The Dual PDE7-GSK3β Inhibitor, VP3.15, as Neuroprotective Disease-Modifying Treatment in a Model of Primary Progressive Multiple Sclerosis
Abstract
1. Introduction
2. Results
2.1. The PDE7/GSK3 Dual Inhibitor VP3.15 Ameliorates Motor Function of TMEV-IDD
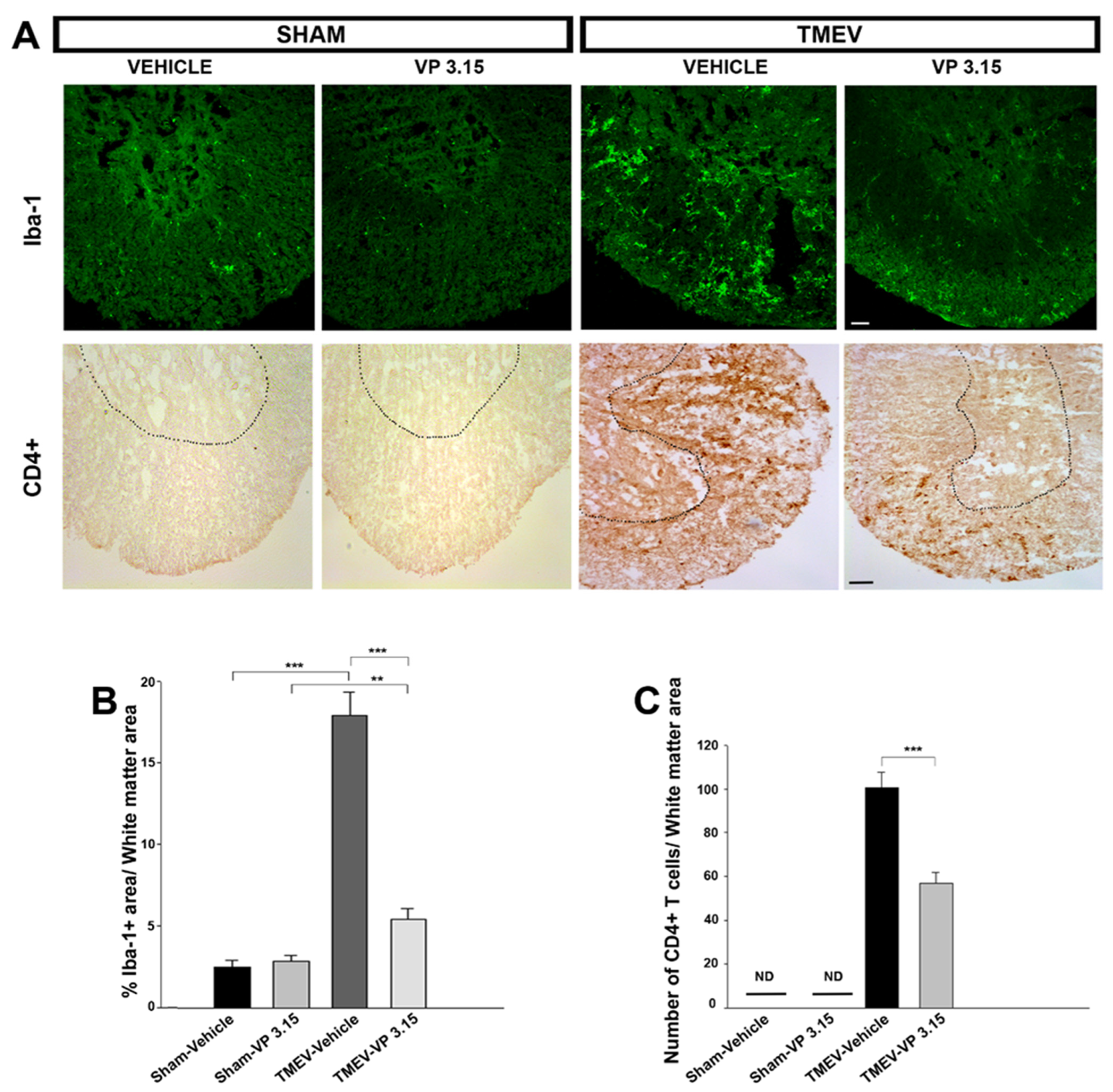
2.2. VP3.15 Limits Microglial Activation and Lymphocyte Infiltration in the Spinal Cord of TMEV Infected Mice
2.3. The Remyelinating Role of VP3.15 and Its Effect in the Integrity of Axons of TMEV Mice
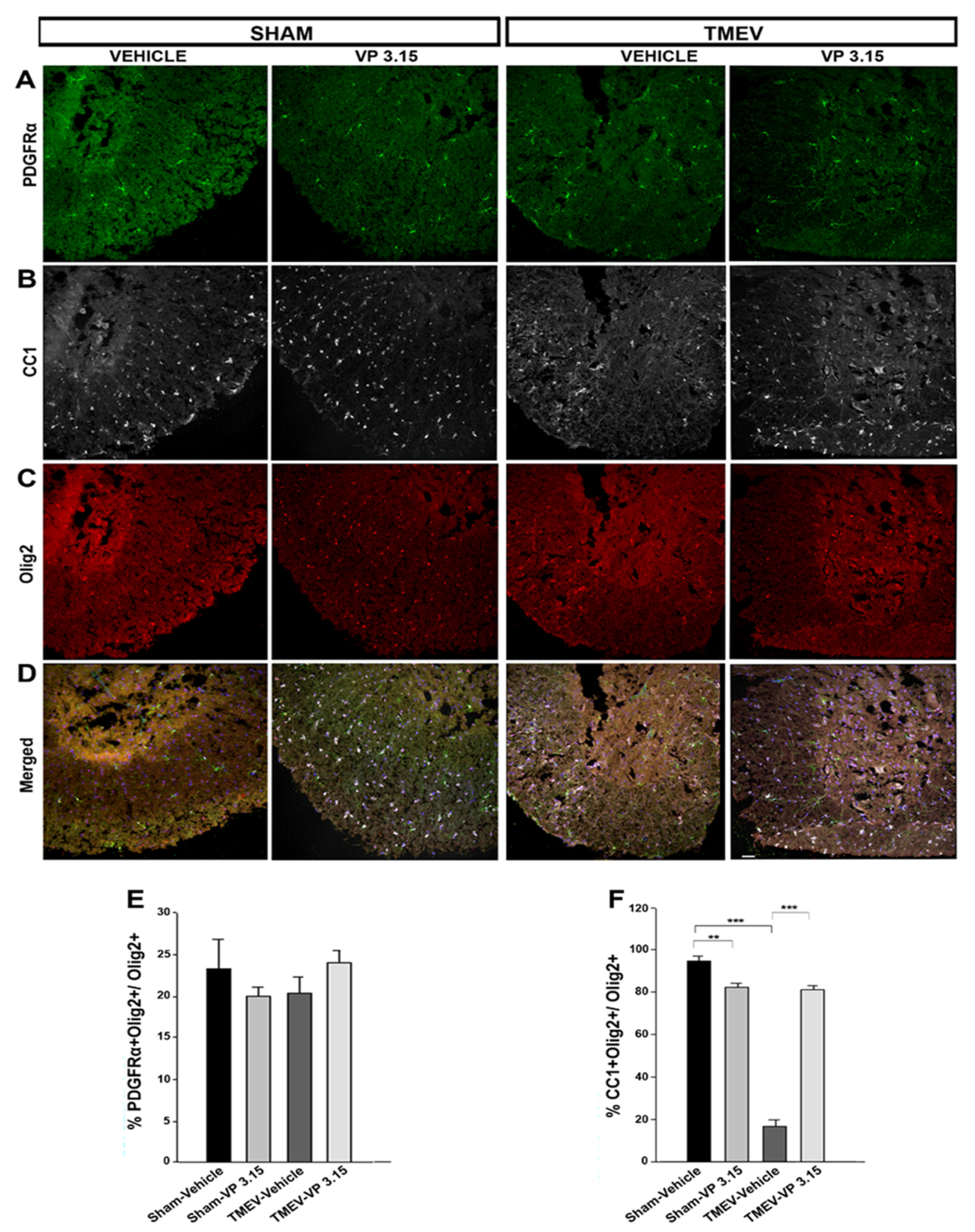
2.4. VP3.15 Promotes the Presence of Mature Oligodendrocytes in Spinal Cord
3. Discussion
4. Materials and Methods
4.1. Animal and Theiler’s Virus Inoculation
4.2. Treatments and Evaluation of Motor Function
4.3. Tissue Processing
4.4. Eriochrome Cyanine Staining
4.5. Immunofluorescence Analysis
4.6. Immunohistochemistry Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Ann. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Weber, M.S.; Tumani, H. Primary Progressive Multiple Sclerosis: Putting Together the Puzzle. Front. Neurol. 2017, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef]
- Lipton, H.L. Theiler’s virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect. Immun. 1975, 11, 1147–11554. [Google Scholar] [CrossRef]
- Lipton, H.L.; Dal Canto, M.C. Chronic neurologic disease in Theiler’s virus infection of SJL/J mice. J. Neurol. Sci. 1976, 30, 201–207. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Machín, I.; Cordero, C.; Carrillo-Salinas, F.; Mestre, L.; Hernández-Torres, G.; Ortega-Gutiérrez, S.; López-Rodríguez, M.L.; de Castro, F.; et al. 2-AG limits Theiler’s virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia 2018, 66, 1447–1463. [Google Scholar] [CrossRef]
- Feliú, A.; Moreno-Martet, M.; Mecha, M.; Carrillo-Salinas, F.J.; de Lago, E.; Fernández-Ruiz, J.; Guaza, C. A Sativex(®)-like combination of phytocannabinoids as a disease-modifying therapy in a viral model of multiple sclerosis. Br. J. Pharm. 2015, 172, 3579–3595. [Google Scholar] [CrossRef]
