Mobilization of Hematopoietic Stem and Progenitor Cells during Dengue Virus Infection
Abstract
1. Introduction
2. Results
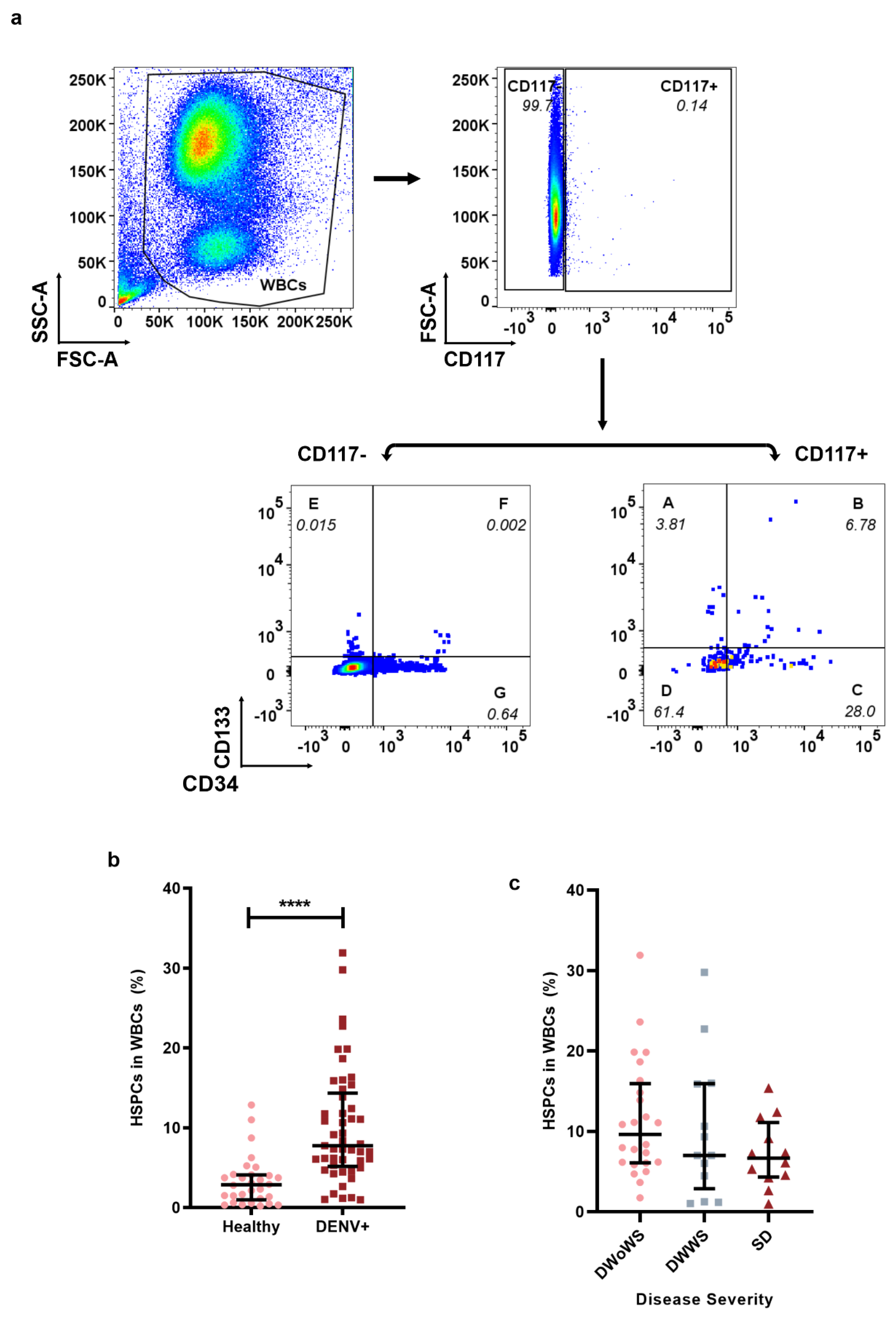
2.1. Mobilization of HSPCs in Dengue-Infected Patients
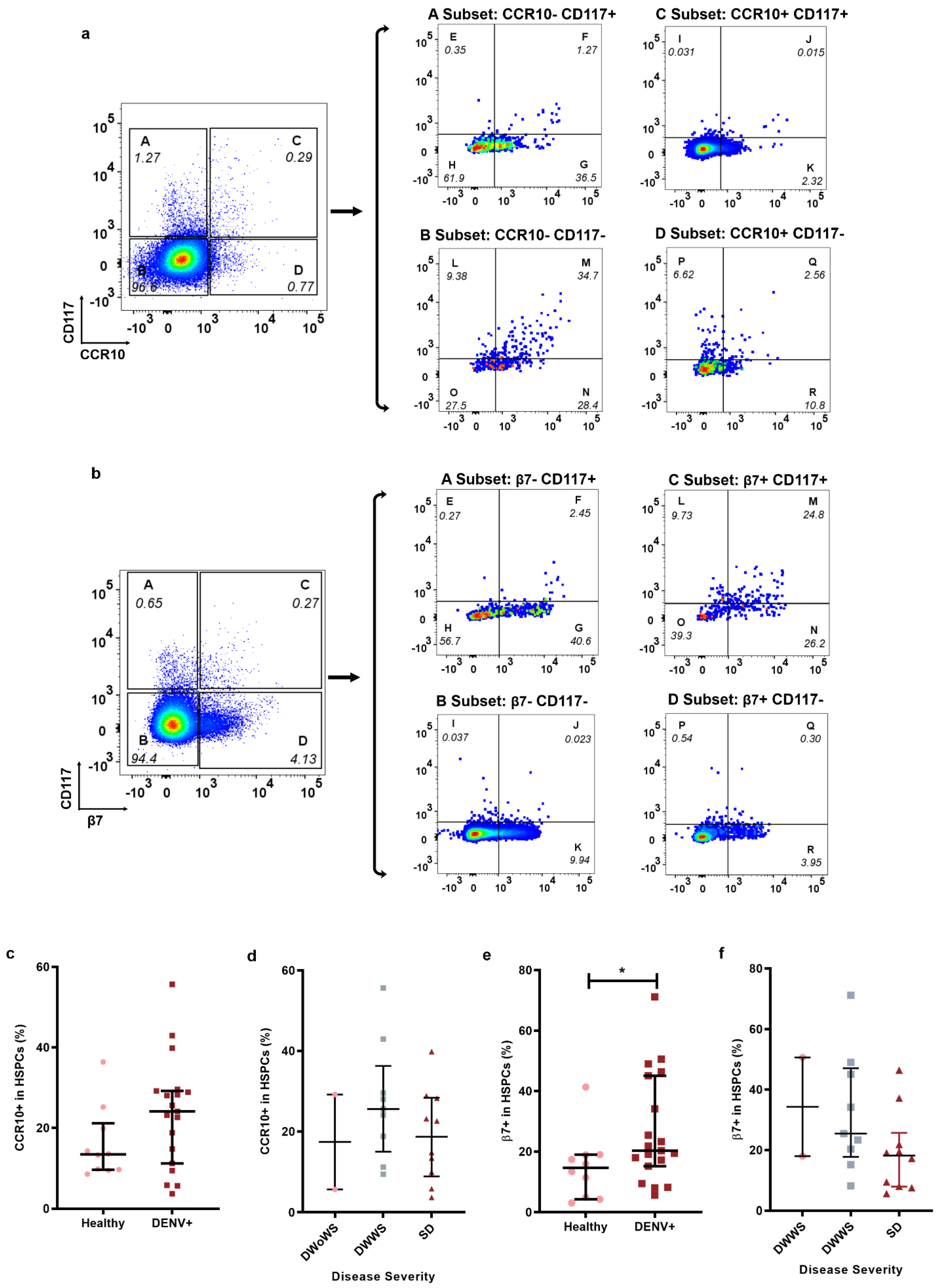
2.2. Homing Markers Expression in HSPCs during Dengue Infection
2.3. HSPCs in Dengue Patients Were Permissive for DENV Infection
3. Discussion
4. Materials and Methods
4.1. Ethical Statement and Study Cohort
4.2. White Blood Cells Preparation
4.3. Fluorochrome Conjugated DENV NS1 Antibody
4.4. Flow Cytometric Analysis
4.5. Gating Strategy for HSPCs, Homing HSPCs, and DENV Infected or Uninfected HSPCs
4.6. HSPCs Isolation
4.7. Virus Titration
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perera, R.; Kuhn, R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Strategy for Dengue Prevention and Control, 2012–2020; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Tuiskunen Bäck, A.; Lundkvist, Å. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef] [PubMed]
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Granick, J.L.; Falahee, P.C.; Dahmubed, D.; Borjesson, D.L.; Miller, L.S.; Simon, S.I. Staphylococcus aureus recognition by hematopoietic stem and progenitor cells via TLR2/MyD88/PGE2 stimulates granulopoiesis in wounds. Blood 2013, 122, 1770–1778. [Google Scholar] [CrossRef]
- Schulz, C.; Von Andrian, U.H.; Massberg, S. Hematopoietic stem and progenitor cells: Their mobilization and homing to bone marrow and peripheral tissue. Immunol. Res. 2009, 44, 160–168. [Google Scholar] [CrossRef]
- Lu, X.-J.; Chen, Q.; Rong, Y.-J.; Chen, J. Mobilisation and dysfunction of haematopoietic stem/progenitor cells after Listonella anguillarum infection in ayu, Plecoglossus altivelis. Sci. Rep. 2016, 6, 28082. [Google Scholar] [CrossRef]
- Costa, V.V.; Fagundes, C.T.; Souza, D.G.; Teixeira, M.M. Inflammatory and Innate Immune Responses in Dengue Infection: Protection versus Disease Induction. Am. J. Pathol. 2013, 182, 1950–1961. [Google Scholar] [CrossRef]
