The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review
Abstract
:1. Introduction
1.1. Genesis of Combination Therapy
1.1.1. Antibiotic Combination Therapy
- (i).
- Targeting diverse pathways as it has been “for the combination of isoniazid, rifampicin, ethambutol, and pyrazinamide for use in the treatment of tuberculosis” [35,36]. This combination comprises two or more drugs that individually target various facets of the infection, as many ailments have been discovered to be multicomponent or multifactorial. Also, drugs A and B’s mechanisms are very much liable to be impartial in their exact activities. Nevertheless, they could have interactive impacts on patient status’s general upturn [37].
- (ii).
- Inhibiting different targets through a single pathway. The mechanism involves several drug combinations that aim at only one “disease component and usually a single cell type or even a single response pathway in the cell type, but with various targeted sites” [36]. This precise targeting reduced drug doses and toxicities, thus allowing more noteworthy results. For example, the drug cotrimoxazole (sulfamethoxazole + trimethoprim) utilizes two medicines functioning at various stages in a single pathway to achieve better inhibition than using either of those drugs alone [37]. “It is a combination of sulfamethoxazole, inhibits folic acid production by suppressing dihydropteroate synthetase, and trimethoprim, which functions at a later step in nucleotide production to subdue dihydrofolate reductase” [37].
- (iii).
- Inhibiting the same target in different ways, for example, in cases regarding streptogramins and virginiamycin [35,36]. “The mode of action involves one drug that is useful on its own but is not sufficiently efficient or too toxic and a second drug that does not share the same activity as the first drug and may have no beneficial effect on its own, but that can improve the efficacy of the first drug by either pharmacokinetic/pharmacodynamic mechanism” [37].
1.1.2. The Pros and Cons of Antibiotic Combination Therapy
The Pros (Benefits) of Combination Antibiotic Therapy
- (a)
- Synergy in action: The first and most important justification for the application of combined antibiotic treatment is to generate synergistic drug interactions [37]. Synergistic combinations of antibiotics can exterminate MDR maladies much more efficiently than antibiotics taken independently [37]. For example, the tigecycline and carbapenem combination has been used to efficiently treat carbapenemase-containing Klebsiella pneumonia-infected patients [34]. Moreso, the combination of ceftazidime with tobramycin has been demonstrated to be a potent therapy for patients with cystic fibrosis [35,38].
- (b)
- Polymicrobial infections: Combination therapy effectively treats polymicrobial infections—diseases that comprise multiple bacterial pathogens that are usually detected in the pelvic region, intra-abdominal and urogenital tract A diseases. Combinations of ciprofloxacin with metronidazole drugs are the basis for this treatment [39].
- (c)
- Improved uptake and sequential blockage: In the improvement of the uptake and inhibition of consecutive steps, combining treatments also helps. Beta-lactam combined with aminoglycoside drugs results in antibacterial synergy with increased uptake. This behavior is facilitated by way of beta-lactam bringing about cell membrane impairment, which enables the aminoglycoside movement into the bacteria cells, thus improving the bactericidal activity [18].
- (d)
- Reduced toxic effects and diminished death rate: Coherent drug combination therapy reduces the concentration necessary for therapeutics and minimizes the dose associated with toxicity and reduced mortality rate. Though, there is no information “from clinical trials that prove beyond a reasonable doubt that combination therapy with different agents allows for a reduction of the drug dose sufficient to decrease dose-related toxicity” [40].
- (e)
- Prevention of Drug Resistance: Different molecular targets of individual agents permit the investigation of the use of drug combination therapy and thus, widen the activity spectrum. With their broad ranges of action and multimodal activity, antimicrobial substances may impede the emergence of drug resistance [18].
- (f)
- Empirical Treatment: In a situation where the nature of the infectious disease is not evident, i.e., undiagnosed infections, empiric antibiotic combinations help begin the treatment. Utilizing empiric antibiotic therapy with an agent to which bacteria is sensitive has been linked to a decrease in death rate and improvement in the results [41,42].
Adverse Effects of Combination Antibiotic Therapy
- (i).
- Antagonism: In contrast to synergistic drug interactions, some combinations might show antagonism, where one drug can either invalidate or diminish the effect of the other on organisms [38]. For example, the initiation of Beta-lactamase by one agent leaves the second agent unsuccessful in treating Enterobacter, Serratia, or Pseudomonas with combination therapy. Antagonism may make bactericidal agent bacteriostatic [18].
- (ii).
- Clostridium difficile infection (CDI): This is another known unfavorable effect of antibiotic use. Any wide-spectrum antibiotic (aminoglycosides and beta-lactams) can cause an overgrowth of C. difficile. “Among these, fluoroquinolones were documented being an independent risk factor for CDI’’ [43,44].
- (iii).
- Other effects of combination therapy include: drug toxicity such as nephrotoxicity and ototoxicity, fungal overgrowth, drug interactions, irrational drug use, and a rise in the cost of treatment [42].
2. Drug Interactions
“The fractional inhibitory concentration (FIC) is the Minimum Inhibitory concentration (MIC) of drug A in the company of B divided by the MIC of A”.
