Alicyclobacillus mali FL18 as a Novel Source of Glycosyl Hydrolases: Characterization of a New Thermophilic β-Xylosidase Tolerant to Monosaccharides
Abstract
1. Introduction
2. Results
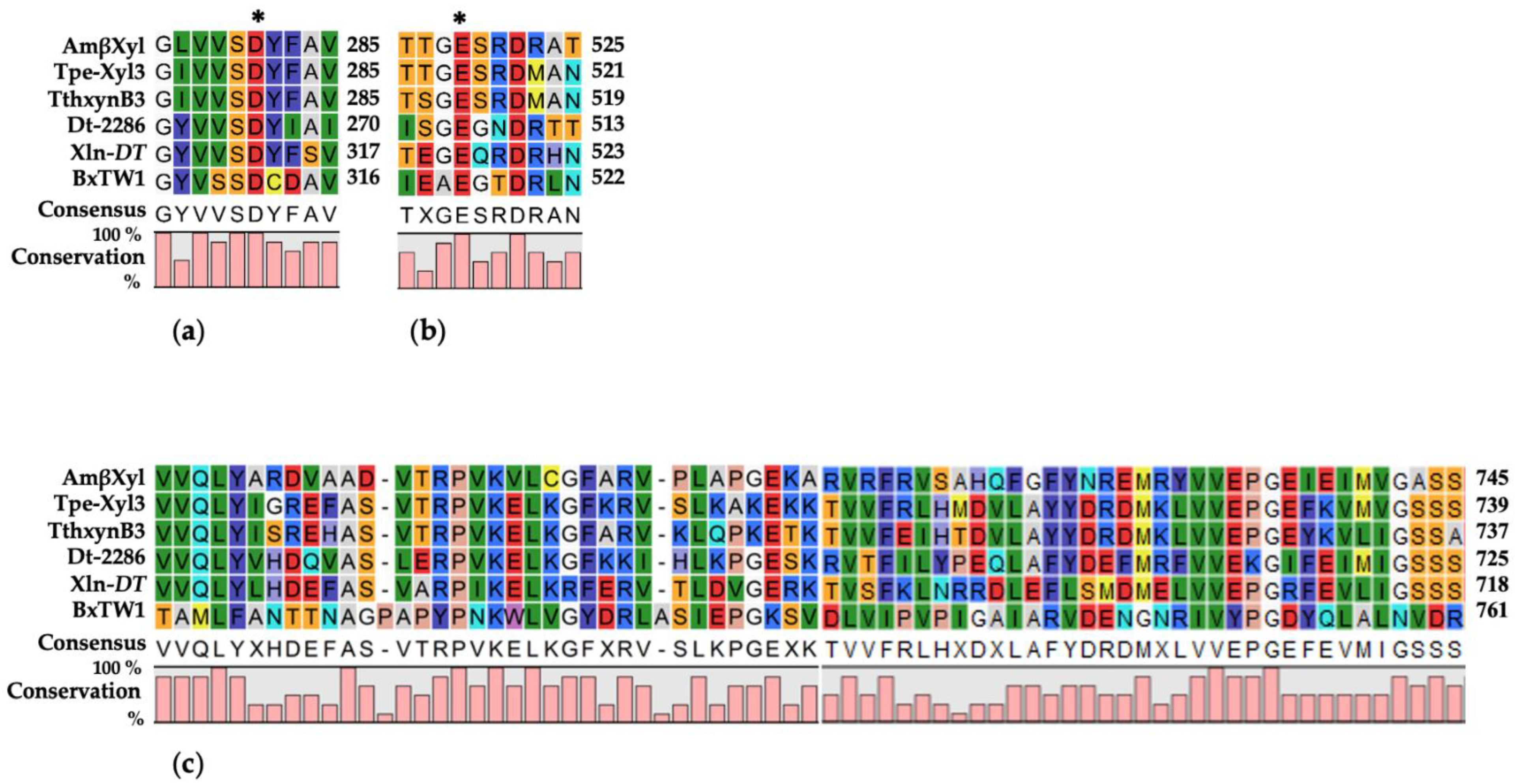
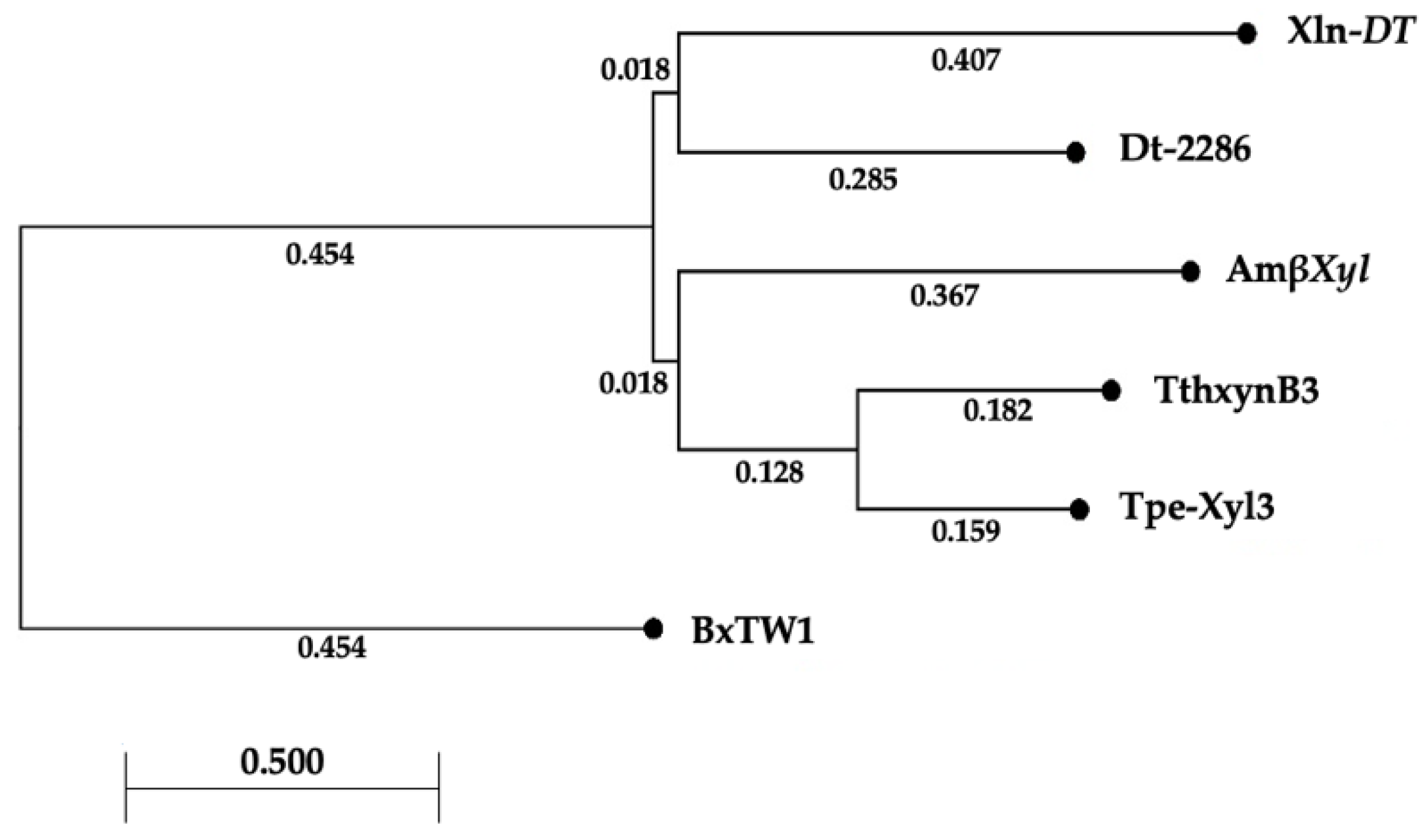
2.1. Sequence Analysis
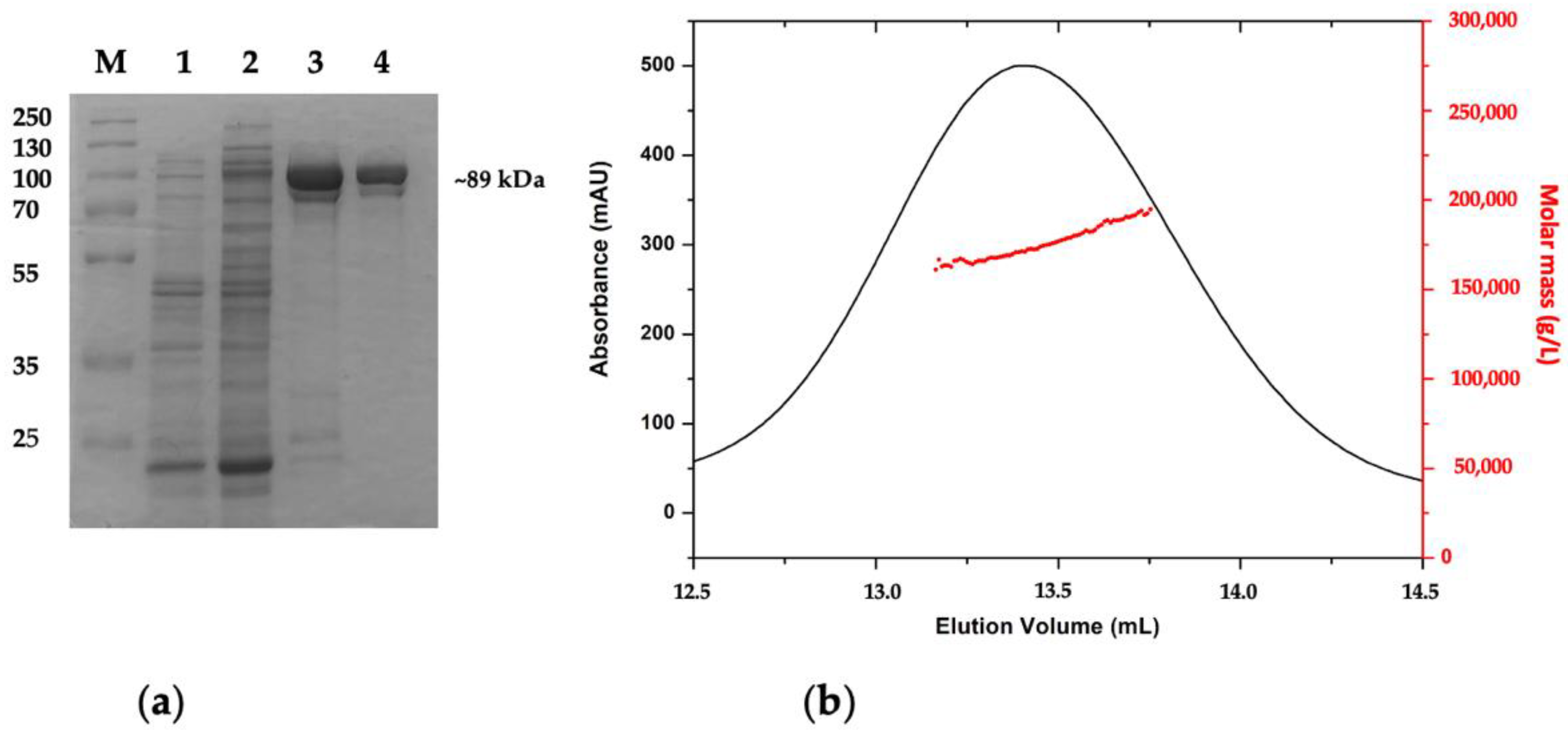
2.2. Purification and Quaternary Structure of AmβXyl
2.3. Characterization of AmβXyl
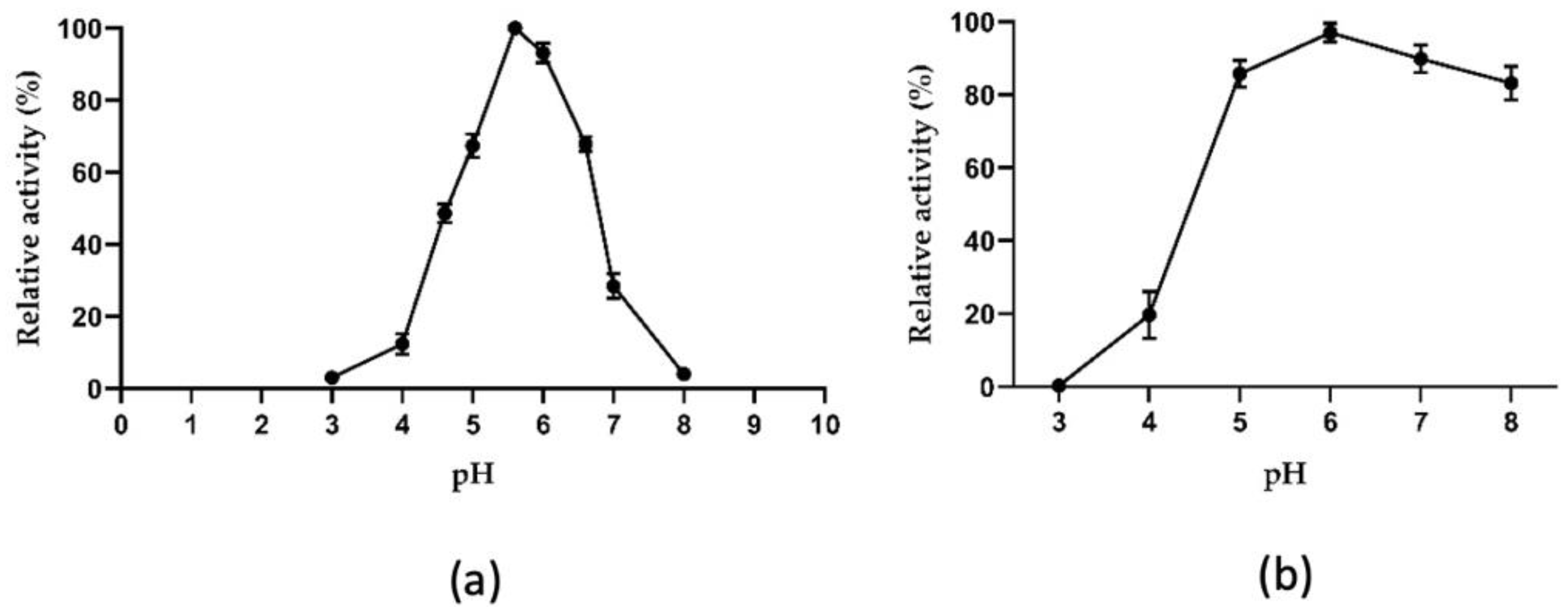
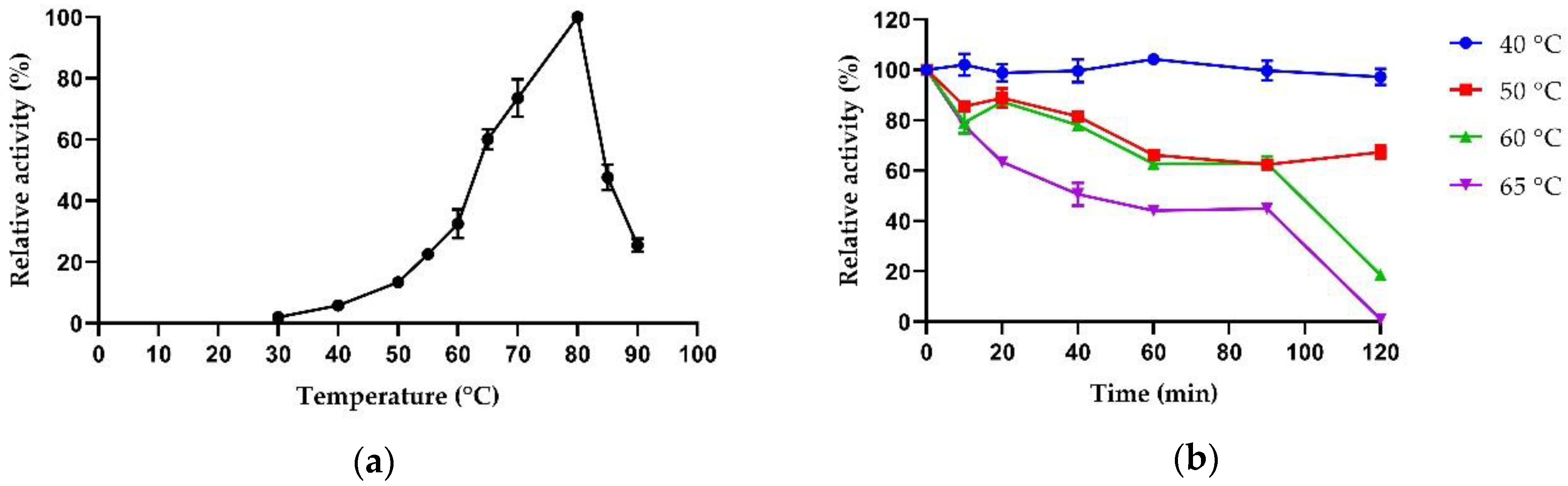
2.3.1. Biochemical Properties
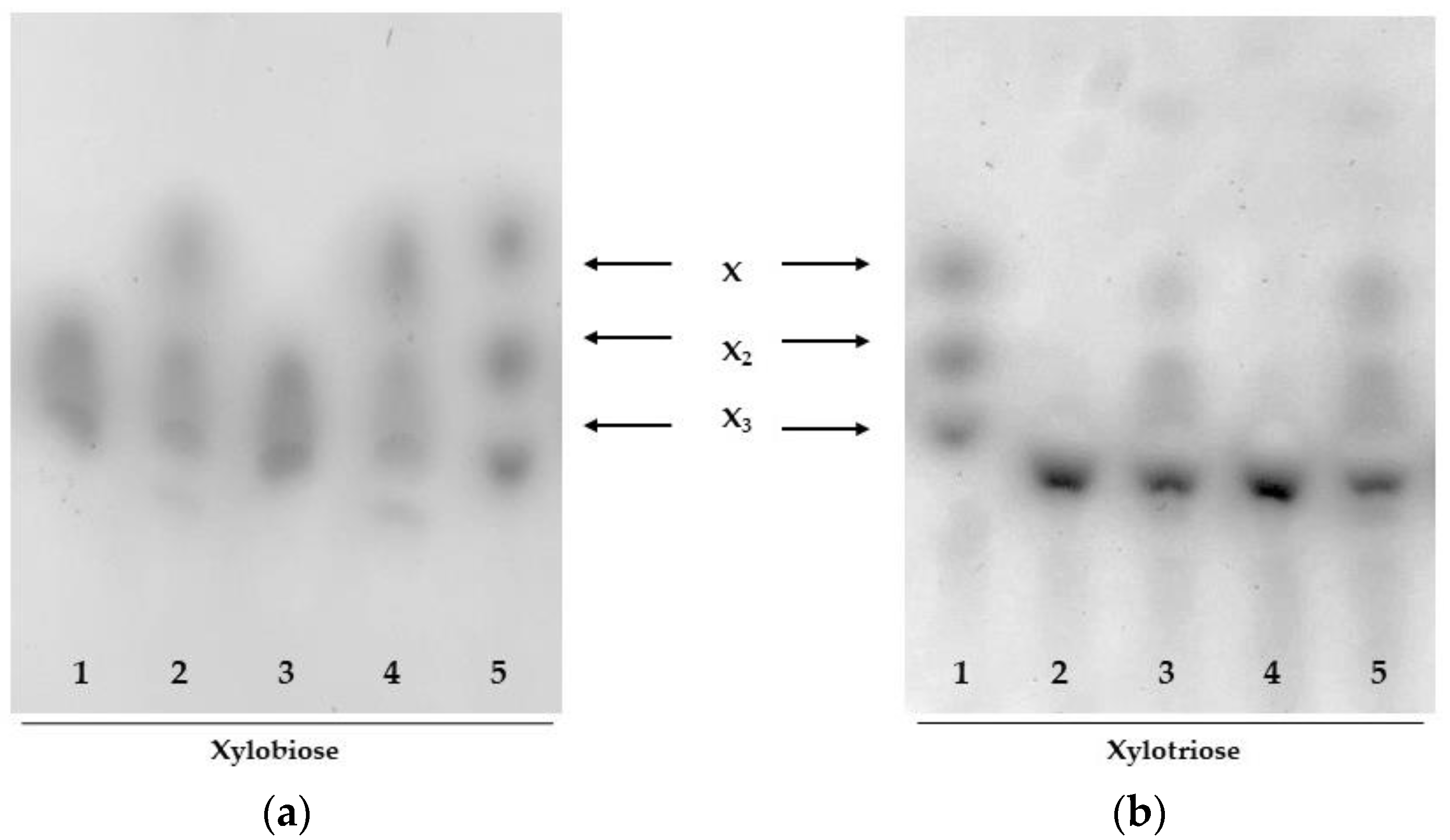
2.3.2. Substrate Specificity
2.3.3. Kinetic Parameters
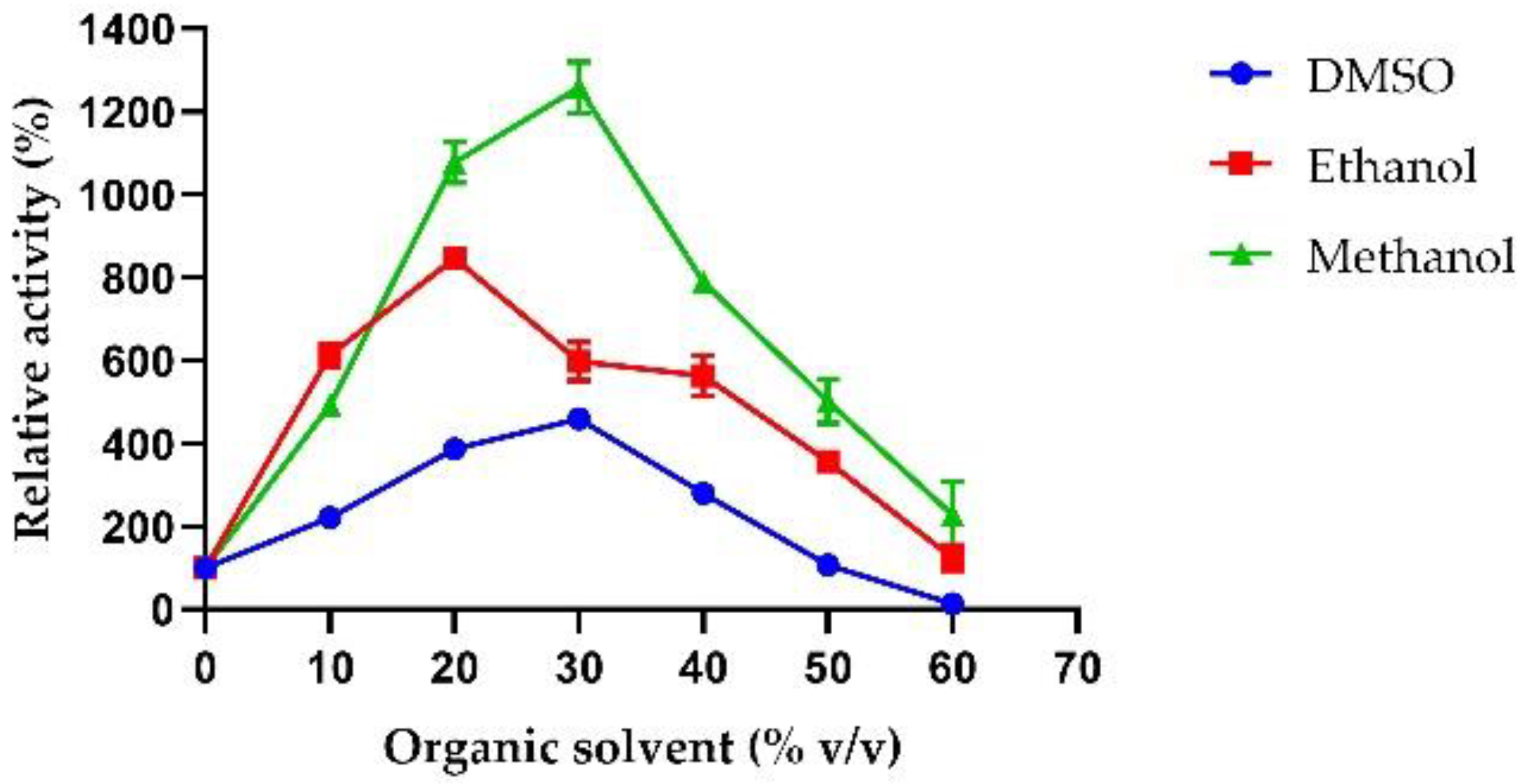
2.3.4. Effect of Chemical Agents
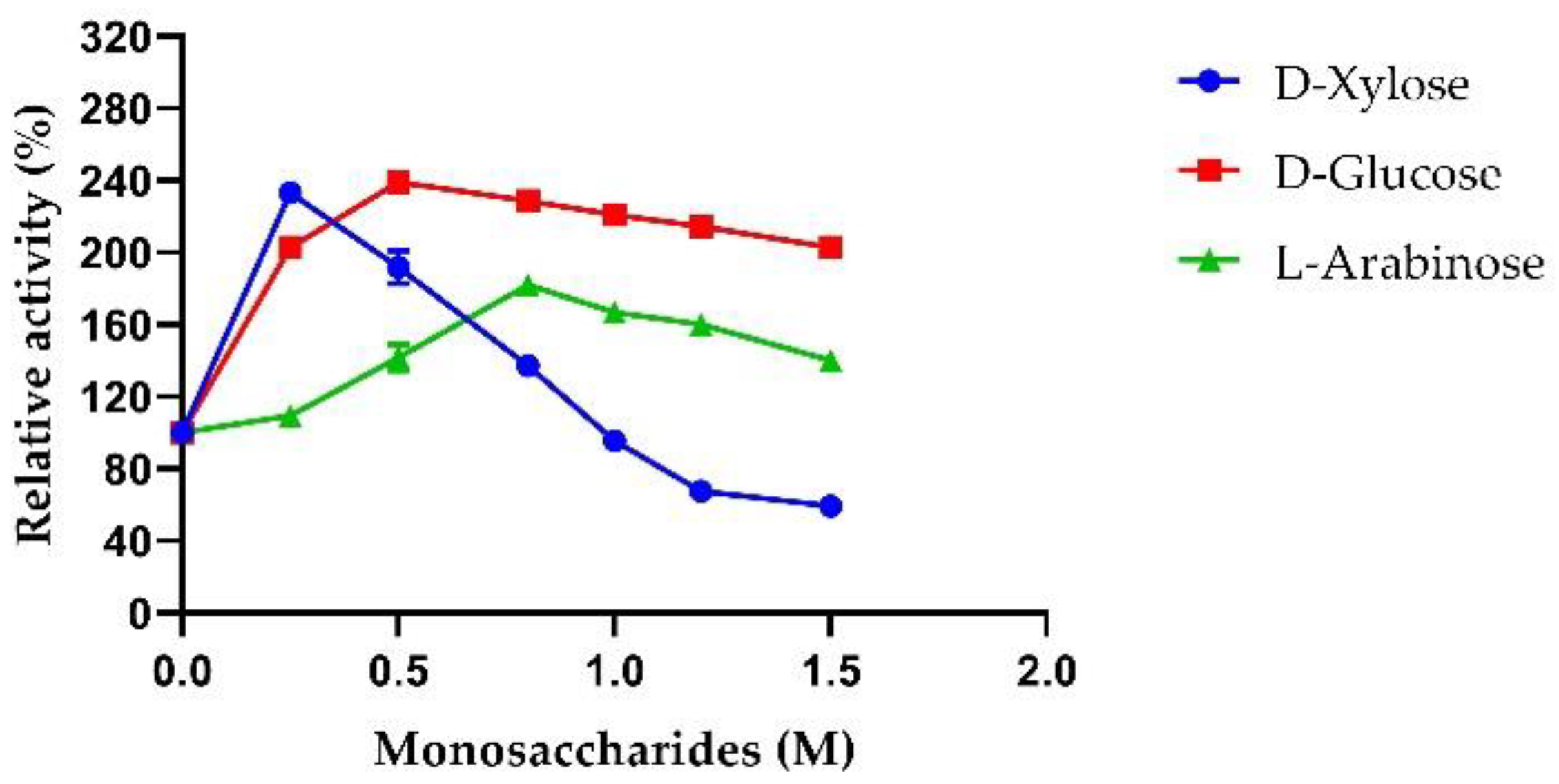
2.3.5. Sugar Tolerance of AmβXyl
3. Materials and Methods
3.1. Gene Synthesis
3.2. Sequence Analyses of AmβXyl
3.3. Expression and Purification of AmβXyl in E. coli
3.4. AmβXyl Biochemical Characterization
3.4.1. Quaternary Structure Determination
3.4.2. β-Xylosidase Activity
3.4.3. Substrate Specificity and Kinetic Parameters
3.4.4. TLC Analysis
3.4.5. pH and Temperature Effects on Activity and Stability of the Enzyme
3.4.6. Effect of Chemicals and Monosaccharides on Enzyme Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A Review on the Pretreatment of Lignocellulose for High-Value Chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent Advances in Lignocellulosic Biomass for Biofuels and Value-Added Bioproducts—A Critical Review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef] [PubMed]
- Ebringerová, A.; Hromádková, Z.; Heinze, T. Hemicellulose. Adv. Polym. Sci. 2005, 186, 1–67. [Google Scholar]
- Juturu, V.; Wu, J.C. Microbial Xylanases: Engineering, Production and Industrial Applications. Biotechnol. Adv. 2012, 30, 1219–1227. [Google Scholar] [CrossRef]
- Goldbeck, R.; Gonçalves, T.A.; Damásio, A.R.L.; Brenelli, L.B.; Wolf, L.D.; Paixão, D.A.A.; Rocha, G.J.M.; Squina, F.M. Effect of Hemicellulolytic Enzymes to Improve Sugarcane Bagasse Saccharification and Xylooligosaccharides Production. J. Mol. Catal. B Enzym. 2016, 131, 36–46. [Google Scholar] [CrossRef]