- Burberry, A.; Zeng, M.Y.; Ding, L.; Wicks, I.; Inohara, N.; Morrison, S.; Núñez, G. Infection Mobilizes Hematopoietic Stem Cells through Cooperative NOD-like Receptor and Toll-like Receptor Signaling. Cell Host Microbe 2014, 15, 779–791. [Google Scholar] [CrossRef]
- Kwon, S.G.; Park, I.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Role of stem cell mobilization in the treatment of ischemic diseases. Arch. Pharmacal Res. 2019, 42, 224–231. [Google Scholar] [CrossRef]
- Peeples, E.S.; Parry, S.M. The impact of hypoxic-ischemic brain injury on stem cell mobilization, migration, adhesion, and proliferation. Neural Regen. Res. 2018, 13, 1125–1135. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Adamiak, M.; Thapa, A.; Bujko, K.; Brzeźniakiewicz-Janus, K.; Lenkiewicz, A.M. NLRP3 inflammasome couples purinergic signaling with activation of the complement cascade for the optimal release of cells from bone marrow. Leukemia 2019, 33, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Petit, I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002, 30, 973–981. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Le, H.T.; Moon, U.J.; Tran, V.G.; Kim, H.J.; Jung, S.; Nguyen, Q.-T.; Kim, B.-S.; Jun, J.-B.; et al. IL-33–Induced Hematopoietic Stem and Progenitor Cell Mobilization Depends upon CCR2. J. Immunol. 2014, 193, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Zhang, S.-Y.; Chen, Y.-Y.; Shi, K.; Zou, B.; Liu, J.; Yang, Q.; Jiang, H.; Wei, L.; Li, C.-Z.; et al. ICAM-1 Deficiency in the Bone Marrow Niche Impairs Quiescence and Repopulation of Hematopoietic Stem Cells. Stem Cell Rep. 2018, 11, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Becquart, P.; Wauquier, N.; Nkoghe, D.; Ndjoyi-Mbiguino, A.; Padilla, C.; Souris, M.; Leroy, E.M. Acute dengue virus 2 infection in Gabonese patients is associated with an early innate immune response, including strong interferon alpha production. BMC Infect. Dis. 2010, 10, 356. [Google Scholar] [CrossRef]
- Huang, J.; Liang, W.; Chen, S.; Zhu, Y.; Chen, H.; Mok, C.K.P.; Zhou, Y. Serum Cytokine Profiles in Patients with Dengue Fever at the Acute Infection Phase. Dis. Markers 2018, 2018, 8403937. [Google Scholar] [CrossRef] [PubMed]