- Kim, Y.T.; Hur, E.M.; Snider, W.D.; Zhou, F.Q. Role of GSK3 Signaling in Neuronal Morphogenesis. Front. Mol. Neurosci. 2011, 4, 48. [Google Scholar] [CrossRef]
- Sánchez-Cruz, A.; Villarejo-Zori, B.; Marchena, M.; Zaldivar-Díez, J.; Palomo, V.; Gil, C.; Lizasoain, I.; de la Villa, P.; Martínez, A.; de la Rosa, E.J.; et al. Modulation of GSK-3 provides cellular and functional neuroprotection in the rd10 mouse model of retinitis pigmentosa. Mol. Neurodegener. 2018, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; James, J.L.; Parker, S.J.; Lidgerwood, G.E.; Duncan, C.; Meyerowitz, J.; Nonaka, T.; Hasegawa, M.; Kanninen, K.M.; Grubman, A.; et al. Kinase Inhibitor Screening Identifies Cyclin-Dependent Kinases and Glycogen Synthase Kinase 3 as Potential Modulators of TDP-43 Cytosolic Accumulation during Cell Stress. PLoS ONE 2013, 8, e67433. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, J.A.; Susín, C.; Alonso-Gil, S.; Pérez, D.I.; Palomo, V.; Pérez, C.; Conde, S.; Santos, A.; Gil, C.; Martínez, A.; et al. Glycogen synthase kinase-3 inhibitors as potent therapeutic agents for the treatment of Parkinson disease. ACSC Chem. Neurosci. 2013, 4, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Kaidanovich-Beilin, O.; Yeh, W.I.; Song, L.; Palomo, V.; Michalek, S.M.; Woodgett, J.R.; Harrington, L.E.; Eldar-Finkelman, H.; Martinez, A.; et al. Regulation of Th1 cells and experimental autoimmune encephalomyelitis by glycogen synthase kinase-3. J. Immunol. 2013, 190, 5000–5011. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodríguez, E.M.; Bribián, A.; Boyd, A.; Palomo, V.; Pastor, J.; Lagares, A.; Gil, C.; Martínez, A.; Williams, A.; de Castro, F. Promoting in vivo remyelination with small molecules: A neuroreparative pharmacological treatment for Multiple Sclerosis. Sci. Rep. 2017, 7, 43545. [Google Scholar] [CrossRef]
- Giembycz, M.A. Life after PDE4: Overcoming adverse events with dual-specificity phosphodiesterase inhibitors. Curr. Opin. Pharmacol. 2005, 5, 238–244. [Google Scholar] [CrossRef]
