Oxidative Precipitation Synthesis of Calcium-Doped Manganese Ferrite Nanoparticles for Magnetic Hyperthermia
Abstract
1. Introduction
2. Results and Discussion
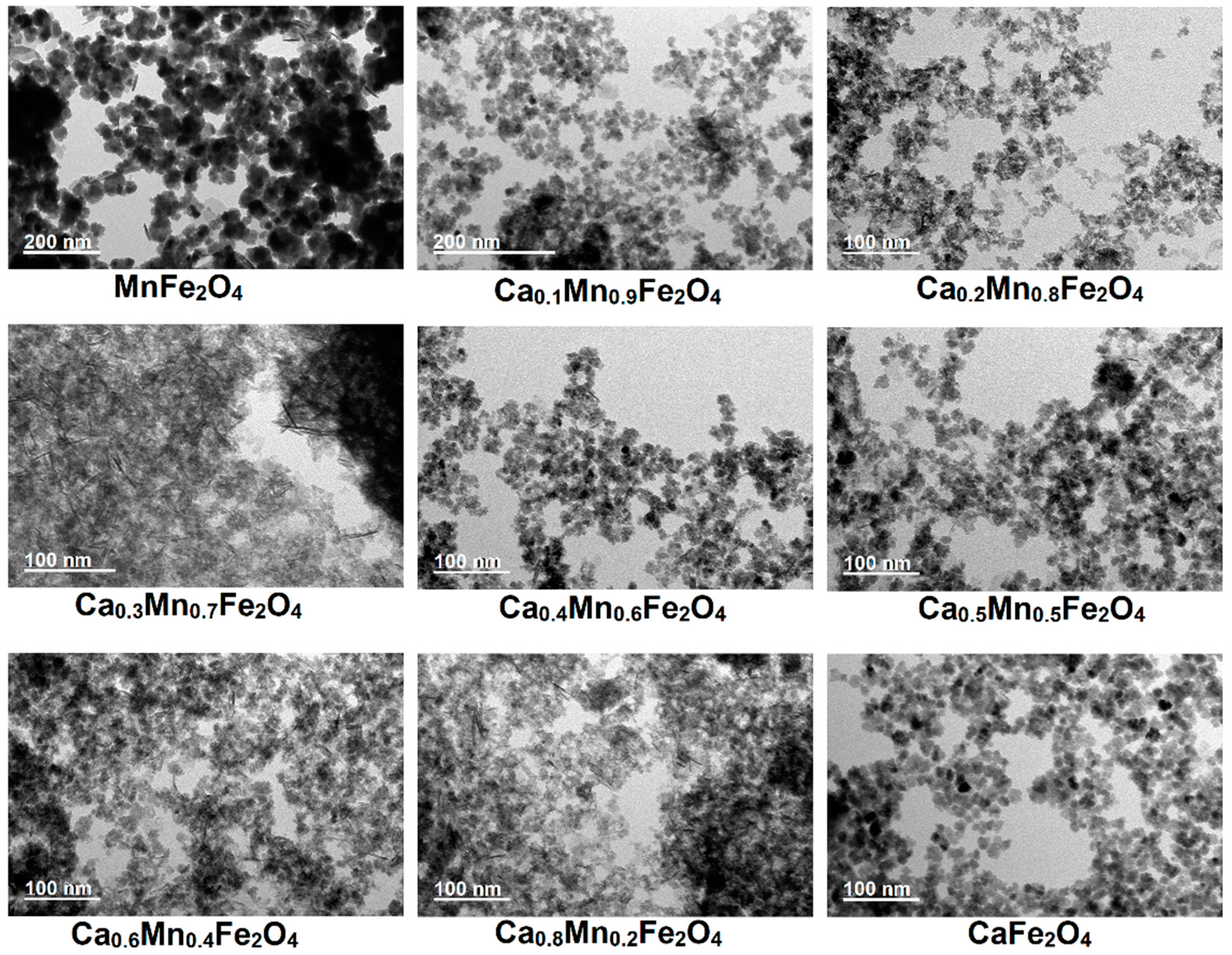
2.1. Nanoparticles Morphology
2.2. Microstructure Characterization
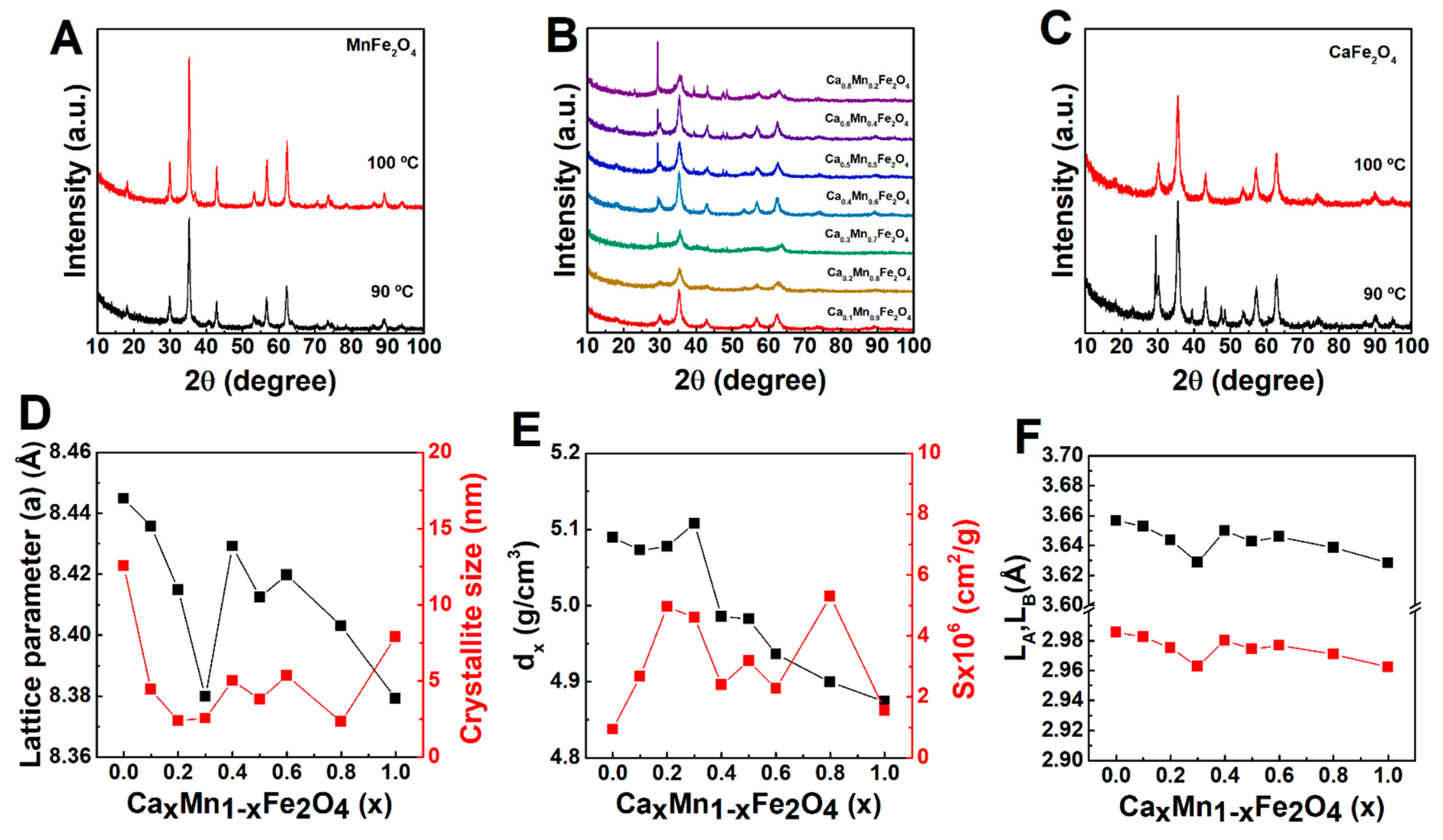
2.2.1. X-ray Diffraction Analysis
2.2.2. Raman Spectroscopy Characterization
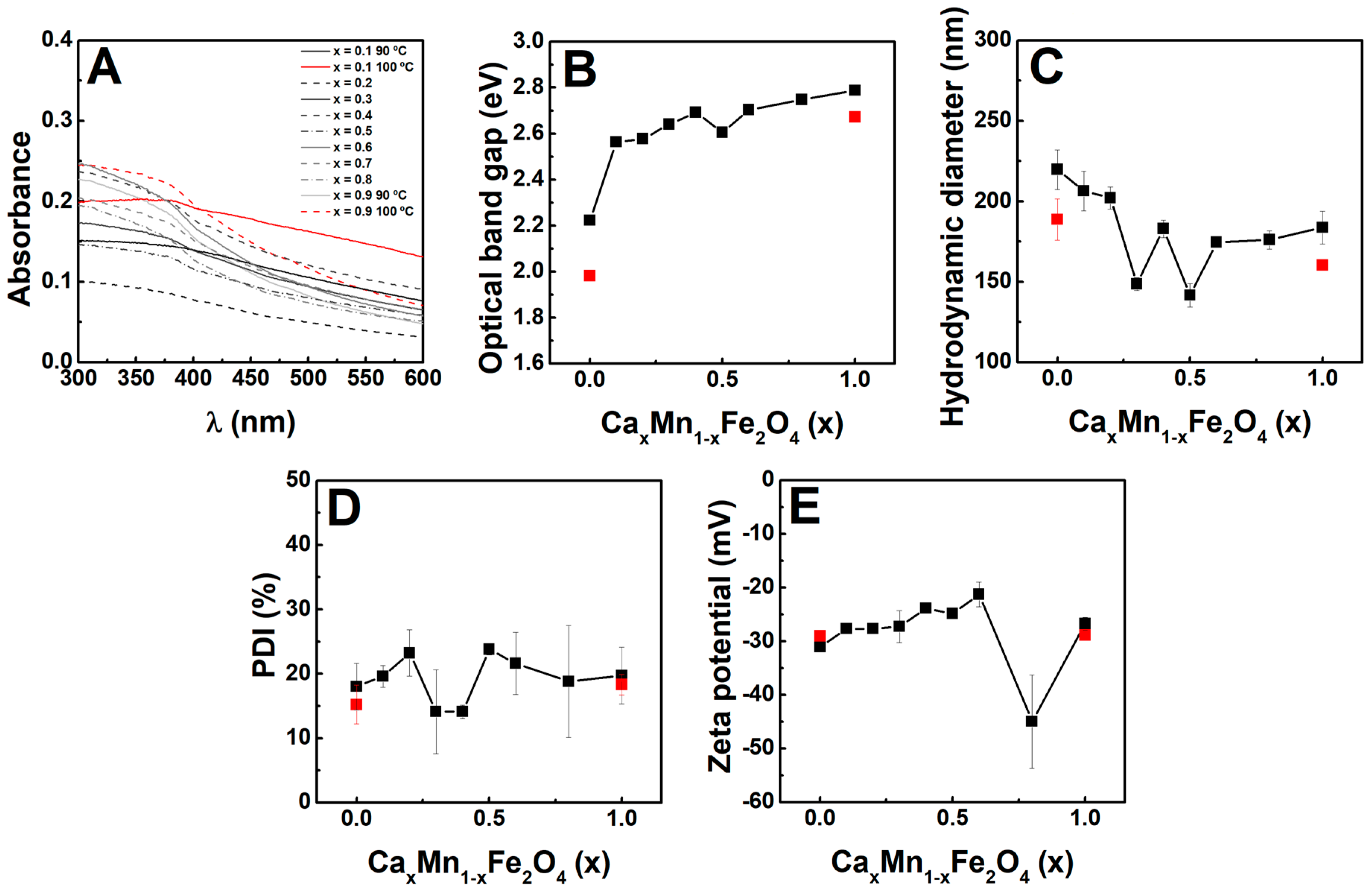
2.3. Optical Properties
2.4. Hydrodynamic Diameter and Zeta Potential
2.5. Magnetic Properties
2.6. Magnetic Hyperthermia
3. Materials and Methods
3.1. Synthesis of Magnetic Nanoparticles
3.2. General Spectroscopic Methods
3.3. Transmission Electron Microscopy (TEM)
3.4. Magnetic Properties
3.5. Hyperthermia Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Kurian, J.; Lahiri, B.B.; Mathew, M.J.; Philip, J. High Magnetic Fluid Hyperthermia Efficiency in Copper Ferrite Nanoparticles Prepared by Solvothermal and Hydrothermal Methods. J. Magn. Magn. Mater. 2021, 538, 168233. [Google Scholar] [CrossRef]
- Gahrouei, Z.E.; Labbaf, S.; Kermanpur, A. Cobalt Doped Magnetite Nanoparticles: Synthesis, Characterization, Optimization and Suitability Evaluations for Magnetic Hyperthermia Applications. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 116, 113759. [Google Scholar] [CrossRef]
- Chand, P.; Vaish, S.; Kumar, P. Structural, Optical and Dielectric Properties of Transition Metal (MFe2O4; M = Co, Ni and Zn) Nanoferrites. Phys. B Condens. Matter 2017, 524, 53–63. [Google Scholar] [CrossRef]
- Doaga, A.; Cojocariu, A.M.; Amin, W.; Heib, F.; Bender, P.; Hempelmann, R.; Caltun, O.F. Synthesis and Characterizations of Manganese Ferrites for Hyperthermia Applications. Mater. Chem. Phys. 2013, 143, 305–310. [Google Scholar] [CrossRef]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Mahapatra, S.; Raghunath, R.; Kumar, A.V.; Sadasivan, S. Daily Intake of Manganese by Adult Population of Mumbai, India. Sci. Total Environ. 2002, 299, 73–77. [Google Scholar] [CrossRef]
- Ma, H.; Guo, L.; Zhang, H.; Wang, Y.; Miao, Y.; Liu, X.; Peng, M.; Deng, X.; Peng, Y.; Fan, H. The Metal Ion Release of Manganese Ferrite Nanoparticles: Kinetics, Effects on Magnetic Resonance Relaxivities, and Toxicity. ACS Appl. Bio Mater. 2022, 5, 3067–3074. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, S.; Kang, X.; Sun, T.; Zhou, C.; Xu, Z.; Du, M.; Zhang, Y.; Wang, G.; Liu, Y.; et al. Metabolic Conversion and Removal of Manganese Ferrite Nanoparticles in Raw264.7 Cells and Induced Alteration of Metal Transporter Gene Expression. Int. J. Nanomed. 2021, 16, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Khanna, L.; Verma, N.K. Biocompatibility and Superparamagnetism in Novel Silica/CaFe2O4 Nanocomposite. Mater. Lett. 2014, 128, 376–379. [Google Scholar] [CrossRef]