2.1. Combination of Beta-Lactams with Aminoglycosides and Fluoroquinolones
2.1.1. Combination Therapy of Beta-Lactams with Lipopeptide and Glycopeptide
2.1.2. Beta-Lactam/Beta-Lactamase Inhibitor Combinations
2.1.3. Clinical Cases of Combination Therapy
2.2. Nano-Materials Combinations with Antibacterial Drugs
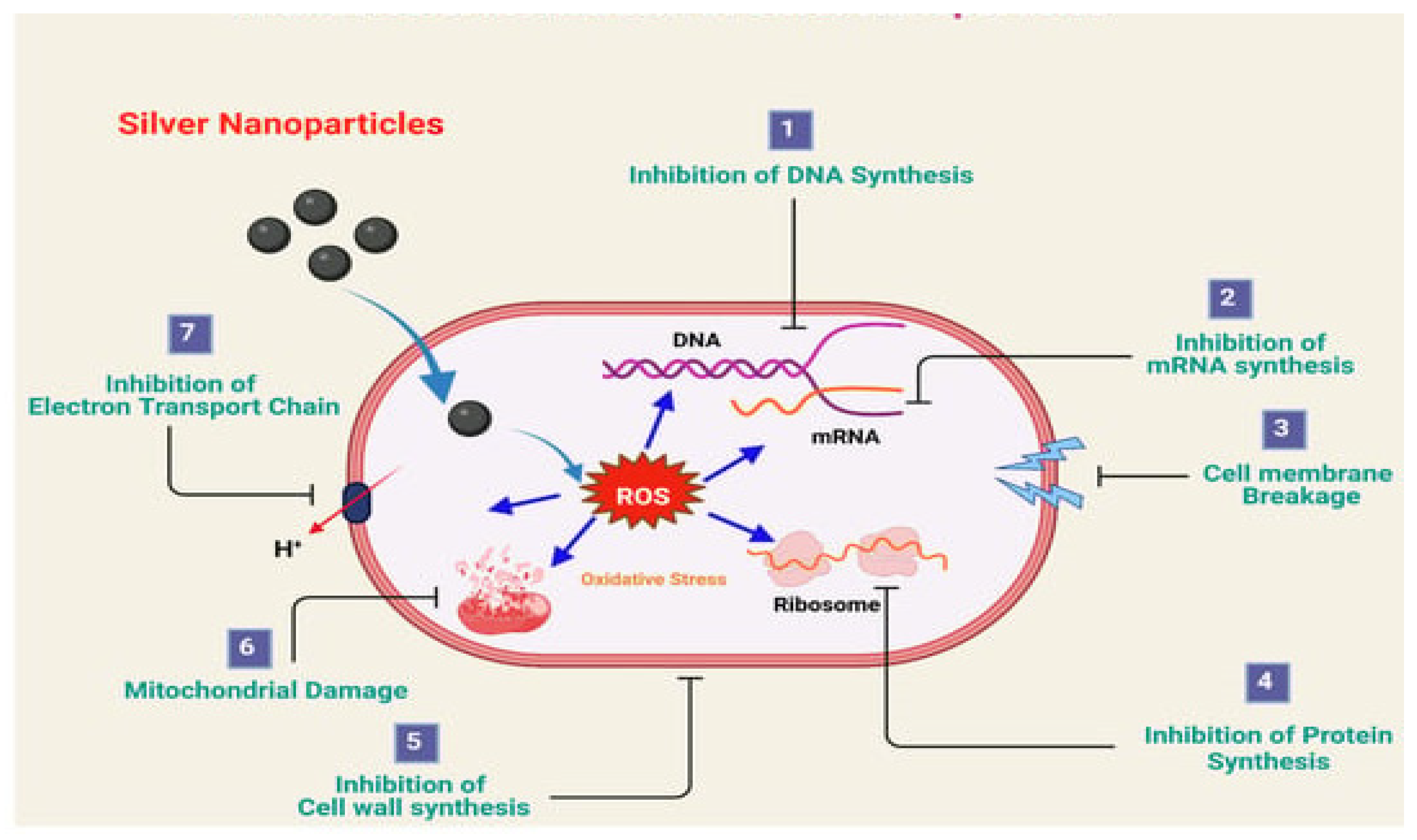
3. Effect of Nanomaterials on Bacteria
- They can simply infiltrate the cell membrane of bacteria and potentially harm its composition, which eventually causes cell lysis [85].
- The modality of action of nanomaterials’ antimicrobial effect is similar to the mechanism of antibiotics, such as cell membrane disruption, “reactive oxygen species (ROS)-facilitated oxidative stress, intracellular protein production inhibition, and leaking of intracellular components” [86].
- Several nanoparticles can serve as carriers for antibiotic drugs to distribute them efficiently to their action sites by reducing the drugs’ likely negative results [33].
- The retaining power of NPs in the body far exceeds that of antibiotics, and this could be beneficial for lasting therapeutic results [87].
- Nanomaterials can be highly functional with respect to their target and their goal because they may be efficient against the cells of bacteria without actually being noxious to animal cells [33].
3.1. Organic versus Inorganic Nanoparticles
3.2. Types of Inorganic Nanoparticles
3.3. Silver Nanoparticles
3.4. Zinc Oxide Nanoparticles (ZnO NPs)
3.5. Other Nanoparticles
4. Mode of Action of Nanomaterials
4.1. Advantages of Nanomaterials in Combating MDR Pathogens
- The time of drug retention in the blood can be enhanced as a result of using NPs as an antimicrobial drug delivery vehicle [153].
- The solubility of nanomaterials in the bloodstream is allowed by the surface chemistry of NPs [33].
- Opsonization is an additional biological impediment where the physicochemical properties of nanomaterials have been well applied for the efficient delivery of antibacterial drugs to the site of action. It also allows a high measure at the site of infection site and therefore reduces the harmful effects [154,155]. For example, vancomycin is an effective Gram-positive bacteria drug but can be harmful to the kidney and ear [85]. In that regard, “Qi et al. [156] documented that the vancomycin-modified mesoporous silica NPs can be aimed at a particular Gram-positive disease-causing microorganism and selectively exterminate them over macrophage-like cells” [130].
- Antibiotics can be protected from damage to the chemical reaction and resistance against targeted bacteria by nanomaterials. It has been proven by many researchers that several NPs can “overcome the traditional efflux mechanism of bacteria cells that often obstruct the uptake of antibiotics by the cells” [157]. For example, “Liu et al. [158] reported that the dendrimers could impede P-glycoprotein-mediated efflux in the gastrointestinal tract”.
