Activity and Synergy of Cu-ATCUN Antimicrobial Peptides
Abstract
1. Introduction
1.1. Cathelicidins
1.2. Clawed-Toe Frog Peptides
2. Results
2.1. MIC Activity
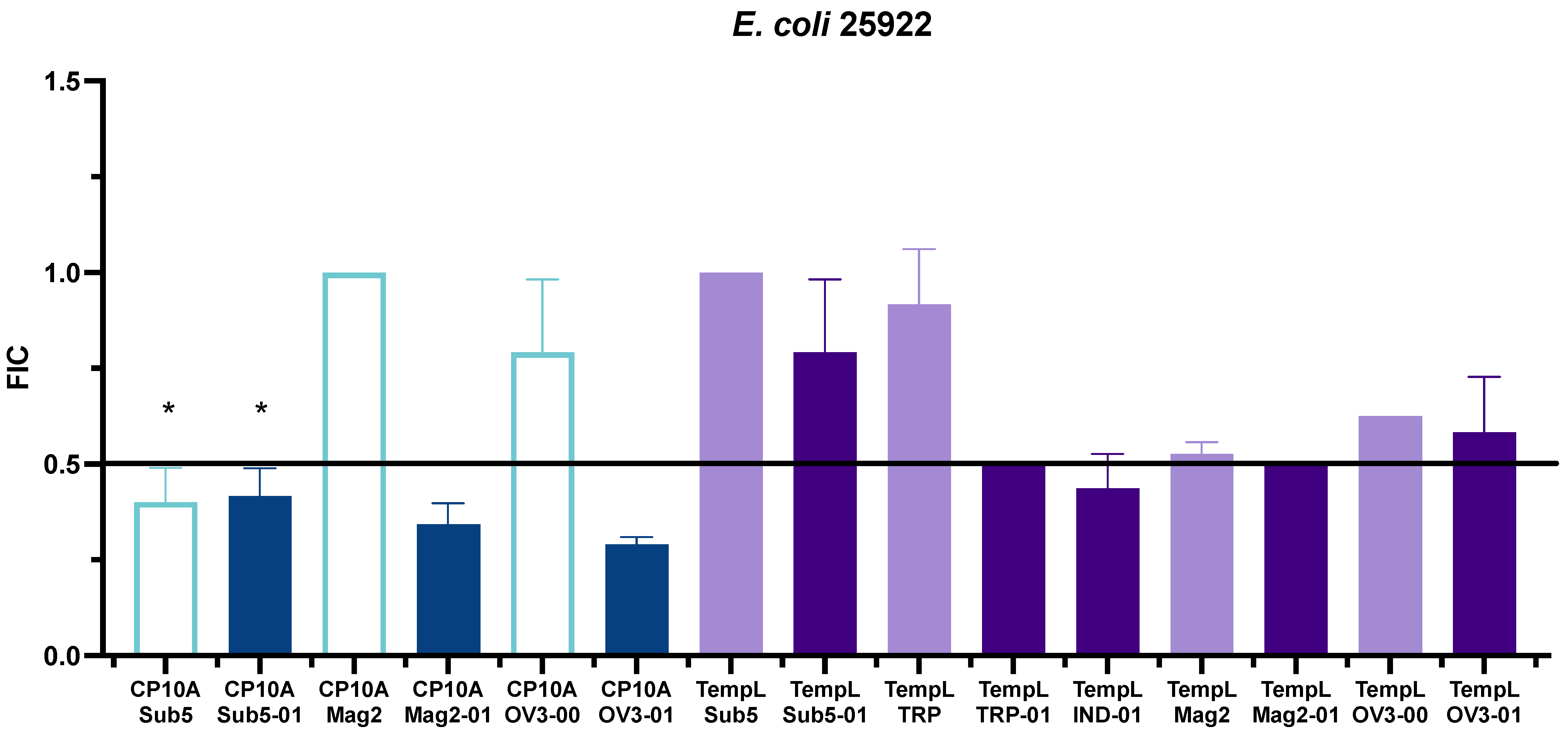
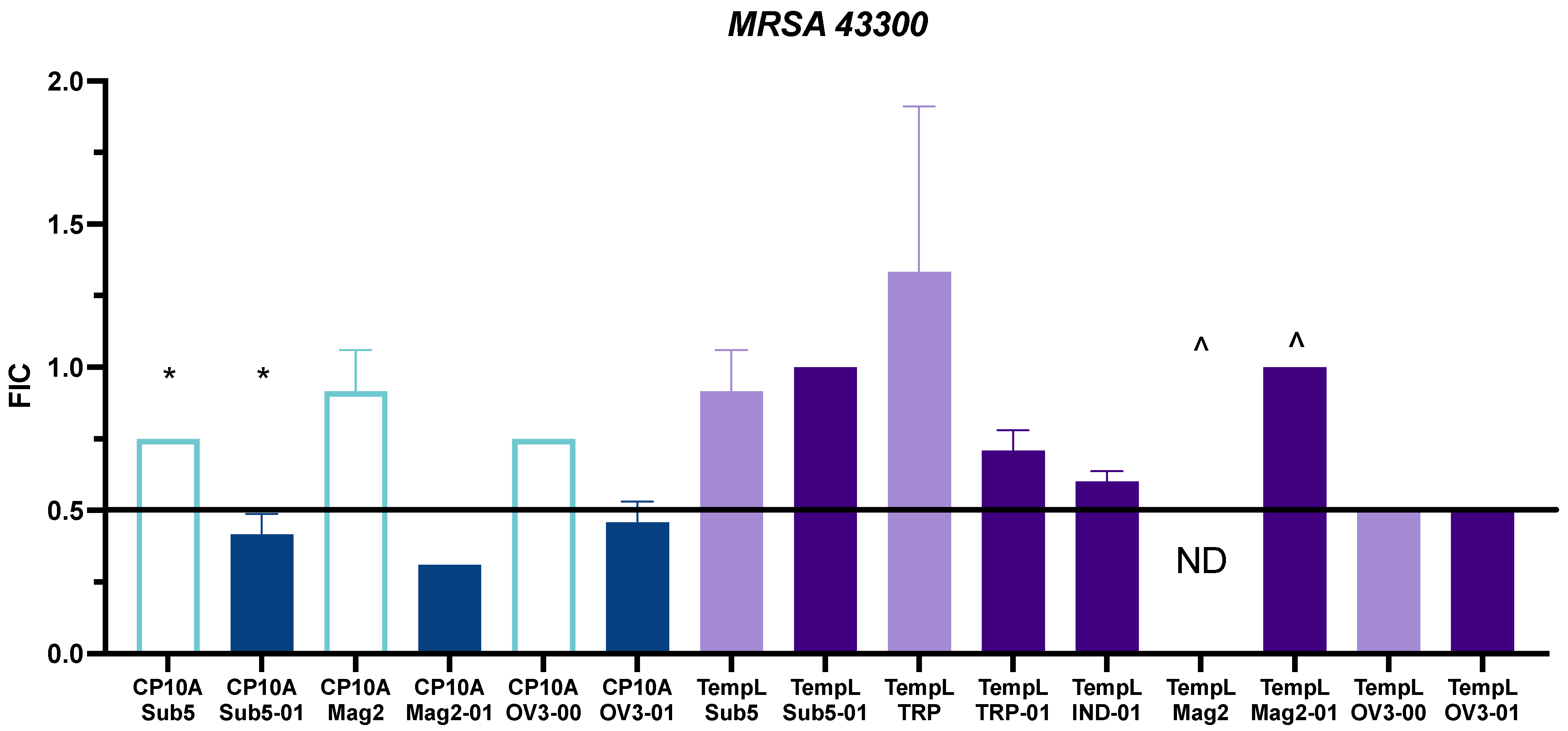
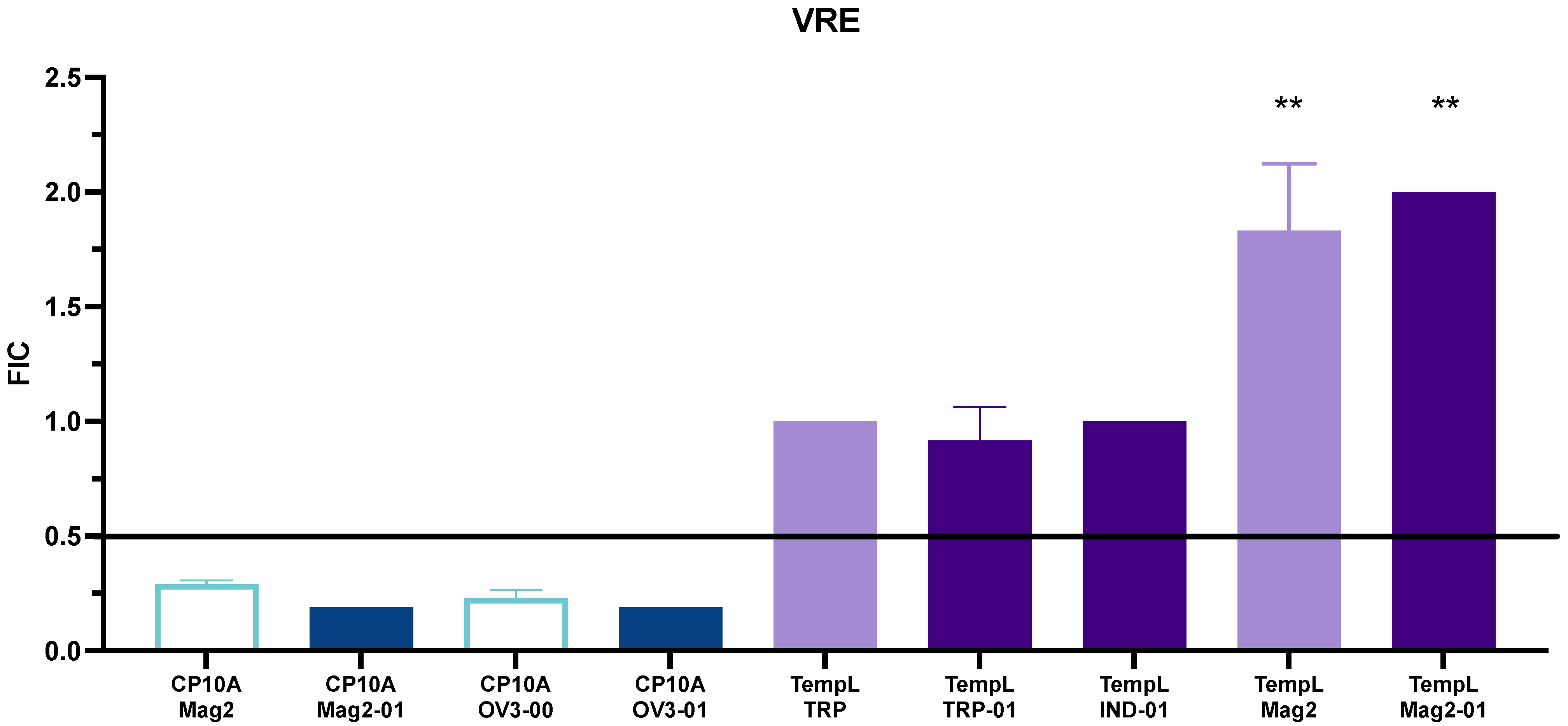
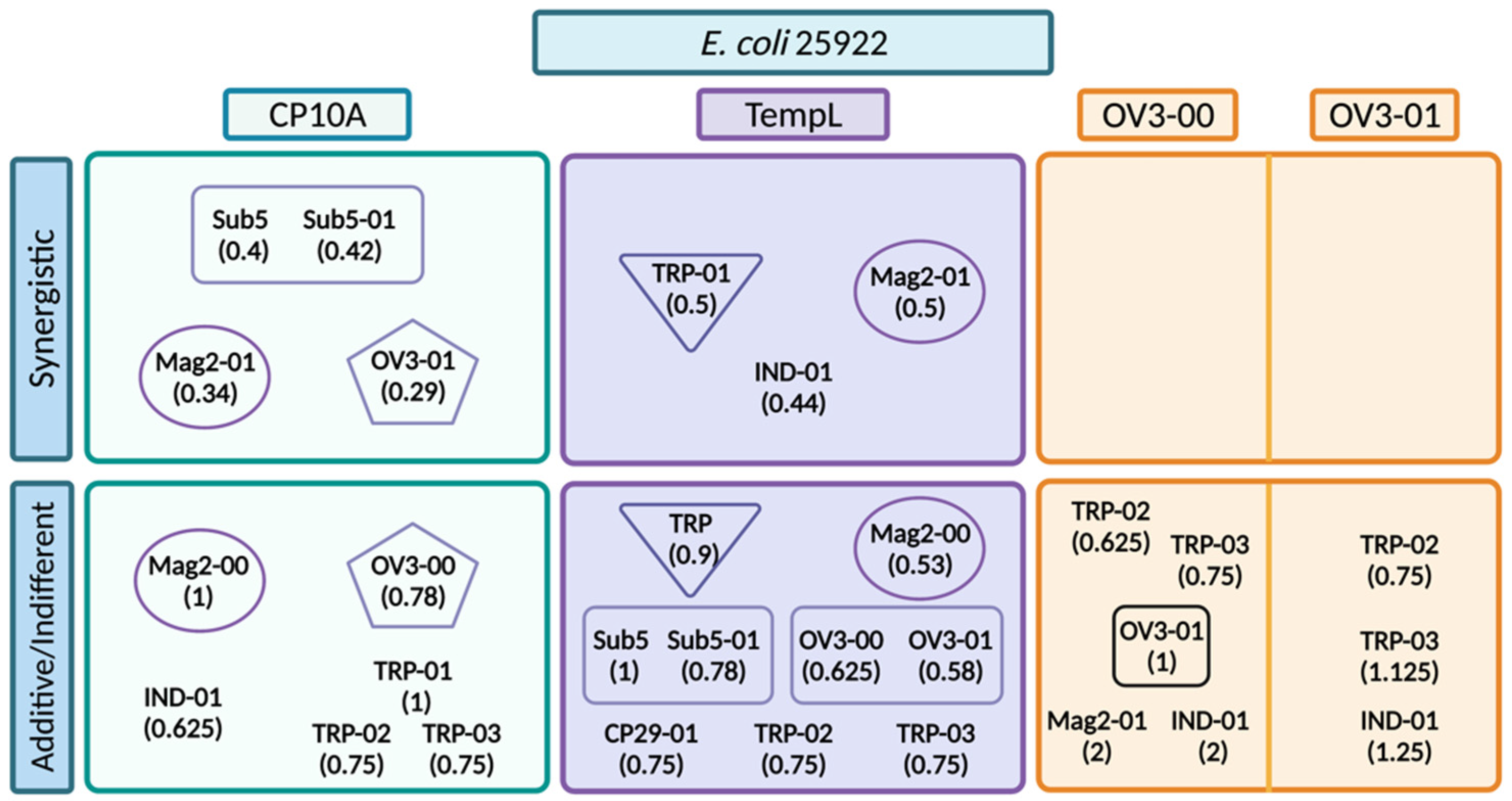
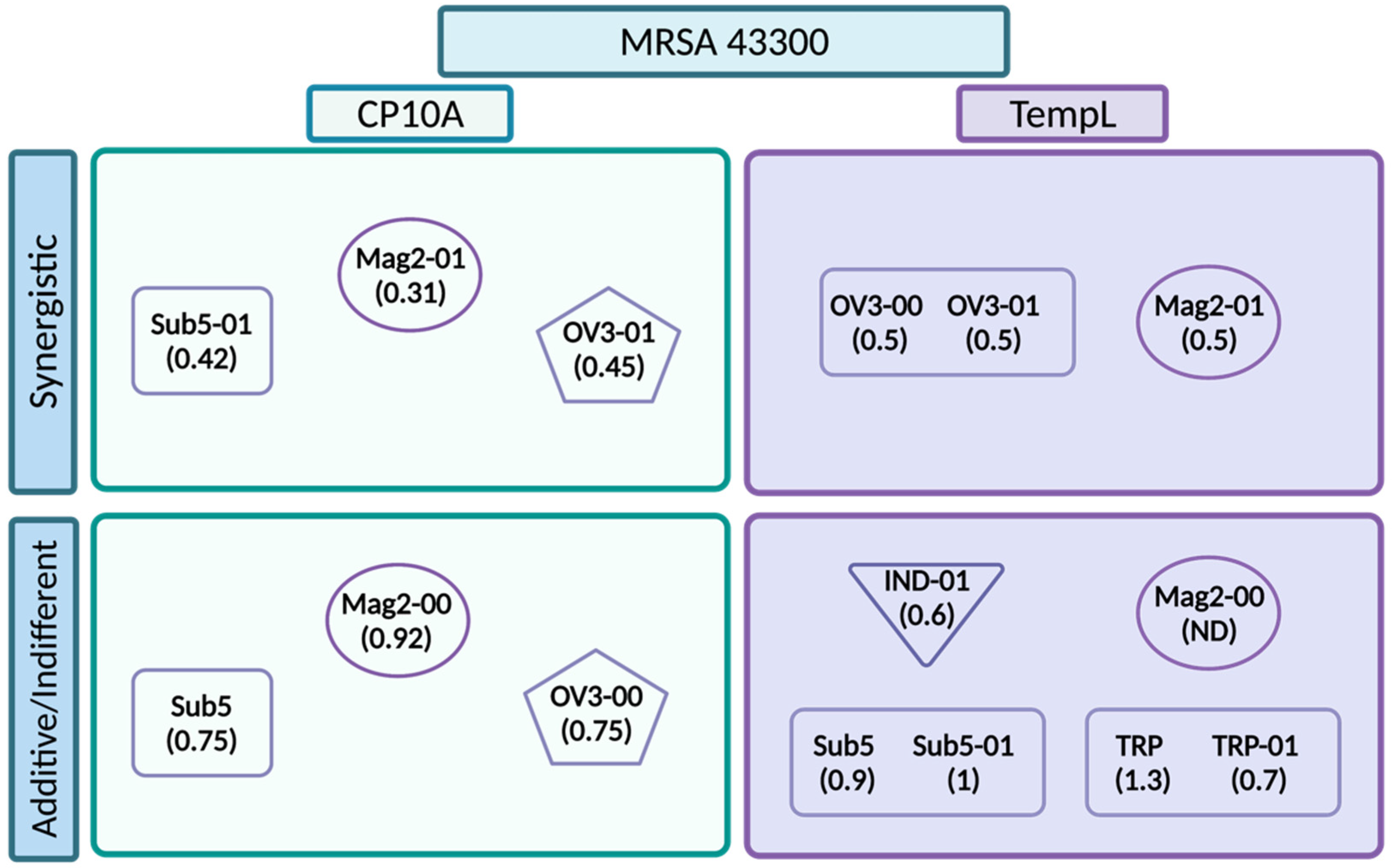
2.2. FIC Activity
3. Discussion
3.1. Role of MICs in Determining Therapeutic Potential
3.2. Synergy
3.3. AR Resistance
3.4. Conclusions
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Minimum Inhibitory Concentrations
4.3. Fractional Inhibitory Concentrations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019. [CrossRef]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updates 2016, 26, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.R.; Coleman, P.J.; Alvarez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hu, J.; Ke, F. Experimental Induction of Bacterial Resistance to the Antimicrobial Peptide Tachyplesin I and Investigation of the Resistance Mechanisms. Antimicrob. Agents Chemother. 2016, 60, 6067–6075. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Thompson, Z.; Yu, Z.; Cowan, J.A. Cu-ATCUN Derivatives of Sub5 Exhibit Enhanced Antimicrobial Activity via Multiple Modes of Action. ACS Chem. Biol. 2019, 14, 449–458. [Google Scholar] [CrossRef]
- Alexander, J.L.; Yu, Z.; Cowan, J.A. Amino Terminal Copper and Nickel Binding Motif Derivatives of Ovispirin-3 Display Increased Antimicrobial Activity via Lipid Oxidation. J. Med. Chem. 2017, 60, 10047–10055. [Google Scholar] [CrossRef]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Libardo, M.D.J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities. Chem. Rev. 2021, 121, 2648–2712. [Google Scholar] [CrossRef]
- Pinkham, A.M.; Yu, Z.; Cowan, J.A. Attenuation of West Nile Virus NS2B/NS3 Protease by Amino Terminal Copper and Nickel Binding (ATCUN) Peptides. J. Med. Chem. 2018, 61, 980–988. [Google Scholar] [CrossRef]
- Thompson, Z.; Greve, J.M.; Cowan, J.A. Enhanced Synergism and Mechanism of Action Studies of Synthetic Antimicrobial Metallopeptides. ChemMedChem 2021, 16, 2112–2120. [Google Scholar] [CrossRef]
- Libardo, M.D.; Cervantes, J.L.; Salazar, J.C.; Angeles-Boza, A.M. Improved bioactivity of antimicrobial peptides by addition of amino-terminal copper and nickel (ATCUN) binding motifs. ChemMedChem 2014, 9, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Thompson, Z.; Cowan, J.A. Antimicrobial Metallopeptides. ACS Chem. Biol. 2018, 13, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef]
