microbiol. 2017, 30, 1214. [Google Scholar] [CrossRef]
- Murphy, K.; Curley, D.; O’Callaghan, T.F.; O’Shea, C.A.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. The composition of human milk and infant faecal microbiota over the first three months of life: A pilot study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [PubMed]
- Banić, M. Potential Therapeutic Biomolecules of Probiotic Strains of Autochthonous Lactic acid Bacteria. Doctoral dissertation, Faculty of Food Technology and Biotechnology, University of Zagreb, Zagreb, Croatia, 2021. [Google Scholar]
- Coeuret, V.; Gueguen, M.; Vernoux, J.P. In vitro screening of potential probiotic activities of selected lactobacilli isolated from unpasteurized milk products for incorporation into soft cheese. J. Dairy Res. 2004, 71, 451–460. [Google Scholar] [CrossRef]
- Martín, R.; Langa, S.; Reviriego, C.; Jiménez, E.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003, 143, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Aassociation between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Gómez-Gallego, C.; Pohl, S.; Salminen, S.; De Vos, W.M.; Kneifel, W. Akkermansia muciniphila: A novel functional microbe with probiotic properties. Benef. Microbes 2016, 7, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020, 81, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Łubiech, K.; Twarużek, M. Lactobacillus bacteria in breast milk. Nutrients 2020, 12, 3783. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Wan, Y.; Jiang, J.; Lu, M.; Tong, W.; Zhou, R.; Li, J.; Yuan, J.; Wang, F.; Li, D. Human milk microbiota development during lactation and its relation to maternal geographic location and gestational hypertensive status. Gut Microbes 2020, 11, 1438–1449. [Google Scholar] [CrossRef]
- Gil, A.; Rueda, R.; Ozanne, S.; Beek, E.; Van Loo-Bouwman, C.; Schoemaker, M.; Marinello, V.; Venema, K.; Stanton, C.; Schelkle, B.; et al. Is there evidence for bacterial transfer via the placenta and any role in the colonization of the infant gut? A systematic review. Crit. Rev. Microbiol. 2020, 46, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Avershina, E.; Lundgård, K.; Sekelja, M.; Dotterud, C.; Storrø, O.; Øien, T.; Johnsen, R.; Rudi, K. Transition from infant- to adult-like gut microbiota. Environ. Microbiol. 2016, 18, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef]
- Pärnänen, K.; Karkman, A.; Hultman, J.; Lyra, C.; Bengtsson-Palme, J.; Larsson, J.; Rautava, S.; Isolauri, E.; Salminen, S.; Kumar, H.; et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 2018, 9, 3891. [Google Scholar] [CrossRef]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar] [CrossRef]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef]