4.2. Antibacterial Effect of Nanomaterials-Antibiotics Combination
5. Cytotoxicity of Nanomaterials
Approaches to Addressing Nanomaterials Toxicity Dilemma
6. Limitations and Strengths of the Application of Nanoparticles
7. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, M.E.; Bilal, N.E.; Hamid, M.E. Increased multi-drug resistant Escherichia coli from hospitals in Khartoum state, Sudan. Afr. Health Sci. 2012, 12, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Hsieh, Y.-H.; Powers, Z.M.; Kao, C.-Y. Defeating Antibiotic-Resistant Bacteria: Exploring Alternative Therapies for a Post-Antibiotic Era. Int. J. Mol. Sci. 2020, 21, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, V.; Dupont, H.; Glorion, S.; Aupée, M.; Kipnis, E.; Gérard, J.; Hanouz, J.; Fischer, M. Influence of bacterial resistance on mortality in intensive care units: A registry study from 2000 to 2013 (IICU Study). J. Hosp. Infect. 2019, 102, 317–324. [Google Scholar] [CrossRef]
- Naylor, N.R.; Pouwels, K.B.; Hope, R.; Green, N.; Henderson, K.L.; Knight, G.M.; Atun, R.; Robotham, J.V.; Deeny, S.R. The health and cost burden of antibiotic-resistant and susceptible Escherichia coli bacteraemia in the English hospital setting: A national retro-spective cohort study. PLoS ONE 2019, 14, e0221944. [Google Scholar] [CrossRef]
- Hwang, C.S.; Lee, H.; Hong, M.P.; Kim, J.H.; Kim, K.S. Brain abscess caused by chronic invasive actinomycosis in the nasopharynx: A case report and literature review. Medicine 2018, 97, e0406. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Govinden, U.; Bester, L.; Essack, S. Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: Emerging resistance mechanisms and detection methods. J. Appl. Microbiol. 2016, 121, 601–617. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, P.; Zhao, D.; Jiang, Y.; Zhao, F.; Wang, Y.; Li, X.; Du, X.; Yu, Y. Emergence of tigecycline resistance in Escherichia coli co-producing MCR-1 and NDM-5 during tigecycline salvage treatment. Infect. Drug Resist. 2018, 11, 2241–2248. [Google Scholar] [CrossRef]
- Spellberg, B.; Miller, L.G.; Kuo, M.N.; Bradley, J.; Scheld, W.M.; Edwards, J.E. Societal Costs Versus Savings from Wild-Card Patent Extension Legislation to Spur Critically Needed Antibiotic Development. Infection 2007, 35, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Ginocchio, F.; Mikulska, M. New Treatment Options against Gram-Negative Organisms. In Annual Update in Intensive Care and Emergency Medicine; Springer: Berlin/Heidelberg, Germany, 2011; pp. 501–515. [Google Scholar]
- World Health Organization. WHO Priority Pathogens List for R&D of New Antibiotics. 2017. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 27 February 2017).
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr.; Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente-Salcido, N.M.; López-De la Cruz, D.; Andrade, A.A. Bacterial Resistance Challenged by Binary Antimicrobial Combinations. In Antimicrobial Research: Novel Bioknowledge and Educational Programs; Méndez-Vilas, A., Ed.; Bioprospecting and Bioprocessing; Autonomous University of Coahuila: Saltillo, Mexico, 2017. [Google Scholar]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [Green Version]
- Maenza, J.; Flexner, C. Combination antiretroviral therapy for HIV infection. Am. Fam. Physician 1998, 57, 2789–2798. [Google Scholar]
- Forrest, G.N.; Tamura, K. Rifampin Combination Therapy for Nonmycobacterial Infections. Clin. Microbiol. Rev. 2010, 23, 14–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Iseman, M.D. Extensively Drug-Resistant Mycobacterium tuberculosis: Charles Darwin Would Understand. Clin. Infect. Dis. 2007, 45, 1415–1416. [Google Scholar] [CrossRef]
- Jawetz, E.; Gunnison, J.B.; Bruff, J.B.; Coleman, V.R. Studies on Antibiotic Synergism and Antagonism: Synergism among Seven Antibiotics against Various Bacteria in vitro. J. Bacteriol. 1952, 64, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Bushby, S.R.M.; Hitchings, G.H. Trimethoprim, a Sulphonamide Potentiator. Br. J. Pharmacol. Chemother. 1968, 33, 72–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerantzas, C.A.; Jacobs, W.R., Jr. Origins of Combination Therapy for Tuberculosis: Lessons for Future Antimicrobial development and application. MBio 2017, 8, e01586-16. [Google Scholar] [CrossRef] [PubMed]
- Noordeen, S.K. History of chemotherapy of leprosy. Clin. Dermatol. 2016, 34, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.I.; Chauhdary, Z. Antibacterial Activity of Novel Strains of Bacteriophages: An Experimental Approach. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pelfrene, E.; Mura, M.; Sanches, A.C.; Cavaleri, M. Monoclonal antibodies as anti-infective products: A promising future? Clin. Microbiol. Infect. 2019, 25, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Xu, J.-W.; Yao, K.; Xu, Z.-K. Nanomaterials with a photothermal effect for antibacterial activities: An overview. Nanoscale 2019, 11, 8680–8691. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Lipeeva, A.V.; Zakharov, D.O.; Burova, L.G.; Frolova, T.S.; Baev, D.S.; Shirokikh, I.V.; Evstropov, A.N.; Sinitsyna, O.I.; Tolsikova, T.G.; Shults, E.E. Design, Synthesis and Antibacterial Activity of Coumarin-1,2,3-triazole Hybrids Obtained from Natural Furocoumarin Peucedanin. Molecules 2019, 24, 2126. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Ahmed, A.; Azim, A.; Gurjar, M.; Baronia, A.K. Current concepts in combination antibiotic therapy for critically ill patients. Indian J. Crit. Care Med. 2014, 18, 310–314. [Google Scholar] [PubMed] [Green Version]
