Overexpression of RuFLS2 Enhances Flavonol-Related Substance Contents and Gene Expression Levels
Abstract
1. Introduction
2. Results
2.1. Gene Structure, Physicochemical Properties and Domains of RuFLSs
2.2. Multiple Sequence Alignment, Phylogenetic Analysis, and Motif Composition of RuFLSs
2.3. Expression Levels of RuFLSs in Different Tissues and Fruit Developmental Stages of Blackberry
2.4. Changes in Flavonoid, Phenol, and Anthocyanin Contents in Blackberry Fruits at Different Developmental Stages
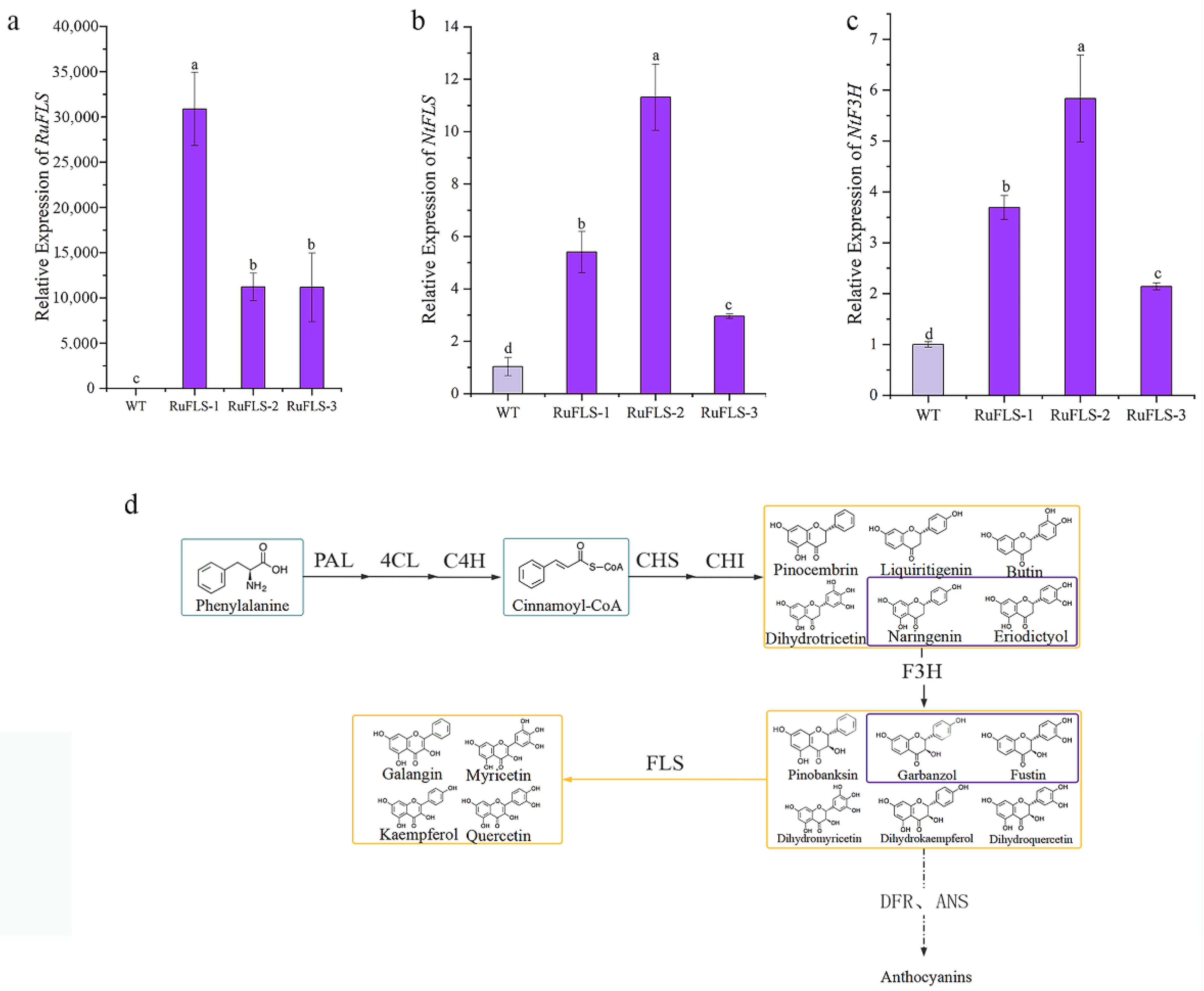
2.5. Heterologous Overexpression of RuFLS2 in Tobacco
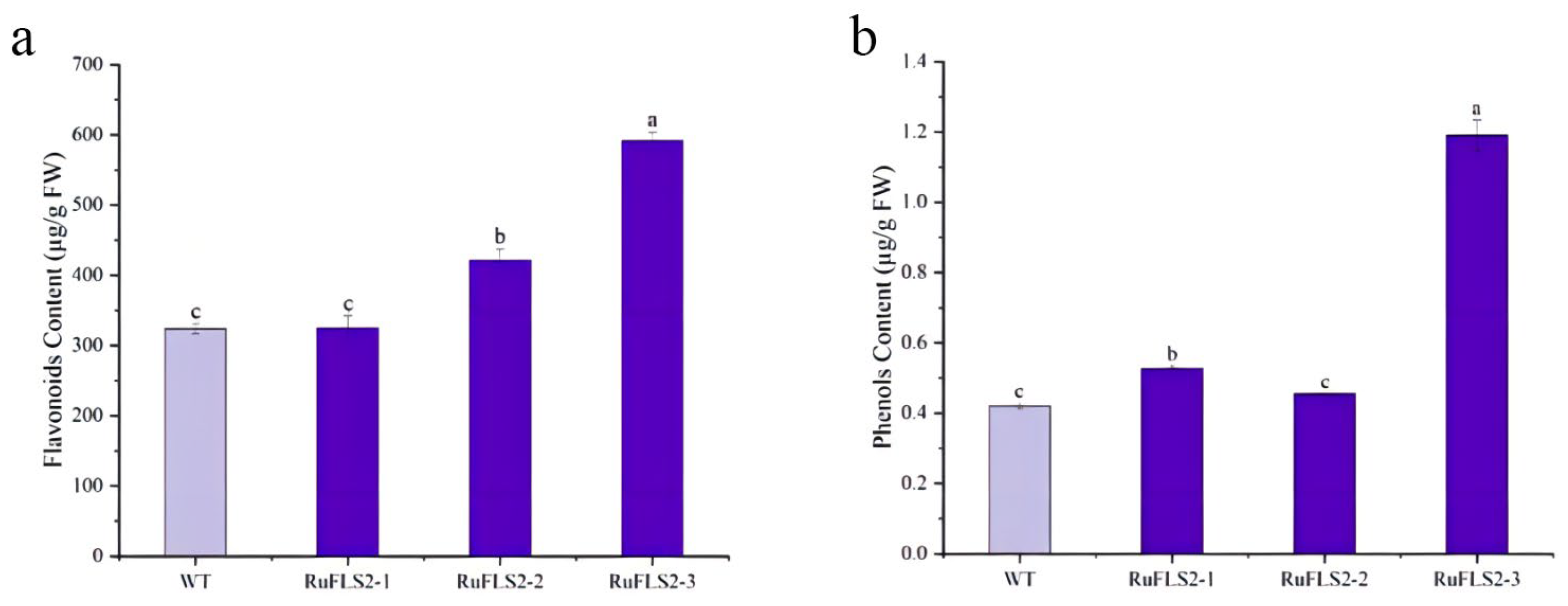
2.6. Flavonoid and Phenol Content Analysis in Genetically Modified Tobacco
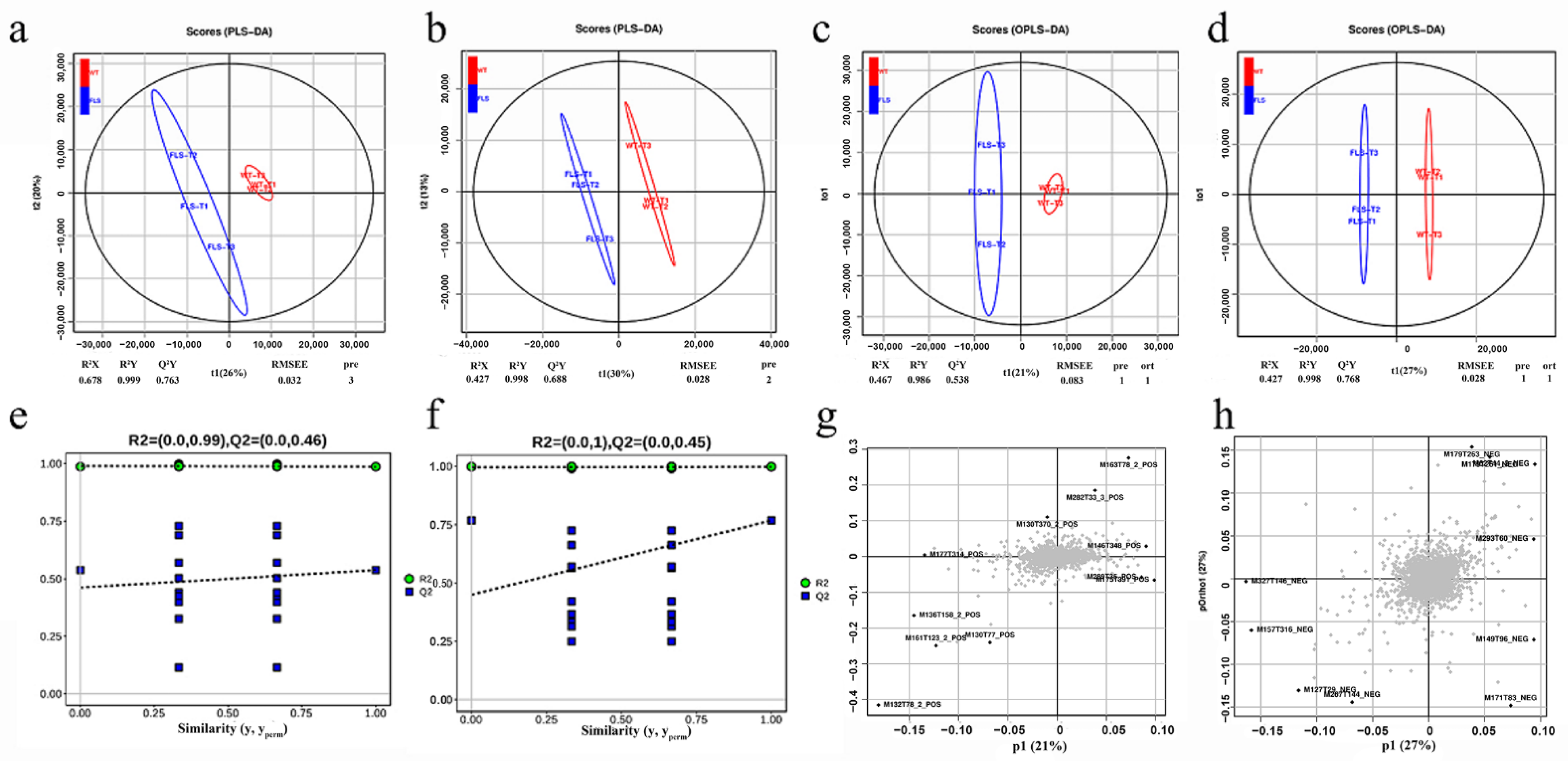
2.7. Multivariate Statistical Analysis of Metabolites in Genetically Modified Tobacco
2.8. DEM-Related Flavonoid Synthesis in Tobacco
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Plant Materials and Growth Conditions
5.2. Total RNA Extraction and cDNA Reverse Transcription
5.3. Cloning of the RuFLS Gene
5.4. Bioinformatics Analysis
5.5. qRT–PCR Analysis
5.6. Determination of Polyphenol, Flavonoid and Anthocyanin Contents
5.7. qRT–PCR Analysis
5.8. LC–MS/MS Analysis of Tobacco
5.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edees, E.S.; Newton, A.; Kent, D.H. Brambles of British Isles; The Ray Scociety: Lymington, UK, 1988. [Google Scholar]
- Wu, W.; Li, W. Cultivation and Utiliazation of Blackberry in China; Jiangsu Science and Technology Press: Nanjing, China, 2010. [Google Scholar]