- Guo, J.; Watson, A.; Kempson, J.; Carlsen, M.; Barbosa, J.; Stebbins, K.; Lee, D.; Dodd, J.; Nadler, S.G.; McKinnon, M.; et al. Identification of potent pyrimidine inhibitors of phosphodiesterase 7 (PDE7) and their ability to inhibit T cell proliferation. Bioorg. Med. Chem. Lett. 2009, 19, 1935–1938. [Google Scholar] [CrossRef]
- Medina-Rodríguez, E.M.; Arenzana, F.J.; Pastor, J.; Redondo, M.; Palomo, V.; García de Sola, R.; Gil, C.; Martínez, A.; Bribián, A.; de Castro, F. Inhibition of endogenous phosphodiesterase 7 promotes oligodendrocyte precursor differentiation and survival. Cell Mol. Life Sci. 2013, 70, 3449–3462. [Google Scholar] [CrossRef] [PubMed]
- Morales-Garcia, J.A.; Redondo, M.; Alonso-Gil, S.; Gil, C.; Perez, C.; Martinez, A.; Santos, A.; Perez-Castillo, A. Phosphodiesterase 7 inhibition preserves dopaminergic neurons in cellular and rodent models of Parkinson disease. PLoS ONE 2011, 6, e17240. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Palomo, V.; Redondo, M.; Alonso-Gil, S.; Gil, C.; Martinez, A.; Perez-Castillo, A. Crosstalk between phosphodiesterase 7 and glycogen synthase kinase-3: Two relevant therapeutic targets for neurological disorders. ACS Chem. Neurosci. 2014, 5, 194–204. [Google Scholar] [CrossRef]
- Baolognesi, M.L. Harnessing polypharmacology with medicinal chemistry. ACS Med. Chem. Lett. 2019, 10, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Palomo, V.; Perez, D.I.; Perez, C.; Morales-Garcia, J.A.; Soteras, I.; Alonso-Gil, S.; Encinas, A.; Castro, A.; Campillo, N.E.; Perez-Castillo, A.; et al. 5-imino-1,2,4-thiadiazoles: First small molecules as substrate competitive inhibitors of glycogen synthase kinase 3. J. Med. Chem. 2012, 55, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Benner, B.; Martorell, A.J.; Mahadevan, P.; Najm, F.J.; Tesar, P.J.; Freundt, E.C. Depletion of Olig2 in oligodendrocyte progenitor cells infected by Theiler’s murine encephalomyelitis virus. J. Neurovirol. 2016, 22, 336–348. [Google Scholar] [CrossRef]
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72 (Suppl. 1), 1–5. [Google Scholar] [CrossRef]