- Fischbach, M.A. Combination therapies for combating antimicrobial resistance. Curr. Opin. Microbiol. 2011, 14, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Toews, M.L.; Bylund, D.B. Pharmacologic principles for combination therapy. Proc. Am. Thorac. Soc. 2005, 2, 282–289. [Google Scholar] [CrossRef]
- Rybak, M.J.; McGrath, B.J. Combination Antimicrobial Therapy for Bacterial Infections. Drugs 1996, 52, 390–405. [Google Scholar] [CrossRef]
- Caballero, J.; Rello, J. Combination antibiotic therapy for community-acquired pneumonia. Ann. Intensive Care 2011, 1, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, K.; Low, D.; Cortes, L.; Beaupre, A.; Gauthier, R.; Gregoire, P.; Legare, M.; Neoveu, F.; Thibert, D.; Tremblay, C. Clinical Characteristics at Initial Presentation and Impact of Dual Therapy on the Outcome of Bacteremic Streptococcus pneumoniae Pneumonia in Adults. Can. Respir. J. 2004, 11, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Zarychanski, R.; Light, B.; Parrillo, J.; Maki, D.; Simon, D.; LaPorta, D.; Lapinsky, S.; Ellis, P.; Mirzanejad, Y.; et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: A propensity-matched analysis. Crit. Care Med. 2010, 38, 1773–1785. [Google Scholar] [CrossRef] [Green Version]
- Mehta, K.C.; Dargad, R.R.; Borade, D.M.; Swami, O.C. Burden of antibiotic resistance in common infectious diseases: Role of antibiotic combination therapy. J. Clin. Diagn. Res. 2014, 8, ME05–ME08. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.; Saheb, N.; Coulombe, M.-A.; Alary, M.-E.; Corriveau, M.-P.; Authier, S.; Leblanc, M.; Rivard, G.; Bettez, M.; Primeau, V.; et al. Emergence of Fluoroquinolones as the Predominant Risk Factor for Clostridium difficile-Associated Diarrhea: A Cohort Study during an Epidemic in Quebec. Clin. Infect. Dis. 2005, 41, 1254–1260. [Google Scholar] [CrossRef] [Green Version]
- Sandora, T.J.; Fung, M.; Flaherty, K.; Helsing, L.; Scanlon, P.; Potter-Bynoe, G.; Gidengil, C.A.; Lee, G.M. Epidemiology and Risk Factors for Clostridium difficile Infection in Children. Pediatr. Infect. Dis. J. 2011, 30, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.T.; Borisy, A.A.; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef]
- Loewe, S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 1953, 3, 285–290. [Google Scholar] [PubMed]
- Chait, R.; Craney, A.; Kishony, R. Antibiotic interactions that select against resistance. Nature 2007, 446, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, G.M.; Eliopoulos, C.T. Antibiotic combinations: Should they be tested? Clin. Microbiol. Rev. 1988, 1, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Cerda, J.; Espinoza-Gómez, H.; Alonso-Núñez, G.; Rivero, I.A.; Gochi-Ponce, Y.; Flores-López, L.Z. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J. Saudi Chem. Soc. 2017, 21, 341–348. [Google Scholar] [CrossRef]
- Faleiro, M.; Miguel, M. Use of Essential Oils and Their Components against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Academic Press: Cambridge, MA, USA, 2013; pp. 65–94. [Google Scholar]
- American Thoracic Society and Infectious Diseases Society of America (ATS). Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [Green Version]
- Glew, R.H.; Pavuk, R.A. Early synergistic interaction between semisynthetic penicillins and aminoglycosidic aminocyclitols against Enterobacteriaceae. Antimicrob. Agents Chemother. 1983, 23, 902–906. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Kanno, H. Synergistic activities of combinations of beta-lactams, fosfomycin, and tobramycin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 789–791. [Google Scholar] [CrossRef] [Green Version]
- Hancock, R.E.; Raffle, V.J.; Nicas, T.I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1981, 19, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.H.; Feinstein, S.A.; Chow, R.T. Early effects of beta-lactams on aminoglycoside uptake, bactericidal rates, and turbidimetrically measured growth inhibition in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1987, 31, 108–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, D.N.; Choi, M.K.; Jung, R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J. Antimicrob. Chemother. 2002, 50, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Gradelski, E.; Kolek, B.; Bonner, D.P.; Valera, L.; Minassian, B.; Fung-Tomc, J. Activity of gatifloxacin and ciprofloxacin in combination with other antimicrobial agents. Int. J. Antimicrob. Agents 2001, 17, 103–107. [Google Scholar] [CrossRef]