- Patro, A.R.K.; Mohanty, S.; Prusty, B.K.; Singh, D.K.; Gaikwad, S.; Saswat, T.; Chattopadhyay, S.; Das, B.K.; Tripathy, R.; Ravindran, B. Cytokine Signature Associated with Disease Severity in Dengue. Viruses 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Baltimore, D. Regulation of stress-induced hematopoiesis. Curr. Opin. Hematol. 2015, 22, 286–292. [Google Scholar] [CrossRef]
- Basu, A.; Jain, P.; Gangodkar, S.V.; Shetty, S.; Ghosh, K. Dengue 2 virus inhibits in vitro megakaryocytic colony formation and induces apoptosis in thrombopoietin-inducible megakaryocytic differentiation from cord blood CD34+ cells. FEMS Immunol. Med. Microbiol. 2008, 53, 46–51. [Google Scholar] [CrossRef]
- Hsu, A.Y.; Ho, T.; Lai, M.; Tan, S.S.; Chen, T.; Lee, M.; Chien, Y.; Chen, Y.; Perng, G.C. Identification and characterization of permissive cells to dengue virus infection in human hematopoietic stem and progenitor cells. Transfusion 2019, 59, 2938–2951. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, V.; Donahue, A.; Uitto, J.; Igoucheva, O. Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues. Cytotherapy 2013, 15, 171–184.e1. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, D.; Abe, R.; Fujita, Y.; Sasaki, M.; Shibaki, A.; Nakamura, H.; McMillan, J.R.; Shimizu, T.; Shimizu, H. CTACK/CCL27 Accelerates Skin Regeneration via Accumulation of Bone Marrow-Derived Keratinocytes. Stem Cells 2006, 24, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Nguyen, L.P.; Hadeiba, H.; Butcher, E.C. Leukocyte Trafficking to the Small Intestine and Colon. Gastroenterology 2016, 150, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Gorfu, G.; Rivera-Nieves, J.; Ley, K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr. Mol. Med. 2009, 9, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.R.V.; Berbel, A.C.E.R.; Papa, M.P.; De Morais, A.T.S.; Peçanha, L.M.T.; De Arruda, L.B. Dengue Virus Directly Stimulates Polyclonal B Cell Activation. PLoS ONE 2015, 10, e0143391. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, Y.-L.; Herve, M.; Gu, F.; Shi, P.-Y.; Blasco, F. Development of a FACS-based assay for evaluating antiviral potency of compound in dengue infected peripheral blood mononuclear cells. J. Virol. Methods 2014, 196, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef]