- Zhang, C.; Yang, H.; Wu, W.; Li, W. Effect of drought stress on physiological changes and leaf surface morphology in the blackberry. Braz. J. Bot. 2017, 40, 625–634. [Google Scholar] [CrossRef]
- Amina, B.; Kirkin, C.; Chatterjee, R.; Elmaliklis, I.-N.; Rao, G.N.; Skotti, E.; Xagoraris, M.; Datta, J.; Ratnasekera, D.; Iqbal, M.A.; et al. Mediterranean Fruits and Berries with Bioactive and Toxic Components. A Review. Curr. Top. Nutraceutical Res. 2022, 20, 113–128. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- D’Angelo, R.W.O.; Gonçalves, M.M.; Fachi, M.M.; Vilhena, R.D.O.; Pontarolo, R.; Maluf, D.F. UPLC–QToF-MS Characterization of Blackberry Extracts of Cultivars ‘Tupy’, ‘Guarani’, and ‘Xavante’: Development of Extract-Loaded Niosomes. Rev. Bras. Farm. 2020, 30, 519–527. [Google Scholar] [CrossRef]
- Goodman, C.; Lyon, K.; Sebrell, T.A.; Balasubramanian, G.; Stoner, G.D.; Bimczok, D. Su1396 a high-throughput metabolic assay and a human gastric organoid model reveal antibacterial effects of black raspberry and blackberry extracts against helicobacter pylori infection. Gastroenterology 2020, 158, S575. [Google Scholar] [CrossRef]
- Aoki, T.; Akashi, T.; Ayabe, S.-I. Flavonoids of Leguminous Plants: Structure, Biological Activity, and Biosynthesis. J. Plant Res. 2000, 113, 475–488. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Anzellotti, D.; Ibrahim, R.K. Molecular characterization and functional expression of flavonol 6-hydroxylase. BMC Plant Biol. 2004, 4, 20. [Google Scholar] [CrossRef]