- Bosetto, A.; Justo, P.I.; Zanardi, B.; Venzon, S.S.; Graciano, L.; dos Santos, E.L.; de Cássia Garcia Simão, R. Research Progress Concerning Fungal and Bacterial β-Xylosidases. Appl. Biochem. Biotechnol. 2016, 178, 766–795. [Google Scholar] [CrossRef]
- Rohman, A.; Dijkstra, B.W.; Puspaningsih, N.N.T. β-Xylosidases: Structural Diversity, Catalytic Mechanism, and Inhibition by Monosaccharides. Int. J. Mol. Sci. 2019, 20, 5524. [Google Scholar] [CrossRef]
- Aulitto, M.; Martinez-Alvarez, L.; Fiorentino, G.; Limauro, D.; Peng, X.; Contursi, P. A Comparative Analysis of Weizmannia coagulans Genomes Unravels the Genetic Potential for Biotechnological Applications. Int. J. Mol. Sci. 2022, 23, 3135. [Google Scholar] [CrossRef]
- Aulitto, M.; Fusco, S.; Nickel, D.B.; Bartolucci, S.; Contursi, P.; Franzén, C.J. Seed Culture Pre-Adaptation of Bacillus coagulans MA-13 Improves Lactic Acid Production in Simultaneous Saccharification and Fermentation. Biotechnol. Biofuels 2019, 12, 45. [Google Scholar] [CrossRef]
- Marcolongo, L.; la Cara, F.; del Monaco, G.; Paixão, S.M.; Alves, L.; Marques, I.P.; Ionata, E. A Novel β-Xylosidase from Anoxybacillus Sp. 3M towards an Improved Agro-Industrial Residues Saccharification. Int. J. Biol. Macromol. 2019, 122, 1224–1234. [Google Scholar] [CrossRef]
- Ing, N.; Deng, K.; Chen, Y.; Aulitto, M.; Gin, J.W.; Pham, T.L.M.; Petzold, C.J.; Singer, S.W.; Bowen, B.; Sale, K.L.; et al. A Multiplexed Nanostructure-Initiator Mass Spectrometry (NIMS) Assay for Simultaneously Detecting Glycosyl Hydrolase and Lignin Modifying Enzyme Activities. Sci. Rep. 2021, 11, 11803. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose Bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Fusco, F.A.; Fiorentino, G.; Bartolucci, S.; Contursi, P.; Limauro, D. A Thermophilic Enzymatic Cocktail for Galactomannans Degradation. Enzym. Microb. Technol. 2018, 111, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Fusco, S.; Limauro, D.; Fiorentino, G.; Bartolucci, S.; Contursi, P. Galactomannan Degradation by Thermophilic Enzymes: A Hot Topic for Biotechnological Applications. World J. Microbiol. Biotechnol. 2019, 35, 32. [Google Scholar] [CrossRef]