- Džidić, M.; Boix-Amorós, A.; Selma-Royo, M.; Mira, A.; Collado, M. Gut microbiota and mucosal immunity in the neonate. Med. Sci. 2018, 6, 56. [Google Scholar]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Prado Acosta, M.; Goyette-Desjardins, G.; Scheffel, J.; Dudeck, A.; Ruland, J.; Lepenies, B. S-Layer from Lactobacillus brevis Modulates Antigen-Presenting Cell Functions via the Mincle-Syk-Card9 Axis. Front. Immunol. 2021, 12, 602067. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Schuster, B. Electrochemical biosensors based on S-layer proteins. Sensors 2020, 20, 1721. [Google Scholar] [CrossRef] [PubMed]
- Lotfollahzadeh, S.; Hosseini, E.S.; Mahmoudi Aznaveh, H.; Nikkhah, M.; Hosseinkhani, S. TRAIL/S-layer/graphene quantum dot nanohybrid enhanced stability and anticancer activity of TRAIL on colon cancer cells. Sci. Rep. 2022, 12, 5851. [Google Scholar] [CrossRef]
- Šušković, J.; Kos, B.; Beganović, J.; Leboš Pavunc, A.; Habjanič, K.; Matošić, S. Antimicrobial activity of lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Kos, B.; Beganović, J.; Jurašić, L.; Švađumović, M.; Leboš Pavunc, A.; Uroić, K.; Šušković, J. Coculture-inducible bacteriocin biosynthesis of different probiotic strains by dairy starter culture Lactococcus lactis. Mljekarstvo 2011, 61, 273–282. [Google Scholar]
- De Giani, A.; Bovio, F.; Forcella, M.; Fusi, P.; Sello, G.; Di Gennaro, P. Identification of a bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express 2019, 9, 88. [Google Scholar] [CrossRef]
- Van Kranenburg, R.; Golić, N.; Bongers, R.; Leer, R.J.; de Vos, W.M.; Siezen, R.J.; Kleerebezem, M. Functional analysis of three plasmids from Lactobacillus plantarum. Appl. Environ. Microbiol. 2005, 71, 1223–1230. [Google Scholar] [CrossRef]
- Diep, D.B.; Straume, D.; Kjos, M.; Torres, C.; Nes, I.F. An overview of the mosaic bacteriocin pln loci from Lactobacillus plantarum. Peptides 2009, 30, 1562–1574. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Tabernero, A.; Cardea, S. Microbial Exopolysaccharides as Drug Carriers. Polymers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Banić, M.; Uroić, K.; Leboš Pavunc, A.; Novak, J.; Zorić, K.; Durgo, K.; Petković, H.; Jamnik, P.; Kazazić, S.; Kazazić, S.; et al. Characterization of S-layer proteins of potential probiotic starter culture Lactobacillus brevis SF9B isolated from sauerkraut. LWT-Food Sci. Technol. 2018, 93, 257–267. [Google Scholar] [CrossRef]