- Noor, A.; Akhtar, M.N.; Khan, S.N.; Nazir, M.S.; Yousaf, M. Synthesis, Morphological and Electromagnetic Evaluations of Ca Doped Mn Spinel Nanoferrites for GHz Regime Applications. Ceram. Int. 2020, 46, 13961–13968. [Google Scholar] [CrossRef]
- Khanna, L.; Verma, N.K. Size-Dependent Magnetic Properties of Calcium Ferrite Nanoparticles. J. Magn. Magn. Mater. 2013, 336, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Mak, K.Y.; Leung, C.W.; Chan, K.Y.; Chan, W.K.; Zhong, W.; Pong, P.W.T. Effect of Synthesis Conditions on the Properties of Citric-Acid Coated Iron Oxide Nanoparticles. Microelectron. Eng. 2013, 110, 329–334. [Google Scholar] [CrossRef]
- Poller, W.C.; Löwa, N.; Schleicher, M.; Münster-Wandowski, A.; Taupitz, M.; Stangl, V.; Ludwig, A.; Wiekhorst, F. Initial Interaction of Citrate-Coated Iron Oxide Nanoparticles with the Glycocalyx of THP-1 Monocytes Assessed by Real-Time Magnetic Particle Spectroscopy and Electron Microscopy. Sci. Rep. 2020, 10, 3591. [Google Scholar] [CrossRef] [PubMed]
- Granath, T.; Mandel, K.; Löbmann, P. Overcoming the Inhibition Effects of Citrate: Precipitation of Ferromagnetic Magnetite Nanoparticles with Tunable Morphology, Magnetic Properties, and Surface Charge via Ferrous Citrate Oxidation. Part. Part. Syst. Charact. 2021, 38, 2100098. [Google Scholar] [CrossRef]
- Maurizi, L.; Bouyer, F.; Paris, J.; Demoisson, F.; Saviot, L.; Millot, N. One Step Continuous Hydrothermal Synthesis of Very Fine Stabilized Superparamagnetic Nanoparticles of Magnetite. Chem. Commun. 2011, 47, 11706–11708. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The Design and Utility of Polymer-Stabilized Iron-Oxide Nanoparticles for Nanomedicine Applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef]
- Jawad, A.; Al-Abodi, E.E. Investigating (Fe3O4) Magnetic Nanoparticles Impregnated onto Tri-Sodium Citrate to Remove, of Methylene Blue Dye from Aqueous Solutions. AIP Conf. Proc. 2019, 2123, 020026. [Google Scholar] [CrossRef]