- Wilson, A.; Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006, 6, 93–106. [Google Scholar] [CrossRef]
- Singh, P.; Yao, Y.; Weliver, A.; Broxmeyer, H.E.; Hong, S.-C.; Chang, C.-H. Vaccinia Virus Infection Modulates the Hematopoietic Cell Compartments in the Bone Marrow. Stem Cells 2008, 26, 1009–1016. [Google Scholar] [CrossRef]
- Pascutti, M.F.; Erkelens, M.N.; Nolte, M.A. Impact of Viral Infections on Hematopoiesis: From Beneficial to Detrimental Effects on Bone Marrow Output. Front. Immunol. 2016, 7, 364. [Google Scholar] [CrossRef]
- Skirecki, T.; Mikaszewska-Sokolewicz, M.; Godlewska, M.; Dołęgowska, B.; Czubak, J.; Hoser, G.; Kawiak, J.; Zielińska-Borkowska, U. Mobilization of Stem and Progenitor Cells in Septic Shock Patients. Sci. Rep. 2019, 9, 3289. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.; Kunstár, A.; Fajka-Boja, R.; Dudics, V.; Tóvári, J.; Légrádi, Á.; Monostori, Ė.; Uher, F.A. Novel Anti-Inflammatory Function of Human Galectin-1: Inhibition of Hematopoietic Progenitor Cell Mobilization. Exp. Hematol. 2007, 35, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P. Galectins as regulators of cell survival in the leukemia niche. Adv. Biol. Regul. 2019, 71, 41–54. [Google Scholar] [CrossRef]
- Liu, K.T.; Liu, Y.H.; Chen, Y.H.; Lin, C.Y.; Huang, C.H.; Yen, M.C.; Kuo, P.L. Serum Galectin-9 and Galectin-3-Binding Protein in Acute Dengue Virus Infection. Int. J. Mol. Sci. 2016, 17, 832. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Clinton, J.L.S.; Rico-Hesse, R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Neglected Trop. Dis. 2019, 13, e0007837. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Chen, Q.; Tang, K.F.; Ooi, E.E.; Hibberd, M.L.; Chen, J. Inhibition of Megakaryocyte Development in the Bone Marrow Underlies Dengue Virus-Induced Thrombocytopenia in Humanized Mice. J. Virol. 2013, 87, 11648–11658. [Google Scholar] [CrossRef]
