Mycosynthesis of Metal-Containing Nanoparticles—Fungal Metal Resistance and Mechanisms of Synthesis
Abstract
1. Introduction
2. Toxic Effects of Metals and Metal-Based NPs on Fungi
2.1. Effects on Cell Growth
2.2. Effects on a Biochemical Level
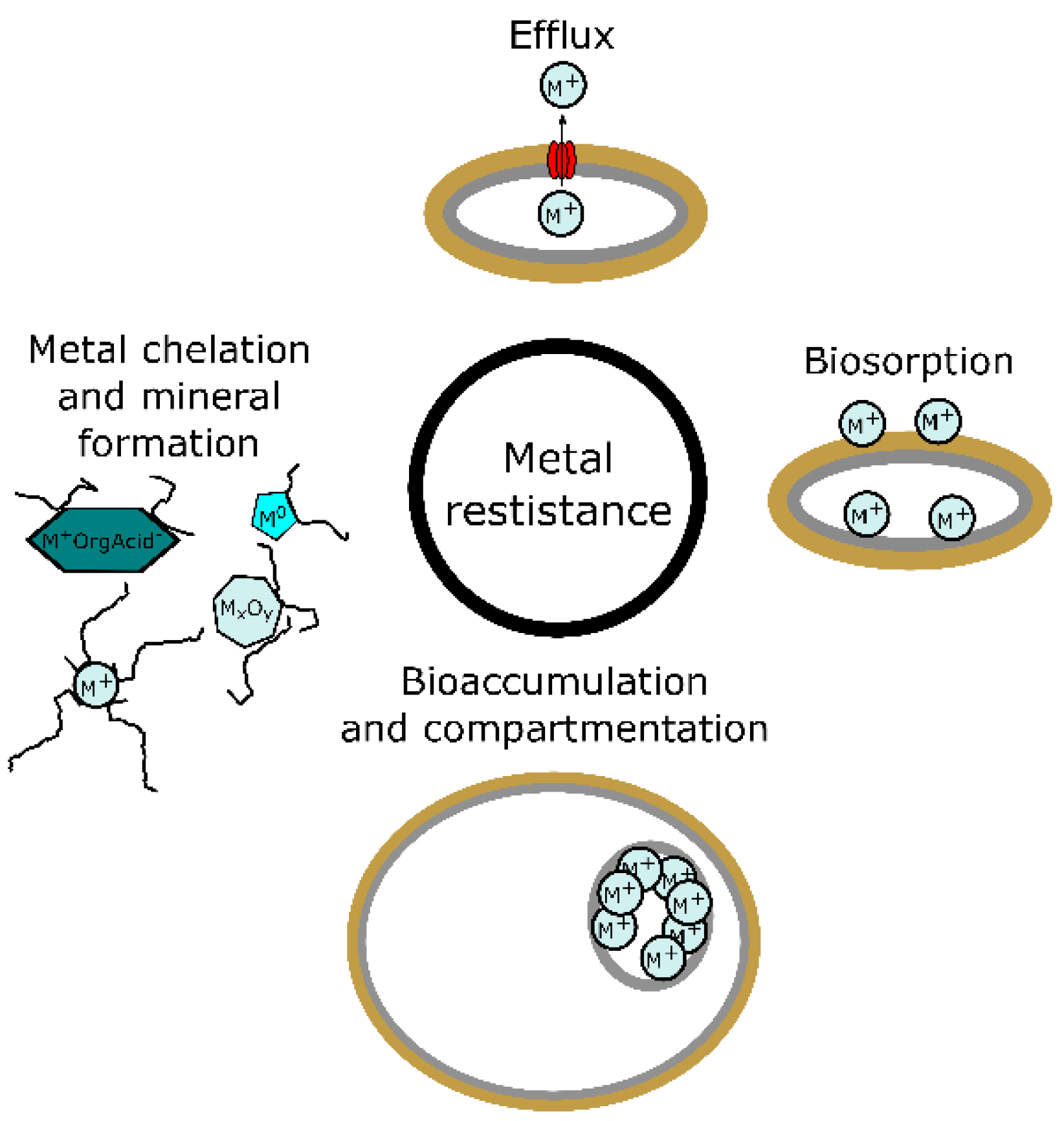
3. Metal Resistance in Fungi
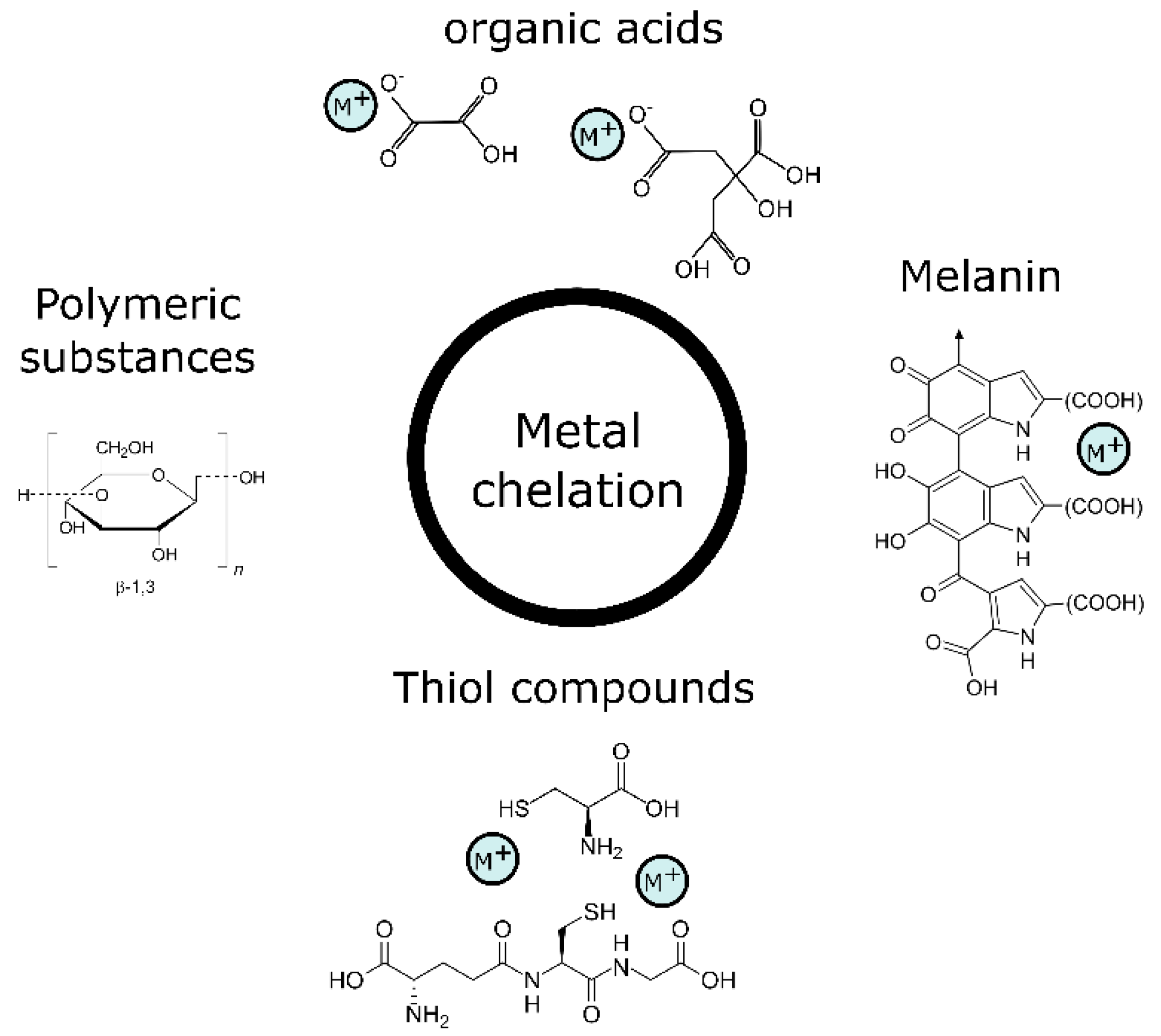
3.1. Metal Chelation and Intra- and Extracellular Mineral Formation
3.1.1. Thiol-Containing Compounds
3.1.2. Polymeric Substances
3.1.3. Melanins
3.1.4. Organic Acids
3.2. Biosorption
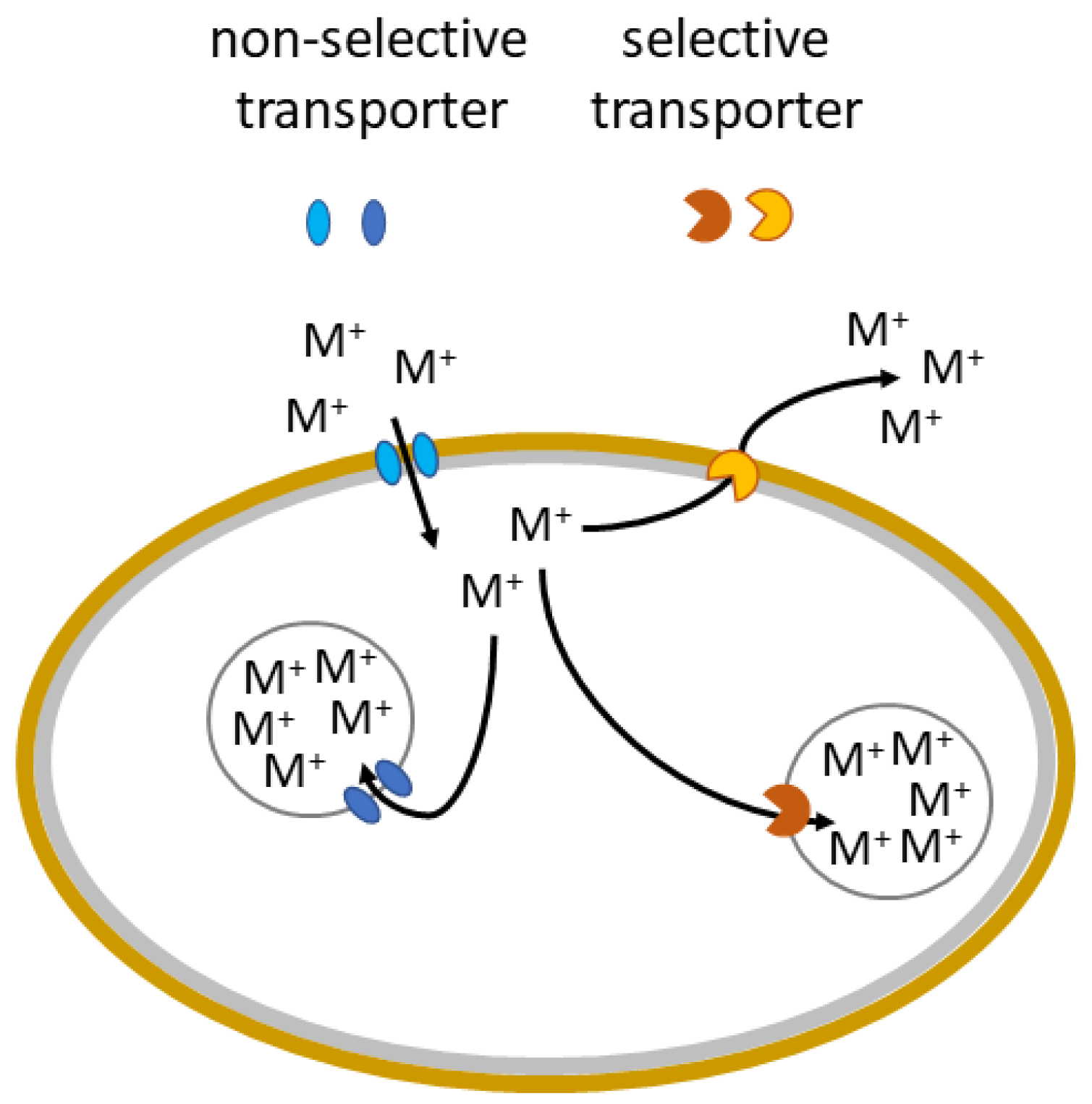
3.3. Bioaccumulation and Compartmentation
3.4. Efflux of Metals
4. Fungal Synthesis of Metal-Containing Nanoparticles
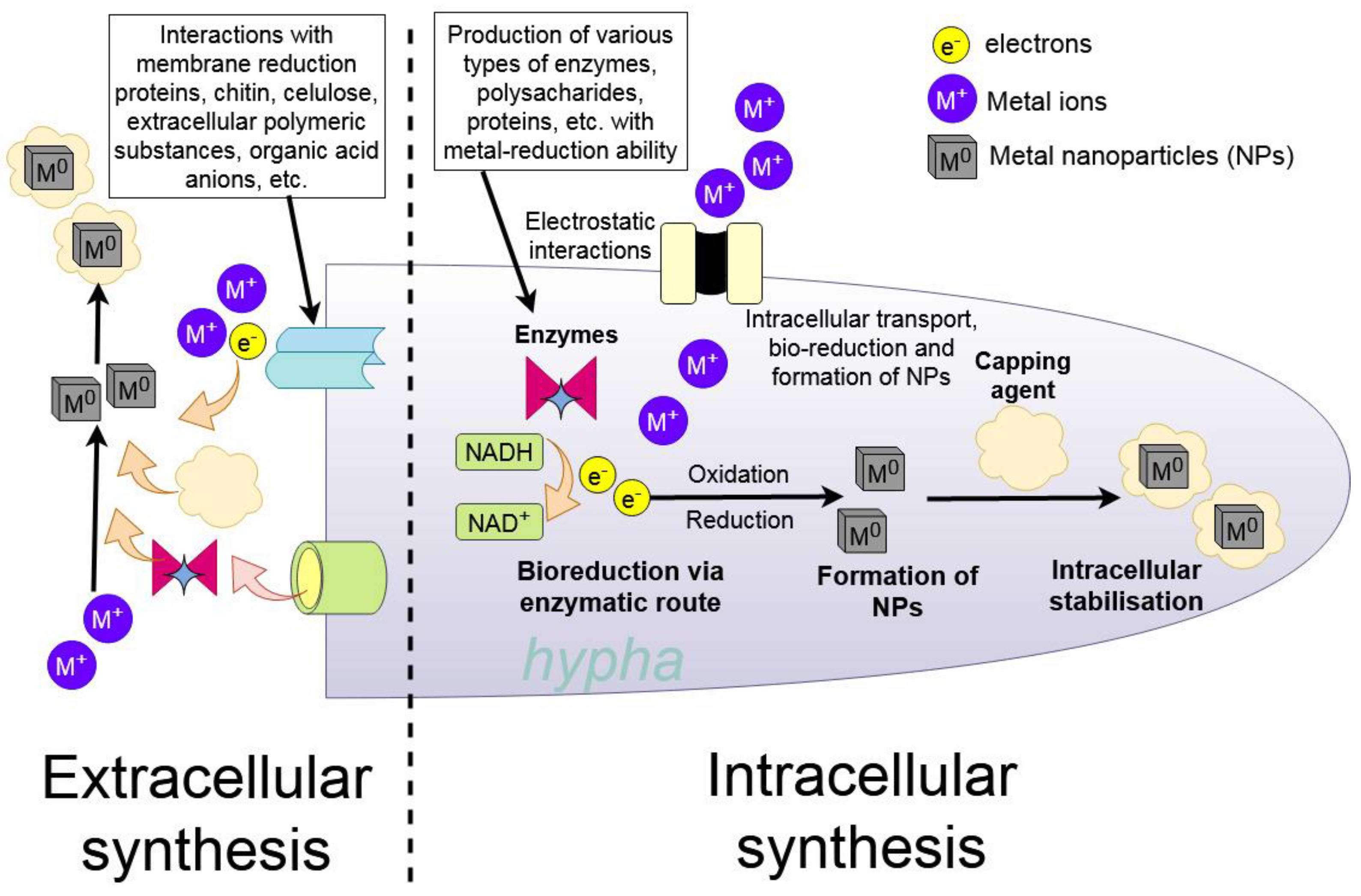
4.1. Mechanisms of Synthesis
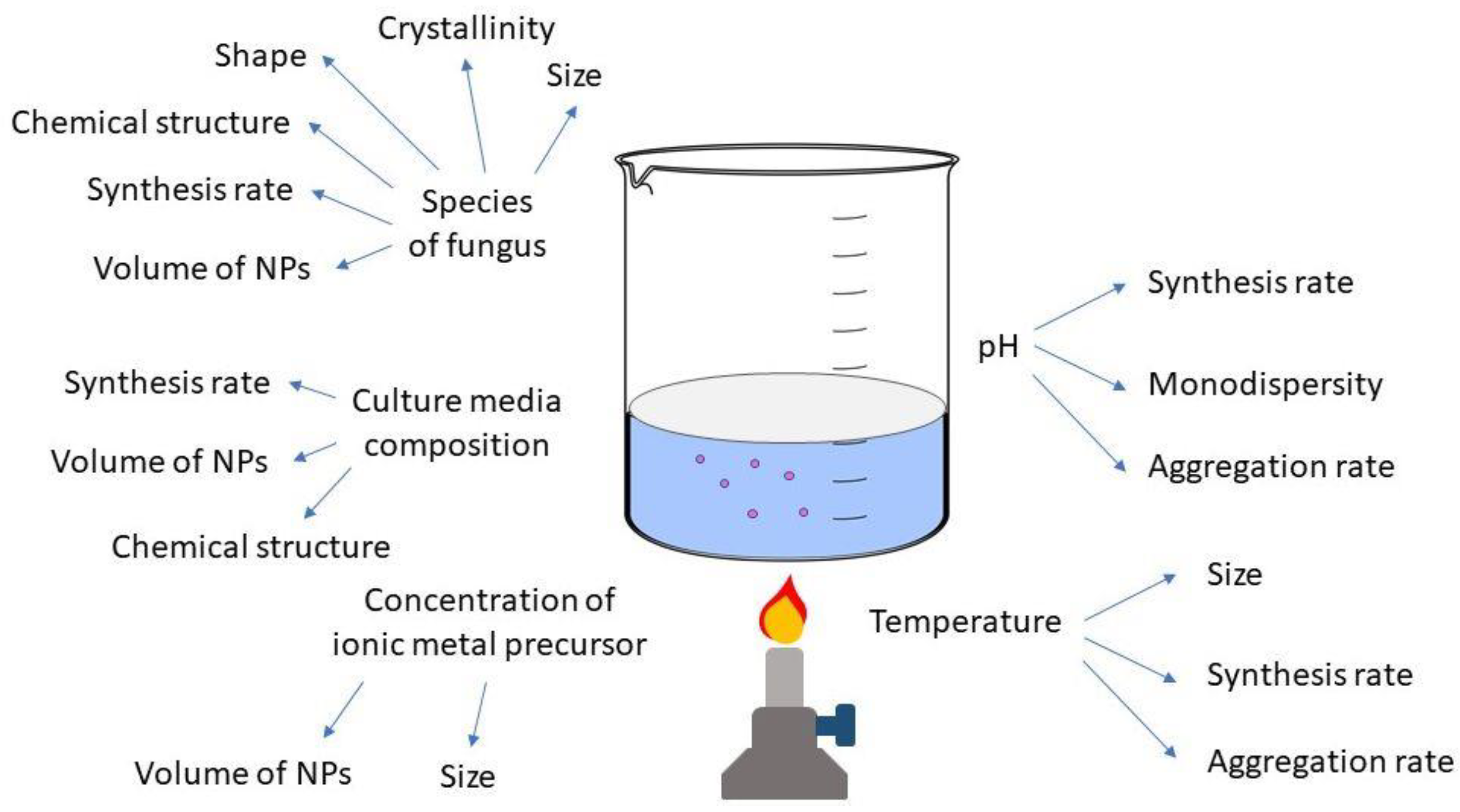
4.2. Factors Affecting Nanoparticle Synthesis
4.2.1. Temperature
4.2.2. pH
4.2.3. Culture Media and Growth Parameters
4.2.4. Metal Precursors
4.2.5. Biomolecules
4.3. Intracellular and Extracellular Synthesis
5. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alavi, M.; Nokhodchi, A. Synthesis and modification of bio-derived antibacterial Ag and ZnO nanoparticles by plants, fungi, and bacteria. Drug Discov. Today 2021, 26, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Urík, M.; Ďurišová, Ľ.; Bujdoš, M.; Šebesta, M.; Dobročka, E.; Kšiňan, S.; Illa, R.; Yu, Q.; et al. Foliar Application of Low Concentrations of Titanium Dioxide and Zinc Oxide Nanoparticles to the Common Sunflower under Field Conditions. Nanomaterials 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Ďurišová, Ľ.; Bujdoš, M.; Černý, I.; Chlpík, J.; Juriga, M.; et al. Effects of Foliar Application of ZnO Nanoparticles on Lentil Production, Stress Level and Nutritional Seed Quality under Field Conditions. Nanomaterials 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, N.; Tatenda, M.; Clemence, M.; Munyengwa, N. Nanotechnology Applications in Crop Production and Food Systems. Int. J. Plant Breed. Crop Sci. 2020, 7, 603–613. [Google Scholar]
- Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-323-99922-9. [Google Scholar]
- Wu, X.; Chen, G.; Shen, J.; Li, Z.; Zhang, Y.; Han, G. Upconversion Nanoparticles: A Versatile Solution to Multiscale Biological Imaging. Bioconjugate Chem. 2015, 26, 166–175. [Google Scholar] [CrossRef]
- Holišová, V.; Urban, M.; Kolenčík, M.; Němcová, Y.; Schröfel, A.; Peikertová, P.; Slabotinský, J.; Kratošová, G. Biosilica-nanogold composite: Easy-to-prepare catalyst for soman degradation. Arab. J. Chem. 2019, 12, 262–271. [Google Scholar] [CrossRef]
- Holišová, V.; Urban, M.; Konvičková, Z.; Kolenčík, M.; Mančík, P.; Slabotinský, J.; Kratošová, G.; Plachá, D. Colloidal stability of phytosynthesised gold nanoparticles and their catalytic effects for nerve agent degradation. Sci. Rep. 2021, 11, 4071. [Google Scholar] [CrossRef]
- Bala, R.; Kalia, A.; Dhaliwal, S.S. Evaluation of Efficacy of ZnO Nanoparticles as Remedial Zinc Nanofertilizer for Rice. J. Soil Sci. Plant Nutr. 2019, 19, 379–389. [Google Scholar] [CrossRef]
- Pištora, J.; Vlček, J.; Lesňák, M.; Blažek, D.; Kolenčík, M. Optical Methods in Diagnostics of Nanostructured Materials, 1st ed.; Akademické nakladatelství CERM: Brno, Czech Republic, 2015. [Google Scholar]