- Ur Rahman, Z.; Dong, Y.L.; Ren, C.; Zhang, Z.Y.; Chen, X. Protein Adsorption on Citrate Modified Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, A.; Nazeer, S.S.; Jeevan, M.; Nimi, N.; Arumugam, S.; Harikrishnan, V.S.; Varma, P.R.H.; Jayasree, R.S. Citrate Coated Iron Oxide Nanoparticles with Enhanced Relaxivity for in Vivo Magnetic Resonance Imaging of Liver Fibrosis. Colloids Surf. B Biointerfaces 2014, 117, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Cheraghipour, E.; Javadpour, S.; Mehdizadeh, A.R. Citrate Capped Superparamagnetic Iron Oxide Nanoparticles Used for Hyperthermia Therapy. J. Biomed. Sci. Eng. 2012, 5, 715–719. [Google Scholar] [CrossRef]
- Asimakidou, T.; Makridis, A.; Veintemillas-Verdaguer, S.; Morales, M.P.; Kellartzis, I.; Mitrakas, M.; Vourlias, G.; Angelakeris, M.; Simeonidis, K. Continuous Production of Magnetic Iron Oxide Nanocrystals by Oxidative Precipitation. Chem. Eng. J. 2020, 393, 124593. [Google Scholar] [CrossRef]
- Granath, T.; Löbmann, P.; Mandel, K. Oxidative Precipitation as a Versatile Method to Obtain Ferromagnetic Fe3O4 Nano- and Mesocrystals Adjustable in Morphology and Magnetic Properties. Part. Part. Syst. Charact. 2021, 38, 2000307. [Google Scholar] [CrossRef]
- Hui, C.; Shen, C.; Yang, T.; Bao, L.; Tian, J.; Ding, H.; Li, C.; Gao, H.-J. Large-Scale Fe 3 O 4 Nanoparticles Soluble in Water Synthesized by a Facile Method. J. Phys. Chem. C 2008, 112, 11336–11339. [Google Scholar] [CrossRef]
- Sleiman, N.; Deluchat, V.; Wazne, M.; Courtin, A.; Saad, Z.; Kazpard, V.; Baudu, M. Role of Iron Oxidation Byproducts in the Removal of Phosphate from Aqueous Solution. RSC Adv. 2016, 6, 1627–1636. [Google Scholar] [CrossRef]
- Gavilán, H.; Kowalski, A.; Heinke, D.; Sugunan, A.; Sommertune, J.; Varón, M.; Bogart, L.K.; Posth, O.; Zeng, L.; González-Alonso, D.; et al. Colloidal Flower-Shaped Iron Oxide Nanoparticles: Synthesis Strategies and Coatings. Part. Part. Syst. Charact. 2017, 34, 1700094. [Google Scholar] [CrossRef]
- Hirazawa, H.; Kusamoto, S.; Aono, H.; Naohara, T.; Mori, K.; Hattori, Y.; Maehara, T.; Watanabe, Y. Preparation of Fine Mg1-XCaXFe2O4 Powder Using Reverse Coprecipitation Method for Thermal Coagulation Therapy in an Ac Magnetic Field. J. Alloys Compd. 2008, 461, 467–473. [Google Scholar] [CrossRef]
- Pereira, D.S.M.; Cardoso, B.D.; Rodrigues, A.R.O.; Amorim, C.O.; Amaral, V.S.A.; Almeida, B.G.; Queiroz, M.-J.R.P.; Martinho, O.; Baltazar, F.; Calhelha, R.C.; et al. Magnetoliposomes Containing Calcium Ferrite Nanoparticles for Applications in Breast Cancer Therapy. Pharmaceutics 2019, 11, 477. [Google Scholar] [CrossRef]
- Satalkar, M.; Kane, S.N. On the Study of Structural Properties and Cation Distribution of Zn0.75-xNixMg0.15Cu0.1Fe2O4 Nano Ferrite: Effect of Ni Addition. J. Phys. Conf. Ser. 2016, 755, 012050. [Google Scholar] [CrossRef]