- Martín-Platero, A.M.; Valdivia, E.; Maqueda, M.; Martín-Sánchez, I.; Martínez-Bueno, M. Polyphasic Approach to Bacterial Dynamics during the Ripening of Spanish Farmhouse Cheese, Using Culture-Dependent and -Independent Methods. App. Environ. Microbiol. 2008, 74, 5662–5673. [Google Scholar] [CrossRef] [PubMed]
- Beganović, J.; Kos, B.; Leboš Pavunc, A.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef]
- Žugić Petrović, T.D.; Ilić, P.D.; Grujović, M.Ž.; Mladenović, K.G.; Kocić-Tanackov, S.D.; Čomić, L.R. Assessment of safety aspect and probiotic potential of autochthonous Enterococcus faecium strains isolated from spontaneous fermented sausage. Biotechnol. Lett. 2020, 42, 1513–1525. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Uroić, K.; Novak, J.; Hynӧnen, U.; Pietilä, T.E.; Leboš Pavunc, A.; Kant, R.; Kos, B.; Palva, A.; Šušković, J. The role of S-layer in adhesive and immunomodulating properties of probiotic starter culture Lactobacillus brevis D6 isolated from artisanal smoked fresh cheese. LWT-Food Sci. Technol. 2016, 69, 625–632. [Google Scholar] [CrossRef]
- Kos, B.; Šušković, J.; Vuković, S.; Šimpraga, M.; Frece, J.; Matošić, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef]
- Ferrari, M.; Hameleers, L.; Stuart, M.C.A.; Oerlemans, M.M.P.; de Vos, P.; Jurak, E.; Walvoort, M.T.C. Efficient isolation of membrane-associated exopolysaccharides of four commercial bifidobacterial strains. Carbohyd. Polym. 2022, 278, 118913. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Pearson, T.; Caporaso, J.G.; Yellowhair, M.; Bokulich, N.A.; Padi, M.; Roe, D.J.; Wertheim, B.C.; Linhart, M.; Martinez, J.A.; Bilagody, C.; et al. Effects of ursodeoxycholic acid on the gut microbiome and colorectal adenoma development. Cancer Med. 2019, 8, 617–628. [Google Scholar] [CrossRef] [PubMed]

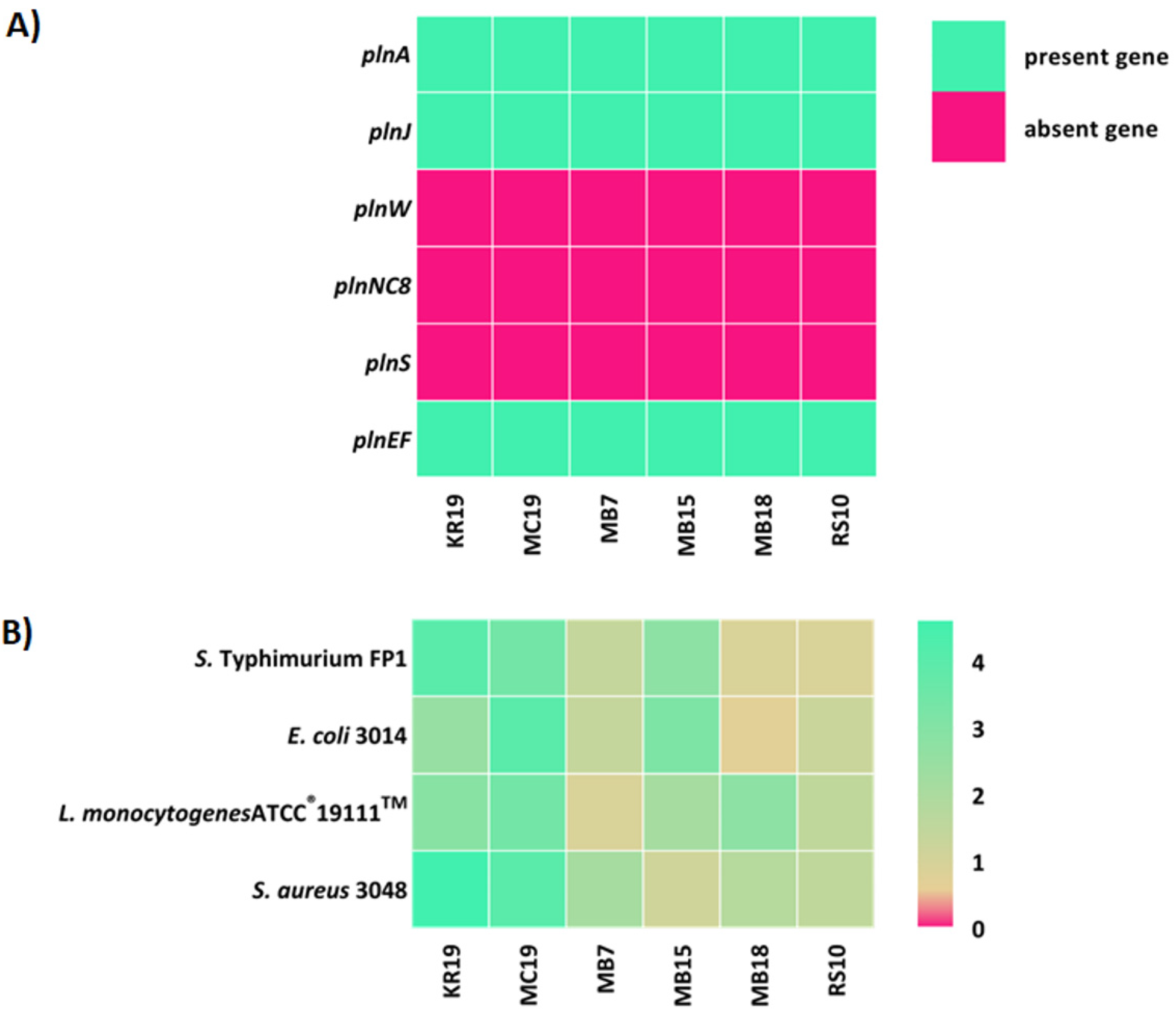

| Strain | Identification | % identity | Accession Number | e-Value |
|---|---|---|---|---|
| KR19 | Lactiplantibacillus plantarum | 98 | MG550988.1 | 0.0 |
| KR20 | Enterococcus faecium | 98 | MN566092.1 | 0.0 |
| MC1 | Limosilactobacillus fermentum | 98 | KY435712.1 | 0.0 |
| MC2 | Enterococcus faecalis | 98 | KC113205.1 | 0.0 |
| MC5 | Staphylococcus epidermis | 98 | CP035643.1 | 0.0 |
| MC13 | Enterococcus faecium | 97 | CP014529.1 | 0.0 |
| MC19 | Lactiplantibacillus plantarum | 97 | HM130542.1 | 0.0 |
| AF2 | Staphylococcus epidermidis | 96 | AY030340.1 | 0.0 |
| AF4 | Staphylococcus epidermidis | 97 | CP014119.1 | 0.0 |
| AF5 | Staphylococcus epidermis | 98 | CP043804.1 | 0.0 |
| AF12 | Enterococcus durans | 97 | MF405179.1 | 0.0 |
| AF16 | Enterococcus faecium | 98 | JN560856.1 | 0.0 |
| MB1 | Levilactobacillus brevis | 85 | MK774569.1 | 0.0 |
| MB2 | Levilactobacillus brevis | 84 | CP031208.1 | 3 × 10−7 |