- Illa, R.; Ješko, R.; Silber, R.; Životský, O.; Kutláková, K.M.; Matějová, L.; Kolenčík, M.; Pištora, J.; Hamrle, J. Structural, magnetic, optical, and magneto-optical properties of CoFe2O4 thin films fabricated by a chemical approach. Mater. Res. Bull. 2019, 117, 96–102. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Konvičková, Z.; Schröfel, A.; Kolenčík, M.; Dědková, K.; Peikertová, P.; Žídek, M.; Seidlerová, J.; Kratošová, G. Antimicrobial bionanocomposite–from precursors to the functional material in one simple step. J. Nanoparticle Res. 2016, 18, 368. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2011, 32, 49–73. [Google Scholar] [CrossRef]
- Horváthová, H.; Dercová, K.; Tlčíková, M.; Hurbanová, M. Biological Synthesis of Nanoparticles: Iron-based Plant Bionanoparticles and Their Use for Remediation of the Contaminated Environment. Chem. Listy 2022, 116, 405–415. [Google Scholar] [CrossRef]
- Sastry, M.; Ahmad, A.; Khan, M.I. Biosynthesis of Metal Nanoparticles Using Fungi and Actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Element Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kon, K.; Kratošová, G.; Durán, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Šebesta, M.; Urík, M.; Bujdoš, M.; Kolenčík, M.; Vávra, I.; Dobročka, E.; Kim, H.; Matúš, P. Fungus Aspergillus niger Processes Exogenous Zinc Nanoparticles into a Biogenic Oxalate Mineral. J. Fungi 2020, 6, 210. [Google Scholar] [CrossRef]
- Gadd, G.M. Mycotransformation of organic and inorganic substrates. Mycologist 1999, 18, 60–70. [Google Scholar] [CrossRef]
- Kang, X.; Csetenyi, L.; Gadd, G.M. Colonization and bioweathering of monazite by Aspergillus niger: Solubilization and precipitation of rare earth elements. Environ. Microbiol. 2021, 23, 3970–3986. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Urík, M.; Štubna, J. Heterotrophic Leaching and Its Application in Biohydrometallurgy. Chem. Listy 2014, 108, 1040–1045. [Google Scholar]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Ramani, R.; Pasricha, R.; Ajayakumar, P.V.; Alam, M.; et al. Bioreduction of AuCl4−Ions by the Fungus, Verticillium sp. and Surface Trapping of the Gold Nanoparticles Formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Silva, T.; Cardoso, J.; Albuquerque-Júnior, R.; Zielinska, A.; Souto, E.; Severino, P.; Mendonça, M. Biosynthesis of Silver Nanoparticles Mediated by Entomopathogenic Fungi: Antimicrobial Resistance, Nanopesticides, and Toxicity. Antibiotics 2021, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; El-Sayed, E.-S.R.; Mohamed, S.S.; El-Seoud, M.A.A.; Elmehlawy, A.A.; Abdou, D.A.M. Novel mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles by the endophytic Aspergillus terreus and evaluation of their antioxidant and antimicrobial activities. Appl. Microbiol. Biotechnol. 2021, 105, 741–753. [Google Scholar] [CrossRef]
- Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Synthesis of Gold Nanoparticles by the Fungus Fusarium oxysporum. ChemBioChem 2002, 3, 461–463. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Sood, R.; Chopra, D.S. Metal–plant frameworks in nanotechnology: An overview. Phytomedicine 2017, 50, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Hariram, M.; Vivekanandhan, S. Phytochemical Process for the Functionalization of Materials with Metal Nanoparticles: Current Trends and Future Perspectives. ChemistrySelect 2018, 3, 13561–13585. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Pradhan, N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol. 2019, 103, 3297–3316. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Ali, N.; Wang, L.; Waseem, H.; Pan, G. Revisiting the mechanistic pathways for bacterial mediated synthesis of noble metal nanoparticles. J. Microbiol. Methods 2019, 159, 18–25. [Google Scholar] [CrossRef]
