Insights into the Biocompatibility and Biological Potential of a Chitosan Nanoencapsulated Textile Dye
Abstract
1. Introduction
2. Results
2.1. Size and Zeta Potential Determination
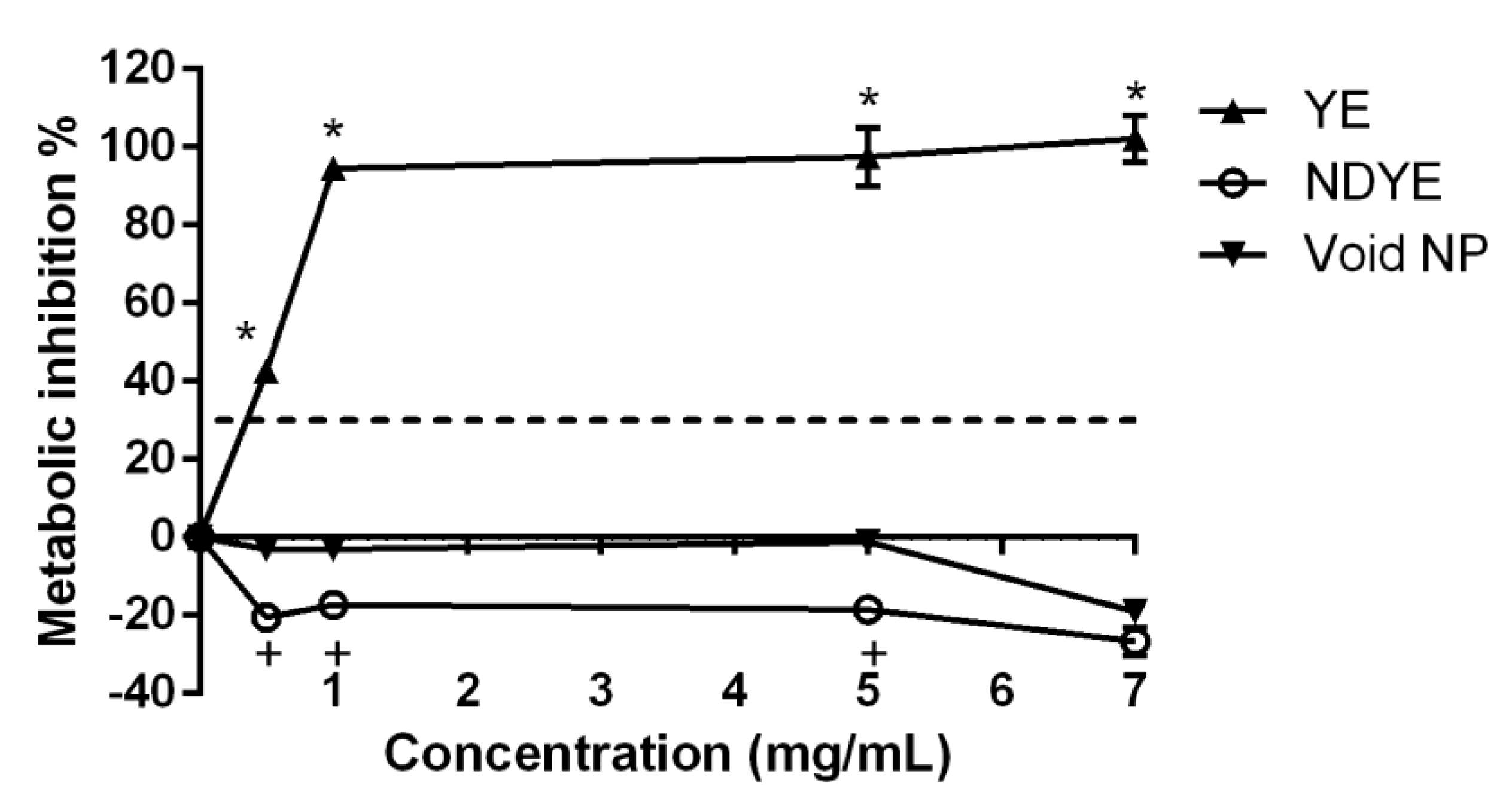
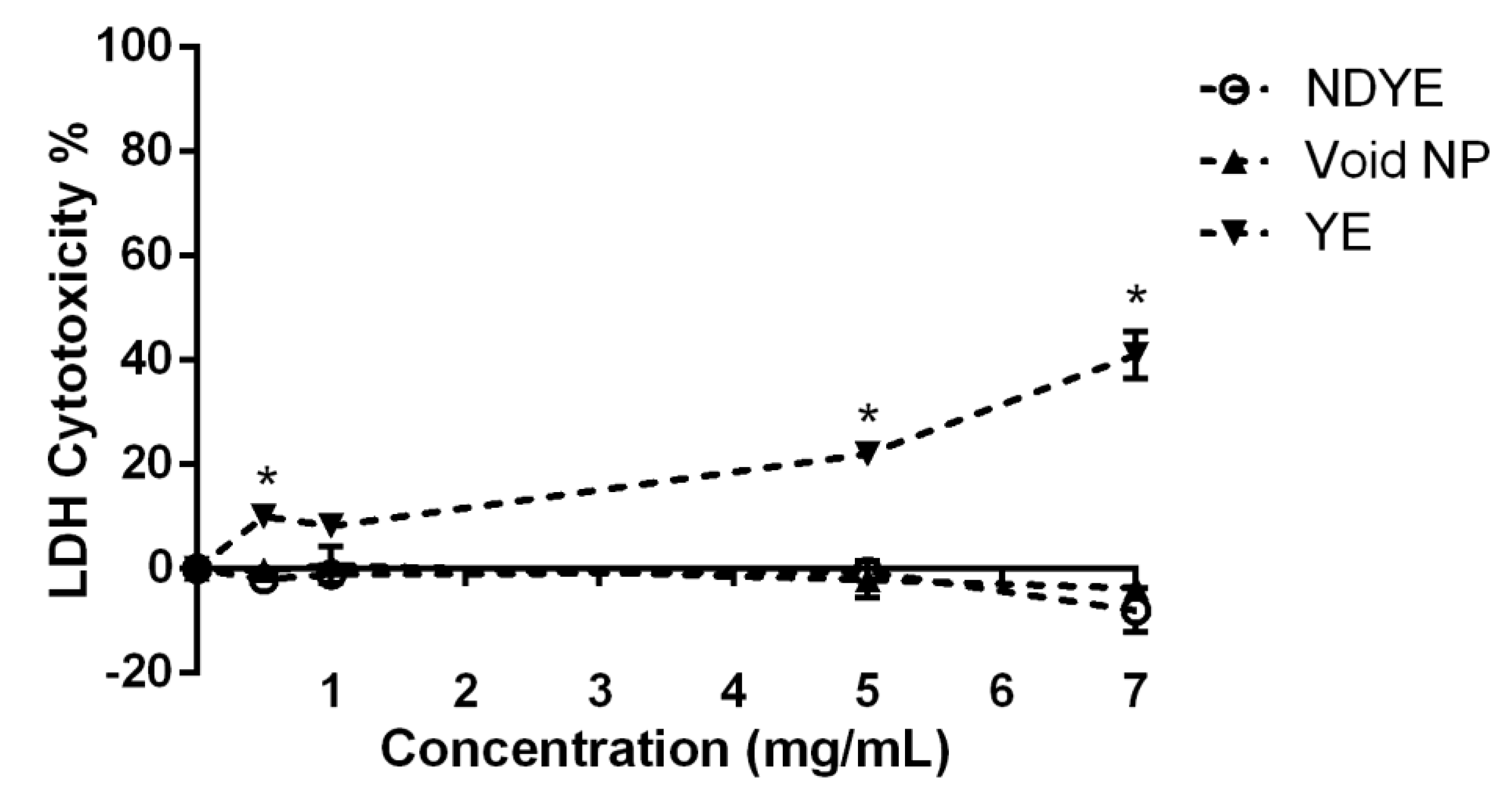
2.2. Biocompatibility Assays
2.3. MIC and MBC Determination
2.4. Biofilm Formation Inhibition
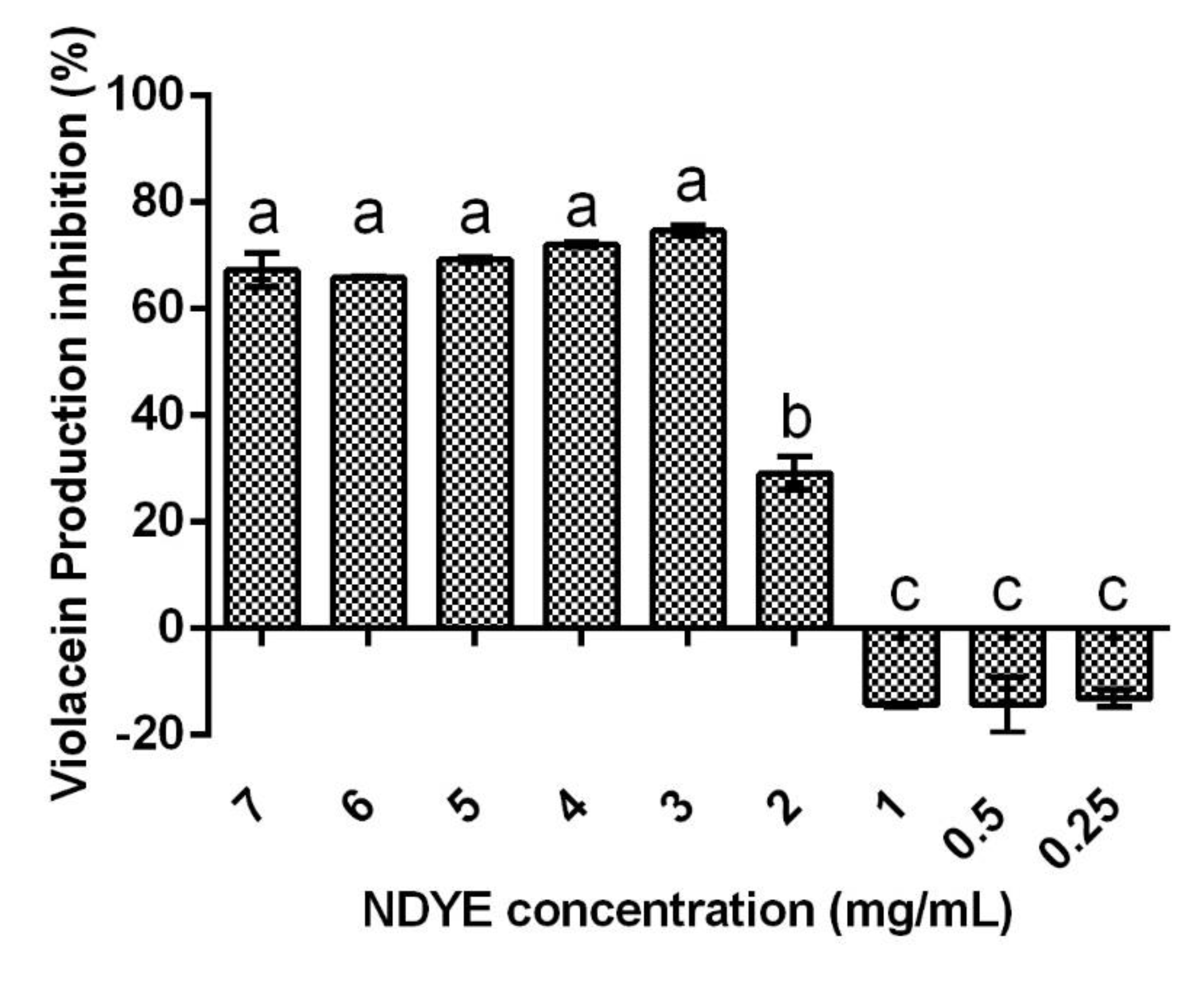
2.5. Quorum Sensing Inhibition
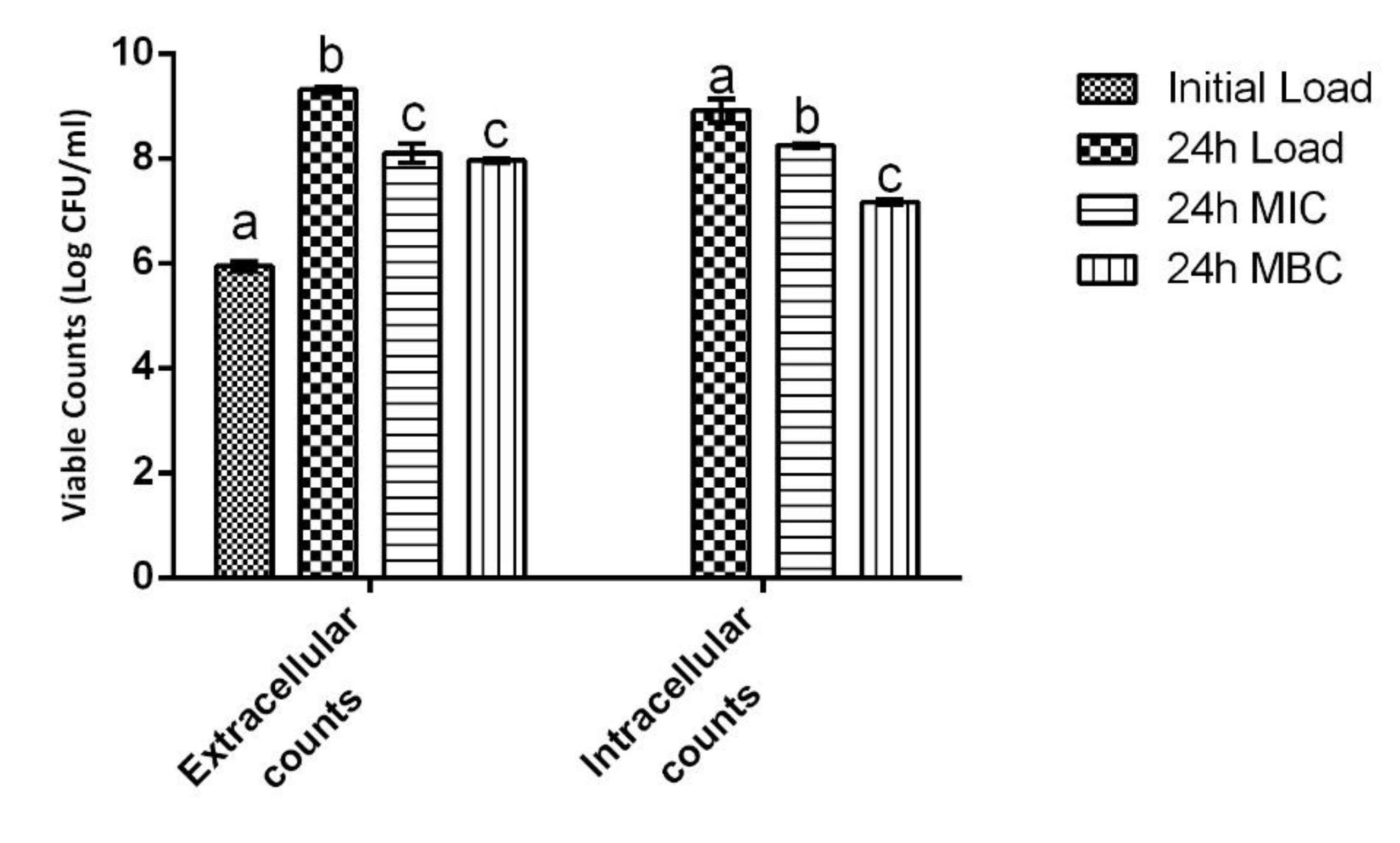
2.6. Cell Infection Assays
3. Discussion
4. Materials and Methods
4.1. Sources of Chemicals and Solutions Preparation
4.2. Microorganisms and Cellular Line
4.3. Nanoencapsulated Dyes Production and Characterization
4.4. Biocompatibility Assays
4.4.1. XTT Assay
4.4.2. LDH Leakage Assay
4.5. Antimicrobial Activity
4.6. Antibiofilm Activity
4.7. Quorum Sensing (QS) Inhibition Assay
4.8. Cell Infection Assays