- Shin, S.Y.; Park, E.J.; Yang, S.-T.; Jung, H.J.; Eom, S.H.; Song, W.K.; Kim, Y.; Hahm, K.-S.; Kim, J.I. Structure–Activity Analysis of SMAP-29, a Sheep Leukocytes-Derived Antimicrobial Peptide. Biochem. Biophys. Res. Commun. 2001, 285, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Tack, B.F.; Sawai, M.V.; Kearney, W.R.; Robertson, A.D.; Sherman, M.A.; Wang, W.; Hong, T.; Boo, L.M.; Wu, H.; Waring, A.J.; et al. SMAP-29 has two LPS-binding sites and a central hinge. Eur. J. Biochem. 2002, 269, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Sawai, M.V.; Waring, A.J.; Kearney, W.R.; McCray, P.B., Jr.; Forsyth, W.R.; Lehrer, R.I.; Tack, B.F. Impact of single-residue mutations on the structure and function of ovispirin/novispirin antimicrobial peptides. Protein Eng. Des. Sel. 2002, 15, 225–232. [Google Scholar] [CrossRef]
- Kalfa, V.C.; Jia, H.P.; Kunkle, R.A.; McCray, P.B.; Tack, B.F.; Brogden, K.A. Congeners of SMAP29 kill ovine pathogens and induce ultrastructural damage in bacterial cells. Antimicrob. Agents Chemother. 2001, 45, 3256–3261. [Google Scholar] [CrossRef]
- Dawson, R.M.; Liu, C.-Q. Cathelicidin peptide SMAP-29: Comprehensive review of its properties and potential as a novel class of antibiotics. Drug Dev. Res. 2009, 70, 481–498. [Google Scholar] [CrossRef]
- Anderson, R.C.; Hancock, R.E.W.; Yu, P.-L. Antimicrobial activity and bacterial-membrane interaction of ovine-derived cathelicidins. Antimicrob. Agents Chemother. 2004, 48, 673–676. [Google Scholar] [CrossRef]
- van der Linden, D.S.; Short, D.; Dittmann, A.; Yu, P.-L. Synergistic effects of ovine-derived cathelicidins and other antimicrobials against Escherichia coli O157:H7 and Staphylococcus aureus 1056 MRSA. Biotechnol. Lett. 2009, 31, 1265–1267. [Google Scholar] [CrossRef]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Perkins, W.R.; Lupan, D.M.; Selsted, M.E.; Janoff, A.S. Liposomal entrapment of the neutrophil-derived peptide indolicidin endows it with in vivo antifungal activity. Biochim. Biophys. Acta (BBA)-Biomembr. 1995, 1237, 109–114. [Google Scholar] [CrossRef]
- Aley, S.B.; Zimmerman, M.; Hetsko, M.; Selsted, M.E.; Gillin, F.D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect. Immun. 1994, 62, 5397–5403. [Google Scholar] [CrossRef]
- Friedrich, C.; Scott Monisha, G.; Karunaratne, N.; Yan, H.; Hancock Robert, E.W. Salt-Resistant Alpha-Helical Cationic Antimicrobial Peptides. Antimicrob. Agents Chemother. 1999, 43, 1542–1548. [Google Scholar] [CrossRef]