- Saxena, P. Harish Phyco-Nanotechnology: New Horizons of Gold Nano-Factories. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2016, 89, 1–11. [Google Scholar] [CrossRef]
- Saw, P.E.; Lee, S.; Jon, S. Naturally Occurring Bioactive Compound-Derived Nanoparticles for Biomedical Applications. Adv. Ther. 2019, 2, 1800146. [Google Scholar] [CrossRef]
- An overview on the green synthesis of nanoparticles and other nano-materials using enzymes and their potential applications. Biointerface Res. Appl. Chem. 2019, 9, 4255–4271. [CrossRef]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Prasad, B.N.; Kumar, T.P.; Rajasimman, M.; Hosseini-Bandegharaei, A.; et al. A focus to green synthesis of metal/metal based oxide nanoparticles: Various mechanisms and applications towards ecological approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Berta, L.; Coman, N.-A.; Rusu, A.; Tanase, C. A Review on Plant-Mediated Synthesis of Bimetallic Nanoparticles, Characterisation and Their Biological Applications. Materials 2021, 14, 7677. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Elzaki, A.; Tirth, V.; Kajoak, S.; Osman, H.; Algahtani, A.; Islam, S.; Faizo, N.L.; Khandaker, M.U.; Islam, M.N.; et al. Biological Synthesis of Nanocatalysts and Their Applications. Catalysts 2021, 11, 1494. [Google Scholar] [CrossRef]
- Agrawal, K.; Gupta, V.K.; Verma, P. Microbial cell factories a new dimension in bio-nanotechnology: Exploring the robustness of nature. Crit. Rev. Microbiol. 2021, 48, 397–427. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Abdal-Hay, A.; Rather, S.-U.; Macossay, J.; Sheikh, F.A. Recent progress in the green fabrication of cadmium sulfide and cadmium oxide nanoparticles: Synthesis, antimicrobial and cytotoxic studies. Mater. Sci. Eng. B 2022, 286, 116022. [Google Scholar] [CrossRef]
- Vargas, G.; Cypriano, J.; Correa, T.; Leão, P.; Bazylinski, D.A.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef]
- Timoszyk, A. A review of the biological synthesis of gold nanoparticles using fruit extracts: Scientific potential and application. Bull. Mater. Sci. 2018, 41, 154. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Mantravadi, K.M.; Rao, G.V.S.N. Strategies to synthesise copper oxide nanoparticles and their bio applications—A review. Mater. Sci. Technol. 2018, 34, 2214–2222. [Google Scholar] [CrossRef]
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Appl. Microbiol. Biotechnol. 2019, 103, 2551–2569. [Google Scholar] [CrossRef]
- Puja, P.; Kumar, P. A perspective on biogenic synthesis of platinum nanoparticles and their biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 211, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, M.; Kannappan, S. An overview of a sustainable approach to the biosynthesis of AgNPs for electrochemical sensors. Arab. J. Chem. 2022, 15, 104324. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Hembram, K.C.; Kumar, R.; Kandha, L.; Parhi, P.; Kundu, C.N.; Bindhani, B.K. Therapeutic prospective of plant-induced silver nanoparticles: Application as antimicrobial and anticancer agent. Artif. Cells Nanomed. Biotechnol. 2018, 46, S38–S51. [Google Scholar] [CrossRef]
- Nandhini, N.; Rajeshkumar, S.; Mythili, S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol. 2019, 19, 101138. [Google Scholar] [CrossRef]
- Andra, S.; Balu, S.K.; Jeevanandham, J.; Muthalagu, M.; Vidyavathy, M.; Chan, Y.S.; Danquah, M.K. Phytosynthesized metal oxide nanoparticles for pharmaceutical applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 755–771. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G. New frontiers in the biosynthesis of metal oxide nanoparticles and their environmental applications: An overview. SN Appl. Sci. 2019, 1, 928. [Google Scholar] [CrossRef]
