Abstract
Herein, a novel approach used to enhance the conversion of electrochemical CO2 reduction (CO2R), as well as the capacity to produce C2 products, is reported. A copper oxide catalyst supported by graphite phase carbon nitride (CuO/g-C3N4) was prepared using a one-step hydrothermal method and exhibited a better performance than pure copper oxide nanosheets (CuO NSs) and spherical copper oxide particles (CuO SPs). The Faradaic efficiency reached 64.7% for all the C2 products, specifically 37.0% for C2H4, with a good durability at −1.0 V vs. RHE. The results suggest that the interaction between CuO and the two-dimensional g-C3N4 planes promoted CO2 adsorption, its activation and C-C coupling. This work offers a practical method that can be used to enhance the activity of electrochemical CO2R and the selectivity of C2 products through synergistic effects.
1. Introduction
According to statistics, the level of CO2 in the air is rising, which has led to global warming [1]. Compared with other measures, the chemical transformation of CO2 into carbon-containing chemicals is a promising option for CO2 mitigation. Among these options, electrocatalysis is considered to have high potential because it uses clean energy generated by electrical power and reacts in an aqueous solution at room temperature and pressure under mild reducing conditions [2,3]. As a result, it is environmentally friendly and transforms CO2 into fuels and chemical materials with significant added value [4].
Among the numerous metal catalysts, copper is the only one that has a moderate binding energy to intermediates, which can generate numerous C1 and high-value C2 and C2+ products [5,6]. Compared to copper, as a simple substance, oxide-derived copper catalysts at lower potentials exhibit a considerably enhanced CO2 electroreduction to C2 [7]. However, in conventional thermal conversion, it is difficult to meet the need for the increased reaction rates and selectivity of the target products at present. Although several novel synthetic strategies for copper-based catalysts have been reported, such as electrodeposition and plasma treatments [7,8,9,10], these complex synthesis methods often require the adoption of harsh reaction conditions and the use of expensive equipment, which hinder the widespread application of the related technologies. Consequently, it is necessary to create new methods that are simple and environmentally friendly for the preparation of catalysts.
Thus far, a wide range of CO2R techniques for oxide-derived copper catalysts have been reported. Ager et al. [11] prepared oxide-derived Cu catalysts based on Cu2O through electroreduction and obtained a selectivity of the C2+ products (FE = 60%) for at least 5 h at −1.0 V in 0.1 M potassium bicarbonate. Cui et al. [12] introduced N into Cu2O to produce nitrogen-doped Cu2O, which resulted in the enhanced CO2 adsorption and doubled Faradaic efficiency of ethylene (10%) compared to Cu2O. However, despite the excellent electrocatalytic performance of copper metal in regard to CO2, it is still affected by its complex reduction products and poor single-product selectivity and CO2R activity. Moreover, CO2 is poorly soluble in water. Thus, the overpotentials related to C2 generation reactions are high, which makes it more difficult for CO2 to be adsorbed on the catalyst surface and renders the competing hydrogen precipitation side reactions significant [13,14]. In this regard, Luo et al. [15] proposed that high CO2 pressure at the same concentration of the electrolyte can lead to a lower local pH, which will increase the surface coverage of CO, thus promoting the formation of C2 products. Wang et al. [16] created a Cu/NxC (nitrogen-doped carbon) interface and found that CO2 has a strong interaction with the Cu/NxC interface and is enriched on NxC, which increases the selectivity of C2 by 200–300%. Hence, there is a need to develop a novel catalyst that is more efficient and highly selective, following this line of reasoning.
Density functional theory (DFT) indicates that, for multi-carbon products, the ability of multiple *CO intermediates to engage in a C-C coupling process and influence the strength of *CO intermediate binding located in the catalytic active center are the determining factors [17]. In order to promote such an interaction, a number of enhancement studies have been undertaken, including the study of g-C3N4. It has a high thermal and chemical stability and special laminar structure, as well as a low cost, and its heterostructure provides a high CO2 adsorption and activation efficiency [18,19]. g-C3N4 contains a large amount of pyridine N. Theoretical calculations indicate that, as the main active site for electrocatalytic reactions, pyridine N, as a substrate, can complex with the metal nanoparticles to stabilize them and also provides an active center for CO2R. The interaction of g-C3N4 with metal also causes the metal surface to be highly electron-rich, thus enhancing the adsorption of reaction intermediates [20]. However, few experiments have been conducted to systematically verify this process. Jiao et al. [21] synthesized Cu-C3N4 to provide experimental evidence for the calculation of the g-C3N4 scaffold. In the synthesized samples, the analysis based on the N K-edge NEXAFS spectra and Cu 2p XPS confirmed the significant chemical interaction between N and Cu atoms. However, the main product of CO2R of Cu-C3N4 was still hydrogen (>50%). This suggests that the g-C3N4-loaded Cu-based catalysts still require further improvement. To date, there is no research that has been carried out to capitalize on the synergistic effects of g-C3N4 and copper oxide to enhance electrochemical CO2R and its selectivity.