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, M.C.; Isloor, A.M.; Moslehyani, A.; Ismail, N.; Ismail, A. Fabrication of novel PPSU/ZSM-5 ultrafiltration hollow fiber membranes for separation of proteins and hazardous reactive dyes. J. Taiwan Inst. Chem. Eng. 2018, 82, 342–350. [Google Scholar] [CrossRef]
- Alver, E.; Bulut, M.; Metin, A.; Çiftçi, H. One step effective removal of Congo Red in chitosan nanoparticles by encapsulation. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2017, 171, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wasim, M.; Sagar, S.; Sabir, A.; Shafiq, M.; Jamil, T. Decoration of open pore network in Polyvinylidene fluoride/MWCNTs with chitosan for the removal of reactive orange 16 dye. Carbohydr. Polym. 2017, 174, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.U.; Paul, D.; Dhotre, D.; Kodam, K.M. Effective biotransformation and detoxification of anthraquinone dye reactive blue 4 by using aerobic bacterial granules. Water Res. 2017, 122, 603–613. [Google Scholar] [CrossRef]
- Jha, P.; Jobby, R.; Desai, N. Remediation of textile azo dye acid red 114 by hairy roots of Ipomoea carnea Jacq. and assessment of degraded dye toxicity with human keratinocyte cell line. J. Hazard. Mater. 2016, 311, 158–167. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- Luo, S.; Van Ooij, W.J. Surface modification of textile fibers for improvement of adhesion to polymeric matrices: A review. J. Adhes. Sci. Technol. 2002, 16, 1715–1735. [Google Scholar] [CrossRef]
- Shahidi, S.; Wiener, J.; Ghoranneviss, M. Surface Modification Methods for Improving the Dyeability of Textile Fabrics. In Eco-Friendly Textile Dyeing and Finishing; Gunay, M., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Shahid, M.; Mohammad, F. Mohammad. Green Chemistry Approaches to Develop Antimicrobial Textiles Based on Sustainable Biopolymers—A Review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Kasiri, M.B.; Safapour, S. Natural dyes and antimicrobials for green treatment of textiles. Environ. Chem. Lett. 2013, 12, 1–13. [Google Scholar] [CrossRef]