- Dalmaso, G.Z.L.; Ferreira, D.; Vermelho, A.B. Marine Extremophiles a Source of Hydrolases for Biotechnological Applications. Mar. Drugs 2015, 13, 1925–1965. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Aulitto, M.; Contursi, P.; Limauro, D.; Bartolucci, S.; Fiorentino, G. Bioprospecting of Extremophilic Microorganisms to Address Environmental Pollution. J. Vis. Exp. 2021, 178, e63453. [Google Scholar] [CrossRef]
- Aulitto, M.; Fusco, S.; Franzén, C.J.; Strazzulli, A.; Moracci, M.; Bartolucci, S.; Contursi, P. Draft Genome Sequence of Bacillus coagulans MA-13, a Thermophilic Lactic Acid Producer from Lignocellulose. Microbiol. Resour. Announc. 2019, 8, 13–15. [Google Scholar] [CrossRef]
- Aulitto, M.; Tom, L.M.; Ceja-Navarro, J.A.; Simmons, B.A.; Singer, S.W. Whole-Genome Sequence of Brevibacillus borstelensis SDM, Isolated from a Sorghum-Adapted Microbial Community. Microbiol. Resour. Announc. 2020, 9, 15–16. [Google Scholar] [CrossRef]
- Shao, W.; Xue, Y.; Wu, A.; Kataeva, I.; Pei, J.; Wu, H.; Wiegel, J. Characterization of a Novel β-Xylosidase, XylC, from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl. Environ. Microbiol. 2011, 77, 719–726. [Google Scholar] [CrossRef]
- Yan, Q.J.; Wang, L.; Jiang, Z.Q.; Yang, S.Q.; Zhu, H.F.; Li, L.T. A Xylose-Tolerant β-Xylosidase from Paecilomyces thermophila: Characterization and Its Co-Action with the Endogenous Xylanase. Bioresour. Technol. 2008, 99, 5402–5410. [Google Scholar] [CrossRef]
- Aulitto, M.; Gallo, G.; Puopolo, R.; Mormone, A.; Limauro, D.; Contursi, P.; Piochi, M.; Bartolucci, S.; Fiorentino, G. Genomic Insight of Alicyclobacillus mali FL18 Isolated From an Arsenic-Rich Hot Spring. Front. Microbiol. 2021, 12, 639697. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Strazzulli, A.; Sansone, F.; Cozzolino, F.; Monti, M.; Moracci, M.; Fiorentino, G.; Limauro, D.; Bartolucci, S.; Contursi, P. Prebiotic Properties of Bacillus coagulans MA-13: Production of Galactoside Hydrolyzing Enzymes and Characterization of the Transglycosylation Properties of a GH42 β-Galactosidase. Microb. Cell Fact. 2021, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Bertram, R.; Schlicht, M.; Mahr, K.; Nothaft, H.; Saier, M.H.; Titgemeyer, F. In Silico and Transcriptional Analysis of Carbohydrate Uptake Systems of Streptomyces coelicolor A3(2). J. Bacteriol. 2004, 186, 1362–1373. [Google Scholar] [CrossRef]