- Melino, S.; Santone, C.; Di Nardo, P.; Sarkar, B. Histatins: Salivary peptides with copper(II)- and zinc(II)-binding motifs: Perspectives for biomedical applications. FEBS J. 2014, 281, 657–672. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef]
- Ghosh, A.; Kar, R.K.; Jana, J.; Saha, A.; Jana, B.; Krishnamoorthy, J.; Kumar, D.; Ghosh, S.; Chatterjee, S.; Bhunia, A. Indolicidin Targets Duplex DNA: Structural and Mechanistic Insight through a Combination of Spectroscopy and Microscopy. ChemMedChem 2014, 9, 2052–2058. [Google Scholar] [CrossRef]
- Boehr, D.D.; Draker, K.A.; Koteva, K.; Bains, M.; Hancock, R.E.; Wright, G.D. Broad-spectrum peptide inhibitors of aminoglycoside antibiotic resistance enzymes. Chem. Biol. 2003, 10, 189–196. [Google Scholar] [CrossRef]
- Falla, T.J.; Karunaratne, D.N.; Hancock, R.E. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 1996, 271, 19298–19303. [Google Scholar] [CrossRef] [PubMed]
- Halevy, R.; Rozek, A.; Kolusheva, S.; Hancock, R.E.; Jelinek, R. Membrane binding and permeation by indolicidin analogs studied by a biomimetic lipid/polydiacetylene vesicle assay. Peptides 2003, 24, 1753–1761. [Google Scholar] [CrossRef]
- Lawyer, C.; Pai, S.; Watabe, M.; Borgia, P.; Mashimo, T.; Eagleton, L.; Watabe, K. Antimicrobial activity of a 13 amino acid tryptophan-rich peptide derived from a putative porcine precursor protein of a novel family of antibacterial peptides. FEBS Lett. 1996, 390, 95–98. [Google Scholar] [CrossRef]
- Yang, S.-T.; Yub Shin, S.; Kim, Y.-C.; Kim, Y.; Hahm, K.-S.; Kim, J.I. Conformation-dependent antibiotic activity of tritrpticin, a cathelicidin-derived antimicrobial peptide. Biochem. Biophys. Res. Commun. 2002, 296, 1044–1050. [Google Scholar] [CrossRef]
- Arias, M.; Piga, K.B.; Hyndman, M.E.; Vogel, H.J. Improving the Activity of Trp-Rich Antimicrobial Peptides by Arg/Lys Substitutions and Changing the Length of Cationic Residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef]
- Yang, S.-T.; Shin, S.Y.; Lee, C.W.; Kim, Y.-C.; Hahm, K.-S.; Kim, J.I. Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Lett. 2003, 540, 229–233. [Google Scholar] [CrossRef]
- Shagaghi, N.; Palombo, E.A.; Clayton, A.H.A.; Bhave, M. Archetypal tryptophan-rich antimicrobial peptides: Properties and applications. World J. Microbiol. Biotechnol. 2016, 32, 31. [Google Scholar] [CrossRef]
- Gennaro, R.; Skerlavaj, B.; Romeo, D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect. Immun. 1989, 57, 3142–3146. [Google Scholar] [CrossRef]