In this work, starting with the material structure of electrocatalysts, CuO/g-C3N4 was prepared based on copper oxide catalysts using g-C3N4 as a carrier by a straightforward hydrothermal method combined with calcination. The morphology and components were analyzed, and the electrochemical performance and catalytic activity were investigated by drop coating the catalysts on carbon paper. During the discussion, the control experiments were performed using CuO NSs and CuO SPs, and the reasons for the efficient catalytic reduction to C2 products by the CuO/g-C3N4 electrode were comprehensively analyzed.
2. Results and Discussion
2.1. Electrochemical Activity Tests
LSV curves of the CuO SP, CuO NS and CuO/g-C3N4 catalysts in Ar- and CO2-saturated 0.1 M KHCO3 solution are displayed in Figure 1a. In the CO2-saturated electrolyte, clearly, all of the investigated catalysts showed higher current densities compared to those in the Ar-saturated electrolyte, demonstrating their great inherent activity for electrochemical CO2R. Under the same solution conditions, CuO/g-C3N4 also exhibited a smaller onset potential than the CuO NSs and CuO SPs. Additionally, CuO/g-C3N4 exhibited a noticeably improved current density from onset potential to −1.3 V vs. RHE relative to the other two CuO catalysts, thus implying its higher CO2R performance. Tafel curves for the overpotential-log (C2 current density) are plotted in Figure 1b. Compared with the CuO NSs and CuO SPs (27.8 mV·dec−1 and 33.6 mV·dec−1), the slope of CuO/g-C3N4 exhibits a significant decrease (17.2 mV·dec−1), which provides the further evidence of the better intrinsic properties of the CuO/g-C3N4 surface. The advantageous current density is also attributed to the lower charge transfer resistance of CuO/g-C3N4, reflecting the improved charge transfer process at the interface of the electrode and surrounding electrolyte, according to the EIS measurements and the fitting results in Figure S1 and Table S1.
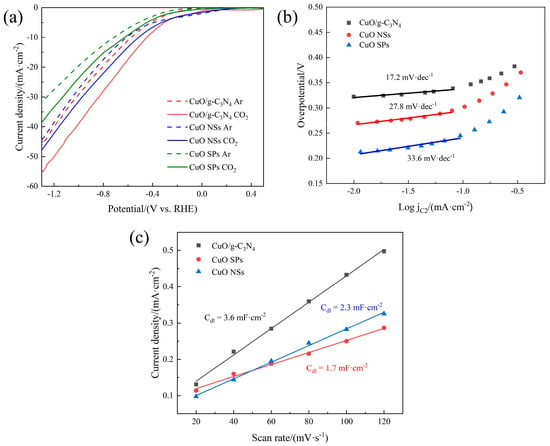
Figure 1.
(a) LSV curves of CuO SPs, CuO NSs and CuO/g-C3N4 in Ar- and CO2-saturated 0.1 M KHCO3 solution, (b) Tafel slope calculation curves of CuO SPs, CuO NSs and CuO/g-C3N4, and (c) capacitive current at OCP as a function of the scan rate of CuO SPs, CuO NSs and CuO/g-C3N4 in CO2-saturated KHCO3 solution.
The ECSA of the CuO SP, CuO NS and CuO/g-C3N4 electrodes were studied by contrast with the corresponding Cdl (double-layer capacitance). The CVs of these three electrodes are presented in Figure S2. The slope of the non-faradaic capacitive current (current density at OCP) versus the scan rate was used to calculate the Cdl value. In Figure 1c, it is clear that the Cdl of CuO/g-C3N4 (3.6 mF·cm−2) is much higher than that of the CuO NSs (2.3 mF·cm−2), indicating that CuO/g-C3N4 has a larger electrochemical active surface area and more exposed active sites, which are advantageous for boosting the CO2R activity. This also proves that the larger active surface area is caused by the g-C3N4 layer. Meanwhile, the Cdl of the CuO NSs is larger than that of the CuO SPs (1.7 mF·cm−2), suggesting that the nanosheets of CuO have a larger active surface area than the spherical nanoparticles of CuO.
2.2. Electrochemical CO2 Reduction Performance Tests
Furthermore, the CO2RR gaseous product distributions of the CuO SPs, CuO NSs and CuO/g-C3N4 were comparatively studied using a potential region of −0.8~−1.2 V vs. RHE. As given in Figure 2a, the CuO SPs consistently preferred CH4 production in all the testing potential regions, while the C2H4 selectivity was very low (<20%). This result indicates that regardless of the applied potentials, CuO SPs do not exhibit a particularly high C-C coupling activity. Comparatively to the CuO SPs, whose Faradaic efficiency was not higher than 5% at −0.8~−1.2 V, that of CH4 was suppressed for the CuO NSs. At the best applied potential of −1.0 V, the C2H4 production increased from 16.5% to 31.7%, and some ethane was produced as well (Figure 2b). However, in terms of the CuO/g-C3N4 electrocatalyst, the CH4 and CO productions were considerably further suppressed, totaling less than 6% at the potential of −1.0 V, as Figure 2c shows, while the CuO NS catalyst had an 8.1% Faradaic efficiency of C1 gaseous products at the same potential. Meanwhile, the C2H4 selectivity in CuO/g-C3N4 was significantly improved, whereas the H2 production caused by the HER side reaction clearly decreased. In particular, at the potential of −1.0 V, the C2H4 Faradaic efficiency reached as high as 37.0%, which was accompanied with H2 formation at 25.8%. A very small amount of HCOOH (8.8%) was also detected at this potential in the liquid products. Ethanol was reliably detected as well. At −0.8~−1.0 V, its Faradaic efficiency varied in the small range of 27.3~28.2%. The Faradaic efficiency for C2 was 64.7% for CuO/g-C3N4 at the respective optimal potential for C2 electroproduction. In brief, the CuO/g-C3N4 catalyst achieves a further improvement in the selectivity of C2 products.