- Bigaut, K.; De Seze, J.; Collongues, N. Ocrelizumab for the treatment of multiple sclerosis. Expert Rev. Neurother. 2019, 19, 97–108. [Google Scholar] [CrossRef]
- Kolind, S.; Vavasour, I.; Tang, L.; Tam, R.; Rauscher, A.; Clayton, D.; Levesque, V.; Carruthers, R.; White, R.; Li, D.; et al. Advanced Myelin-related MRI Measures in Relapsing Multiple Sclerosis Patients treated with Ocrelizumab or Interferon Beta-1a Over 96 Weeks (P6.371). Neurology 2017, 88 (Suppl. 16). [Google Scholar]
- Mestre, L.; Carrillo-Salinas, F.J.; Mecha, M.; Feliú, A.; Espejo, C.; Álvarez-Cermeño, J.C.; Villar, L.M.; Guaza, C. Manipulation of Gut Microbiota Influences Immune Responses, Axon Preservation, and Motor Disability in a Model of Progressive Multiple Sclerosis. Front. Immunol. 2019, 10, 1374. [Google Scholar] [CrossRef]
- Mestre, L.; Redondo, M.; Carrillo-Salinas, F.J.; Morales-García, J.A.; Alonso-Gil, S.; Pérez-Castillo, A.; Gil, C.; Martínez, A.; Guaza, C. PDE7 inhibitor TC3.6 ameliorates symptomatology in a model of primary progressive multiple sclerosis. Br. J. Pharmacol. 2015, 172, 4277–4290. [Google Scholar] [CrossRef]
- Li, L.; Matsumoto, M.; Seabrook, T.J.; Cojean, C.; Brinkman, V.; Pachner, A.R. The effect of FTY720 in the Theiler’s virus model of multiple sclerosis. J. Neurol. Sci. 2011, 308, 41–48. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin Ameliorates Lead-Induced Hepatotoxicity by Suppressing Oxidative Stress and Inflammation, and Modulating Akt/GSK-3β Signaling Pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, I.; Kuang, L.Q.; Libbey, J.E.; Fujinami, R.S. Axonal injury heralds virus-induced demyelination. Am. J. Pathol. 2003, 162, 1259–1269. [Google Scholar] [CrossRef]
- Arévalo-Martín, A.; Vela, J.M.; Molina-Holgado, E.; Borrell, J.; Guaza, C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci. 2003, 23, 2511–2516. [Google Scholar]
- Moliné-Velázquez, V.; Cuervo, H.; Vila-Del Sol, V.; Ortega, M.C.; Clemente, D.; de Castro, F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011, 21, 678–691. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Target | Cellular Location | Dilution | Host Species | Class | Manufacturer | Antibody ID |