- Granick, J.L.; Simon, S.I.; Borjesson, D.L. Hematopoietic Stem and Progenitor Cells as Effectors in Innate Immunity. Bone Marrow Res. 2012, 2012, 165107. [Google Scholar] [CrossRef]
- Puc, I.; Ho, T.-C.; Yen, K.-L.; Vats, A.; Tsai, J.-J.; Chen, P.-L.; Chien, Y.-W.; Lo, Y.-C.; Perng, G. Cytokine Signature of Dengue Patients at Different Severity of the Disease. Int. J. Mol. Sci. 2021, 22, 2879. [Google Scholar] [CrossRef]
- Murgue, B.; Cassar, O.; Guigon, M.; Chungue, E. Dengue Virus Inhibits Human Hematopoietic Progenitor Growth In Vitro. J. Infect. Dis. 1997, 175, 1497–1501. [Google Scholar] [CrossRef]
- Costantini, A.; Giuliodoro, S.; Mancini, S.; Butini, L.; Regnery, C.M.; Silvestri, G.; Greco, F.; Leoni, P.; Montroni, M. Impaired in-vitro growth of megakaryocytic colonies derived from CD34 cells of HIV-1-infected patients with active viral replication. AIDS 2006, 20, 1713–1720. [Google Scholar] [CrossRef]
- Cunin, P.; Nigrovic, P.A. Megakaryocytes as immune cells. J. Leukoc. Biol. 2019, 105, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Chora, F.; Goumnerov, B.; Mumaw, C.; Goebel, W.S.; Fernandez, L.; Baydoun, H.; HogenEsch, H.; Dombkowski, D.M.; Karlewicz, C.A.; et al. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood 2009, 114, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Turner, A.R.; Sridharan, S.; Ratajczak, M.Z.; Janowska-Wieczorek, A. The Ins and Outs of Hematopoietic Stem Cells: Studies to Improve Transplantation Outcomes. Stem Cell Rev. Rep. 2011, 7, 590–607. [Google Scholar] [CrossRef] [PubMed]
- To, L.B.; Levesque, J.-P.; Herbert, K.E. How I treat patients who mobilize hematopoietic stem cells poorly. Blood 2011, 118, 4530–4540. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Rastogi, N.; Upasana, K.; Arora, S.; Thakkar, D.; Yadav, S.P. Dengue virus transmission from donor to recipient during haploidentical stem cell transplantation. IDCases 2021, 25, e01220. [Google Scholar] [CrossRef] [PubMed]
- Punzel, M.; Korukluoğlu, G.; Caglayik, D.Y.; Menemenlioglu, D.; Bozdag, S.C.; Tekgündüz, E.; Altuntas, F.; Campos, R.D.M.; Burde, B.; Günther, S.; et al. Dengue Virus Transmission by Blood Stem Cell Donor after Travel to Sri Lanka; Germany, 2013. Emerg. Infect. Dis. 2014, 20, 1366–1369. [Google Scholar] [CrossRef]