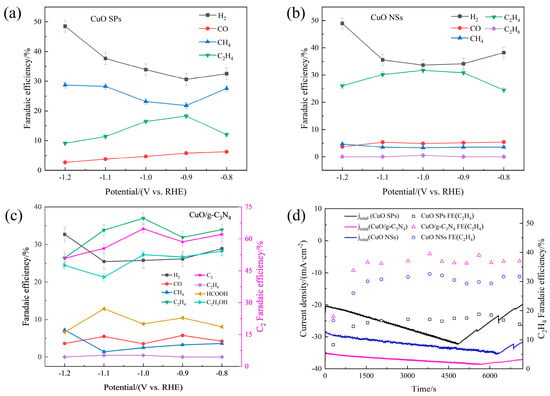
Figure 2.
Faradaic efficiencies of CO2 electroreduction products as a function of the potential: (a) CuO SPs, (b) CuO NSs and (c) CuO/g-C3N4. (d) Stability tests for CuO SPs, CuO NSs and CuO/g-C3N4 at −1.0 V vs. RHE.
Figure S3 compares the geometric partial current densities of several products for various electrodes. At each potential, the H2 formation rate for the electrodes follows the trend CuO/g-C3N4 < Cu NSs < CuO SPs, illustrating the H2 production caused by the competing HER was well controlled on CuO/g-C3N4. Meanwhile, the maximum C2H4 and C2 partial current densities occurred at −1.0 V in CuO/g-C3N4, reaching 14.0 mA·cm−2 and 24.5 mA cm−2, respectively. The above results show the exceptional performance of CO2R compared to that of the previously reported Cu-based electrocatalysts (Table S2).
CuO/g-C3N4 has an excellent activity and selectivity in addition to a good stability. Figure 2d shows its chronoamperometric responses after being biased for two hours at −1.0 V (corresponding to the highest C2H4 selectivity, especially for CuO/g-C3N4). The CuO/g-C3N4 and Cu NSs displayed an excellent stability in these two hours. In terms of CuO/g-C3N4, the total cathodic current density tended to smooth out and was still maintained at 37.0 mA·cm−2 at the end of the stability test. Its corresponding C2H4 selectivity experienced an initial increase and was then retained at over 37.0% throughout the electroreduction. The CuO SPs catalyst, on the other hand, demonstrated a quick catalytic deactivation. The current density dropped rapidly by 40% after 5000s, and there was also a downward trend in the Faradaic efficiency of C2H4 at the end, despite the fact that it experienced a spike during the previous experiment.
2.3. Structure and Morphology Characterizations
The surface characteristics of nano-adsorbents can be identified using N2 adsorption/desorption measurements. In Figure 3, for the CuO/g-C3N4 material, there is a mesoporous structure, as evidenced by the large hysteresis loop from 0.47 to 1.00 of P/P0, which can also be proved by its pore size distribution using the BJH method. Remarkably, the adsorbed quantity of CuO/g-C3N4 is more than that of the CuO NSs, demonstrating that CuO/g-C3N4 has a more consistent pore structure and a greater surface area [22,23]. The BET surface areas of the CuO NSs and CuO/g-C3N4, as obtained, are 7.62 m2/g and 11.2 m2/g, respectively. Meanwhile, a higher average pore volume of 0.048 cm3·g−1 is observed in CuO/g-C3N4 (versus 0.029 cm3·g−1 of the CuO NSs sample). In a word, g-C3N4 contributes to the increase in the surface area as well as the pore volume. Thus, CO2 can be better adsorbed, combined with the synergistic effects between CuO and g-C3N4.
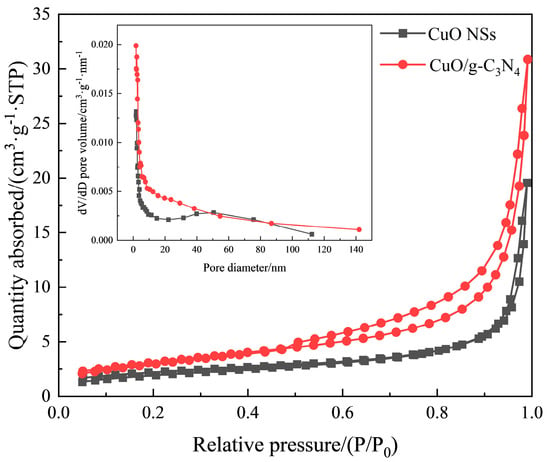
Figure 3.