- Nikam, D.S.; Jadhav, S.V.; Khot, V.M.; Bohara, R.A.; Hong, C.K.; Mali, S.S.; Pawar, S.H. Cation Distribution, Structural, Morphological and Magnetic Properties of Co1−xZnxFe2O4 (x = 0–1) Nanoparticles. RSC Adv. 2015, 5, 2338–2345. [Google Scholar] [CrossRef]
- Bamzai, K.K.; Kour, G.; Kaur, B.; Kulkarni, S.D. Preparation, and Structural and Magnetic Properties of Ca Substituted Magnesium Ferrite with Composition MgCaxFe2−xO4 (x = 0.00, 0.01, 0.03, 0.05, 0.07). J. Mater. 2014, 2014, 184340. [Google Scholar] [CrossRef]
- Gomes, H.C.; Teixeira, S.S.; Graça, M.P.F. Synthesis of Calcium Ferrite for Energy Storage Applications. J. Alloys Compd. 2022, 921, 166026. [Google Scholar] [CrossRef]
- Hashhash, A.; Kaiser, M. Synthesis and Characterization of Calcium-Substituted Mg-Co-Cr Ferrite Nanoparticles with a Crystallite Size Less Than 10 Nm. J. Supercond. Nov. Magn. 2021, 34, 3403–3412. [Google Scholar] [CrossRef]
- Chhaya, S.D.; Pandya, M.P.; Chhantbar, M.C.; Modi, K.B.; Baldha, G.J.; Joshi, H.H. Study of Substitution Limit, Structural, Bulk Magnetic and Electrical Properties of Ca2+ Substituted Magnesium Ferrite. J. Alloys Compd. 2004, 377, 155–161. [Google Scholar] [CrossRef]
- Weil, L.; Bertaut, F.; Bochirol, L. Propriétés Magnétiques et Structure de La Phase Quadratique Du Ferrite de Cuivre. J. Phys. Radium 1950, 11, 208–212. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 Nano- and Micro-Particles by Dry Ice Carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef]
- Massoudi, J.; Smari, M.; Nouri, K.; Dhahri, E.; Khirouni, K.; Bertaina, S.; Bessais, L.; Hlil, E.K. Magnetic and Spectroscopic Properties of Ni-Zn-Al Ferrite Spinel: From the Nanoscale to Microscale. RSC Adv. 2020, 10, 34556–34580. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman Spectroscopy to Unravel the Magnetic Properties of Iron Oxide Nanocrystals for Bio-Related Applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Wang, Z.; Schiferl, D.; Zhao, Y.; O’Neill, H.S.C. High Pressure Raman Spectroscopy of Spinel-Type Ferrite ZnFe2O4. J. Phys. Chem. Solids 2003, 64, 2517–2523. [Google Scholar] [CrossRef]
- Freire, R.M.; Ribeiro, T.S.; Vasconcelos, I.F.; Denardin, J.C.; Barros, E.B.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Fechine, P.B.A. MZnFe2O4 (M = Ni, Mn) Cubic Superparamagnetic Nanoparticles Obtained by Hydrothermal Synthesis. J. Nanoparticle Res. 2013, 15, 1616. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Z.; Zhao, X.; Wu, S.; Li, F.; Yue, M.; Liu, J.P. Microstructure and Magnetic Properties of MFe2O4 (M = Co, Ni, and Mn) Ferrite Nanocrystals Prepared Using Colloid Mill and Hydrothermal Method. J. Appl. Phys. 2015, 117, 17A328. [Google Scholar] [CrossRef]