- Castillo-Henriquez, L.; Alfaro-Aguilar, K.; Ugalde-Alvarez, J.; Vega-Fernandez, L.; Montes de Oca-Vasquez, G.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Zare, E.N.; Padil, V.V.; Mokhtari, B.; Venkateshaiah, A.; Wacławek, S.; Černík, M.; Tay, F.R.; Varma, R.S.; Makvandi, P. Advances in biogenically synthesized shaped metal- and carbon-based nanoarchitectures and their medicinal applications. Adv. Colloid Interface Sci. 2020, 283, 102236. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Hanafy, M.H. Myconanotechnology in veterinary sector: Status quo and future perspectives. Int. J. Vet. Sci. Med. 2018, 6, 270–273. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostructure Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Chauhan, A.; Anand, J.; Parkash, V.; Rai, N. Biogenic synthesis: A sustainable approach for nanoparticles synthesis mediated by fungi. Inorg. Nano-Metal Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Owaid, M.N.; Ibraheem, I. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Guilger Casagrande, M.; De Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2017, 41, 1–20. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Graz, M.; Pawlikowska-Pawlęga, B.; Jarosz-Wilkołazka, A. Growth inhibition and intracellular distribution of Pb ions by the white-rot fungus Abortiporus biennis. Int. Biodeterior. Biodegrad. 2011, 65, 124–129. [Google Scholar] [CrossRef]
- Liaquat, F.; Munis, M.F.H.; Haroon, U.; Arif, S.; Saqib, S.; Zaman, W.; Khan, A.R.; Shi, J.; Che, S.; Liu, Q. Evaluation of Metal Tolerance of Fungal Strains Isolated from Contaminated Mining Soil of Nanjing, China. Biology 2020, 9, 469. [Google Scholar] [CrossRef]
- Rose, P.K.; Devi, R. Heavy metal tolerance and adaptability assessment of indigenous filamentous fungi isolated from industrial wastewater and sludge samples. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 688–694. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Van Assche, J.A. The effects of cadmium and the cadmium-zinc interaction on the axenic growth of ectomycorrhizal fungi. Plant Soil 1992, 145, 237–243. [Google Scholar] [CrossRef]
- Traxler, L.; Shrestha, J.; Richter, M.; Krause, K.; Schäfer, T.; Kothe, E. Metal adaptation and transport in hyphae of the wood-rot fungus Schizophyllum commune. J. Hazard. Mater. 2021, 425, 127978. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Barros, D.; Pradhan, A.; Pascoal, C.; Cássio, F. Proteomic responses to silver nanoparticles vary with the fungal ecotype. Sci. Total Environ. 2020, 704, 135385. [Google Scholar] [CrossRef]
- Sun, M.; Yu, Q.; Hu, M.; Hao, Z.; Zhang, C.; Li, M. Lead sulfide nanoparticles increase cell wall chitin content and induce apoptosis in Saccharomyces cerevisiae. J. Hazard. Mater. 2014, 273, 7–16. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Mohan, P.M.; Sastry, K.S. Excretion of pyruvate in nickel toxicity in wild type and Ni2+ resistant mutants of Neurospora crassa. J. Biosci. 1984, 6, 283–288. [Google Scholar] [CrossRef]
- Ramadan, S.E.; Razak, A.A.; Soliman, H.G. Influence of cadmium on certain biological activities in a cadmium-tolerant fungi. Biol. Trace Element Res. 1988, 18, 179–190. [Google Scholar] [CrossRef]
- Faller, P.; Kienzler, K.; Krieger-Liszkay, A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochim. Biophys. Acta 2005, 1706, 158–164. [Google Scholar] [CrossRef]
