Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications
Abstract
1. Introduction
2. Results
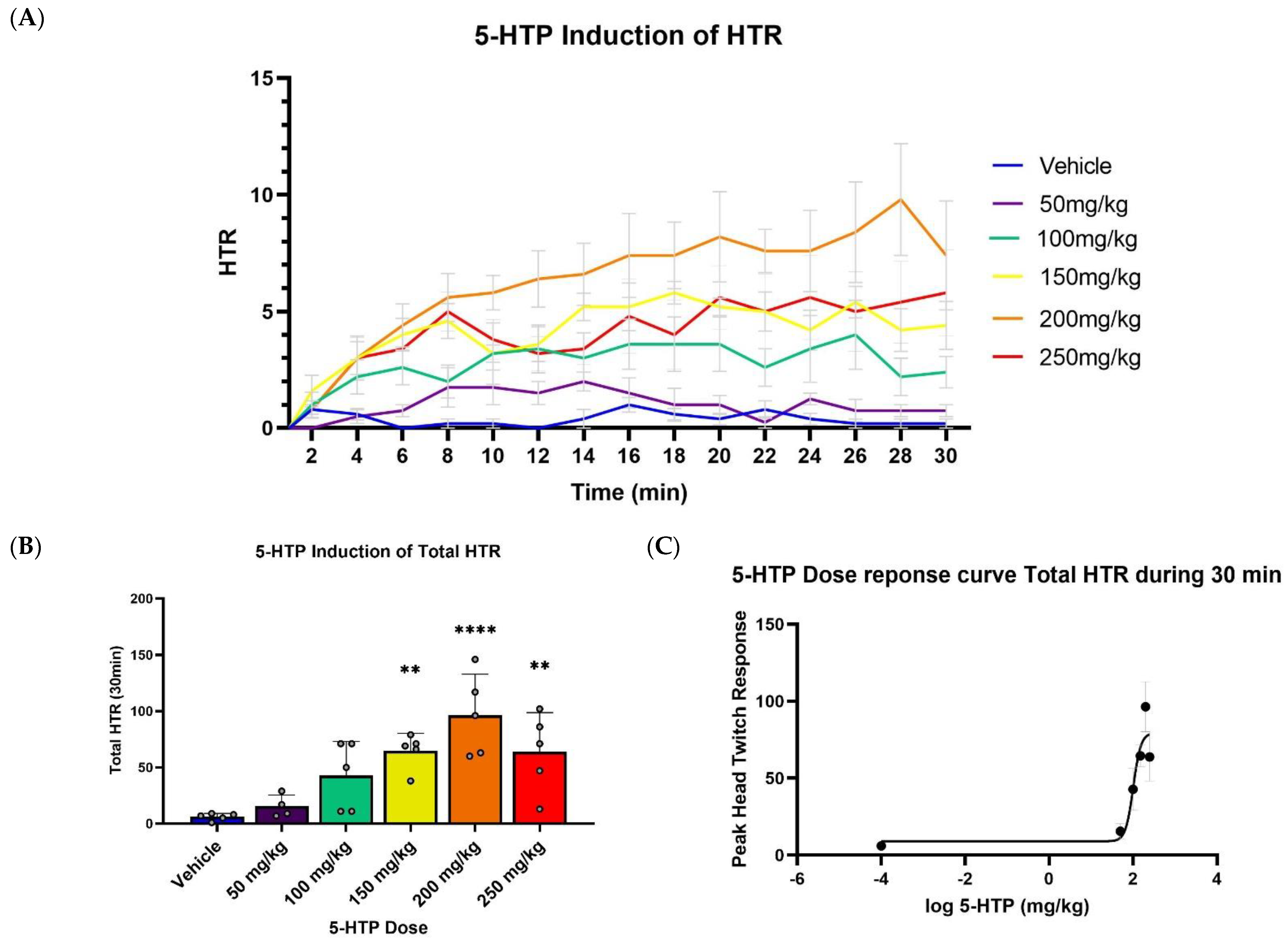
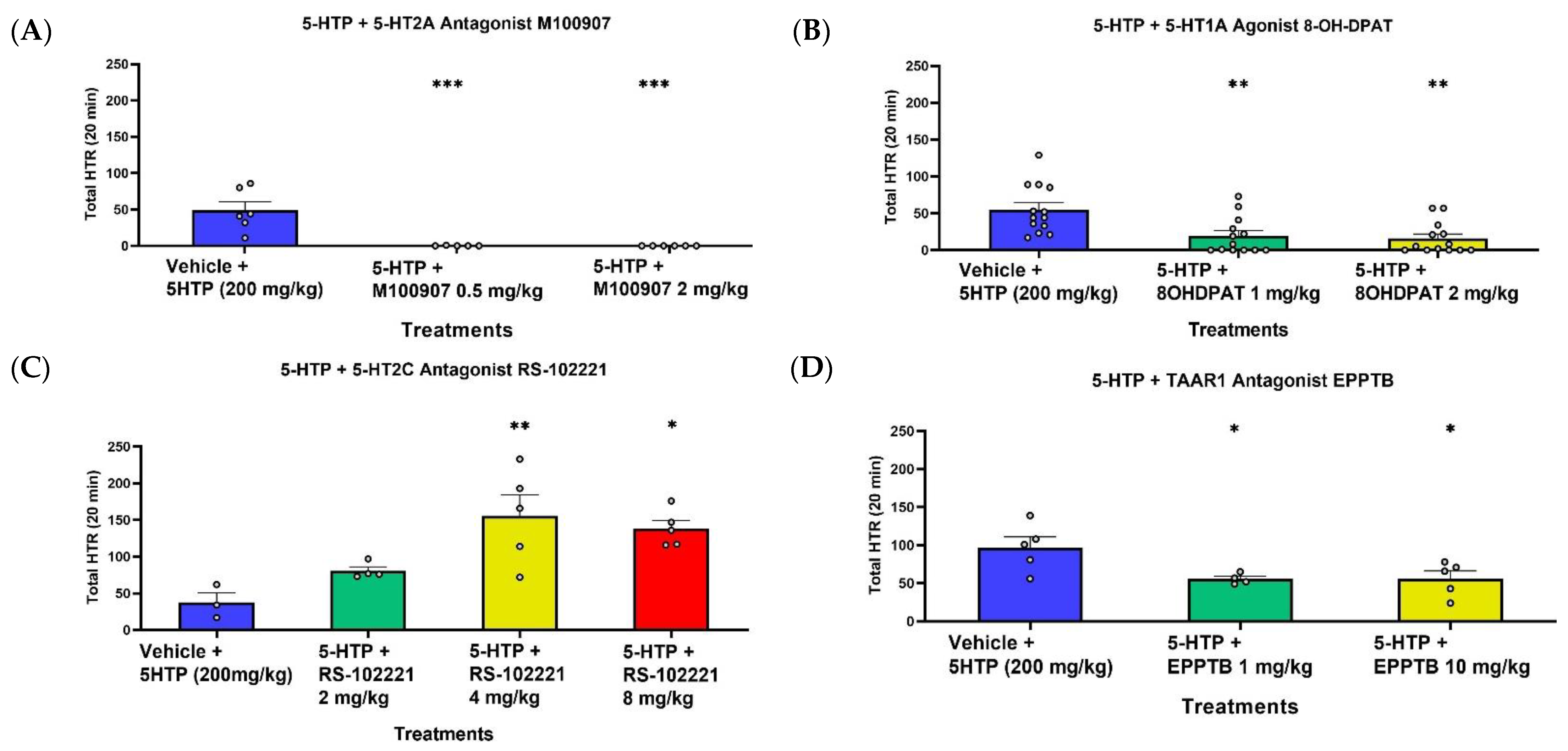
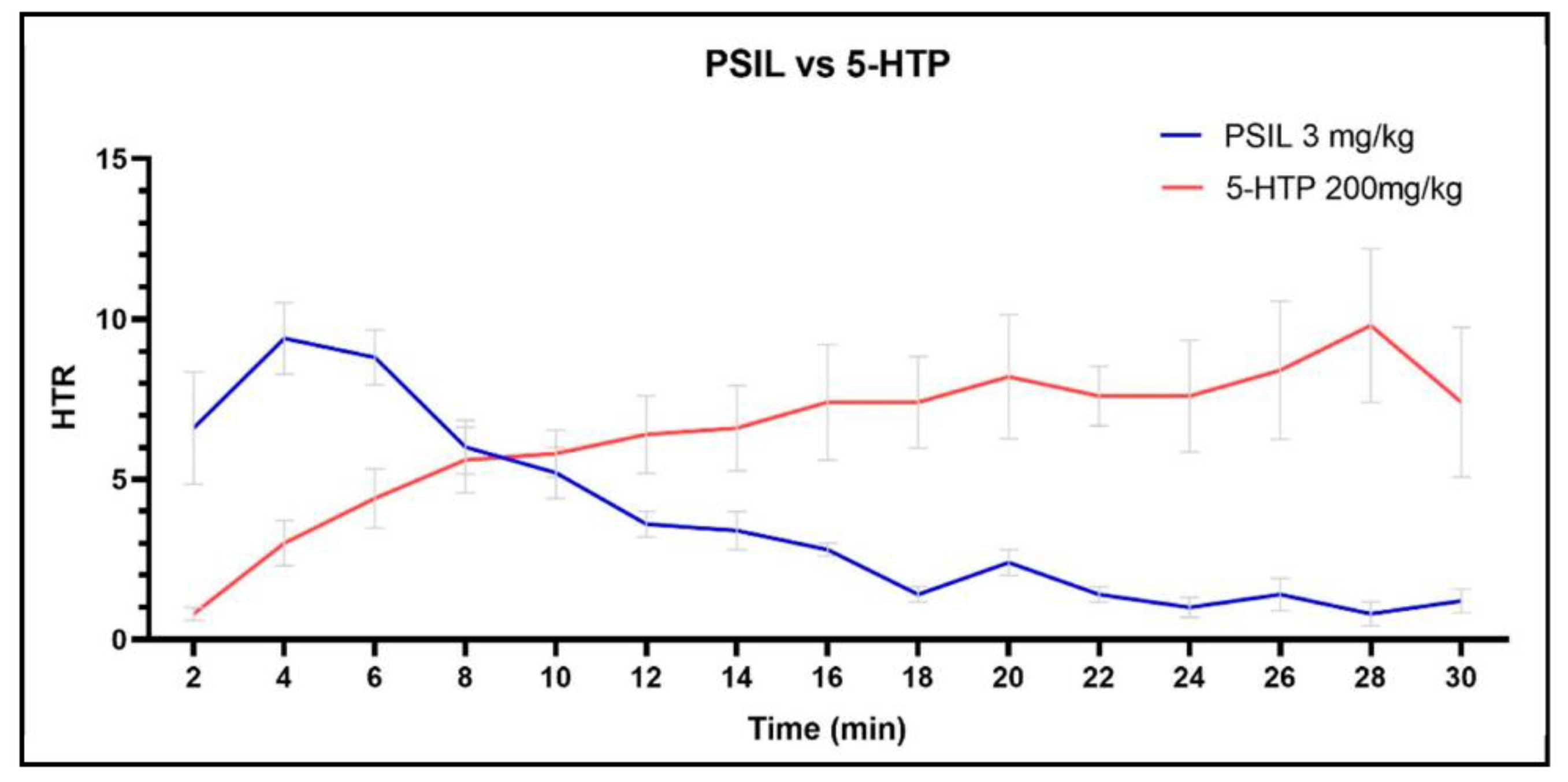
2.1. Effect of 5-HTP on HTR
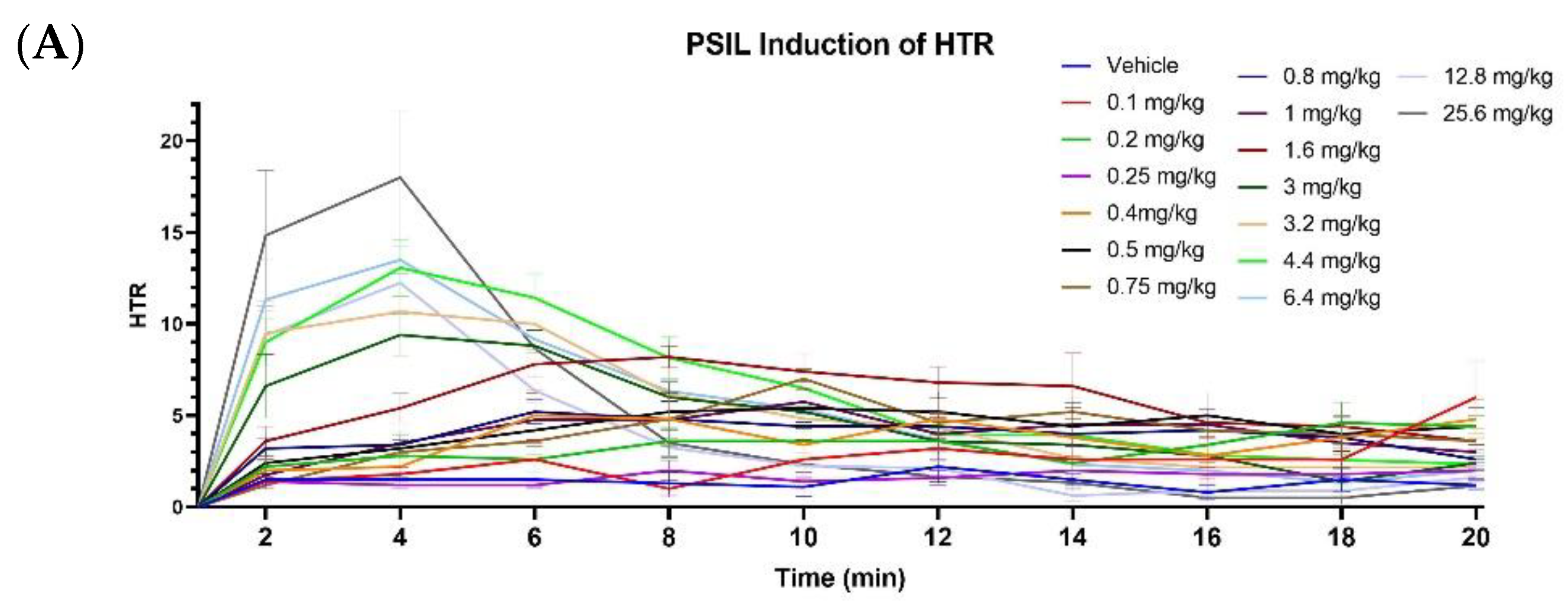
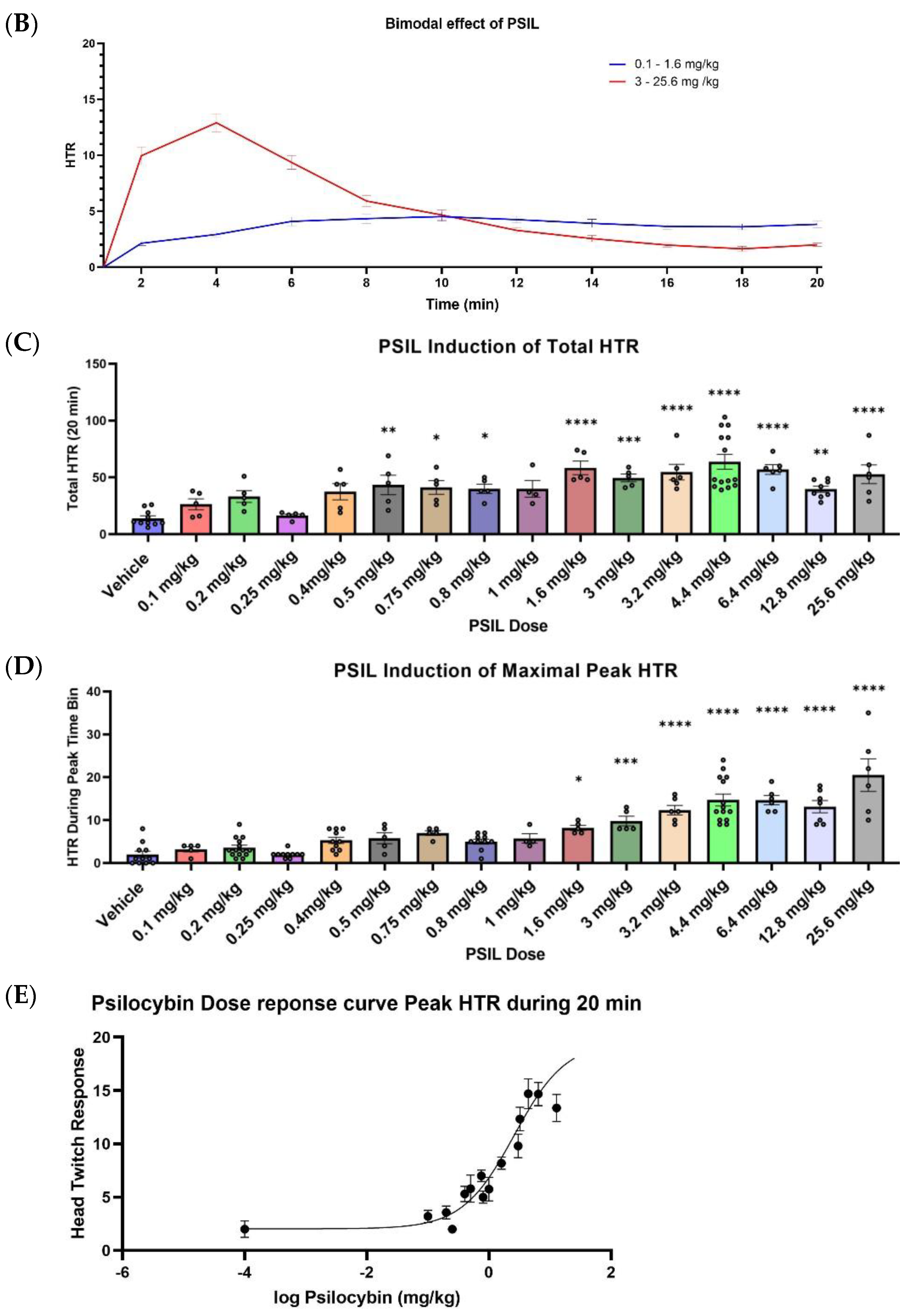
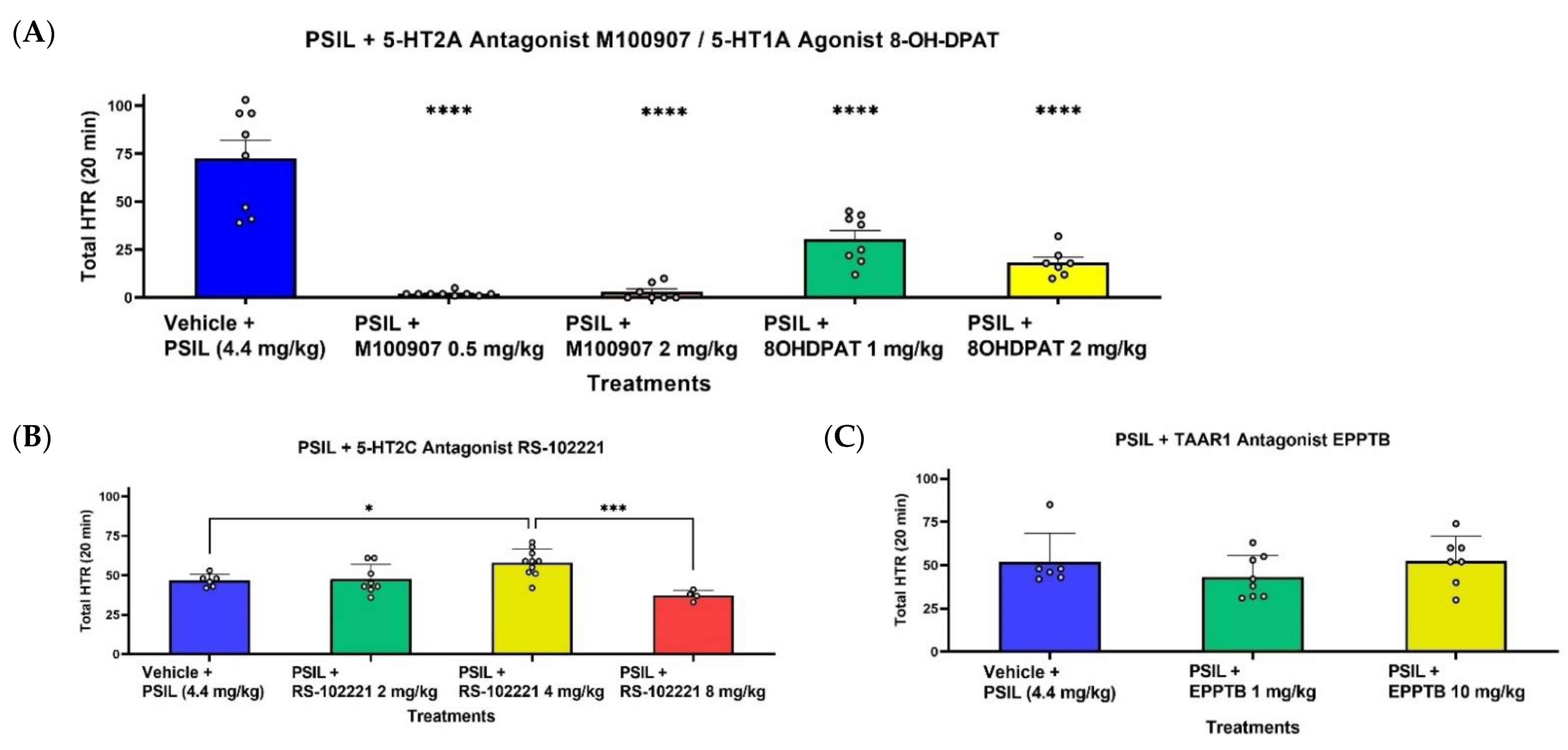
2.2. Effect of Psilocybin on HTR
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
4.3. Ear Tagging
4.4. Head Twitch Acquisition
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knapp, M.; Wong, G. Economics and mental health: The current scenario. World Psychiatry 2020, 19, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Castelpietra, G.; Knudsen, A.K.S.; Agardh, E.E.; Armocida, B.; Beghi, M.; Iburg, K.M.; Logroscino, G.; Ma, R.; Starace, F.; Steel, N.; et al. The burden of mental disorders, substance use disorders and self-harm among young people in Europe, 1990–2019: Findings from the Global Burden of Disease Study 2019. Lancet Reg. Health Eur. 2022, 16, 100341. [Google Scholar] [CrossRef] [PubMed]
- Gershkovich, M.; Wheaton, M.G.; Simpson, H.B. Management of Treatment-Resistant Obsessive-Compulsive Disorder. Curr. Treat. Options Psychiatry 2017, 4, 357–370. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Reps, J.; Ryan, P. Finding factors that predict treatment-resistant depression: Results of a cohort study. Depress. Anxiety 2018, 35, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D. Psychedelic drugs—A new era in psychiatry? Dialogues Clin. Neurosci. 2019, 21, 139–147. [Google Scholar] [CrossRef]