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. Anticancer Potential of Selected Flavonols: Fisetin, Kaempferol, and Quercetin on Head and Neck Cancers. Nutrients 2021, 13, 845. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Ketha, A.; Hieu, H.V.; Tatipamula, V.B. In vitro antimycobacterial studies of flavonols from Bauhinia vahlii Wight and Arn. 3 Biotech 2021, 11, 128. [Google Scholar] [CrossRef]
- Xiao, Z.; He, L.; Hou, X.; Wei, J.; Ma, X.; Gao, Z.; Yuan, Y.; Xiao, J.; Li, P.; Yue, T. Relationships between Structure and Antioxidant Capacity and Activity of Glycosylated Flavonols. Foods 2021, 10, 849. [Google Scholar] [CrossRef]
- Laoué, J.; Fernandez, C.; Ormeño, E. Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress. Plants 2022, 11, 172. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Ma, B.; Wu, Z.; Zheng, L.; Qi, Z.; Wang, Y. A leucoanthocyanidin dioxygenase gene (RtLDOX2) from the feral forage plant Reaumuria trigyna promotes the accumulation of flavonoids and improves tolerance to abiotic stresses. J. Plant Res. 2021, 134, 1121–1138. [Google Scholar] [CrossRef]
- Liu, H.; Su, B.; Zhang, H.; Gong, J.; Zhang, B.; Liu, Y.; Du, L. Identification and Functional Analysis of a Flavonol Synthase Gene from Grape Hyacinth. Molecules 2019, 24, 1579. [Google Scholar] [CrossRef]