- Tsujibo, H.; Takada, C.; Tsuji, A.; Kosaka, M.; Miyamoto, K.; Inamori, Y. Cloning, Sequencing, and Expression of the Gene Encoding an Intracellular β-D-Xylosidase from Streptomyces thermoviolaceus OPC-520. Biosci. Biotechnol. Biochem. 2001, 65, 1824–1831. [Google Scholar] [CrossRef]
- Pinphanichakarn, P.; Tangsakul, T.; Thongnumwon, T.; Talawanich, Y.; Thamchaipenet, A. Purification and Characterization of β-Xylosidase from Streptomyces Sp. CH7 and Its Gene Sequence Analysis. World J. Microbiol. Biotechnol. 2004, 20, 727–733. [Google Scholar] [CrossRef]
- Eisenhuber, K.; Krennhuber, K.; Steinmüller, V.; Jäger, A. Comparison of Different Pre-Treatment Methods for Separating Hemicellulose from Straw during Lignocellulose Bioethanol Production. Energy Procedia 2013, 40, 172–181. [Google Scholar] [CrossRef]
- Tong, X.; Qi, Z.; Zheng, D.; Pei, J.; Li, Q.; Zhao, L. High-Level Expression of a Novel Multifunctional GH3 Family β-Xylosidase/α-Arabinosidase/β-Glucosidase from Dictyoglomus turgidum in Escherichia Coli. Bioorg. Chem. 2021, 111, 104906. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Y.; Tong, X.; Pei, J.; Xiao, W.; Wang, Z.; Zhao, L. Cloning and Characterization of the β-Xylosidase from Dictyoglomus turgidum for High Efficient Biotransformation of 10-Deacetyl-7-Xylosltaxol. Bioorg. Chem. 2020, 94, 103357. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.; Gu, H.; Zhang, Y.; Huang, Y.; Wang, L.; Wang, F. Biochemical Properties of a Novel Thermostable and Highly Xylose-Tolerant β-Xylosidase/α-Arabinosidase from Thermotoga thermarum. Biotechnol. Biofuels 2013, 6, 27. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, J.; Zhao, L.; Pei, J.; Su, E.; Xiao, W.; Wang, Z. Cloning, Overexpression and Characterization of a Thermostable β-Xylosidase from Thermotoga petrophila and Cooperated Transformation of Ginsenoside Extract to Ginsenoside 20(S)-Rg3 with a β-Glucosidase. Bioorg. Chem. 2019, 85, 159–167. [Google Scholar] [CrossRef]