- Pankuch, G.A.; Lin, G.; Seifert, H.; Appelbaum, P.C. Activity of Meropenem with and without Ciprofloxacin and Colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.S.; van Hal, S.; Tong, S.Y. Combination Antibiotic Treatment of Serious Methicillin-Resistant Staphylococcus aureus Infections. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2015; Volume 36, pp. 3–16. [Google Scholar]
- Leonard, S.N.; Rolek, K.M. Evaluation of the combination of daptomycin and nafcillin against vancomycin-intermediate Staphylococcus aureus. J. Antimicrob. Chemother. 2013, 68, 644–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.; Singh, C.; Plata, K.B.; Chanda, P.K.; Paul, A.; Riosa, S.; Rosato, R.R.; Rosato, A.E. β-Lactams Increase the Antibacterial Activity of Daptomycin against Clinical Methicillin-Resistant Staphylococcus aureus Strains and Prevent Selection of Daptomycin-Resistant Derivatives. Antimicrob. Agents Chemother. 2012, 56, 6192–6200. [Google Scholar] [CrossRef] [Green Version]
- Gritsenko, D.; Fedorenko, M.; Ruhe, J.J.; Altshuler, J. Combination Therapy With Vancomycin and Ceftaroline for Refractory Methicillin-resistant Staphylococcus aureus Bacteremia: A Case Series. Clin. Ther. 2017, 39, 212–218. [Google Scholar] [CrossRef]
- Dhand, A.; Sakoulas, G. Daptomycin in Combination With Other Antibiotics for the Treatment of Complicated Methicillin-Resistant Staphylococcus aureus Bacteremia. Clin. Ther. 2014, 36, 1303–1316. [Google Scholar] [CrossRef]
- Van Duin, D.; Bonomo, R.A. Ceftazidime/avibactam and ceftolozane/tazobactam: Second-generation beta-Lactam/beta-lactamase inhibitor combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Jean, S.-S.; Gould, I.M.; Lee, W.-S.; Hsueh, P.-R.; International Society of Antimicrobial Chemotherapy (ISAC). New Drugs for Multidrug-Resistant Gram-Negative Organisms: Time for Stewardship. Drugs 2019, 79, 705–714. [Google Scholar] [CrossRef]
- Cheng, I.-L.; Chen, Y.H.; Lai, C.-C.; Tang, H.-J. The use of ceftolozane-tazobactam in the treatment of complicated intra-abdominal infections and urinary tract infections—A meta-analysis of randomized controlled trials. Int. J. Antimicrob. Agents 2020, 55, 105858. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Chen, C.-C.; Tang, H.-J. Meropenem-Vaborbactam in the Treatment of Acute Bacterial Infections. J. Clin. Med. 2019, 8, 1650. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-K.; Lai, C.-C.; Chao, C.-M. Ceftazidime–Avibactam versus Meropenem for the Treatment of Complicated Intra-Abdominal Infections. Antibiotics 2019, 8, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagacé-Wiens, P.R.S.; Walkty, A.; Denisuik, A.; Golden, A.; et al. Imipenem-relebactam and meropenem-vaborbactam: Two novel carbapenem-beta-lactamase inhibitor combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef] [PubMed]
- Mirza, H.C.; Hortaç, E.; Koçak, A.A.; Demirkaya, M.H.; Yayla, B.; Güçlü, A.Ü.; Başustaoğlu, A. In vitro activity of ceftolozane-tazobactam and ceftazidime-avibactam against clinical Isolates of meropenem-non-susceptible Pseudomonas aeruginosa: A two-center study. J. Glob. Antimicrob. Resist. 2019, 20, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulou, I.; Kazmierczak, K.; Stone, G.G. In vitro activity of ceftazidime/avibactam against isolates of carbapenem-non-susceptible Enterobacteriaceae collected during the INFORM global surveillance programme (2015–17). J. Antimicrob. Chemother. 2019, 75, 384–391. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, A.; Ammerman, N.C.; Lee, J.; Martins, O.; Kreiswirth, B.N.; Lamichhane, G.; Parrish, N.M.; Nuermberger, E.L. In vitro activity of the new beta-lactamase inhibitors relebactam and vaborbactam in combination with beta-lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob. Agents Chemother. 2019, 63, e02623-18. [Google Scholar]
- Petty, L.A.; Henig, O.; Patel, T.S.; Pogue, J.M.; Kaye, K.S. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect. Drug Resist. 2018, 11, 1461–1472. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Jun, Y.H.; Kim, Y.R.; Park, K.G.; Park, Y.J.; Kang, J.Y.; Kim, S.I. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; Retrospective study of impact of combination antimicrobial therapy. BMC Infect. Dis. 2014, 14, 161. [Google Scholar] [CrossRef] [Green Version]
- Ceravolo, F.; Grisolia, M.; Sestito, S.; Falvo, F.; Moricca, M.T.; Concolino, D. Combination therapy in a patient with chronic neu-ronopathic Gaucher disease: A case report. J. Med. Case Rep. 2017, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- González-Bello, C. Antibiotic adjuvants—A strategy to unlock bacterial resistance to antibiotics. Bioorganic Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13, e5. [Google Scholar] [CrossRef]
- Bernal, P.; Molina-Santiago, C.; Daddaoua, A.; Llamas, M.A. Antibiotic adjuvants: Identification and clinical use. Microb. Biotechnol. 2013, 6, 445–449. [Google Scholar] [CrossRef]
- Food and Drug Administration; US Department of Health and Human Services. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology; Food and Drug Administration: Silver Spring, MD, USA, 2014.