N2 adsorption–desorption isotherm and BJH pore size distribution plots (insets) of CuO NSs and CuO/g-C3N4.
The crystal phase of the synthesized catalysts was revealed by the XRD patterns. According to Figure 4a, the graphitic materials with two specific diffraction planes, (100) and (002), correspond to the two typical diffraction peaks of pure g-C3N4 at 13.08° and 27.17°, which are caused by the interlayer stacking of conjugated aromatic rings and the in-plane structure of tri-s-triazine motifs [24,25]. In terms of the CuO NSs, the main peaks at 32.50°, 35.50°, 38.73°, 38.96° and 48.73° can be attributed to the crystal facets (−110), (002), (111), (200) and (−202) of CuO (JCPDS#45-0937), some of which are also depicted in the HRTEM and SAED patterns (Figure S4). The CuO/g-C3N4 composite allows for the observation of both g-C3N4 and CuO XRD diffraction peaks, and the absence of any additional distinctive peaks indicates the high purity of the samples immediately after preparation. Meanwhile, the cluster bands between 1248 and 1631 cm−1 in the FTIR spectra of g-C3N4 (Figure 4b) can be classified as the classic stretching mode of C-N heterocycles, and the heptazine ring system is the source of the 805 cm−1 sharp peak. The peaks of Cu-O stretching vibrations are also very obvious. The distinctive bands seen in CuO NSs at 425 cm−1 correspond to the CuO Au mode, and those at the slightly higher wavenumber position of 496 cm−1 correspond to the CuO Bu mode [26,27,28,29]. All of them can be seen in CuO/g-C3N4. This evidence prove that the two elements, g-C3N4 and CuO, coexist in the CuO/g-C3N4 material.
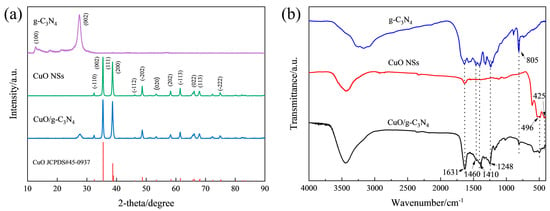
Figure 4.
(a) XRD patterns of g-C3N4, CuO NSs and CuO/g-C3N4, (b) FTIR spectra of g-C3N4, CuO NSs and CuO/g-C3N4.
The elemental compositions of g-C3N4 and CuO/g-C3N4 were examined by XPS spectroscopy, and the binding energies obtained for each of them were compared. The investigated spectra in Figure 5a confirm that all the anticipated elements are present, namely N 1s and C 1s for g-C3N4 and O 1s, Cu 2p, N 1s and C 1s for CuO/g-C3N4. In Figure 5b, the Cu 2p high-resolution peak of the composite CuO/g-C3N4 is observed. The pattern of CuO/g-C3N4 presents with a pair of peaks discovered at 932.7 and 952.5 eV that are associated with two typical energy levels of copper: 2p3/2 and 2p1/2. CuO crystals are present, according to the nearly 20 eV spin-orbit energy difference. This also indicates that the Cu in the sample is in the +II oxidation state. The small peaks at 940~945 eV are satellite peaks, which are generally seen in Cu ions in the oxidation state of +II. Furthermore, in Figure 5c, a C 1s binding energy peak is shown at 284.8 eV, which refers to g-C3N4 and CuO/g-C3N4, belonging to the C=N sp2 bond and the interaction of the metal oxide with g-C3N4 in the mixture. Similarly, on the s-triazine ring of graphitic nitride of g-C3N4 and CuO/g-C3N4, there are sp2 N-C=N bonds, which can be represented by the C 1s peaks at 288.1 and 287.9 eV. Assigned to pyridine nitrogen, pyrrolic nitrogen and graphitic nitrogen, respectively, the N 1s peak of g-C3N4 can be deconvoluted into three chemical states, i.e., the peaks at 398.5, 399.0 and 400.6 eV. In terms of pyridine N, the binding energy of the CuO/g-C3N4 sample (398.3 eV) is 0.2 eV lower than that of pure g-C3N4 (398.5 eV), impacting the interaction between the two molecules at the interface (Figure 5d) [30,31,32,33,34,35].
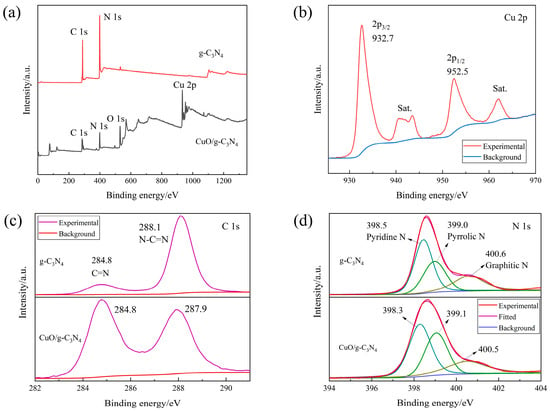
Figure 5.