- Hartwig, A. Zinc Finger Proteins as Potential Targets for Toxic Metal Ions: Differential Effects on Structure and Function. Antioxid. Redox Signal. 2001, 3, 625–634. [Google Scholar] [CrossRef]
- Jin, Y.H.; Clark, A.B.; Slebos, R.J.C.; Al-Refai, H.; Taylor, J.; Kunkel, T.; Resnick, M.; Gordenin, D.A. Cadmium is a mutagen that acts by inhibiting mismatch repair. Nat. Genet. 2003, 34, 326–329. [Google Scholar] [CrossRef]
- Naganuma, A.; Miura, N.; Kaneko, S.; Mishina, T.; Hosoya, S.; Miyairi, S.; Furuchi, T.; Kuge, S. GFAT as a target molecule of methylmercury toxicity in Saccharomyces cerevisiae. FASEB J. 2000, 14, 968–972. [Google Scholar] [CrossRef]
- Sharma, S.K.; Goloubinoff, P.; Christen, P. Heavy metal ions are potent inhibitors of protein folding. Biochem. Biophys. Res. Commun. 2008, 372, 341–345. [Google Scholar] [CrossRef]
- Robinson, J.; Isikhuemhen, O.; Anike, F. Fungal–Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zeng, G.; Chen, G.; Yan, M.; Chen, A.; Du, J.; Huang, J.; Yi, B.; Zhou, Y.; He, X.; et al. The Effect of Heavy Metal-Induced Oxidative Stress on the Enzymes in White Rot Fungus Phanerochaete chrysosporium. Appl. Biochem. Biotechnol. 2014, 175, 1281–1293. [Google Scholar] [CrossRef]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; Alnadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef] [PubMed]
- García-Saucedo, C.; Field, J.A.; Otero-Gonzalez, L.; Sierra-Álvarez, R. Low toxicity of HfO2, SiO2, Al2O3 and CeO2 nanoparticles to the yeast, Saccharomyces cerevisiae. J. Hazard. Mater. 2011, 192, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Otero-González, L.; García-Saucedo, C.; Field, J.A.; Sierra-Álvarez, R. Toxicity of TiO2, ZrO2, Fe0, Fe2O3, and Mn2O3 nanoparticles to the yeast, Saccharomyces cerevisiae. Chemosphere 2013, 93, 1201–1206. [Google Scholar] [CrossRef]
- Ezzouhri, L.; Castro, E.; Moya, M.; Espinola, F.; Lairini, K. Heavy Metal Tolerance of Filamentous Fungi Isolated from Polluted Sites in Tangier, Morocco. Afr. J. Microbiol. Res. 2009, 3, 35–48. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Vandenkoornhuyse, P.; Adriaensen, K.; Vangronsveld, J. Genetic variation and heavy metal tolerance in the ectomycorrhizal basidiomycete Suillus Luteus. New Phytol. 2000, 147, 367–379. [Google Scholar] [CrossRef]
- Howe, R.; Evans, R.L.; Ketteridge, S.W. Copper-binding proteins in ectomycorrhizal fungi. New Phytol. 1997, 135, 123–131. [Google Scholar] [CrossRef]
- Baldrian, P.; Gabriel, J. Intraspecific Variability in Growth Response to Cadmium of the Wood-Rotting Fungus Piptoporus Betulinus. Mycologia 2002, 94, 428–436. [Google Scholar] [CrossRef]
- Canovas, D.; Vooijs, R.; Schat, H.; de Lorenzo, V. The Role of Thiol Species in the Hypertolerance of Aspergillus sp. P37 to Arsenic. J. Biol. Chem. 2004, 279, 51234–51240. [Google Scholar] [CrossRef]
- Geetha, N.; Bhavya, G.; Abhijith, P.; Shekhar, R.; Dayananda, K.; Jogaiah, S. Insights into nanomycoremediation: Secretomics and mycogenic biopolymer nanocomposites for heavy metal detoxification. J. Hazard. Mater. 2021, 409, 124541. [Google Scholar] [CrossRef]
- Ott, T.; Fritz, E.; Polle, A.; Schützendübel, A. Characterisation of antioxidative systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol. Ecol. 2002, 42, 359–366. [Google Scholar] [CrossRef]
- Courbot, M.; Diez, L.; Ruotolo, R.; Chalot, M.; Leroy, P. Cadmium-Responsive Thiols in the Ectomycorrhizal Fungus Paxillus involutus. Appl. Environ. Microbiol. 2004, 70, 7413–7417. [Google Scholar] [CrossRef]