- Plank, C. Silence the target. Nat. Nanotechnol. 2009, 4, 544–545. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale 2013, 5, 8340–8350. [Google Scholar] [CrossRef]
- Aabed, K.; Mohammed, A.E. Synergistic and Antagonistic Effects of Biogenic Silver Nanoparticles in Combination With Antibiotics Against Some Pathogenic Microbes. Front. Bioeng. Biotechnol. 2021, 9, 652362. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, O.O.; Ojemaye, M.O.; Okoh, A.I. Antibacterial Activity of Metallic Nanoparticles against Multidrug-Resistant Pathogens Isolated from Environmental Samples: Nanoparticles/Antibiotic Combination Therapy and Cytotoxicity Study. ACS Appl. Bio Mater. 2022, 5, 4814–4826. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Watt, J.; Cheong, S.; Tilley, R.D. How to control the shape of metal nanostructures in organic solution phase synthesis for plasmonics and catalysis. Nano Today 2013, 8, 198–215. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, T.J.; Seil, J.T. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle Adhesion to the Cell Membrane and Its Effect on Nanoparticle Uptake Efficiency. J. Am. Chem. Soc. 2013, 135, 8–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial Activity of Nanosilver Ions and Particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for green synthesis of silver nanoparticles: Toward antimicrobial applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of Silver in the Prevention and Treatment of Infections: Silver Review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, Y.; Chen, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implantswa by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, O.M.; Sihtmäe, M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavičius, R. Plasma membrane is the target of rapid antibacterial action of silver nanoparticles in Escherichia coli and Pseudomonas aeruginosa. Int. J. Nanomed. 2018, 13, 6779–6790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral an-aerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Poulose, S.; Panda, T.; Nair, P.P.; Theodore, T. Biosynthesis of silver nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 2038–2049. [Google Scholar] [CrossRef]
- Prasannakumar, J.B.; Vidya, Y.S.; Anantharaju, K.S.; Ramgopal, G.; Nagabhushana, H.; Sharma, S.C.; Prasad, B.D.; Prashantha, S.C.; Basavaraj, R.B.; Rajanaik, H.; et al. Bio-mediated route for the synthesis of shape tunable Y2O3: Tb3+ nano-particles: Photoluminescence and antibacterial properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 131–140. [Google Scholar] [CrossRef]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Actis, L.; Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K.; Ong, J.L. Effect of silver nanoparticle geometry on methicillin susceptible and resistant Staphylococcus aureus, and osteoblast viability. J. Mater. Sci. Mater. Med. 2015, 26, 215. [Google Scholar] [CrossRef]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface Charge-Switching Polymeric Nanoparticles for Bacterial Cell Wall-Targeted Delivery of Antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, N.B.; Jensen, K.A.; Baun, A.; Rasmussen, K.; Rauscher, H.; Tantra, R.; Cupi, D.; Gilliland, D.; Pianella, F.; Riego Sintes, J.M. Techniques and protocols for dispersing nanoparticle powders in aqueous media—Is there a rationale for har-monization? J. Toxicol. Environ. 2015, 18, 299–326. [Google Scholar]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef]
- Reshma, V.G.; Syama, S.; Sruthi, S.; Reshma, S.C.; Remya, N.S.; Mohanan, P.V. Engineered nanoparticles with Antimicrobial property. Curr. Drug Metab. 2018, 18, 1040–1054. [Google Scholar] [CrossRef]
- Kotov, N.A.; VanEpps, J.S. Antibacterial Metal Oxide Nanoparticles: Challenges in Interpreting the Literature. Curr. Pharm. Des. 2018, 24, 896–903. [Google Scholar]
- Sinha, R.; Karan, R.; Sinha, A.; Khare, S.K. Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 2011, 102, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Du, F.; Wu, W.; Wu, R.; Liu, F.; Liang, Y. Enhanced reactive oxygen species (ROS) yields and antibacterial activity of spongy ZnO/ZnFe2O4 hybrid micro-hexahedra selectively synthesized through a versatile glucose-engineered co-precipitation/annealing process. J. Mater. Chem. B 2013, 1, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green silver nanoparticles of Phyllanthus amarus: As an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Gelabert, A.; Sivry, Y.; Gobbi, P.; Mansouri-Guilani, N.; Menguy, N.; Brayner, R.; Siron, V.; Benedetti, M.F.; Ferrari, R. Testing nanoeffect onto model bacteria: Impact of speciation and genotypes. Nanotoxicology 2016, 10, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Nagvenkar, A.P.; Deokar, A.; Perelshtein, I.; Gedanken, A. A one-step sonochemical synthesis of stable ZnO–PVA nano-colloid as a potential biocidal agent. J. Mater. Chem. B 2016, 4, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, W.; Li, Y.; Wang, G.; Yang, L.; Jin, J.; Chen, Q.; Huang, M. Synthesis, characterization, antimicrobial activity and mechanism of a novel hydroxyapatite whisker/nano zinc oxide biomaterial. Biomed. Mater. 2014, 10, 015001. [Google Scholar] [CrossRef] [PubMed]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94B, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria “A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Miri, A.; Mahdinejad, N.; Ebrahimy, O.; Khatami, M.; Sarani, M. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater. Sci. Eng. C 2019, 104, 109981. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Shi, H.; Fu, L. Green biosynthesis of ZnO nanoparticles by Plectranthus amboinicus leaf extract and their application for electrochemical determination of norfloxacin. Inorg. Nano-Met. Chem. 2019, 49, 277–282. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Hatami, Z.; Ragheb, E.; Jalali, F.; Tabrizi, M.A.; Shamsipur, M. Zinc oxide-gold nanocomposite as a proper platform for label-free DNA biosensor. Bioelectrochemistry 2020, 133, 107458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.H.; Markus, J.; Soshnikova, V.; Oh, K.H.; Anandapadmanaban, G.; Perez, Z.E.J.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.C. Facile and green synthesis of zinc oxide particles by Stevia Rebaudiana and its in vitro photocatalytic activity. Inorg. Nano-Met. Chem. 2019, 49, 1–6. [Google Scholar]