XPS spectra of g-C3N4 and CuO/g-C3N4: (a) full scan, (b) Cu 2p, (c) C 1s, (d) N 1s.
The morphology of g-C3N4, CuO and CuO/g-C3N4 were confirmed using FESEM (Figure 6) and TEM (Figure S5). In Figure 6a, the pure g-C3N4 is composed of irregular and loose aggregates of sheet-like structures. The lamellae are formed as a result of the thermal breakdown of the urea fracture while producing a large number of pores, and this rough appearance confers a very high functionalization on the g-C3N4 sheets. In Figure 6b, it can be seen that a large number of CuO nanosheets of varying lengths were synthesized, with an average width of about 300 nm. Figure 6c demonstrates that, in the case of the CuO/g-C3N4 composites, it is evident that the particles with smooth surfaces in the connected sheets of agglomerates are considered as CuO NSs loaded onto the g-C3N4 matrix. Meanwhile, the corresponding elemental mapping (Figure 6d–g) also shows that the C, N, O and Cu atoms are evenly distributed throughout the composite, indicating the emergence of the CuO/g-C3N4 structure and the close contact between them.
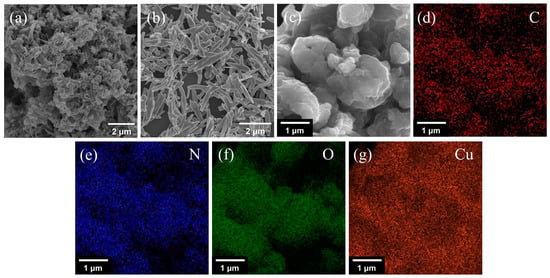
Figure 6.
FESEM images of (a) g-C3N4, (b) CuO NSs, (c) CuO/g-C3N4, and (d–g) elemental mapping results of C (red), N (blue), O (green), and Cu (orange) of CuO/g-C3N4.
3. Materials and Methods
3.1. Materials
Urea, polyvinyl pyrrolidone K30 (PVP-K30), Cu(NO3)2·3H2O, sodium acetate (C2H3NaO2), NaOH, KHCO3, isopropanol (C3H8O) and acetone (C3H6O) were purchased from Sinopharm Chemical Reagent. Sodium dodecyl sulfate (SDS), ethanol (99.7%), Nafion-117 solution (~5%) and CuO SPs (40 nm, 99.5%) were purchased from Macklin. Carbon paper (GDS180S) was purchased from Ce Tech. Each chemical was used directly as received, and UP water (>18.2 × 106 Ω·cm) was employed to dispense the whole aqueous solution.
3.2. Preparation of Catalysts
3.2.1. Preparation of g-C3N4
A total of 20 g urea was placed into a 50 mL crucible, and after that, the prepared sample was placed in a muffle furnace, directly heated for 2 h in air conditions at 540 °C and a rate of 10 °C min−1. After allowing it to naturally drop to an ambient temperature, the g-C3N4 sample was obtained.
3.2.2. Preparation of CuO NSs
A total of 0.238 g Cu(NO3)2·3H2O, 0.173 g C2H3NaO2, 0.500 g PVP-K30 and 0.295 g SDS were dissolved in 100 mL H2O. Then, under continuous rapid stirring, 0.1 M NaOH was added dropwise until a pH > 12 was obtained. The suspension was filled in a 200 mL stainless-steel autoclave with a Teflon lining and heated for 24 h at 170 °C. The resultant sample was obtained by centrifuging the suspension, repeatedly washing it in H2O and EtOH, and then heating it to 550 °C (5 °C min−1) and holding it there for two hours in air.
3.2.3. Preparation of g-C3N4
The procedure was similar to that of CuO NSs, except for the fact that 0.100 g g-C3N4 was introduced in the first instance.
3.3. Characterization
The morphology and structure of the studied samples were characterized by field emission scanning electron microscopy (FESEM, Regulus 8100, 5 kV) and transmission electron microscopy (TEM, Tecnai G2 F20, 200 kV). Energy-dispersive X-ray spectroscopy (EDS) mapping was conducted using FESEM Regulus 8100. The atomic valence states and some molecular structures were investigated by X-ray photoelectron spectroscopy (XPS, Thermo Escalab 250), and the source gun type was Al Kα. X-ray diffraction (XRD) was carried out using D8 Advance, produced by German Bruker-AXS, operating at 40 kV and 40 mA with an accuracy of 0.01° (2θ) at room temperature. Fourier transform infrared spectrometer (FTIR) spectra were measured in the 400–4000 cm−1 range using a Nicolet iS50 FT-IR spectrometer, with the samples prepared as KBr pellets. Brunauer–Emmett–Teller (BET) surface area measurements were performed at 77 K using a TriStar II 3020 adsorption analyzer in the N2 adsorption mode.