- Barroso, K.S.N.; Kaufman, J.; Brunetta, D.; Araújo, F.M.D.C.; Barroso-Duarte, F. Dengue encephalitis in allogenic hematopoietic stem cell transplantation recipient. Bone Marrow Transplant. 2017, 52, 1455–1456. [Google Scholar] [CrossRef]
- Vicente, C.R.; Herbinger, K.-H.; Fröschl, G.; Romano, C.M.; Cabidelle, A.D.S.A.; Junior, C.C. Serotype influences on dengue severity: A cross-sectional study on 485 confirmed dengue cases in Vitória, Brazil. BMC Infect. Dis. 2016, 16, 320. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Su, W.; Cao, Y.; Jing, Q.; Wu, X.; Yang, Z. Circulation of genotypes of dengue virus serotype 2 in Guangzhou over a period of 20 years. Virol. J. 2022, 19, 47. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puc, I.; Ho, T.-C.; Chien, Y.-W.; Tan, S.-S.; Fong, Y.-C.; Chen, Y.-J.; Wang, S.-H.; Li, Y.-H.; Chen, C.-H.; Chen, P.-L.; et al. Mobilization of Hematopoietic Stem and Progenitor Cells during Dengue Virus Infection. Int. J. Mol. Sci. 2022, 23, 14330. https://doi.org/10.3390/ijms232214330
Puc I, Ho T-C, Chien Y-W, Tan S-S, Fong Y-C, Chen Y-J, Wang S-H, Li Y-H, Chen C-H, Chen P-L, et al. Mobilization of Hematopoietic Stem and Progenitor Cells during Dengue Virus Infection. International Journal of Molecular Sciences. 2022; 23(22):14330. https://doi.org/10.3390/ijms232214330
Chicago/Turabian StylePuc, Irwin, Tzu-Chuan Ho, Yu-Wen Chien, Sia-Seng Tan, Yu-Cin Fong, Yi-Ju Chen, Sheng-Hsuan Wang, Yun-Hsuan Li, Chun-Hong Chen, Po-Lin Chen, and et al. 2022. "Mobilization of Hematopoietic Stem and Progenitor Cells during Dengue Virus Infection" International Journal of Molecular Sciences 23, no. 22: 14330. https://doi.org/10.3390/ijms232214330
APA StylePuc, I., Ho, T.-C., Chien, Y.-W., Tan, S.-S., Fong, Y.-C., Chen, Y.-J., Wang, S.-H., Li, Y.-H., Chen, C.-H., Chen, P.-L., Perng, G.-C., & Tsai, J.-J. (2022). Mobilization of Hematopoietic Stem and Progenitor Cells during Dengue Virus Infection. International Journal of Molecular Sciences, 23(22), 14330. https://doi.org/10.3390/ijms232214330