- Nekvapil, F.; Bunge, A.; Radu, T.; Cinta Pinzaru, S.; Turcu, R. Raman Spectra Tell Us so Much More: Raman Features and Saturation Magnetization for Efficient Analysis of Manganese Zinc Ferrite Nanoparticles. J. Raman Spectrosc. 2020, 51, 959–968. [Google Scholar] [CrossRef]
- Babu, K.V.; Kumar, G.V.S.; Satyanarayana, G.; Sailaja, B.; Lakshmi, C.C.S. Microstructural and Magnetic Properties of Ni1−xCuxFe2O4 (x = 0.05, 0.1 and 0.15) Nano-Crystalline Ferrites. J. Sci. Adv. Mater. Devices 2018, 3, 236–242. [Google Scholar] [CrossRef]
- Galinetto, P.; Albini, B.; Bini, M.; Mozzati, M.C. Raman Spectroscopy in Zinc Ferrites Nanoparticles. In Raman Spectroscopy; Nascimento, G., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Sharifi, S.; Yazdani, A.; Rahimi, K. Incremental Substitution of Ni with Mn in NiFe2O4 to Largely Enhance Its Supercapacitance Properties. Sci. Rep. 2020, 10, 10916. [Google Scholar] [CrossRef]
- Baig, M.M.; Zulfiqar, S.; Yousuf, M.A.; Touqeer, M.; Ullah, S.; Agboola, P.O.; Warsi, M.F.; Shakir, I. Structural and Photocatalytic Properties of New Rare Earth La3+ Substituted MnFe2O4 Ferrite Nanoparticles. Ceram. Int. 2020, 46, 23208–23217. [Google Scholar] [CrossRef]
- Hashemi, A.; Naseri, M.; Ghiyasvand, S.; Naderi, E.; Vafai, S. Evaluation of Physical Properties, Cytotoxicity, and Antibacterial Activities of Calcium–Cadmium Ferrite Nanoparticles. Appl. Phys. A Mater. Sci. Process. 2022, 128, 236. [Google Scholar] [CrossRef]
- Jitkang, L.; Pin, Y.S.; Xin, C.H.; Chun, L.S. Characterization of Magnetic Nanoparticle by Dynamic Light Scattering. Nanoscale Res. Lett. 2013, 8, 308–381. [Google Scholar]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of Magnetic Iron Oxide Nanoparticles for Medical Applications. J. Nanobiotechnology 2022, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Fizeșan, I.; Pop, A.; Scorus, L.; Dudric, R.; Stiufiuc, G.; Vedeanu, N.; Tetean, R.; Loghin, F.; Stiufiuc, R.; et al. In Vitro Intracellular Hyperthermia of Iron Oxide Magnetic Nanoparticles, Synthesized at High Temperature by a Polyol Process. Pharmaceutics 2020, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Jedlovszky-Hajdú, A.; Bombelli, F.B.; Monopoli, M.P.; Tombácz, E.; Dawson, K.A. Surface Coatings Shape the Protein Corona of SPIONs with