- Graciano, L.; Corrêa, J.M.; Gandra, R.F.; Seixas, F.A.V.; Kadowaki, M.K.; Sampaio, S.C.; da Conceição Silva, J.L.; Osaku, C.A.; de Simão, R.C.G. The Cloning, Expression, Purification, Characterization and Modeled Structure of Caulobacter crescentus β-Xylosidase I. World J. Microbiol. Biotechnol. 2012, 28, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.A.; Fiorentino, G.; Pedone, E.; Contursi, P.; Bartolucci, S.; Limauro, D. Biochemical Characterization of a Novel Thermostable β-Glucosidase from Dictyoglomus turgidum. Int. J. Biol. Macromol. 2018, 113, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Jia, H.; Yan, Q.; Zhou, P.; Jiang, Z. High-Level Expression of Extracellular Secretion of a β-Xylosidase Gene from Paecilomyces thermophila in Escherichia Coli. Bioresour. Technol. 2011, 102, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Seo, M.J.; Oh, D.K. Characterization of β-Xylosidase from Thermoanaerobacterium thermosaccharolyticum and Its Application to the Production of Ginsenosides Rg1 and Rh1 from Notoginsenosides R1 and R2. Biotechnol. Lett. 2014, 36, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Shi, P.; Xu, B.; Bai, Y.; Luo, H.; Yao, B. Cloning, Expression, and Characterization of a Thermostable β-Xylosidase from Thermoacidophilic alicyclobacillus Sp. A4. Process. Biochem. 2014, 49, 1422–1428. [Google Scholar] [CrossRef]
- la Grange, D.C.; Pretorius, I.S.; Claeyssens, M.; van Zyl, W.H. Degradation of Xylan to D-Xylose by Recombinant Saccharomyces cerevisiae Coexpressing the Aspergillus niger β-Xylosidase (XlnD) and the Trichoderma Reesei Xylanase II (Xyn2) Genes. Appl. Environ. Microbiol. 2001, 67, 5512–5519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, T.; Li, X. Screening and Identification of an Enterobacter ludwigii Strain Expressing an Active β-Xylosidase. Ann. Microbiol. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Sharma, M.; Soni, R.; Nazir, A.; Oberoi, H.S.; Chadha, B.S. Evaluation of Glycosyl Hydrolases in the Secretome of Aspergillus fumigatus and Saccharification of Alkali-Treated Rice Straw. Appl. Biochem. Biotechnol. 2011, 163, 577–591. [Google Scholar] [CrossRef]