- Vicente, A.; Sohm, B.; Flayac, J.; Rousselle, P.; Bauda, P.; Pagnout, C. Toxicity mechanisms of ZnO UV-filters used in sunscreens toward the model cyanobacteria Synechococcus elongatus PCC 7942. Environ. Sci. Pollut. Res. 2019, 26, 22450–22463. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alloisio, M.; Vicini, S.; Castellano, M. Preparation of composite alginate-based electrospun membranes loaded with ZnO nanoparticles. Carbohydr. Polym. 2020, 227, 115371. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Mahjoub, S.; Elahi, S.M.; Zabihi, E.; Tashakkorian, H. Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf. B Biointerfaces 2020, 186, 110686. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.-S. Nanomaterials as Delivery Vehicles and Components of New Strategies to Combat Bacterial Infections: Advantages and Limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Rajasekar, K.; Dinesh, A.; Durka, M.; Muthukumaravel, K. Facile synthesis and in vitro biological screening of Pd@ SiO2 core–shell nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 3536–3543. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H.N. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [Green Version]
- Ijaz, U.; Bhatti, I.A.; Mirza, S.; Ashar, A. Characterization and evaluation of antibacterial activity of plant mediated calcium oxide (CaO) nanoparticles by employing Mentha pipertia extract. Mater. Res. Express 2017, 4, 105402. [Google Scholar] [CrossRef]
- Manikandan, V.; Jayanthi, P.; Priyadharsan, A.; Vijayaprathap, E.; Anbarasan, P.M.; Velmurugan, P. Green synthesis of pH-responsive Al2O3 nanoparticles: Application to rapid removal of nitrate ions with enhanced antibacterial activity. J. Photochem. Photobiol. A Chem. 2019, 371, 205–215. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Yamamoto, O.; Ohira, T.; Alvarez, K.; Fukuda, M. Antibacterial characteristics of CaCO3–MgO composites. Mater. Sci. Eng. B 2010, 173, 208–212. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Saquib, Q.; Musarrat, J. Interaction of Al2O3 nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Appl. Microbiol. 2014, 116, 772–783. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Simor, A.E. Antimicrobial resistance in hospitals: How concerned should we be? CMAJ 2009, 180, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-W.; Hsu, C.-Y.; Lai, S.-M.; Syu, W.-J.; Wang, T.-Y.; Lai, P.-S. Metal nanobullets for multidrug resistant bacteria and biofilms. Adv. Drug Deliv. Rev. 2014, 78, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Q.; Zhao, J.; Urmila, K. Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci. Rep. 2015, 5, 11033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, Z.; Yasmeen, F.; Komatsu, S. Nanoparticles: Synthesis, morphophysiological effects, and proteomic responses of crop plants. Int. J. Mol. Sci. 2020, 21, 3056. [Google Scholar] [CrossRef] [PubMed]
- Stensberg, M.C.; Wei, Q.S.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition via Zinc Oxide Nanoparticles and Their Statistical Determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yu, F.; Park, Y.-S.; Wang, J.; Shin, M.-C.; Chung, H.S.; Yang, V.C. Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery. Biomaterials 2010, 31, 9086–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Rafael, D.; Videira, M.; Ferreira, D.; Sosnik, A.; Sarmento, B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 2013, 65, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.; Lawlor, A.; Whelan, A.; Regan, F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst 2008, 133, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, F.; Tao, Y.; Chen, D.; Qu, W.; Huang, L.; Liu, Z.; Pan, Y.; Yuan, Z. Enhanced intracellular delivery and antibacterial efficacy of enrofloxacin-loaded docosanoic acid solid lipid nanoparticles against intracellular Salmonella. Sci. Rep. 2017, 7, 41104. [Google Scholar] [CrossRef]
- Chu, L.; Gao, H.; Cheng, T.; Zhang, Y.; Liu, J.; Huang, F.; Yang, C.; Shi, L.; Liu, J. A charge-adaptive nanosystem for prolonged and enhanced in vivo antibiotic delivery. Chem. Commun. 2016, 52, 6265–6268. [Google Scholar] [CrossRef]
- Xiong, M.-H.; Li, Y.-J.; Bao, Y.; Yang, X.-Z.; Hu, B.; Wang, J. Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [Green Version]
- Qi, G.; Li, L.; Yu, F.; Wang, H. Vancomycin-Modified Mesoporous Silica Nanoparticles for Selective Recognition and Killing of Pathogenic Gram-Positive Bacteria Over Macrophage-Like Cells. ACS Appl. Mater. Interfaces 2013, 5, 10874–10881. [Google Scholar] [CrossRef]
- Mühling, M.; Bradford, A.; Readman, J.W.; Somerfield, P.; Handy, R.D. An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar. Environ. Res. 2009, 68, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tee, J.K.; Chiu, G.N.C. Dendrimers in oral drug delivery application: Current explorations, toxicity issues and strategies for improvement. Curr. Pharm. Des. 2015, 21, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Smékalová, M.; Kilianová, M.; Prucek, R.; Bogdanová, K.; Večeřová, R.; Kolář, M.; Havrdová, M.; Płaza, G.A.; Chojniak, J.; et al. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules 2016, 21, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scandorieiro, S.; De Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindhu, P.D.; Mukherjee, A.; Chandrasekaran, N. Synergistic effect of biogenic silver nanocolloid in combination with antibiotics: A potent therapeutic agent. Int. J. Pharm. Pharm. Sci. 2013, 51, 292–295. [Google Scholar]
- Esmaeillou, M.; Zarrini, G.; Rezaee, M.A.; Mojarrad, J.S.; Bahadori, A. Vancomycin Capped with Silver Nanoparticles as an Antibacterial Agent against Multi-Drug Resistance Bacteria. Adv. Pharm. Bull. 2017, 7, 479–483. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sonawane, S.J.; Sikwal, D.R.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf. B Biointerfaces 2015, 136, 651–658. [Google Scholar] [CrossRef]
- Hur, Y.E.; Park, Y. Vancomycin-Functionalized Gold and Silver Nanoparticles as an Antibacterial Nanoplatform Against Methicillin-Resistant Staphylococcus aureus. J. Nanosci. Nanotechnol. 2016, 16, 6393–6399. [Google Scholar] [CrossRef]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles Functionalized with Ampicillin Destroy Multiple-Antibiotic-Resistant Isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and Methicillin-Resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, F.; Jalal, R. Antimicrobial action of zinc oxide nanoparticles in combination with ciprofloxacin and ceftazidime against multidrug-resistant Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2016, 6, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their anti-microbial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandi, V.; Kandi, S. Antimicrobial properties of nanomolecules: Potential candidates as antibiotics in the era of multi-drug resistance. Epidemiol. Health 2015, 37, e2015020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver Nanoparticles: Therapeutical Uses, Toxicity, and Safety Issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Dalal, C.; Saha, A.; Jana, N.R. Nanoparticle Multivalency Directed Shifting of Cellular Uptake Mechanism. J. Phys. Chem. C 2016, 120, 6778–6786. [Google Scholar] [CrossRef] [Green Version]