| MB5 | Streptococcus oralis subsp. dentisani | 83 | CP034442.1 | 0.0 |
| MB6 | Staphylococcus epidermis | 98 | CP043804.1 | 0.0 |
| MB7 | Lactiplantibacillus plantarum | 95 | MF197402.1 | 0.0 |
| MB10 | Streptococcus salivarius | 97 | CP014144.1 | 0.0 |
| MB11 | Streptococcus oralis | 98 | LR134336.1 | 0.0 |
| MB13 | Levilactobacillus brevis | 88 | MT512175.1 | 0.0 |
| MB15 | Lactiplantibacillus plantarum | 97 | JQ801725.1 | 0.0 |
| MB18 | Lactiplantibacillus plantarum | 90 | MT604681.1 | 0.0 |
| MB20 | Levilactobacillus brevis | 88 | JQ805655.1 | 0.0 |
| RS4 | Streptococcus oralis | 92 | CP019562.1 | 0.0 |
| RS8 | Staphylococcus epidermis | 98 | AP019721.1 | 0.0 |
| RS10 | Lactiplantibacillus plantarum | 98 | AB362728.1 | 0.0 |
| RS17 | Staphylococcus epidermidis | 89 | MG557813.1 | 0.0 |
| RS19 | Streptococcus mitis | 98 | KX880968.1 | 0.0 |
| Peptide Monoisotopic Neutral Mass | Protein Score | MS/MS Score | Significance | Peptide Sequence |
|---|---|---|---|---|
| L. brevisMB1 | ||||
| 1271.7085 | 484 | 45 | no | K.VNIVDLSGNTIK.S |
| 1466.7405 | 98 | unique | K.AFGPDFAAAITSATK.G | |
| 1635.7781 | 100 | yes | K.AFGPDFAAAITSATK.G | |
| 1694.8363 | 103 | unique | K.DALVAAGVLYDSTSDAK.A | |
| 2499.2969 | 138 | yes | K.ADGWILLSNLTQTNALNEATQVK.V | |
| L. brevisMB2 | ||||
| 1271.7085 | 462 | 36 | no | K.VNIVDLSGNTIK.S |
| 1466.7405 | 87 | unique | K.AFGPDFAAAITSATK.G | |
| 1635.7781 | 106 | yes | K.AFGPDFAAAITSATK.G | |
| 1694.8363 | 92 | unique | K.DALVAAGVLYDSTSDAK.A | |
| 2499.2969 | 140 | yes | K.ADGWILLSNLTQTNALNEATQVK.V | |
| L. brevisMB13 | ||||
| 1271.7085 | 437 | 75 | unique | K.VNIVDLSGNTIK.S |
| 1466.7405 | 77 | unique | K.AFGPDFAAAITSATK.G | |
| 1635.7781 | 109 | unique | K.SATAFAGGLTSYDTFK.E | |
| 1694.8363 | 108 | unique | K.DALVAAGVLYDSTSDAK.A | |
| 2499.2969 | 118 | unique | K.ADGWILLSNLTQTNALNEATQVK.V | |
| L. brevisMB20 | ||||
| 1466.7405 | 647 | 85 | unique | K.AFGPDFAAAITSATK.G |
| 1635.7781 | 107 | unique | K.SATAFAGGLTSYDTFK.E | |
| 1694.8363 | 103 | unique | K.DALVAAGVLYDSTSDAK.A | |
| 1890.0211 | 21 | no | R.NVNLTGTNAIYTKPGTVK.G | |
| 2259.1383 | 21 | no | K.TIADTTAYKDATFSVDKVGTR.T | |
| 2499.2969 | 141 | unique | K.ADGWILLSNLTQTNALNEATQVK.V | |
| Nursing Mother | Sampling Time | Human Milk | Infant’s Faeces |
|---|---|---|---|
| KR | within one week after birth | KRM1 | KRF1 |
| one month after birth | KRM2 | KRF2 | |
| one month after the introduction of solid food into the infant’s diet | KRM3 | KRF3 | |
| MC | within one week after birth | MCM1 | MCF1 |
| one month after birth | MCM2 | MCF2 | |
| one month after the introduction of solid food into the infant’s diet | MCM3 | MCF3 | |