- Yang, X.; Shi, P.; Huang, H.; Luo, H.; Wang, Y.; Zhang, W.; Yao, B. Two Xylose-Tolerant GH43 Bifunctional β-Xylosidase/α- Arabinosidases and One GH11 Xylanase from Humicola insolens and Their Synergy in the Degradation of Xylan. Food Chem. 2014, 148, 381–387. [Google Scholar] [CrossRef]
- Fujii, T.; Yu, G.; Matsushika, A.; Kurita, A.; Yano, S.; Murakami, K.; Sawayama, S. Ethanol Production from Xylo-Oligosaccharides by Xylose-Fermenting Saccharomyces cerevisiae Expressing β-Xylosidase. Biosci. Biotechnol. Biochem. 2011, 75, 1140–1146. [Google Scholar] [CrossRef][Green Version]
- Kont, R.; Kurašin, M.; Teugjas, H.; Väljamäe, P. Strong Cellulase Inhibitors from the Hydrothermal Pretreatment of Wheat Straw. Biotechnol. Biofuels 2013, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and Utilization of Thermophiles and Thermostable Enzymes in Biorefining. Microb. Cell Fact. 2007, 6, 9. [Google Scholar] [CrossRef]
- Chen, Y.A.; Zhou, Y.; Liu, D.; Zhao, X.; Qin, Y. Evaluation of the Action of Tween 20 Non-Ionic Surfactant during Enzymatic Hydrolysis of Lignocellulose: Pretreatment, Hydrolysis Conditions and Lignin Structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, H.; Qi, F.; Zhao, X.; Liu, D. Non-Ionic Surfactants Do Not Consistently Improve the Enzymatic Hydrolysis of Pure Cellulose. Bioresour. Technol. 2015, 182, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, X.; Xu, S.; Li, C.; Wei, X.; Liu, Y.; Zhang, R.; Tang, X.; Zhou, J.; Huang, Z. Glycoside Hydrolase Family 39 β-Xylosidase of Sphingomonas Showing Salt/Ethanol/Trypsin Tolerance, Low-PH/Low-Temperature Activity, and Transxylosylation Activity. J. Agric. Food Chem. 2018, 66, 9465–9472. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dai, L.; Li, J.; Deng, M.; Miao, H.; Zhou, J.; Mu, Y.; Wu, Q.; Tang, X.; Yang, Y.; et al. Molecular and Biochemical Characterization of a Novel Xylanase from Massilia Sp. RBM26 Isolated from the Feces of Rhinopithecus Bieti. J. Microbiol. Biotechnol. 2015, 26, 9–19. [Google Scholar] [CrossRef]