- Fujita, A.; Goto-Yamamoto, N.; Aramaki, I.; Hashizume, K. Organ-Specific Transcription of Putative Flavonol Synthase Genes of Grapevine and Effects of Plant Hormones and Shading on Flavonol Biosynthesis in Grape Berry Skins. Biosci. Biotechnol. Biochem. 2006, 70, 632–638. [Google Scholar] [CrossRef]
- Medda, S.; Sanchez-Ballesta, M.; Romero, I.; Dessena, L.; Mulas, M. Expression of Structural Flavonoid Biosynthesis Genes in Dark-Blue and White Myrtle Berries (Myrtus communis L.). Plants 2021, 10, 316. [Google Scholar] [CrossRef]
- Sun, Y.-J.; He, J.-M.; Kong, J.-Q. Characterization of two flavonol synthases with iron-independent flavanone 3-hydroxylase activity from Ornithogalum caudatum Jacq. BMC Plant Biol. 2019, 19, 195. [Google Scholar] [CrossRef]
- Preuß, A.; Stracke, R.; Weisshaar, B.; Hillebrecht, A.; Matern, U.; Martens, S. Arabidopsis thaliana expresses a second functional flavonol synthase. FEBS Lett. 2009, 583, 1981–1986. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, W.; Wu, W. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of flavonoid biosynthesis in blackberry. Food Res. Int. 2022, 153, 110948. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Yang, H.; Wu, W.; Li, W.; Lyu, L. Single nucleotide polymorphism discovery and functional annotation analysis of blackberry fruit. Trees 2022, 36, 1313–1324. [Google Scholar] [CrossRef]
- Pelletier, M.K.; Burbulis, I.E.; Winkel-Shirley, B. Disruption of specific flavonoid genes enhances the accumulation of flavonoid enzymes and end-products in Arabidopsis seedlings. Plant Mol. Biol. 1999, 40, 45–54. [Google Scholar] [CrossRef]