- Winkelman, M. Introduction: Evidence for entheogen use in prehistory and world religions. J. Psychedelic Stud. 2019, 3, 43–62. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Richards, W.A. Current perspectives on psychedelic therapy: Use of serotonergic hallucinogens in clinical interventions. Int. Rev. Psychiatry 2018, 30, 291–316. [Google Scholar] [CrossRef]
- Hofmann, A.; Heim, R.; Brack, A.; Kobel, H.; Frey, A.; Ott, H.; Petrzilka, T.; Troxler, F. Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen. Helv. Chim. Acta 1959, 42, 1557–1572. [Google Scholar] [CrossRef]
- Galvao-Coelho, N.L.; Marx, W.; Gonzalez, M.; Sinclair, J.; de Manincor, M.; Perkins, D.; Sarris, J. Classic serotonergic psychedelics for mood and depressive symptoms: A meta-analysis of mood disorder patients and healthy participants. Psychopharmacology 2021, 238, 341–354. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Krediet, E.; Bostoen, T.; Breeksema, J.; van Schagen, A.; Passie, T.; Vermetten, E. Reviewing the Potential of Psychedelics for the Treatment of PTSD. Int. J. Neuropsychopharmacol. 2020, 23, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.N.; Meshkat, S.; Benitah, K.; Lipsitz, O.; Gill, H.; Lui, L.M.W.; Teopiz, K.M.; McIntyre, R.S.; Rosenblat, J.D. Registered clinical studies investigating psychedelic drugs for psychiatric disorders. J. Psychiatr. Res. 2021, 139, 71–81. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Nichols, C.D.; Gainetdinov, R.R.; Nichols, D.E.; Kalueff, A.V. Psychedelic Drugs in Biomedicine. Trends Pharmacol. Sci. 2017, 38, 992–1005. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Slocum, S.T.; DiBerto, J.F.; Roth, B.L. Molecular insights into psychedelic drug action. J. Neurochem. 2022, 162, 24–38. [Google Scholar] [CrossRef]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Chatha, M.; Klein, A.K.; Wallach, J.; Brandt, S.D. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 2020, 167, 107933. [Google Scholar] [CrossRef]