- Coradi, M.; Zanetti, M.; Valério, A.; de Oliveira, D.; da Silva, A.; Souza, S.M.D.A.G.U.D.; de Souza, A.A.U. Production of antimicrobial textiles by cotton fabric functionalization and pectinolytic enzyme immobilization. Mater. Chem. Phys. 2018, 208, 28–34. [Google Scholar] [CrossRef]
- Singh, R.; Jain, A.; Panwar, S.; Gupta, D.; Khare, S.K. Antimicrobial activity of some natural dyes. Dye. Pigment. 2005, 66, 99–102. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, J.; Liu, Z.; Liang, X.; Shang, C. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J. Hazard. Mater. 2011, 185, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, J.; Chan, W.; Szeto, Y. The sorption of acid dye onto chitosan nanoparticles. Polymer 2006, 47, 5838–5842. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Veiga, M.; Baptista, P.; Tavaria, F.K.; Pintado, M.E. Textile dyes loaded chitosan nanoparticles: Characterization, biocompatibility and staining capacity. Carbohydr. Polym. 2020, 251, 117120. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.W.; Rajendran, S.; Joshi, M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr. Polym. 2011, 83, 438–446. [Google Scholar] [CrossRef]
- Yang, H.-C.; Wang, W.-H.; Huang, K.-S.; Hon, M.-H. Preparation and application of nanochitosan to finishing treatment with anti-microbial and anti-shrinking properties. Carbohydr. Polym. 2010, 79, 176–179. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Chen, Y.-Y.; Lin, H.; Wang, C.; Yang, Z.-D. Preparation of chitosan nanoparticles and their application to Antheraea pernyi silk. J. Appl. Polym. Sci. 2010, 117, 3362–3369. [Google Scholar] [CrossRef]
- Cheung, A.; Szeto, Y.S.; McKay, G. Enhancing the adsorption capacities of acid dyes by chitosan nano particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Wang, J.J.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices in Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009; p. 34.