- Yegin, S. Single-Step Purification and Characterization of an Extreme Halophilic, Ethanol Tolerant and Acidophilic Xylanase from Aureobasidium pullulans NRRL Y-2311-1 with Application Potential in the Food Industry. Food Chem. 2017, 221, 67–75. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Jain, I.; Kumar, V.; Satyanarayana, T. Applicability of Recombinant β-Xylosidase from the Extremely Thermophilic Bacterium Geobacillus thermodenitrificans in Synthesizing Alkylxylosides. Bioresour. Technol. 2014, 170, 462–469. [Google Scholar] [CrossRef]
- de Ximenes, F.A.; de Paula Silveira, F.Q.; Filho, E.X.F. Production of β-Xylosidase Activity by Trichoderma Harzianum Strains. Curr. Microbiol. 1996, 33, 71–77. [Google Scholar] [CrossRef]
- Xia, W.; Shi, P.; Xu, X.; Qian, L.; Cui, Y.; Xia, M.; Yao, B. High Level Expression of a Novel Family 3 Neutral β-Xylosidase from Humicola insolens Y1 with High Tolerance to D-Xylose. PLoS ONE 2015, 10, e0117578. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Fusco, S.; Fiorentino, G.; Limauro, D.; Pedone, E.; Bartolucci, S.; Contursi, P. Thermus thermophilus as Source of Thermozymes for Biotechnological Applications: Homologous Expression and Biochemical Characterization of an α-Galactosidase. Microb. Cell Fact. 2017, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]

| Purification Step | Total Activity (U) | Total Protein (mg) | Specific Activity (U/mg) | Yield (%) | Fold Purification |
|---|---|---|---|---|---|
| C.E. | 6300.00 | 281.29 | 22.39 | 100.00 | 1.00 |
| A.C. | 1470.60 | 3.87 | 380.00 | 23.30 | 16.97 |
| A.E.C. | 756.00 | 1.26 | 600.00 | 12.00 | 26.80 |
| Substrate | Relative Activity (%) |
|---|---|
| PNP-β-xyl | 100 |
| PNP-α-ara | 59.30 |
| PNP-β-glu | 0.19 |
| PNP-α-glu | 0.73 |
| PNP-β-gal | ND |
| PNP-α-gal | ND |
| Beechwood xylan | 14.59 |
| Wheat arabinoxylan | 20.86 |
| Substrate | Enzyme | KM (mM) | kcat (s−1) | kcat/KM (mM−1·s−1) | Reference |
|---|---|---|---|---|---|
| PNP-β-xyl | Am βXyl | 0.52 | 1606.00 | 3088.46 | This work |
| TthxynB3 | 0.27 | 316.81 | 1173.40 | [29] | |
| Dt-Xyl3 | 0.83 | ND | ND | [28] | |