- Lu, J.; Tjia, M.; Mullen, B.; Cao, B.; Lukasiewicz, K.; Shah-Morales, S.; Weiser, S.; Cameron, L.P.; Olson, D.E.; Chen, L.; et al. An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Mol. Psychiatry 2021, 26, 6237–6252. [Google Scholar] [CrossRef]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Corne, S.J.; Pickering, R.W.; Warner, B.T. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br. J. Pharmacol. Chemother. 1963, 20, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Harrington, A.W.; Kiessel, C.L.; Eckler, J.R.; Rabin, R.A.; Winter, J.C.; Coop, A.; Rice, K.C.; Woods, J.H. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol. Biochem. Behav. 2006, 83, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Harrington, A.W.; Eckler, J.R.; Arshad, S.; Rabin, R.A.; Winter, J.C.; Coop, A.; Rice, K.C.; Woods, J.H. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology 2005, 181, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Simoneau, J.; Cohen, M.S.; Zimmerman, S.M.; Henson, C.M.; Rice, K.C.; Woods, J.H. Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J. Pharmacol. Exp. Ther. 2010, 335, 728–734. [Google Scholar] [CrossRef] [PubMed]
- González-Maeso, J.; Yuen, T.; Ebersole, B.J.; Wurmbach, E.; Lira, A.; Zhou, M.; Weisstaub, N.; Hen, R.; Gingrich, J.A.; Sealfon, S.C. Transcriptome Fingerprints Distinguish Hallucinogenic and Nonhallucinogenic 5-Hydroxytryptamine 2A Receptor Agonist Effects in Mouse Somatosensory Cortex. J. Neurosci. 2003, 23, 8836–8843. [Google Scholar] [CrossRef] [PubMed]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Halberstadt, A.L. Automated detection of the head-twitch response using wavelet scalograms and a deep convolutional neural network. Sci. Rep. 2020, 10, 8344. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef]