- Stracke, R.; De Vos, R.C.H.; Bartelniewoehner, L.; Ishihara, H.; Sagasser, M.; Martens, S.; Weisshaar, B. Metabolomic and genetic analyses of flavonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 2009, 229, 427–445. [Google Scholar] [CrossRef]
- Cheng, S.; Song, Q.; Yu, T.; Liu, X.; Wang, L.; Mao, D.; Zhang, W.; Xu, F. Characterization and expression analysis of four members genes of flavanone 3-hydroxylase families from Chamaemelum nobile. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 102–115. [Google Scholar] [CrossRef]
- Xu, F.; Li, L.; Zhang, W.; Cheng, H.; Sun, N.; Cheng, S.; Wang, Y. Isolation, characterization, and function analysis of a flavonol synthase gene from Ginkgo biloba. Mol. Biol. Rep. 2012, 39, 2285–2296. [Google Scholar] [CrossRef]
- Lukačin, R.; Wellmann, F.; Britsch, L.; Martens, S.; Matern, U. Flavonol synthase from Citrus unshiu is a bifunctional dioxygenase. Phytochemistry 2003, 62, 287–292. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.-J.; Wang, Y.; Liu, S.; Geng, Z.; Song, A.; Jiang, J.; Chen, S.; Chen, F. Functional identification of a flavone synthase and a flavonol synthase genes affecting flower color formation in Chrysanthemum morifolium. Plant Physiol. Biochem. 2021, 166, 1109–1120. [Google Scholar] [CrossRef]
- Fang, F.; Tang, K.; Huang, W.-D. Changes of flavonol synthase and flavonol contents during grape berry development. Eur. Food Res. Technol. 2013, 237, 529–540. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.-H.; Yang, J.-H.; Lee, J.-Y.; Lim, S.-H. Increased Flavonol Levels in Tobacco Expressing AcFLS Affect Flower Color and Root Growth. Int. J. Mol. Sci. 2020, 21, 1011. [Google Scholar] [CrossRef]