3.4. Electrochemical Measurements
The carbon paper was pre-treated with acetone and washed at least 3 times with H2O and EtOH before being air-dried. A total of 4 mg of the sample (CuO/g-C3N4, CuO NSs or CuO SPs) was dispersed in 1 mL isopropanol and 30 μL Nafion solution, followed by sonication for 30 min to create the sample ink. Then, the ink was homogeneously added drop by drop onto the carbon paper (2 × 2 cm2) and dried on a hot plate, and then it was divided into working electrodes with a surface area of 1 × 1 cm2.
The electrochemical measurements were performed using an electrochemistry workstation (Gamry Reference 300) in a three-electrode system. A Nafion-117 membrane divided the two compartments of the H-cell. As the counter electrode and reference electrode, respectively, platinum grid and Ag/AgCl electrode (saturated KCl) were employed. CO2-saturated (pH ≈ 6.8) or Ar-saturated 0.1 M KHCO3 (pH ≈ 8.3) was used as the electrolyte. Additionally, each measurement potential was standardized to the reversible hydrogen electrode (RHE) reference scale, together with manual internal resistance compensation:
The iRu was determined by potentiostatic electrochemical impedance spectroscopy measurements under an open circuit potential (OCP) at frequencies ranging from 105 Hz to 0.1 Hz. The linear sweep voltammetry (LSV) was tested in the same environment at a sweep rate of 10 mV·s−1. The current density equals the testing current divided by the geometric surface area of working electrode. The double-layer capacitance method was used to conduct the electrochemical active surface area (ECSA) tests. The potential range was OCP ± 50 mV, and cyclic voltammetry was performed at different sweep speeds. Gas chromatography (Agilent GC 8890) was used to identify the resulting gas phase products, and NMR (AVANCE III HD 400 MHz) was used to detect the products in the liquid phase.
3.5. Calculation of the Faradaic Efficiency
The following equation was used to calculate the Faradaic efficiency of the gas products:
Above, n is the amount of e− that is transferred to the product formation. C is the concentration (ppm) of the gases revealed by GC. G is rate of CO2. I is the cell current. P = 1.01×105 Pa. R is the universal gas constant. T = 273.15 K.
The Faradaic efficiency of the liquid products was calculated by following equation:
Above, c is the concentration of the product. V is the total volume of the cathodic electrolyte and e is the electron. Q is the number of the transfer charge.
4. Conclusions
In summary, a CuO/g-C3N4 catalyst was fabricated by a simple hydrothermal method and achieved highly active and selective electrochemical CO2R to C2 products. The catalyst demonstrates a significant advantage over pure CuO nanosheets and spherical CuO particles and shows a high Faradaic efficiency of 37.0% for C2H4 at −1.0 V vs. RHE. It also has an ethylene catalytic stability that lasts for at least two hours. Meanwhile, the Faradaic efficiencies of all the C2 products of the composite are 64.7%, performing better than many other Cu-based catalysts, which indicates a synergistic promotion of C-C coupling between CuO and g-C3N4. Moreover, the structure and morphology characterizations demonstrated that the composite is based on g-C3N4-supported uniform polycrystalline copper oxide. The introduction of g-C3N4 increases the specific surface area, which promotes the mass transfer kinetics and provides new opportunities for the adsorption of CO2 and the exposure of active sites. Additionally, the interaction of pyridine N with copper oxide was confirmed, which further increases the reaction activity of CO2 reduction. This work provides an effective strategy that can be used to improve the selectivity and activity of C2 formation during the electrochemical reduction of CO2 and bridges the gap between the laboratory-based conversion of CO2 to economically valuable chemicals and its industrial application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232214381/s1. Refs. [36,37,38,39,40,41,42,43,44,45,46,47] are cited.