- Darmani, N.A.; Reeves, S.L. The mechanism by which the selective 5-HT1A receptor antagonist S-(−)UH 301 produces head-twitches in mice. Pharmacol. Biochem. Behav. 1996, 55, 1–10. [Google Scholar] [CrossRef]
- Sun, H.-L.; Zheng, J.-W.; Wang, K.; Liu, R.-K.; Liang, J.-H. Tramadol reduces the 5-HTP-induced head–twitch response in mice via the activation of μ and κ opioid receptors. Life Sci. 2003, 72, 1221–1230. [Google Scholar] [CrossRef]
- Devi, M.; Sharma, R. Antidepressant activity of aqueous extract of Phaseolus vulgaris (black bean) in rodent models of dep ression. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 118–124. [Google Scholar] [CrossRef]
- Zhuk, O.; Jasicka-Misiak, I.; Poliwoda, A.; Kazakova, A.; Godovan, V.V.; Halama, M.; Wieczorek, P.P. Research on acute toxicity and the behavioral effects of methanolic extract from psilocybin mushrooms and psilocin in mice. Toxins 2015, 7, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente Revenga, M.; Shah, U.H.; Nassehi, N.; Jaster, A.M.; Hemanth, P.; Sierra, S.; Dukat, M.; Gonzalez-Maeso, J. Psychedelic-like Properties of Quipazine and Its Structural Analogues in Mice. ACS Chem. Neurosci. 2021, 12, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.; Babler, A.; Vogel, H.; Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef]
- Olbrich, S.; Preller, K.H.; Vollenweider, F.X. LSD and ketanserin and their impact on the human autonomic nervous system. Psychophysiology 2021, 58, e13822. [Google Scholar] [CrossRef]
- Pokorny, T.; Preller, K.H.; Kraehenmann, R.; Vollenweider, F.X. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur. Neuropsychopharmacol. 2016, 26, 756–766. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B.; Thompson, S.M. Harnessing psilocybin: Antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc. Natl. Acad Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef]