- Wu, M.; Hancock, R.E. Improved derivatives of bactenecin, a cyclic dodecameric antimicrobial cationic peptide. Antimicrob. Agents Chemother. 1999, 43, 1274–1276. [Google Scholar] [CrossRef]
- Hilpert, K.; Volkmer-Engert, R.; Walter, T.; Hancock, R.E. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 2005, 23, 1008–1012. [Google Scholar] [CrossRef]
- Torcato, I.M.; Huang, Y.H.; Franquelim, H.G.; Gaspar, D.D.; Craik, D.J.; Castanho, M.A.; Henriques, S.T. The antimicrobial activity of Sub3 is dependent on membrane binding and cell-penetrating ability. Chembiochem 2013, 14, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Mania, D.; Hilpert, K.; Ruden, S.; Fischer, R.; Takeshita, N. Screening for antifungal peptides and their modes of action in Aspergillus nidulans. Appl. Environ. Microbiol. 2010, 76, 7102–7108. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, K.; McLeod, B.; Yu, J.; Elliott Melissa, R.; Rautenbach, M.; Ruden, S.; Bürck, J.; Muhle-Goll, C.; Ulrich Anne, S.; Keller, S.; et al. Short Cationic Antimicrobial Peptides Interact with ATP. Antimicrob. Agents Chemother. 2010, 54, 4480–4483. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M.; Martin, B.; Chen, H.C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA 1988, 85, 910–913. [Google Scholar] [CrossRef]
- Cruciani, R.A.; Barker, J.L.; Zasloff, M.; Chen, H.C.; Colamonici, O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc. Natl. Acad. Sci. USA 1991, 88, 3792–3796. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Prajeep, M.; Sonnevend, A.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; King, J.D. Host-defense peptides in skin secretions of the tetraploid frog Silurana epitropicalis with potent activity against methicillin-resistant Staphylococcus aureus (MRSA). Peptides 2012, 37, 113–119. [Google Scholar] [CrossRef]
- Roelants, K.; Fry, B.G.; Ye, L.; Stijlemans, B.; Brys, L.; Kok, P.; Clynen, E.; Schoofs, L.; Cornelis, P.; Bossuyt, F. Origin and functional diversification of an amphibian defense peptide arsenal. PLoS Genet. 2013, 9, e1003662. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Grazia Ad Fau-Cappiello, F.; Cappiello F Fau-Casciaro, B.; Casciaro, B.; Fau-Luca, V.; Luca, V. Naturally Occurring Peptides from Rana temporaria: Antimicrobial Properties and More. Curr. Top. Med. Chem. 2016, 16, 54–64. [Google Scholar] [CrossRef]