Author Contributions
Conceptualization, Z.Y.; formal analysis, Z.Y.; supervision, T.W.; validation, T.W.; writing—original draft, Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Ningbo Municipal Science and Technology Program (2018B10023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pires, J.; Martins, F.; Alvim-Ferraz, M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Nielsen, D.U.; Hu, X.-M.; Daasbjerg, K.; Skrydstrup, T. Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nat. Catal. 2018, 1, 244–254. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 reduction: A classification problem. ChemPhysChem 2017, 18, 3266–3273. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zegkinoglou, I.; Divins, N.J.; Scholten, F.; Sinev, I.; Grosse, P.; Roldan Cuenya, B. Plasma-activated copper nanocube catalysts for efficient carbon dioxide electroreduction to hydrocarbons and alcohols. ACS Nano 2017, 11, 4825–4831. [Google Scholar] [CrossRef]
- Scholten, F.; Sinev, I.; Bernal, M.; Roldan Cuenya, B. Plasma-modified dendritic Cu catalyst for CO2 electroreduction. ACS Catal. 2019, 9, 5496–5502. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, X.; Yang, D.; Ma, J.; Kang, X.; Zheng, L.; Zhang, J.; Wu, Z.; Han, B. Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex. Nat. Commun. 2019, 10, 3851. [Google Scholar] [CrossRef]
- Song, Y.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.; Wu, Z.; Meyer, H.M., III; Chi, M.; Ma, C.; Sumpter, B.G. High-selectivity electrochemical conversion of CO2 to ethanol using a copper nanoparticle/N-doped graphene electrode. ChemistrySelect 2016, 1, 6055–6061. [Google Scholar] [CrossRef]
- Lum, Y.; Ager, J.W. Stability of residual oxides in oxide-derived copper catalysts for electrochemical CO2 reduction investigated with 18O labeling. Angew. Chem. Int. Ed. 2018, 57, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Luo, W.; Yuan, H.; Liu, G.; Hao, R.; Qin, N.; Wang, Z.; Liu, K.; Wang, Z.; Cui, D. Stabilizing intermediates and optimizing reaction processes with N doping in Cu2O for enhanced CO2 electroreduction. Appl. Catal. B 2022, 308, 121191. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, S.; Jiang, S.P.; Wang, S. Supported single atoms as new class of catalysts for electrochemical reduction of carbon dioxide. Small Methods 2019, 3, 1800440. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Ma, J.; Zhang, Z.; Hu, W. Recent advances in atomic-level engineering of nanostructured catalysts for electrochemical CO2 reduction. Adv. Funct. Mater. 2020, 30, 1910534. [Google Scholar] [CrossRef]
- Mi, Y.; Peng, X.; Liu, X.; Luo, J. Selective formation of C2 products from electrochemical CO2 reduction over Cu1. 8Se nanowires. ACS Appl. Energy Mater. 2018, 1, 5119–5123. [Google Scholar]
- Li, Z.; Yang, Y.; Yin, Z.; Wei, X.; Peng, H.; Lyu, K.; Wei, F.; Xiao, L.; Wang, G.; Abruna, H.D. Interface-enhanced catalytic selectivity on the C2 products of CO2 electroreduction. ACS Catal. 2021, 11, 2473–2482. [Google Scholar] [CrossRef]
- Chan, Y.-T.; Huang, I.-S.; Tsai, M.-K. Enhancing C–C bond formation by surface strain: A computational investigation for C2 and C3 intermediate formation on strained Cu surfaces. Phys. Chem. Chem. Phys. 2019, 21, 22704–22710. [Google Scholar] [CrossRef]
- Ghosh, U.; Majumdar, A.; Pal, A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures: Recent progress and prospects. J. Environ. Chem. Eng. 2021, 9, 104631. [Google Scholar] [CrossRef]
- Shi, H.; Chen, G.; Zhang, C.; Zou, Z. Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel. ACS Catal. 2014, 4, 3637–3643. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, Z.; Guo, S.; Han, M.; Zhu, C.; Zhou, Y.; Bai, L.; Gao, J.; Huang, H.; Li, Y. Enhanced activity for CO2 electroreduction on a highly active and stable ternary Au-CDots-C3N4 electrocatalyst. ACS Catal. 2018, 8, 188–197. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Chen, P.; Jaroniec, M.; Qiao, S.-Z. Molecular scaffolding strategy with synergistic active centers to facilitate electrocatalytic CO2 reduction to hydrocarbon/alcohol. J. Am. Chem. Soc. 2017, 139, 18093–18100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Zhao, Y.; Xu, Z.; Chen, L.; Zhao, L.; Tian, X.; Sun, W. Superior adsorption of 3D nanoporous architectures for Ni (II) ions adsorption using polyvinyl alcohol as cross-linking agent and adsorption conveyor. RSC Adv. 2018, 8, 7899–7903. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, W.; Li, W.-T.; Song, C.; He, Y.-S.; Razal, J.M.; Ma, Z.-F.; Chen, J. N-doped pierced graphene microparticles as a highly active electrocatalyst for Li-air batteries. 2D Mater. 2015, 2, 024002. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, J.; Yang, Y.; Wang, D.; Bie, L.; Fahlman, B.D. Novel g-C3N4/CoO nanocomposites with significantly enhanced visible-light photocatalytic activity for H2 evolution. ACS Appl. Mater. Interfaces 2017, 9, 12427–12435. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, S.P.; Reddy, B.P.; Byon, C.; Shim, J. Carbon/CuO nanosphere-anchored g-C3N4 nanosheets as ternary electrode material for supercapacitors. J. Solid State Chem. 2018, 262, 106–111. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M. Green synthesis of 2D CuO nanoleaves (NLs) and its application for the reduction of p-nitrophenol. Mater. Lett. 2015, 161, 79–82. [Google Scholar] [CrossRef]