- Tohge, T.; Nishiyama, Y.; Hirai, M.Y.; Yano, M.; Nakajima, J.-I.; Awazuhara, M.; Inoue, E.; Takahashi, H.; Goodenowe, D.B.; Kitayama, M.; et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005, 42, 218–235. [Google Scholar] [CrossRef]
- Mehrtens, F.; Kranz, H.; Bednarek, P.; Weisshaar, B. The Arabidopsis Transcription Factor MYB12 Is a Flavonol-Specific Regulator of Phenylpropanoid Biosynthesis. Plant Physiol. 2005, 138, 1083–1096. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Jiang, S.; Zhang, Z.; Lu, N.; Qiu, H.; Qu, C.; Wang, Y.; Wu, S.; Chen, X. MYB12 and MYB22 play essential roles in proanthocyanidin and flavonol synthesis in red-fleshed apple (Malus sieversiif. niedzwetzkyana). Plant J. 2017, 90, 276–292. [Google Scholar] [CrossRef]
- Zhai, R.; Zhao, Y.; Wu, M.; Yang, J.; Li, X.; Liu, H.; Wu, T.; Liang, F.; Yang, C.; Wang, Z.; et al. The MYB transcription factor PbMYB12b positively regulates flavonol biosynthesis in pear fruit. BMC Plant Biol. 2019, 19, 85. [Google Scholar] [CrossRef]
- Stracke, R.; Favory, J.-J.; Gruber, H.; Bartelniewoehner, L.; Bartels, S.; Binkert, M.; Funk, M.; Weisshaar, B.; Ulm, R. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 2010, 33, 88–103. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Law, C.L. Extraction of Total Phenolic Content from Garcinia mangostana Linn. hull. I. Effects of Solvents and UV–Vis Spectrophotometer Absorbance Method. Food Bioprocess Technol. 