- Graves, J.L., Jr.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today Technol. 2015, 20, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar]
- Sengupta, J.; Ghosh, S.; Datta, P.; Gomes, A.; Gomes, A. Physiologically Important Metal Nanoparticles and Their Toxicity. J. Nanosci. Nanotechnol. 2014, 14, 990–1006. [Google Scholar] [CrossRef]
- Dobrucka, R.; Szymanski, M.; Przekop, R. The study of toxicity effects of biosynthesized silver nanoparticles using Veronica officinalis extract. Int. J. Environ. Sci. Technol. 2019, 16, 8517–8526. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Bera, S.; Bhattacharya, R.; Saha, P.; Roy, S.S.; Sen, T.; Jana, S. Synthesis, characterization and antibacterial activity of Ag incorporated ZnO–graphene nanocomposites. RSC Adv. 2016, 6, 88751–88761. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.N.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB). Antibiotics 2018, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Finley, P.J.; Norton, R.; Austin, C.; Mitchell, A.; Zank, S.; Durham, P. Unprecedented Silver Resistance in Clinically Isolated Enterobacteriaceae: Major Implications for Burn and Wound Management. Antimicrob. Agents Chemother. 2015, 59, 4734–4741. [Google Scholar] [CrossRef] [Green Version]
- Gaillet, S.; Rouanet, J.-M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Cinteza, L.O.; Scomoroscenco, C.; Voicu, S.N.; Nistor, C.L.; Nitu, S.G.; Trica, B.; Jecu, M.-L.; Petcu, C. Chitosan-Stabilized Ag Nanoparticles with Superior Biocompatibility and Their Synergistic Antibacterial Effect in Mixtures with Essential Oils. Nanomaterials 2018, 8, 826. [Google Scholar] [CrossRef] [Green Version]
- Meshram, J.V.; Koli, V.B.; Kumbhar, S.G.; Borde, L.C.; Phadatare, M.R.; Pawar, S.H. Structural, spectroscopic and anti-microbial inspection of PEG capped ZnO nanoparticles for biomedical applications. Mater. Res. Express 2018, 5, 045016. [Google Scholar] [CrossRef]
- Abdallah, O.M.; El-Baghdady, K.Z.; Khalil, M.M.H.; El Borhamy, M.I.; Meligi, G.A. Antibacterial, antibiofilm and cytotoxic activities of biogenic polyvinyl alcohol-silver and chitosan-silver nanocomposites. J. Polym. Res. 2020, 27, 74. [Google Scholar] [CrossRef]
- Chia, S.L.; Leong, D.T. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased Dissolution of ZnO by Iron Doping Yields Nanoparticles with Reduced Toxicity in the Rodent Lung and Zebrafish Embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef] [Green Version]
- Limayem, A.; Micciche, A.; Haller, E.; Zhang, C.; Mohapatra, S. Nanotherapeutics for mutating multi-drug resistant fecal bacteria. J. Nanosci. Nanotechnol. 2015, 1, 100–106. [Google Scholar]
- Mehta, M.; Allen-Gipson, D.; Mohapatra, S.; Kindy, M.; Limayem, A. Study on the therapeutic index and synergistic effect of Chitosan-zinc oxide nanomicellar composites for drug-resistant bacterial biofilm inhibition. Int. J. Pharm. 2019, 565, 472–480. [Google Scholar] [CrossRef]
- Sandhiya, S.; Dkhar, S.A.; Surendiran, A. Emerging trends of nanomedicine—An overview. Fundam. Clin. Pharmacol. 2009, 23, 263–269. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar]

| Property | Functions | References |
|---|---|---|
| Particle Size | “Smaller Ag-NPs have larger specific surface areas, which result in higher antimicrobial activity”. Because there is a higher chance of being in contact with, and transient on, the cellular membrane of the organism than with bigger nanoparticles. | [64,104,105] |
| Particle Shape | Ag-NPs with various forms can result in various microbial cell injury degrees through interacting with periplasmic enzymes. For example, cube-shaped silver nanoparticles demonstrate more potent antimicrobial effect than sphere-shaped and wire-shaped silver nanoparticles with comparable diameters, implying that the shape impact on antimicrobial activities is because of the surface area and facet reactivity. | [105,106,107] |
| Roughness | “As the roughness of Ag-NPs increases, the size and the surface area-to-mass ratio promotes the adsorption of bacterial proteins, which is followed by a reduction in bacterial adhesion”. | [64] |
| Environmental Condition | Temperature and pH cause considerable differences in antimicrobial effect. For example, the temperature of the environment has a strong impact on antimicrobial activity due to its impact on ROS generation rate. Likewise, “at low pH, the surfaces of the NPs were positively charged, which is beneficial to the interaction with the negatively charged groups of the bacterial cell barrier, inducing strong multivalent electrostatic regulation”. | [64,108] |
| Doping Modification | Prevents the assemblage of gaps and permits their dispersion in aqueous conditions or other hydrophilic media. | [109] |
| Zeta Potential | AgNPs with a positive surface charge are susceptible to the fact that they are absorbed on bacterial surfaces, in contrast to their counterparts with negative charge. | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeniji, O.O.; Nontongana, N.; Okoh, J.C.; Okoh, A.I. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. Int. J. Mol. Sci. 2022, 23, 15038. https://doi.org/10.3390/ijms232315038
Adeniji OO, Nontongana N, Okoh JC, Okoh AI. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. International Journal of Molecular Sciences. 2022; 23(23):15038. https://doi.org/10.3390/ijms232315038
Chicago/Turabian StyleAdeniji, Oluwaseun Ola, Nolonwabo Nontongana, Janet Chiyem Okoh, and Anthony Ifeanyi Okoh. 2022. "The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review" International Journal of Molecular Sciences 23, no. 23: 15038. https://doi.org/10.3390/ijms232315038
APA StyleAdeniji, O. O., Nontongana, N., Okoh, J. C., & Okoh, A. I. (2022). The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. International Journal of Molecular Sciences, 23(23), 15038. https://doi.org/10.3390/ijms232315038