- Kliche, G.; Popovic, Z. Far-infrared spectroscopic investigations on CuO. Phys. Rev. B 1990, 42, 10060. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wang, Q.; Jin, Y.; Huang, D.; Cui, Q.; Zou, G. Nitrogen-rich carbon nitride hollow vessels: Synthesis, characterization, and their properties. J. Phys. Chem. B 2010, 114, 9429–9434. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, X. Solution-phase synthesis of CuO hierarchical nanosheets at near-neutral pH and near-room temperature. Mater. Lett. 2007, 61, 2222–2226. [Google Scholar] [CrossRef]
- Duan, Y. Facile preparation of CuO/g-C3N4 with enhanced photocatalytic degradation of salicylic acid. Mater. Res. Bull. 2018, 105, 68–74. [Google Scholar] [CrossRef]
- Zhao, L.; Kuang, X.; Liu, Z.; Hou, Y.; Wang, Z.; Wei, Q.; Lee, J.Y.; Kang, B. Anchoring CuO nanoparticles on C, N-codoped G-C3N4 nanosheets from melamine-entrapped MOF gel for high-efficiency oxygen evolution. ChemNanoMat 2019, 5, 1170–1175. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Tian, W.; Cao, J. Graphitic carbon nitride nanosheets decorated flower-like NiO composites for high-performance triethylamine detection. ACS Omega 2019, 4, 9645–9653. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, C.; Wang, H.; Dou, Y.; Shi, W.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. High-performance NiO/g-C3N4 composites for visible-light-driven photocatalytic overall water splitting. Inorg. Chem. Front. 2018, 5, 1646–1652. [Google Scholar] [CrossRef]
- Cao, S.-W.; Yuan, Y.-P.; Barber, J.; Loo, S.C.J.; Xue, C. Noble-metal-free g-C3N4/Ni(dmgH)2 composite for efficient photocatalytic hydrogen evolution under visible light irradiation. Appl. Surf. Sci. 2014, 319, 344–349. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (0 0 1) vs (1 0 1) facets of TiO2. Appl. Catal. B 2015, 164, 420–427. [Google Scholar]
- Wu, M.; Zhu, C.; Wang, K.; Li, G.; Dong, X.; Song, Y.; Xue, J.; Chen, W.; Wei, W.; Sun, Y. Promotion of CO2 Electrochemical Reduction via Cu Nanodendrites. ACS Appl. Mater. Interfaces 2020, 12, 11562–11569. [Google Scholar] [CrossRef]
- Altaf, N.; Liang, S.; Huang, L.; Wang, Q. Electro-derived Cu-Cu2O nanocluster from LDH for stable and selective C2 hydrocarbons production from CO2 electrochemical reduction. J. Energy Chem. 2019, 48, 169–180. [Google Scholar] [CrossRef]
- Handoko, A.D.; Ong, C.W.; Huang, Y.; Lee, Z.G.; Lin, L.; Panetti, G.B.; Yeo, B.S. Mechanistic insights into the selective electroreduction of carbon dioxide to ethylene on Cu2O-derived copper catalysts. J. Phys. Chem. C 2016, 120, 20058–20067. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.; Wang, J.; Kulinich, S.A.; Du, X.-W. Laser-Prepared CuZn Alloy Catalyst for Selective Electrochemical Reduction of CO2 to Ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef]
- Jeon, H.S.; Kunze, S.; Scholten, F.; Roldan Cuenya, B. Prism-shaped Cu nanocatalysts for electrochemical CO2 reduction to ethylene. ACS Catal. 2018, 8, 531–535. [Google Scholar] [CrossRef]
- Zhuang, T.-T.; Liang, Z.-Q.; Seifitokaldani, A.; Li, Y.; De Luna, P.; Burdyny, T.; Che, F.; Meng, F.; Min, Y.; Quintero-Bermudez, R.; et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 2018, 1, 421–428. [Google Scholar] [CrossRef]
- De Luna, P.; Quintero-Bermudez, R.; Dinh, C.-T.; Ross, M.B.; Bushuyev, O.S.; Todorović, P.; Regier, T.; Kelley, S.O.; Yang, P.; Sargent, E.H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110. [Google Scholar] [CrossRef]
- Kim, D.; Kley, C.S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl. Acad. Sci. USA 2017, 114, 10560–10565. [Google Scholar] [CrossRef] [PubMed]
- Ting LR, L.; Pique, O.; Lim, S.Y.; Tanhaei, M.; Calle-Vallejo, F.; Yeo, B.S. Enhancing CO2 electroreduction to ethanol on copper–silver composites by opening an alternative catalytic pathway. ACS Catal. 2020, 10, 4059–4069. [Google Scholar] [CrossRef]
- Jiang, K.; Sandberg, R.B.; Akey, A.J.; Liu, X.; Bell, D.C.; Nørskov, J.K.; Chan, K.; Wang, H. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 2018, 1, 111–119. [Google Scholar] [CrossRef]
- Torelli, D.A.; Francis, S.A.; Crompton, J.C.; Javier, A.; Thompson, J.R.; Brunschwig, B.S.; Soriaga, M.P.; Lewis, N.S. Nickel–gallium-catalyzed electrochemical reduction of CO2 to highly reduced products at low overpotentials. ACS Catal. 2016, 6, 2100–2104. [Google Scholar] [CrossRef]
- Kim, T.; Palmore, G.T.R. A scalable method for preparing Cu electrocatalysts that convert CO2 into C2+ products. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).