2012, 5, 2928–2933. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Amri, A.; Al-Hadhrami, A.; Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018, 4, e00874. [Google Scholar] [CrossRef]
- Cheng, F.-R.; Cui, H.-X.; Fang, J.-L.; Yuan, K.; Jin, S.-H.; Zhu, X.-T.; Xu, Y. Content determination of functional composition and antioxidant activity from six purple plants. Phcog. Mag. 2021, 17, 342–347. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
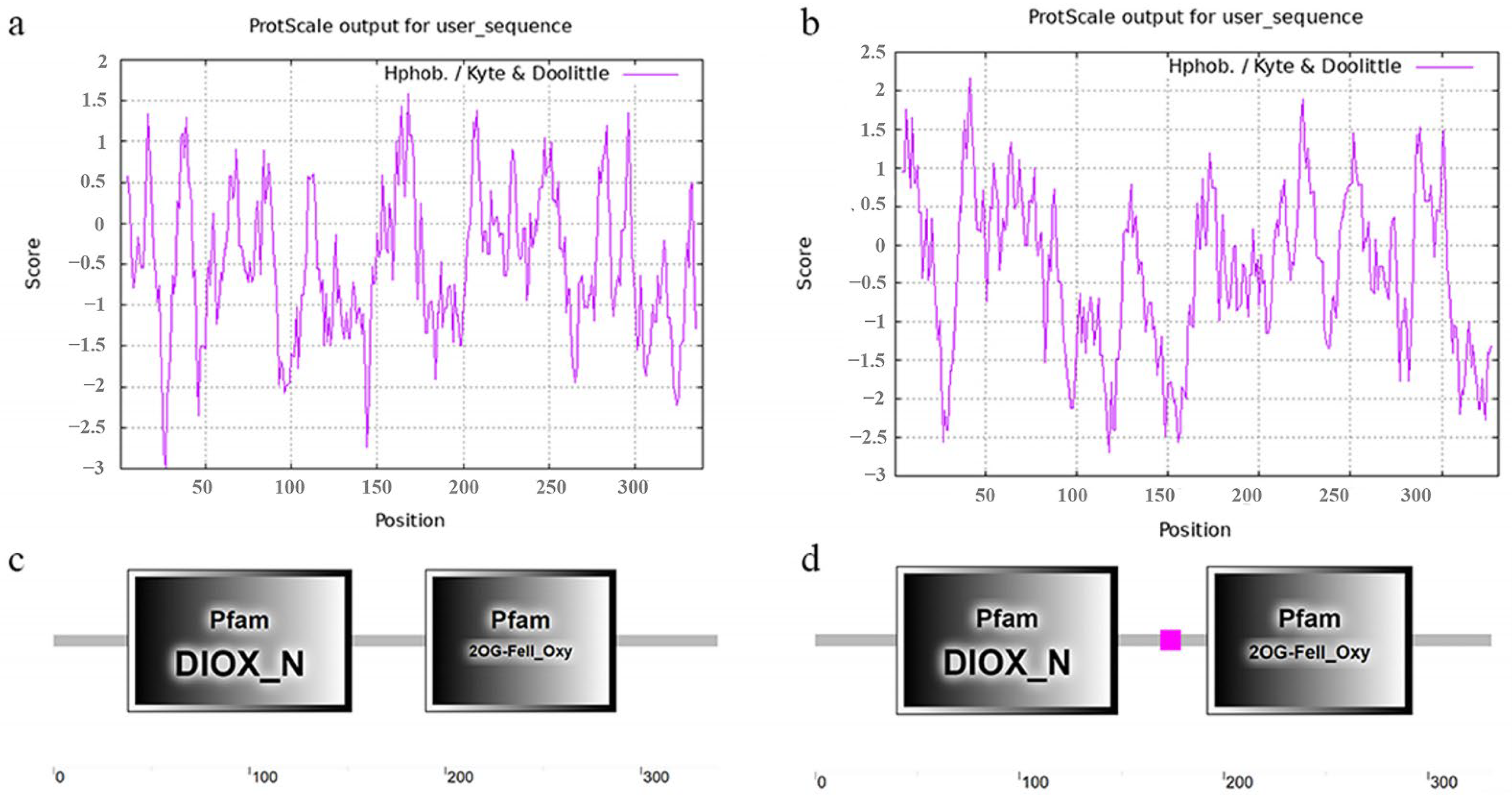
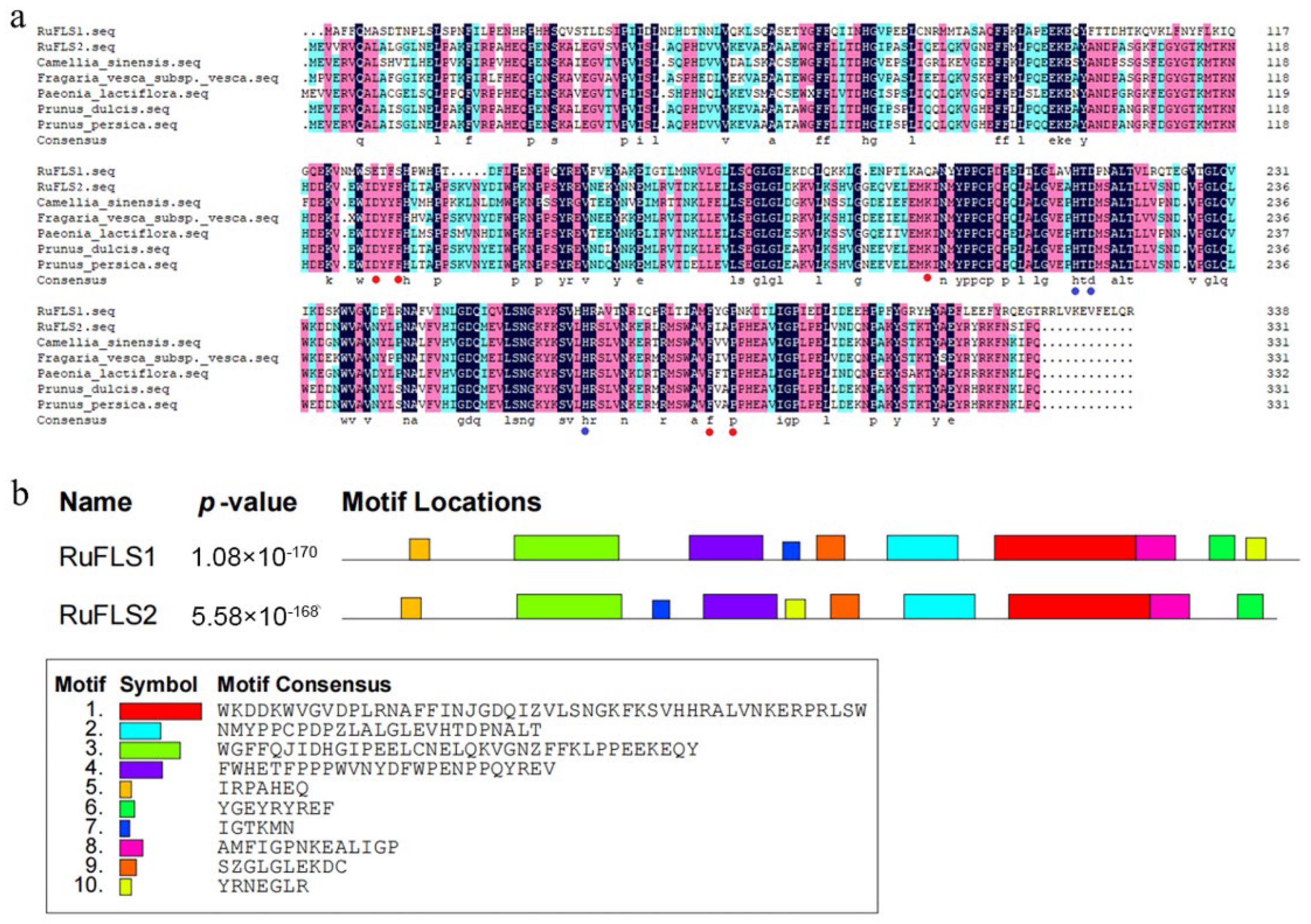



| Name | Number of Amino Acids | Molecular Weight | Theoretical pI | Negatively Charged Residues (Asp + Glu) | Positively Charged Residues (Arg + Lys) | Instability Index (II) | Aliphatic Index | Hydropathicity |
|---|---|---|---|---|---|---|---|---|
| RuFLS1 | 339 | 39.03 | 5.72 | 42 | 32 | 36.45 | 81.36 | −0.494 |
| RuFLS2 | 331 | 37.30 | 5.44 | 44 | 33 | 34.70 | 88.91 | −0.341 |
| Fruit Color | Phenols (mg·g−1 FW) | Flavonoids (mg·g−1 FW) | Anthocyanins (mg·g−1 FW) |
|---|---|---|---|
| Green | 40.68 ± 1.00a | 11.50 ± 0.37a | 0.02 ± 0.002d |
| Green–red | 11.91 ± 1.05b | 6.97 ± 0.11b | 0.05 ± 0.01d |
| Red | 3.28 ± 0.03c | 1.81 ± 0.01c | 0.39 ± 0.01c |
| Red–purple | 2.35 ± 0.01c | 1.80 ± 0.12c | 0.93 ± 0.02b |
| Purple | 3.10 ± 0.01c | 1.95 ± 0.10c | 2.60 ± 0.03a |
| Pathway | Name | Fold Change |
|---|---|---|
| ko00941//Flavonoid biosynthesis | Phlorizin | −0.72 |