Relevance to Their Application in Vivo. Langmuir 2012, 28, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Chauhan, N.; Othman, S.F.; Khalilzad-Sharghi, V.; Ebeling, M.C.; Khan, S.; Jaggi, M.; Chauhan, S.C. Implications of Protein Corona on Physico-Chemical and Biological Properties of Magnetic Nanoparticles. Biomaterials 2015, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nawara, K.; Romiszewski, J.; Kijewska, K.; Szczytko, J.; Twardowski, A.; Mazur, M.; Krysinski, P. Adsorption of Doxorubicin onto Citrate-Stabilized Magnetic Nanoparticles. J. Phys. Chem. C 2012, 116, 5598–5609. [Google Scholar] [CrossRef]
- Caruntu, D.; Caruntu, G.; O’Connor, C.J. Magnetic Properties of Variable-Sized Fe3O4 Nanoparticles Synthesized from Non-Aqueous Homogeneous Solutions of Polyols. J. Phys. D Appl. Phys. 2007, 40, 5801–5809. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Coutinho, P.J.G.; Martins, J.A.; Testa-Anta, M.; Salgueiriño, V.; Correa-Duarte, M.A.; Ferreira, P.M.T.; et al. Impact of Citrate and Lipid-Functionalized Magnetic Nanoparticles in Dehydropeptide Supramolecular Magnetogels: Properties, Design and Drug Release. Nanomaterials 2021, 11, 16. [Google Scholar] [CrossRef]
- Karimi, Z.; Karimi, L.; Shokrollahi, H. Nano-Magnetic Particles Used in Biomedicine: Core and Coating Materials. Mater. Sci. Eng. C 2013, 33, 2465–2475. [Google Scholar] [CrossRef]
- Otero-Lorenzo, R.; Fantechi, E.; Sangregorio, C.; Salgueiriño, V. Solvothermally Driven Mn Doping and Clustering of Iron Oxide Nanoparticles for Heat Delivery Applications. Chem. A Eur. J. 2016, 22, 6666–6675. [Google Scholar] [CrossRef]
- Ouyahia, S.; Rais, A.; Bozzo, B.; Taibi, K.; Addou, A. Cations Distribution by Rietveld Refinement and Magnetic Properties of MgCrxFe2−xO4 Spinel Ferrites. Appl. Phys. A Mater. Sci. Process. 2020, 126, 666. [Google Scholar] [CrossRef]
- Sharma, R.; Thakur, P.; Kumar, M.; Barman, P.B.; Sharma, P.; Sharma, V. Enhancement in A-B Super-Exchange Interaction with Mn Substitution in Mg-Zn Ferrites as a Heating Source in Hyperthermia Applications. Ceram. Int. 2017, 43, 13661–13669. [Google Scholar] [CrossRef]
- Nitika; Rana, A.; Kumar, V. Evaluation of Structural, Magnetic, Optical, Electrical, and Humidity Sensing Properties of Manganese-Substituted Zinc Ferrite Nanoparticles. Appl. Phys. A 2021, 127, 860. [Google Scholar] [CrossRef]
- Kumar, P.; Pathak, S.; Singh, A.; Jain, K.; Khanduri, H.; Wang, L.; Kim, S.-K.; Pant, R.P. Observation of Intrinsic Fluorescence in Cobalt Ferrite Magnetic Nanoparticles by Mn2+ Substitution and Tuning the Spin Dynamics by Cation Distribution. J. Mater. Chem. C 2022, 10, 12652–12679. [Google Scholar] [CrossRef]