- Custodio, R.J.P.; Ortiz, D.M.; Lee, H.J.; Sayson, L.V.; Buctot, D.; Kim, M.; Lee, Y.S.; Kim, K.-M.; Cheong, J.H.; Kim, H.J. 5-HT2CR is as important as 5-HT2AR in inducing hallucinogenic effects in serotonergic compounds. iScience 2022, unpublished. [Google Scholar] [CrossRef]
- Brown, R.T.; Nicholas, C.R.; Cozzi, N.V.; Gassman, M.C.; Cooper, K.M.; Muller, D.; Thomas, C.D.; Hetzel, S.J.; Henriquez, K.M.; Ribaudo, A.S.; et al. Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clin. Pharmacokinet. 2017, 56, 1543–1554. [Google Scholar] [CrossRef]
- van Amsterdam, J.; van den Brink, W. The therapeutic potential of psilocybin: A systematic review. Expert Opin. Drug Saf. 2022, 21, 833–840. [Google Scholar] [CrossRef]
- Janhavi, P.; Divyashree, S.; Sanjailal, K.P.; Muthukumar, S.P. DoseCal: A virtual calculator for dosage conversion between human and different animal species. Arch. Physiol. Biochem. 2022, 128, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Geyer, M.A. Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology 2013, 227, 727–739. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente Revenga, M.; Vohra, H.Z.; Gonzalez-Maeso, J. Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection. J. Neurosci. Methods 2020, 334, 108595. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente Revenga, M.; Shin, J.M.; Vohra, H.Z.; Hideshima, K.S.; Schneck, M.; Poklis, J.L.; Gonzalez-Maeso, J. Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo. Sci. Rep. 2019, 9, 14247. [Google Scholar] [CrossRef]