|---|---|---|---|---|---|---|---|
| MBP | Myelin | Plasma Membrane | 1:500 | Rat | Monoclonal clone 12 | Biorad | aa 82–87 |
| NFH | Neurons/ Axons | Cell Body | 1:1000 | Rabbit | Polyclonal | Abcam | Ab 8135 |
| Iba-1 | Microglia | Plasma membrane | 1:500 | Guinea pig | Polyclonal | Synaptic Systems | 234 004 |
| PDGFRα | OPCs | Plasma membrane | 1:200 | Goat | Polyclonal | RD Systems | AF 1062 |
| CC1 | Mature oligodendrocytes | Cell body | 1:200 | Mouse | Monoclonal clone CC1 | Merck Millipore | OP 80 |
| Olig2 | Oligodendrocyte lineage | Nucleus | 1:200 | Rabbit | Polyclonal | Merck Millipore | AB 9610 |
| CD4 | T cell | Plasma membrane | 1:250 | Rat | Monoclonal | BD Pharmingen | 550278 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez-Fernández, R.; Gil, C.; Guaza, C.; Mestre, L.; Martínez, A. The Dual PDE7-GSK3β Inhibitor, VP3.15, as Neuroprotective Disease-Modifying Treatment in a Model of Primary Progressive Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 14378. https://doi.org/10.3390/ijms232214378
Benítez-Fernández R, Gil C, Guaza C, Mestre L, Martínez A. The Dual PDE7-GSK3β Inhibitor, VP3.15, as Neuroprotective Disease-Modifying Treatment in a Model of Primary Progressive Multiple Sclerosis. International Journal of Molecular Sciences. 2022; 23(22):14378. https://doi.org/10.3390/ijms232214378
Chicago/Turabian StyleBenítez-Fernández, Rocio, Carmen Gil, Carmen Guaza, Leyre Mestre, and Ana Martínez. 2022. "The Dual PDE7-GSK3β Inhibitor, VP3.15, as Neuroprotective Disease-Modifying Treatment in a Model of Primary Progressive Multiple Sclerosis" International Journal of Molecular Sciences 23, no. 22: 14378. https://doi.org/10.3390/ijms232214378
APA StyleBenítez-Fernández, R., Gil, C., Guaza, C., Mestre, L., & Martínez, A. (2022). The Dual PDE7-GSK3β Inhibitor, VP3.15, as Neuroprotective Disease-Modifying Treatment in a Model of Primary Progressive Multiple Sclerosis. International Journal of Molecular Sciences, 23(22), 14378. https://doi.org/10.3390/ijms232214378