- Assar, S.T.; Abosheiasha, H.F. Effect of Ca Substitution on Some Physical Properties of Nano-Structured and Bulk Ni-Ferrite Samples. J. Magn. Magn. Mater. 2015, 374, 264–272. [Google Scholar] [CrossRef]
- Topkaya, R.; Baykal, A.; Demir, A. Yafet–Kittel-Type Magnetic Order in Zn-Substituted Cobalt Ferrite Nanoparticles with Uniaxial Anisotropy. J. Nanopart. Res. 2013, 15, 1359. [Google Scholar] [CrossRef]
- Vargas, J.M.; Nunes, W.C.; Socolovsky, L.M.; Knobel, M.; Zanchet, D. Effect of Dipolar Interaction Observed in Iron-Based Nanoparticles. Phys. Rev. B 2005, 72, 184428. [Google Scholar] [CrossRef]
- Félix, L.L.; Rodriguez Martínez, M.A.; Pacheco Salazar, D.G.; Huamani Coaquira, J.A. One-Step Synthesis of Polyethyleneimine-Coated Magnetite Nanoparticles and Their Structural, Magnetic and Power Absorption Study. RSC Adv. 2020, 10, 41807–41815. [Google Scholar] [CrossRef] [PubMed]
- Bruvera, I.J.; Mendoza Zélis, P.; Pilar Calatayud, M.; Goya, G.F.; Sánchez, F.H. Determination of the Blocking Temperature of Magnetic Nanoparticles: The Good, the Bad, and the Ugly. J. Appl. Phys. 2015, 118, 184304. [Google Scholar] [CrossRef]
- Yelenich, O.; Solopan, S.; Kolodiazhnyi, T.; Tykhonenko, Y.; Tovstolytkin, A.; Belous, A. Magnetic Properties and AC Losses in AFe2O4 (A = Mn, Co, Ni, Zn) Nanoparticles Synthesized from Nonaqueous Solution. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef][Green Version]
- Khanna, L.; Verma, N.K. PEG/CaFe2O4 Nanocomposite: Structural, Morphological, Magnetic and Thermal Analyses. Phys. B Condens. Matter 2013, 427, 68–75. [Google Scholar] [CrossRef]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.-M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef]
- Gupta, R.; Tomar, R.; Chakraverty, S.; Sharma, D. Effect of Manganese Doping on the Hyperthermic Profile of Ferrite Nanoparticles Using Response Surface Methodology. RSC Adv. 2021, 11, 16942–16954. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Eskandarzadeh, N.; Jalili, H.; Ghotbi Varzaneh, A.; Kameli, P.; Orue, I.; Chernenko, V.; Hajalilou, A.; Ferreira, L.P.; Cruz, M.M. Magnetic Hyperthermia Properties of CoFe2O4 Nanoparticles: Effect of Polymer Coating and Interparticle Interactions. Ceram. Int. 2022, 48, 27995–28005. [Google Scholar] [CrossRef]