| AF | within one week after birth | AFM1 | AFF1 |
| one month after birth | AFM2 | AFF2 | |
| one month after the introduction of solid food into the infant’s diet | AFM3 | AFF3 | |
| MB | within one week after birth | MBM1 | MBF1 |
| one month after birth | MBM2 | MBF2 | |
| one month after the introduction of solid food into the infant’s diet | MBM3 | MBF3 | |
| RS | within one week after birth | RSM1 | RSF1 |
| one month after birth | RSM2 | RSF2 | |
| one month after the introduction of solid food into the infant’s diet | RSM3 | RSF3 |
| Strain | Identification | Accession Number | Biomolecule |
|---|---|---|---|
| MB1 | Levilactobacillus brevis | SAMN22155538 | S-layer protein |
| MB2 | Levilactobacillus brevis | SAMN22155539 | S-layer protein |
| MB13 | Levilactobacillus brevis | SAMN22155540 | S-layer protein |
| MB20 | Levilactobacillus brevis | SAMN22155541 | S-layer protein |
| MC19 | Lactiplantibacillus plantarum | SAMN22155542 | Plantaricin |
| KR19 | Lactiplantibacillus plantarum | SAMN22155536 | Plantaricin |
| MC1 | Limosilactobacillus fermentum | SAMN22155537 | EPS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banić, M.; Butorac, K.; Čuljak, N.; Leboš Pavunc, A.; Novak, J.; Bellich, B.; Kazazić, S.; Kazazić, S.; Cescutti, P.; Šušković, J.; et al. The Human Milk Microbiota Produces Potential Therapeutic Biomolecules and Shapes the Intestinal Microbiota of Infants. Int. J. Mol. Sci. 2022, 23, 14382. https://doi.org/10.3390/ijms232214382
Banić M, Butorac K, Čuljak N, Leboš Pavunc A, Novak J, Bellich B, Kazazić S, Kazazić S, Cescutti P, Šušković J, et al. The Human Milk Microbiota Produces Potential Therapeutic Biomolecules and Shapes the Intestinal Microbiota of Infants. International Journal of Molecular Sciences. 2022; 23(22):14382. https://doi.org/10.3390/ijms232214382
Chicago/Turabian StyleBanić, Martina, Katarina Butorac, Nina Čuljak, Andreja Leboš Pavunc, Jasna Novak, Barbara Bellich, Saša Kazazić, Snježana Kazazić, Paola Cescutti, Jagoda Šušković, and et al. 2022. "The Human Milk Microbiota Produces Potential Therapeutic Biomolecules and Shapes the Intestinal Microbiota of Infants" International Journal of Molecular Sciences 23, no. 22: 14382. https://doi.org/10.3390/ijms232214382
APA StyleBanić, M., Butorac, K., Čuljak, N., Leboš Pavunc, A., Novak, J., Bellich, B., Kazazić, S., Kazazić, S., Cescutti, P., Šušković, J., Zucko, J., & Kos, B. (2022). The Human Milk Microbiota Produces Potential Therapeutic Biomolecules and Shapes the Intestinal Microbiota of Infants. International Journal of Molecular Sciences, 23(22), 14382. https://doi.org/10.3390/ijms232214382