- Mangoni, M.L. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. CMLS 2006, 63, 1060–1069. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Marcellini, H.G.L.; Simmaco, M. Biological characterization and modes of action of temporins and bombinins H, multiple forms of short and mildly cationic anti-microbial peptides from amphibian skin. J. Pept. Sci. 2007, 13, 603–613. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.L.; Barra, D. Temporins, Antimicrobial Peptides from the European Red Frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.J.; Bozzi, A.; Di Giulio, A.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Bevins, C.L.; Zasloff, M.A. Magainins: A new family of membrane-active host defense peptides. Biochem. Pharmacol. 1990, 39, 625–629. [Google Scholar] [CrossRef]
- Alexander, J.L. Characterization of Catalytic Metallodrugs: Advances towards Novel Antibiotics. Ph.D. Thesis, Ohio State University, Columbus, OH, USA, 2017. [Google Scholar]
- Friedrich, C.L.; Rozek, A.; Patrzykat, A.; Hancock, R.E. Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J. Biol. Chem. 2001, 276, 24015–24022. [Google Scholar] [CrossRef] [PubMed]
- Annis, D.H.; Craig, B.A. The effect of interlaboratory variability on antimicrobial susceptibility determination. Diagn. Microbiol. Infect. Dis. 2005, 53, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Shim, M.S.; Chung, J.; Lim, D.-Y.; Lee, B.J. Purification and characterization of antimicrobial peptides from the skin secretion of Rana dybowskii. Peptides 2007, 28, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Libardo, M.D.; Gorbatyuk, V.Y.; Angeles-Boza, A.M. Central Role of the Copper-Binding Motif in the Complex Mechanism of Action of Ixosin: Enhancing Oxidative Damage and Promoting Synergy with Ixosin B. ACS Infect. Dis. 2016, 2, 71–81. [Google Scholar] [CrossRef]
- Hayden, R.M.; Goldberg, G.K.; Ferguson, B.M.; Schoeneck, M.W.; Libardo, M.D.; Mayeux, S.E.; Shrestha, A.; Bogardus, K.A.; Hammer, J.; Pryshchep, S.; et al. Complementary Effects of Host Defense Peptides Piscidin 1 and Piscidin 3 on DNA and Lipid Membranes: Biophysical Insights into Contrasting Biological Activities. J. Phys. Chem. B 2015, 119, 15235–15246. [Google Scholar] [CrossRef]
- Zerweck, J.; Strandberg, E.; Kukharenko, O.; Reichert, J.; Bürck, J.; Wadhwani, P.; Ulrich, A.S. Molecular mechanism of synergy between the antimicrobial peptides PGLa and magainin 2. Sci. Rep. 2017, 7, 13153. [Google Scholar] [CrossRef]