- Glatfelter, G.C.; Chojnacki, M.R.; McGriff, S.A.; Wang, T.; Baumann, M.H. Automated Computer Software Assessment of 5-Hydroxytryptamine 2A Receptor-Mediated Head Twitch Responses from Video Recordings of Mice. ACS Pharmacol. Transl. Sci. 2022, 5, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Khumnark, M.; Hines, R.M.; Hines, D.J. Behavioral arrest and a characteristic slow waveform are hallmark responses to selective 5-HT2A receptor activation. Sci. Rep. 2021, 11, 1925. [Google Scholar] [CrossRef]
- Corne, S.; Pickering, R. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 1967, 11, 65–78. [Google Scholar] [CrossRef]
- Turner, E.H.; Loftis, J.M.; Blackwell, A.D. Serotonin a la carte: Supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol. Ther. 2006, 109, 325–338. [Google Scholar] [CrossRef]
- Evans, C.E.; Sebastian, J. Serotonin syndrome. Emerg. Med. J. 2007, 24, e20. [Google Scholar] [CrossRef]
- Birmes, P.; Coppin, D.; Schmitt, L.; Lauque, D. Serotonin syndrome: A brief review. CMAJ 2003, 168, 1439–1442. [Google Scholar]
- Canal, C.E.; Booth, R.G.; Morgan, D. Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 2013, 70, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Geyer, M.A. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 2014, 77, 200–207. [Google Scholar] [CrossRef]
- Fantegrossi, W.E.; Reissig, C.J.; Katz, E.B.; Yarosh, H.L.; Rice, K.C.; Winter, J.C. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): Possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol. Biochem. Behav. 2008, 88, 358–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Elliott, S.P.; Hoang, K.; Wallach, J.; Halberstadt, A.L. Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test. Anal. 2016, 8, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Jaster, A.M.; Elder, H.; Marsh, S.A.; de la Fuente Revenga, M.; Negus, S.S.; Gonzalez-Maeso, J. Effects of the 5-HT2A receptor antagonist volinanserin on head-twitch response and intracranial self-stimulation depression induced by different structural classes of psychedelics in rodents. Psychopharmacology 2022, 239, 1665–1677. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Nadkarni, V.; Means, C.R.; Chiu, Y.-T.; Roth, B.L.; Wetsel, W.C. LSD’s effects are differentially modulated in arrestin knockout mice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Darmani, N.A.; Martin, B.R.; Pandey, U.; Glennon, R.A. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? J. Pharmacol. Biochem. Behav. 1990, 36, 901–906. [Google Scholar] [CrossRef]
- Arnt, J.; Hyttel, J. Facilitation of 8-OHDPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur. J. Pharmacol. 1989, 161, 45–51. [Google Scholar] [CrossRef]
- Canal, C.E.; Olaghere da Silva, U.B.; Gresch, P.J.; Watt, E.E.; Sanders-Bush, E.; Airey, D.C. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology 2010, 209, 163–174. [Google Scholar] [CrossRef]
- Simmler, L.D.; Buchy, D.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J. Pharmacol. Exp. Ther. 2016, 357, 134–144. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; McLaughlin, R.; Maione, S.; Comai, S.; Gobbi, G. The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT1A, D2 and TAAR1 receptors. Pharmacol. Res. 2016, 113 Pt A, 81–91. [Google Scholar] [CrossRef]
- Wallach, J. Endogenous hallucinogens as ligands of the trace amine receptors: A possible role in sensory perception. Med. Hypotheses 2009, 72, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Erkizia-Santamaría, I.; Alles-Pascual, R.; Horrillo, I.; Meana, J.J.; Ortega, J.E. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed. Pharmacother. 2022, 154, 113612. [Google Scholar] [CrossRef] [PubMed]