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, A.; Castro, P.M.; Pintado, M. Production of antimicrobial chitosan nanoparticles against food pathogens. J. Food Eng. 2015, 167, 210–216. [Google Scholar] [CrossRef]
- Costa, E.; Silva, S.; Vicente, S.; Neto, C.; Castro, P.; Veiga, M.; Madureira, R.; Tavaria, F.; Pintado, M. Chitosan nanoparticles as alternative anti-staphylococci agents: Bactericidal, antibiofilm and antiadhesive effects. Mater. Sci. Eng. C 2017, 79, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Veiga, M.; Tavaria, F.; Pintado, M.M. Exploring chitosan nanoparticles as effective inhibitors of antibiotic resistant skin microorganisms–From in vitro to ex vitro testing. Carbohydr. Polym. 2018, 201, 340–346. [Google Scholar] [CrossRef]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Sahu, S.K.; Mahapatra, S.K.; Santra, S.; Bal, M.; Roy, S.; Pramanik, P. Nanoconjugated vancomycin: New opportunities for the development of anti-VRSA agents. Nanotechnology 2010, 21, 105103. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.; Hu, H.; Wang, W.; Zhang, X. Macromolecular agents with antimicrobial potentialities: A drive to combat antimicrobial resistance. Int. J. Biol. Macromol. 2017, 103 (Suppl. C), 554–574. [Google Scholar] [CrossRef] [PubMed]
- Aleanizy, F.S.; Alqahtani, F.Y.; Shazly, G.; Alfaraj, R.; Alsarra, I.; Alshamsan, A.; Abdulhady, H.G. Measurement and evaluation of the effects of pH gradients on the antimicrobial and antivirulence activities of chitosan nanoparticles in Pseudomonas aeruginosa. Saudi Pharm. J. 2017, 26, 79–83. [Google Scholar] [CrossRef]
- Freire, P.L.; Albuquerque, A.J.; Farias, I.A.; da Silva, T.G.; Aguiar, J.S.; Galembeck, A.; Flores, M.A.; Sampaio, F.C.; Stamford, T.C.M.; Rosenblatt, A. Antimicrobial and cytotoxicity evaluation of colloidal chitosan–silver nanoparticles–fluoride nanocomposites. Int. J. Biol. Macromol. 2016, 93, 896–903. [Google Scholar] [CrossRef]
- El-Naggar, M.Y.; Gohar, Y.M.; Sorour, M.A.; Waheeb, M.G. Hydrogel Dressing with a Nano-Formula against Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Diabetic Foot Bacteria. J. Microbiol. Biotechnol. 2016, 26, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Aksoy, M.; Beck, B. Antimicrobial activity of chitosan and a chitosan oligomer against bacterial pathogens of warmwater fish. J. Appl. Microbiol. 2017, 122, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Harjai, K.; Chhibber, S. Antibiotic susceptibility of ica-positive and ica-negative MRSA in different phases of biofilm growth. J. Antibiot. 2014, 68, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Harjai, K.; Chhibber, S. Potential of sequential treatment with minocycline and S. aureus specific phage lysin in eradication of MRSA biofilms: An in vitro study. Appl. Microbiol. Biotechnol. 2015, 99, 3201–3210. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Liu, C.; Yu, W.; Han, F. Enhancing the stability and antibiofilm activity of DspB by immobilization on carboxymethyl chitosan nanoparticles. Microbiol. Res. 2015, 178, 35–41. [Google Scholar] [CrossRef]
- Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Control of biofilm-associated infections by signaling molecules and nanoparticles. Int. J. Pharm. 2016, 510, 409–418. [Google Scholar] [CrossRef]
- Ilk, S.; Sağlam, N.; Özgen, M.; Korkusuz, F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2017, 94, 653–662. [Google Scholar] [CrossRef]