- Su, Z.; Zeng, Y.; Li, X.; Perumal, A.B.; Zhu, J.; Lu, X.; Dai, M.; Liu, X.; Lin, F. The Endophytic Fungus Piriformospora Indica-Assisted Alleviation of Cadmium in Tobacco. J. Fungi 2021, 7, 675. [Google Scholar] [CrossRef]
- Morselt, A.F.W.; Smits, W.T.M.; Limonard, T. Histochemical demonstration of heavy metal tolerance in ectomycorrhizal fungi. Plant Soil 1986, 96, 417–420. [Google Scholar] [CrossRef]
- Leonhardt, T.; Sácký, J.; Šimek, P.; Šantrůček, J.; Kotrba, P. Metallothionein-like peptides involved in sequestration of Zn in the Zn-accumulating ectomycorrhizal fungus Russula atropurpurea. Metallomics 2014, 6, 1693–1701. [Google Scholar] [CrossRef]
- Sardar, U.R.; Bhargavi, E.; Devi, I.; Bhunia, B.; Tiwari, O.N. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018, 199, 353–364. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Jin, Y.; Zhao, X.; Cai, Z. Adsorption of Pb(II), Cd(II) and Zn(II) by extracellular polymeric substances extracted from aerobic granular sludge: Efficiency of protein. J. Environ. Chem. Eng. 2015, 3, 1223–1232. [Google Scholar] [CrossRef]
- Dang, C.; Yang, Z.; Liu, W.; Du, P.; Cui, F.; He, K. Role of extracellular polymeric substances in biosorption of Pb2+ by a high metal ion tolerant fungal strain Aspergillus niger PTN31. J. Environ. Chem. Eng. 2018, 6, 2733–2742. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Noguera, D.; Zhao, N.; Song, Y.; Ding, J.; Zhao, Q.; Cui, F. Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. J. Hazard. Mater. 2017, 321, 473–483. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Vacchina, V.; Baldrián, P.; Gabriel, J.; Szpunar, J. Investigation of the response of wood-rotting fungi to copper stress by size-exclusion chromatography and capillary zone electrophoresis with ICP MS detection. Anal. Bioanal. Chem. 2001, 372, 453–456. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Yang, R.; Wu, L. Distribution, characteristics of extracellular polymeric substances of Phanerochaete chrysosporium under lead ion stress and the influence on Pb removal. Sci. Rep. 2020, 10, 17633. [Google Scholar] [CrossRef]
- Suh, J.H.; Yun, J.W.; Kim, D.S. Effect of extracellular polymeric substances (EPS) on Pb2+ accumulation by Aureobasidium pullulans. Bioprocess Biosyst. Eng. 1999, 21, 1–4. [Google Scholar] [CrossRef]
- Cao, F.; Bourven, I.; Guibaud, G.; Rene, E.R.; Lens, P.N.; Pechaud, Y.; van Hullebusch, E.D. Alteration of the characteristics of extracellular polymeric substances (EPS) extracted from the fungus Phanerochaete chrysosporium when exposed to sub-toxic concentrations of nickel (II). Int. Biodeterior. Biodegrad. 2018, 129, 179–188. [Google Scholar] [CrossRef]
- Mattoon, E.; Cordero, R.; Casadevall, A. Fungal Melanins and Applications in Healthcare, Bioremediation and Industry. J. Fungi 2021, 7, 488. [Google Scholar] [CrossRef]
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzym. Microb. Technol. 1996, 19, 311–317. [Google Scholar] [CrossRef]
- Liu, R.; Meng, X.; Mo, C.; Wei, X.; Ma, A. Melanin of fungi: From classification to application. World J. Microbiol. Biotechnol. 2022, 38, 228. [Google Scholar] [CrossRef]
- García-Rivera, J.; Casadevall, A. Melanization of Cryptococcus neoformans reduces its susceptibility to the antimicrobial effects of silver nitrate. Med. Mycol. 2001, 39, 353–357. [Google Scholar] [CrossRef]
- Gadd, G.M.; De Rome, L. Biosorption of copper by fungal melanin. Appl. Microbiol. Biotechnol. 1988, 29, 610–617. [Google Scholar] [CrossRef]
- Berthelot, C.; Zegeye, A.; Gaber, D.A.; Chalot, M.; Franken, P.; Kovács, G.M.; Leyval, C.; Blaudez, D. Unravelling the Role of Melanin in Cd and Zn Tolerance and Accumulation of Three Dark Septate Endophytic Species. Microorganisms 2020, 8, 537. [Google Scholar] [CrossRef]