- Jaster, A.M.; Younkin, J.; Cuddy, T.; de la Fuente Revenga, M.; Poklis, J.L.; Dozmorov, M.G.; González-Maeso, J.J.N.L. Differences across sexes on head-twitch behavior and 5-HT2A receptor signaling in C57BL/6J mice. Neurosci. Lett. 2022, 788, 136836. [Google Scholar] [CrossRef] [PubMed]
- Yaden, D.B.; Griffiths, R.R. The Subjective Effects of Psychedelics Are Necessary for Their Enduring Therapeutic Effects. ACS Pharmacol. Transl. Sci. 2021, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahar, O.; Botvinnik, A.; Esh-Zuntz, N.; Brownstien, M.; Wolf, R.; Lotan, A.; Wolf, G.; Lerer, B.; Lifschytz, T. Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications. Int. J. Mol. Sci. 2022, 23, 14148. https://doi.org/10.3390/ijms232214148
Shahar O, Botvinnik A, Esh-Zuntz N, Brownstien M, Wolf R, Lotan A, Wolf G, Lerer B, Lifschytz T. Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications. International Journal of Molecular Sciences. 2022; 23(22):14148. https://doi.org/10.3390/ijms232214148
Chicago/Turabian StyleShahar, Orr, Alexander Botvinnik, Noam Esh-Zuntz, Michal Brownstien, Rachel Wolf, Amit Lotan, Gilly Wolf, Bernard Lerer, and Tzuri Lifschytz. 2022. "Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications" International Journal of Molecular Sciences 23, no. 22: 14148. https://doi.org/10.3390/ijms232214148
APA StyleShahar, O., Botvinnik, A., Esh-Zuntz, N., Brownstien, M., Wolf, R., Lotan, A., Wolf, G., Lerer, B., & Lifschytz, T. (2022). Role of 5-HT2A, 5-HT2C, 5-HT1A and TAAR1 Receptors in the Head Twitch Response Induced by 5-Hydroxytryptophan and Psilocybin: Translational Implications. International Journal of Molecular Sciences, 23(22), 14148. https://doi.org/10.3390/ijms232214148