- Ballhausen, B.; Jung, P.; Kriegeskorte, A.; Makgotlho, P.E.; Ruffing, U.; von Müller, L.; Köck, R.; Peters, G.; Herrmann, M.; Ziebuhr, W.; et al. LA-MRSA CC398 differ from classical community acquired-MRSA and hospital acquired-MRSA lineages: Functional analysis of infection and colonization processes. Int. J. Med. Microbiol. 2014, 304, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Bünter, J.P.; Seth-Smith, H.M.; Rüegg, S.; Heikinheimo, A.; Borel, N.; Johler, S. Wild type agr-negative livestock-associated MRSA exhibits high adhesive capacity to human and porcine cells. Res. Microbiol. 2017, 168, 130–138. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular staphylococcus aureus: Live-in and let die. Front. Cell. Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Niu, H.; Wang, D.; Sun, F.; Sun, Y.; Duan, J. Chitosan conjugation enables intracellular bacteria susceptible to aminoglycoside antibiotic. Glycobiology 2016, 26, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Elbi, S.; Nimal, T.R.; Rajan, V.K.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of Salmonella. Colloids Surf. B Biointerfaces 2017, 160 (Suppl. C), 40–47. [Google Scholar]
- Costa, E.M.; Pereira, C.F.; Ribeiro, A.A.; Casanova, F.; Freixo, R.; Pintado, M.; Ramos, O.L. Characterization and Evaluation of Commercial Carboxymethyl Cellulose Potential as an Active Ingredient for Cosmetics. Appl. Sci. 2022, 12, 6560. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Costa, M.R.; Pereira, M.F.; Pereira, J.O.; Soares, J.C.; Pintado, M.M. Aqueous extracts of Vaccinium corymbosum as inhibitors of Staphylococcus aureus. Food Control 2015, 51, 314–320. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Madureira, A.R.; Cardelle-Cobas, A.; Tavaria, F.K.; Pintado, M.M. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr. Polym. 2014, 101, 1081–1086. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M. Antimicrobial Effect of Chitosan against Periodontal Pathogens Biofilms. SOJ Microbiol. Infect. Dis. 2014, 2, 1–6. [Google Scholar]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1195–1208. [Google Scholar] [CrossRef]


| Microorganism | MIC | MBC |
|---|---|---|
| Methicillin-sensitive Staphylococcus aureus | 1 | 1 |
| Methicillin-resistant Staphylococcus aureus | 1 | 3 |
| Vancomycin-resistant Staphylococcus aureus | 2 | 3 |
| Staphylococcus epidermidis | 2 | 3 |
| Methicillin-resistant Staphylococcus epidermidis | 0.5 | 3 |
| Pseudomonas aeruginosa | 1 | Nd |
| Acinetobacter baumannii | 0.5 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M. Insights into the Biocompatibility and Biological Potential of a Chitosan Nanoencapsulated Textile Dye. Int. J. Mol. Sci. 2022, 23, 14234. https://doi.org/10.3390/ijms232214234
Costa EM, Silva S, Tavaria FK, Pintado M. Insights into the Biocompatibility and Biological Potential of a Chitosan Nanoencapsulated Textile Dye. International Journal of Molecular Sciences. 2022; 23(22):14234. https://doi.org/10.3390/ijms232214234
Chicago/Turabian StyleCosta, Eduardo M., Sara Silva, Freni K. Tavaria, and Manuela Pintado. 2022. "Insights into the Biocompatibility and Biological Potential of a Chitosan Nanoencapsulated Textile Dye" International Journal of Molecular Sciences 23, no. 22: 14234. https://doi.org/10.3390/ijms232214234
APA StyleCosta, E. M., Silva, S., Tavaria, F. K., & Pintado, M. (2022). Insights into the Biocompatibility and Biological Potential of a Chitosan Nanoencapsulated Textile Dye. International Journal of Molecular Sciences, 23(22), 14234. https://doi.org/10.3390/ijms232214234