- Oh, J.-J.; Kim, J.Y.; Kim, Y.J.; Kim, S.; Kim, G.-H. Utilization of extracellular fungal melanin as an eco-friendly biosorbent for treatment of metal-contaminated effluents. Chemosphere 2021, 272, 129884. [Google Scholar] [CrossRef]
- Gadd, G.M. (Ed.) Fungi in Biogeochemical Cycles; Cambridge University Press: Cambridge, UK, 2006; ISBN 9780511550522. [Google Scholar]
- Polák, F.; Urík, M.; Bujdoš, M.; Uhlík, P.; Matúš, P. Evaluation of aluminium mobilization from its soil mineral pools by simultaneous effect of Aspergillus strains’ acidic and chelating exometabolites. J. Inorg. Biochem. 2018, 181, 162–168. [Google Scholar] [CrossRef]
- Sayer, J.A.; Gadd, G.M. Solubilization and transformation of insoluble inorganic metal compounds to insoluble metal oxalates by Aspergillus niger. Mycol. Res. 1997, 101, 653–661. [Google Scholar] [CrossRef]
- Sazanova, K.; Osmolovskaya, N.; Schiparev, S.; Yakkonen, K.; Kuchaeva, L.; Vlasov, D. Organic Acids Induce Tolerance to Zinc- and Copper-Exposed Fungi Under Various Growth Conditions. Curr. Microbiol. 2014, 70, 520–527. [Google Scholar] [CrossRef]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of Oxalic Acid Overexcretion in Transformations of Toxic Metal Minerals by Beauveria caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef]
- Ge, W.; Zamri, D.; Mineyama, H.; Valix, M. Bioaccumulation of heavy metals on adapted Aspergillus foetidus. Adsorption 2011, 17, 901–910. [Google Scholar] [CrossRef]
- Magyarosy, A.; Laidlaw, R.; Kilaas, R.; Echer, C.; Clark, D.; Keasling, J. Nickel accumulation and nickel oxalate precipitation by Aspergillus niger. Appl. Microbiol. Biotechnol. 2002, 59, 382–388. [Google Scholar] [CrossRef]
- Jarosz-Wilkolazka, A.; Gadd, G.M. Oxalate production by wood-rotting fungi growing in toxic metal-amended medium. Chemosphere 2003, 52, 541–547. [Google Scholar] [CrossRef]
- Tang, J.D.; Parker, L.A.; Perkins, A.D.; Sonstegard, T.S.; Schroeder, S.G.; Nicholas, D.D.; Diehl, S.V. Gene Expression Analysis of Copper Tolerance and Wood Decay in the Brown Rot Fungus Fibroporia radiculosa. Appl. Environ. Microbiol. 2013, 79, 1523–1533. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of heavy metals: Processes, mechanisms, and affecting factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412. [Google Scholar] [CrossRef]
- Goyal, N.; Jain, S.; Banerjee, U. Comparative studies on the microbial adsorption of heavy metals. Adv. Environ. Res. 2003, 7, 311–319. [Google Scholar] [CrossRef]
- Tan, T.; Cheng, P. Biosorption of Metal Ions with Penicillium chrysogenum. Appl. Biochem. Biotechnol. 2003, 104, 119–128. [Google Scholar] [CrossRef]
- Zapotoczny, S.; Jurkiewicz, A.; Tylko, G.; Anielska, T.; Turnau, K. Accumulation of copper by Acremonium pinkertoniae, a fungus isolated from industrial wastes. Microbiol. Res. 2007, 162, 219–228. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Melville, L.H.; Ferrol, N.; Lott, J.N.; Azcón-Aguilar, C.; Peterson, R.L. Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can. J. Microbiol. 2008, 54, 103–110. [Google Scholar] [CrossRef]
- Wang, H.-R.; Zhao, X.-Y.; Zhang, J.-M.; Lu, C.; Feng, F.-J. Arbuscular mycorrhizal fungus regulates cadmium accumulation, migration, transport, and tolerance in Medicago sativa. J. Hazard. Mater. 2022, 435, 129077. [Google Scholar] [CrossRef]