| Tpe-Xyl3 | 0.12 | 982.32 | 7808.00 | [30] | |
| Dt-2286 | 0.12 | 651.59 | 5245.31 | [27] | |
| PNP-α-ara | Am βXyl | 10.56 | 2395.80 | 226.87 | This work |
| TthxynB3 | 0.21 | 106.23 | 505.90 | [29] | |
| Dt-Xyl3 | 2.01 | ND | ND | [28] | |
| Tpe-Xyl3 | 6.16 | 2201.64 | 357.89 | [30] | |
| Dt-2286 | 0.50 | 1054.33 | 2077.35 | [27] |
| Metal Ions or Chemical Agents | Relative Activity (%) | ||
|---|---|---|---|
| 1 mM | 5 mM | 0.5% | |
| None | 100 | 100 | 100 |
| CuCl2 | 32.10 | ND | |
| ZnCl2 | 105.80 | 93.99 | |
| LiCl | 89.85 | 83.20 | |
| MgCl2 | 91.64 | 77.81 | |
| CaCl2 | 82.97 | 82.46 | |
| MnSO4 | 78.13 | 59.59 | |
| NiCl2 | 104.28 | 86.29 | |
| EDTA | 96.99 | 98.89 | |
| SDS | 1.30 | ||
| Triton X-100 | 91.08 | ||
| Tween 20 | 113.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, F.; Aulitto, M.; Fiorentino, G.; Pedone, E.; Contursi, P.; Limauro, D. Alicyclobacillus mali FL18 as a Novel Source of Glycosyl Hydrolases: Characterization of a New Thermophilic β-Xylosidase Tolerant to Monosaccharides. Int. J. Mol. Sci. 2022, 23, 14310. https://doi.org/10.3390/ijms232214310
Salzano F, Aulitto M, Fiorentino G, Pedone E, Contursi P, Limauro D. Alicyclobacillus mali FL18 as a Novel Source of Glycosyl Hydrolases: Characterization of a New Thermophilic β-Xylosidase Tolerant to Monosaccharides. International Journal of Molecular Sciences. 2022; 23(22):14310. https://doi.org/10.3390/ijms232214310
Chicago/Turabian StyleSalzano, Flora, Martina Aulitto, Gabriella Fiorentino, Emilia Pedone, Patrizia Contursi, and Danila Limauro. 2022. "Alicyclobacillus mali FL18 as a Novel Source of Glycosyl Hydrolases: Characterization of a New Thermophilic β-Xylosidase Tolerant to Monosaccharides" International Journal of Molecular Sciences 23, no. 22: 14310. https://doi.org/10.3390/ijms232214310
APA StyleSalzano, F., Aulitto, M., Fiorentino, G., Pedone, E., Contursi, P., & Limauro, D. (2022). Alicyclobacillus mali FL18 as a Novel Source of Glycosyl Hydrolases: Characterization of a New Thermophilic β-Xylosidase Tolerant to Monosaccharides. International Journal of Molecular Sciences, 23(22), 14310. https://doi.org/10.3390/ijms232214310