- Libardo, M.D.; Paul, T.J.; Prabhakar, R.; Angeles-Boza, A.M. Hybrid peptide ATCUN-sh-Buforin: Influence of the ATCUN charge and stereochemistry on antimicrobial activity. Biochimie 2015, 113, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Han, M.; Cowan, J.A. Toward the design of a catalytic metallodrug: Selective cleavage of G-quadruplex telomeric DNA by an anticancer copper-acridine-ATCUN complex. Angew. Chem. Int. Ed. Engl. 2015, 54, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Chen, C.; Jou, M.L.; Lee, A.Y.; Lin, Y.C.; Yu, Y.P.; Huang, W.T.; Wu, S.H. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: Evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005, 33, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Krajewski, K.; Lee, H.F.; Antony, S.; Johnson, A.A.; Amin, R.; Roller, P.; Kvaratskhelia, M.; Pommier, Y. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 2006, 34, 5157–5165. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Nielsen, C.G.; Aarestrup, F.M.; Hansen, E.B. Comparative Evaluation of the Antimicrobial Activity of Different Antimicrobial Peptides against a Range of Pathogenic Bacteria. PLoS ONE 2015, 10, e0144611. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 289–294. [Google Scholar] [CrossRef]
- Mohanram, H.; Bhattacharjya, S. Resurrecting Inactive Antimicrobial Peptides from the Lipopolysaccharide Trap. Antimicrob. Agents Chemother. 2014, 58, 1987–1996. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J. Biol. Chem. 2005, 280, 10378–10387. [Google Scholar] [CrossRef]
- Snyder, D.S.; McIntosh, T.J. The Lipopolysaccharide Barrier: Correlation of Antibiotic Susceptibility with Antibiotic Permeability and Fluorescent Probe Binding Kinetics. Biochemistry 2000, 39, 11777–11787. [Google Scholar] [CrossRef]
- Allende, D.; McIntosh, T.J. Lipopolysaccharides in Bacterial Membranes Act like Cholesterol in Eukaryotic Plasma Membranes in Providing Protection against Melittin-Induced Bilayer Lysis. Biochemistry 2003, 42, 1101–1108. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, Y.; Barra, D.; Simmaco, M.; Shai, Y.; Mangoni, M.L. A synergism between temporins toward Gram-negative bacteria overcomes resistance imposed by the lipopolysaccharide protective layer. J. Biol. Chem. 2006, 281, 28565–28574. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Sugishita, K.I.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with lipopolysaccharide-containing liposomes as a model for outer membranes of Gram-negative bacteria. FEBS J. 1999, 449, 4. [Google Scholar] [CrossRef]
- Rana, F.R.; Blazyk, J. Interactions between the antimicrobial peptide, magainin 2, and Salmonella typhimurium lipopolysaccharides. FEBS Lett. 1991, 293, 11–15. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Papo, N.; Shai, Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J. Biol. Chem. 2006, 281, 1636–1643. [Google Scholar] [CrossRef]