| Neohesperidin | −0.77 | |
| Naringenin-7-O-glucoside | 0.70 | |
| (+)-catechin | −0.09 | |
| Catechin gallate | −0.59 | |
| Nobiletin | −0.48 | |
| ko00944//Flavone and flavonol biosynthesis | Apigenin 7-glucoside | 0.61 |
| Kaempferol 3-O-rutinoside | 0.58 | |
| Luteolin 7-glucoside | −0.01 | |
| Astragalin | 2.74 | |
| Quercitrin | 0.46 | |
| Quercetin 3-glucoside | −0.07 | |
| Ligustroflavone | −0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Overexpression of RuFLS2 Enhances Flavonol-Related Substance Contents and Gene Expression Levels. Int. J. Mol. Sci. 2022, 23, 14230. https://doi.org/10.3390/ijms232214230
Huang X, Wu Y, Zhang S, Yang H, Wu W, Lyu L, Li W. Overexpression of RuFLS2 Enhances Flavonol-Related Substance Contents and Gene Expression Levels. International Journal of Molecular Sciences. 2022; 23(22):14230. https://doi.org/10.3390/ijms232214230
Chicago/Turabian StyleHuang, Xin, Yaqiong Wu, Shanshan Zhang, Hao Yang, Wenlong Wu, Lianfei Lyu, and Weilin Li. 2022. "Overexpression of RuFLS2 Enhances Flavonol-Related Substance Contents and Gene Expression Levels" International Journal of Molecular Sciences 23, no. 22: 14230. https://doi.org/10.3390/ijms232214230
APA StyleHuang, X., Wu, Y., Zhang, S., Yang, H., Wu, W., Lyu, L., & Li, W. (2022). Overexpression of RuFLS2 Enhances Flavonol-Related Substance Contents and Gene Expression Levels. International Journal of Molecular Sciences, 23(22), 14230. https://doi.org/10.3390/ijms232214230