- Nasrin, S.; Chowdhury, F.U.Z.; Hoque, S.M. Study of Hyperthermia Temperature of Manganese-Substituted Cobalt Nano Ferrites Prepared by Chemical Co-Precipitation Method for Biomedical Application. J. Magn. Magn. Mater. 2019, 479, 126–134. [Google Scholar] [CrossRef]
- Kheradmand, A.; Vahidi, O.; Masoudpanah, S.M. Magnetic, Hyperthermic and Structural Properties of Zn Substituted CaFe2O4 Powders. Appl. Phys. A Mater. Sci. Process. 2018, 124, 255. [Google Scholar] [CrossRef]
- Lopes, F.A.C.; Fernandes, A.V.F.; Rodrigues, J.M.; Queiroz, M.-J.R.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Rodrigues, A.R.O.; et al. Magnetoliposomes Containing Multicore Nanoparticles and a New Antitumor Thienopyridine Compound with Potential Application in Chemo/Thermotherapy. Biomedicines 2022, 10, 1547. [Google Scholar] [CrossRef]
- Amorim, C.O.; Mohseni, F.; Dumas, R.K.; Amaral, V.S.; Amaral, J.S. A Geometry-Independent Moment Correction Method for the MPMS3 SQUID-Based Magnetometer. Meas. Sci. Technol. 2021, 32, 105602. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso, S.R.S.; Andrade, R.G.D.; Gomes, V.; Amorim, C.O.; Amaral, V.S.; Salgueiriño, V.; Coutinho, P.J.G.; Ferreira, P.M.T.; Correa-Duarte, M.A.; Castanheira, E.M.S. Oxidative Precipitation Synthesis of Calcium-Doped Manganese Ferrite Nanoparticles for Magnetic Hyperthermia. Int. J. Mol. Sci. 2022, 23, 14145. https://doi.org/10.3390/ijms232214145
Veloso SRS, Andrade RGD, Gomes V, Amorim CO, Amaral VS, Salgueiriño V, Coutinho PJG, Ferreira PMT, Correa-Duarte MA, Castanheira EMS. Oxidative Precipitation Synthesis of Calcium-Doped Manganese Ferrite Nanoparticles for Magnetic Hyperthermia. International Journal of Molecular Sciences. 2022; 23(22):14145. https://doi.org/10.3390/ijms232214145
Chicago/Turabian StyleVeloso, Sérgio R. S., Raquel G. D. Andrade, Valéria Gomes, Carlos O. Amorim, Vítor S. Amaral, Verónica Salgueiriño, Paulo J. G. Coutinho, Paula M. T. Ferreira, Miguel A. Correa-Duarte, and Elisabete M. S. Castanheira. 2022. "Oxidative Precipitation Synthesis of Calcium-Doped Manganese Ferrite Nanoparticles for Magnetic Hyperthermia" International Journal of Molecular Sciences 23, no. 22: 14145. https://doi.org/10.3390/ijms232214145
APA StyleVeloso, S. R. S., Andrade, R. G. D., Gomes, V., Amorim, C. O., Amaral, V. S., Salgueiriño, V., Coutinho, P. J. G., Ferreira, P. M. T., Correa-Duarte, M. A., & Castanheira, E. M. S. (2022). Oxidative Precipitation Synthesis of Calcium-Doped Manganese Ferrite Nanoparticles for Magnetic Hyperthermia. International Journal of Molecular Sciences, 23(22), 14145. https://doi.org/10.3390/ijms232214145