- Ravensdale, J.; Wong, Z.; O’Brien, F.; Gregg, K. Efficacy of Antibacterial Peptides Against Peptide-Resistant MRSA Is Restored by Permeabilization of Bacteria Membranes. Front. Microbiol. 2016, 7, 1745. [Google Scholar] [CrossRef]
- Komatsuzawa, H.; Sugai, M.; Shirai, C.; Suzuki, J.; Hiramatsu, K.; Suginaka, H. Triton X-100 alters the resistance level of methicillin-resistant Staphylococcus aureus to oxacillin. FEMS Microbiol. Lett. 1995, 134, 209–212. [Google Scholar] [CrossRef]
- Patel, H.; Huynh, Q.; Bärlehner, D.; Heerklotz, H. Additive and Synergistic Membrane Permeabilization by Antimicrobial (Lipo)Peptides and Detergents. Biophys. J. 2014, 106, 2115–2125. [Google Scholar] [CrossRef]
- Jutkina, J.; Marathe, N.P.; Flach, C.F.; Larsson, D.G.J. Antibiotics and common antibacterial biocides stimulate horizontal transfer of resistance at low concentrations. Sci. Total Environ. 2018, 616–617, 172–178. [Google Scholar] [CrossRef]
- Long, H.; Miller, S.F.; Strauss, C.; Zhao, C.; Cheng, L.; Ye, Z.; Griffin, K.; Te, R.; Lee, H.; Chen, C.C.; et al. Antibiotic treatment enhances the genome-wide mutation rate of target cells. Proc. Natl. Acad. Sci. USA 2016, 113, E2498–E2505. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.P.D.; White, P. Fmoc Solid Phase Peptide Synthesis; Oxford University Press: Oxford, NY, USA, 2000; p. 346. [Google Scholar]
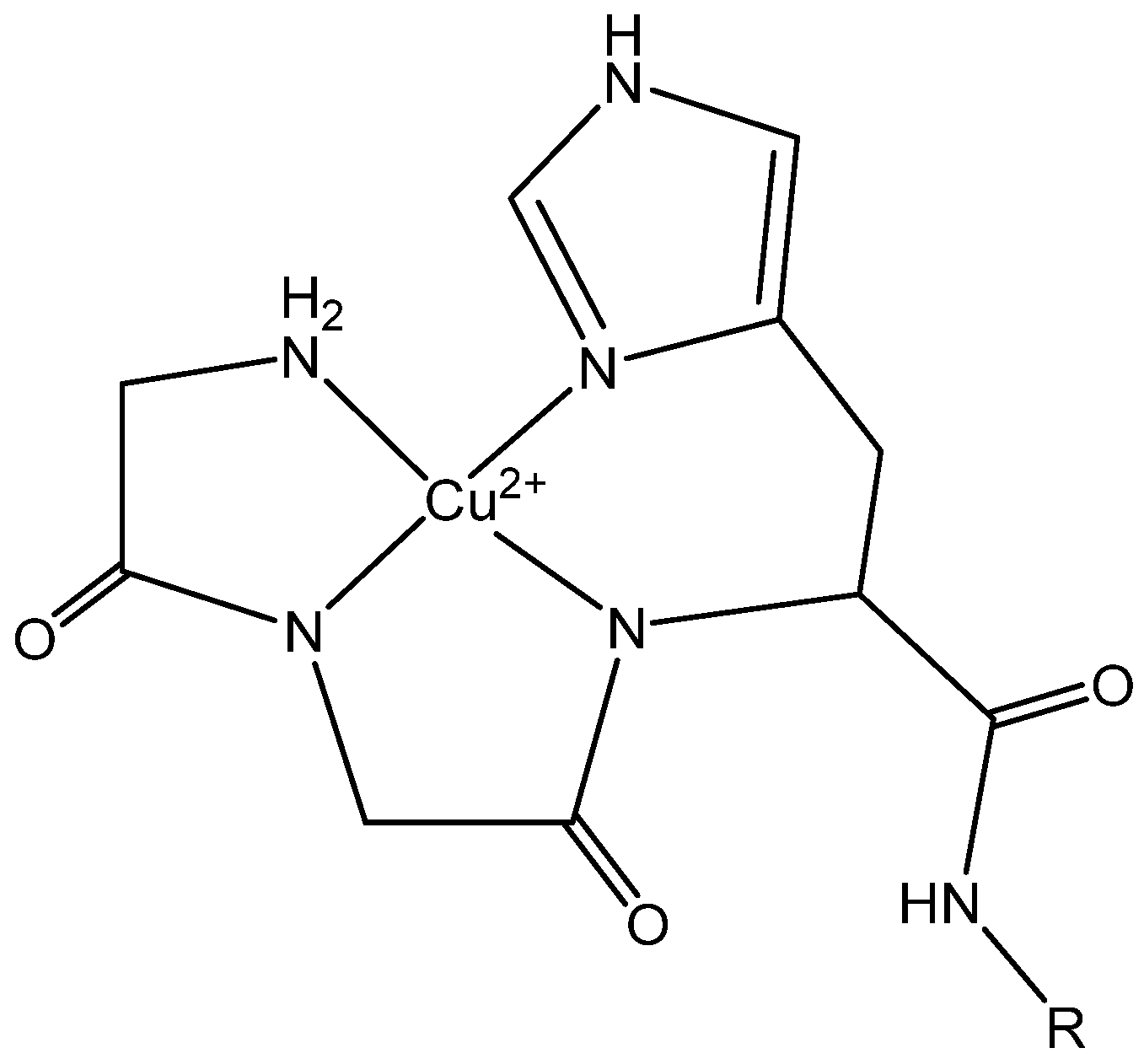
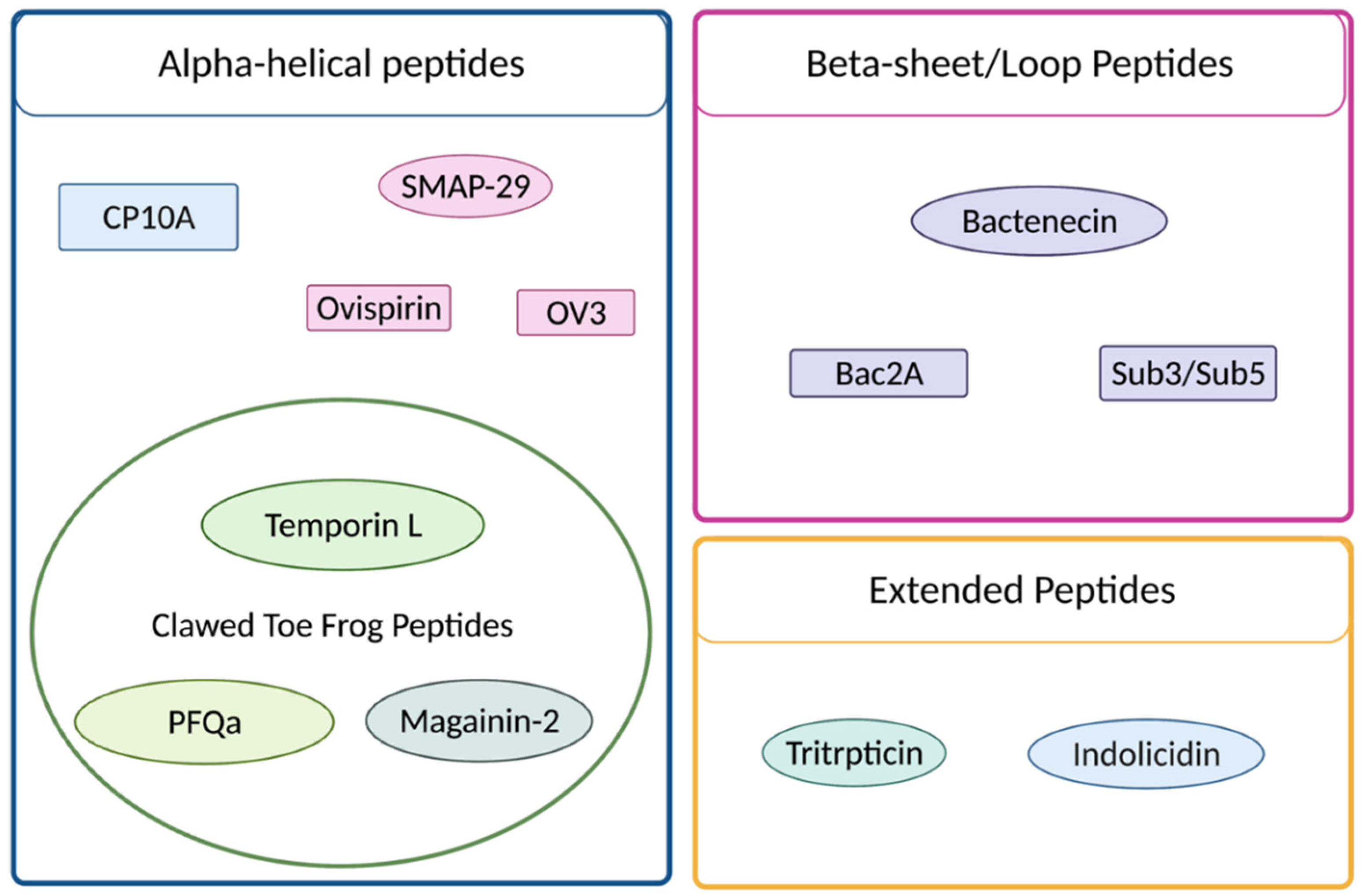
| Peptide | Sequence | AA | Charge pH 7 a | % Helical (SDS) b |
|---|---|---|---|---|
| SMAP-29 | RGLRRLGRKIAHGVKKYGPTVLRIIRIAG | 29 | 10.1 | 58% [19] |
| OV | KNLRRIIRKIIHIIKKYGPTILRIIRIIG | 29 | 10.1 | 86% [17] |
| OV3 | IRRIIRKIIHIIKK-NH2 | 14 | 7.1 | 38% [8] |
| OV3-00 | IRRIIRKIIHIIKKYG-NH2 | 16 | 7.1 | ND |
| OV3-01 | GGHIRRIIRKIIHIIKKYG-NH2 | 19 | 7.2 | ND |
| Cu-1 | GGHGIRRIIRKIIHIKK-NH2 | 18 | 7.2 | 14% [8] |
| Cu-2 | GGHGIRRIIRKIIHIIKKGGC-NH2 | 21 | 7.1 | 56% [8] |
| Cu-3 | GGHIRRIIRKIIHIIKK-NH2 | 17 | 7.2 | 35% [8] |
| Cu-4 | GGHIRRIIRKIIHIIKKGGC-NH2 | 20 | 7.1 | 64% [8] |
| MIC (µM) | Gram Stain | GGH | OV3-00 | OV3-01 | OV3 * | Cu-1 * | Cu-2 * | Cu-3 * | Cu-4 * |
|---|---|---|---|---|---|---|---|---|---|
| E. coli 25922 | (-) | >128 | 2 | 4 | >16 | >16 | 3 | >16 | 1.3 |
| A. baumannii BAA747 | (-) | >128 | 4 | 8 | 1 | 2.7 | 9.3 | 5.3 | 10.7 |
| P. aeruginosa (27583) | (-) | >128 | 16 | 16 | 16 | >16 | 12 | >16 | 4 |
| K. pneumoniae 700603 | (-) | >128 | 4 | 16 | >16 | 16 | >16 | 16 | 8 |
| S. aureus 43300 (MRSA) | (+) | >128 | 8 | 16 | >16 | 16 | 4 | 16 | 1.5 |
| E. faecium 700221 (VRE) | (+) | >128 | 4 | 4 | 16 | 16 | 8 | 8 | 3.3 |
| Peptide | Sequence | AA | Charge pH 7 * | Structure |
|---|---|---|---|---|
| Mag2 | GIGKFLHSAKKWGKAFVGEIMNS-NH2 | 23 | 4.1 | helix [54] |
| Mag2-01 | GGHGIGKFLHSAKKWGKAFVGEIMNS-NH2 | 26 | 4.2 | helix |
| CP29 | KWKSFIKKLTTAVKKVLTTGLPALIS-NH2 | 26 | 7 | helix [27] |
| CP29-01 | GGHKWKSFIKKLTTAVKKVLTTGLPALIS-NH2 | 29 | 7.1 | helix |
| TRP | VRRFPWWWPFLRR-NH2 | 13 | 5 | β-turn [35] |
| TRP-01 | GGHVRRFPWWWPFLRRGGC-NH2 | 19 | 5 | β-turn [55] |
| TRP-02 | GGHGVRRFPWWWPFLRR-NH2 | 17 | 5 | β-turn [55] |
| TRP-03 | GGHGVRRFPWWWPFLRRGGC-NH2 | 20 | 5 | β-turn [55] |
| IND-01 | GGHILPWKWPWWPWRRGGC-NH2 | 19 | 4 | extended [55] |
| CP10A | GILAWKWAWWAWRR-NH2 | 14 | 4 | helix [56] |
| TempL | FVQWFSKFLGRIL-NH2 | 13 | 3 | helix [50] |
| PFQα | FLGALLGPLMNLLQ-NH2 | 14 | 1 | helix [47] |
| PFQα-01 | GGHYLGALLGPLMNLLQ-NH2 | 17 | 1.1 | helix |
| Sub5 | RRWKIVVIRWRR-NH2 | 12 | 7 | β-loop [7] |
| Sub5-01 | GGHRRWKIVVIRWRR-NH2 | 15 | 7.1 | β-loop [7] |
| MIC (μM) | Gram Stain | GGH | CP29-01 | Mag2 | Mag2-01 | TRP | IND-01 | TRP-01 | TempL | PFQα-01 | CP10A | Sub5 | Sub5-01 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli 25922 | (-) | >128 | 8 | 8 | 16 | 8 | 32 | 32 | 4 | >128 | 16 | 3.3 | 2.7 |
| A. baumannii BAA747 | (-) | >128 | 1 | 8 | 8 | 8 | 32 | 16 | 4 | 128 | 8 | 0.8 | 1 |
| P. aeruginosa 27583 | (-) | >128 | 2 | 32 | 64 | 16 | >64 | 64 | 32 | >128 | 64 | 4 | 2 |
| K. pneumoniae 700603 | (-) | >128 | 8 | 64 | 64 | 32 | >64 | 32 | 16 | >128 | 32 | 1.7 | 2 |
| S. aureus 43300 (MRSA) | (+) | >128 | 16 | >128 | >128 | 8 | 64 | 32 | 4 | 16 | 4 | 2 | 1 |
| E. faecium 700221 (VRE) | (+) | >128 | 4 | 16 | 32 | 2 | 8 | 8 | 8 | >128 | 8 | 0.5 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greve, J.M.; Cowan, J.A. Activity and Synergy of Cu-ATCUN Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 14151. https://doi.org/10.3390/ijms232214151
Greve JM, Cowan JA. Activity and Synergy of Cu-ATCUN Antimicrobial Peptides. International Journal of Molecular Sciences. 2022; 23(22):14151. https://doi.org/10.3390/ijms232214151
Chicago/Turabian StyleGreve, Jenna M., and J. A. Cowan. 2022. "Activity and Synergy of Cu-ATCUN Antimicrobial Peptides" International Journal of Molecular Sciences 23, no. 22: 14151. https://doi.org/10.3390/ijms232214151
APA StyleGreve, J. M., & Cowan, J. A. (2022). Activity and Synergy of Cu-ATCUN Antimicrobial Peptides. International Journal of Molecular Sciences, 23(22), 14151. https://doi.org/10.3390/ijms232214151