- Boriová, K.; Čerňanský, S.; Matúš, P.; Bujdoš, M.; Šimonovičová, A. Bioaccumulation and biovolatilization of various elements using filamentous fungus Scopulariopsis brevicaulis. Lett. Appl. Microbiol. 2014, 59, 217–223. [Google Scholar] [CrossRef]
- Sintuprapa, W.; Thiravetyan, P.; Tanticharoen, M. A possible mechanism of Zn2+ uptake by living cells of Penicillium sp. Biotechnol. Lett. 2000, 22, 1709–1712. [Google Scholar] [CrossRef]
- Lapinskas, P.J.; Lin, S.-J.; Culotta, V.C. The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol. Microbiol. 1996, 21, 519–528. [Google Scholar] [CrossRef]
- Schmidt, K.; Wolfe, D.M.; Stiller, B.; Pearce, D.A. Cd2+, Mn2+, Ni2+ and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem. Biophys. Res. Commun. 2009, 383, 198–202. [Google Scholar] [CrossRef]
- Devirgiliis, C.; Murgia, C.; Danscher, G.; Perozzi, G. Exchangeable zinc ions transiently accumulate in a vesicular compartment in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2004, 323, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.; Pradhan, A.; Pascoal, C.; Cássio, F. Transcriptomics reveals the action mechanisms and cellular targets of citrate-coated silver nanoparticles in a ubiquitous aquatic fungus. Environ. Pollut. 2020, 268, 115913. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Giri, R.; Sharma, R. Lead, cadmium and nickel removal efficiency of white-rot fungus Phlebia brevispora. Lett. Appl. Microbiol. 2020, 71, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Ruytinx, J.; Nguyen, H.; Van Hees, M.; De Beeck, M.O.; Vangronsveld, J.; Carleer, R.; Colpaert, J.V.; Adriaensen, K. Zinc export results in adaptive zinc tolerance in the ectomycorrhizal basidiomycete Suillus bovinus. Metallomics 2013, 5, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Blaudez, D.; Botton, B.; Chalot, M. Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 2000, 146, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M.; Courbot, M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Logeman, B.L.; Thiele, D.J. Copper Acquisition and Utilization in Fungi. Annu. Rev. Microbiol. 2017, 71, 597–623. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; He, D.; Fang, X.; Xu, J.; Lee, Y.-W.; Keller, N.P.; Shi, J. Copper Tolerance Mediated by FgAceA and FgCrpA in Fusarium graminearum. Front. Microbiol. 2020, 11, 1392. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, D.; Mondal, S.K.; Biswas, R.; Das, T.K.; Boujedaini, N.; Khuda-Bukhsh, A.R. Tolerance of arsenate-induced stress in Aspergillus niger, a possible candidate for bioremediation. Ecotoxicol. Environ. Saf. 2010, 73, 172–182. [Google Scholar] [CrossRef]
- Sharples, J.M.; Meharg, A.A.; Chambers, S.M.; Cairney, J.W. Mechanism of Arsenate Resistance in the Ericoid Mycorrhizal Fungus Hymenoscyphus ericae. Plant Physiol. 2000, 124, 1327–1334. [Google Scholar] [CrossRef]
- Culotta, V.C.; Yang, M.; Hall, M.D. Manganese Transport and Trafficking: Lessons Learned from Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1159–1165. [Google Scholar] [CrossRef]
- Farcasanu, I.C.; Mizunuma, M.; Hirata, D.; Miyakawa, T. Involvement of histidine permease (Hip1p) in manganese transport in Saccharomyces cerevisiae. Mol. Gen. Genet. 1998, 259, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Diss, L.; Blaudez, D.; Gelhaye, E.; Chalot, M. Genome-wide analysis of fungal manganese transporters, with an emphasis on Phanerochaete chrysosporium. Environ. Microbiol. Rep. 2011, 3, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Antsotegi-Uskola, M.; Markina-Iñarrairaegui, A.; Ugalde, U. Copper Resistance in Aspergillus nidulans Relies on the PI-Type ATPase CrpA, Regulated by the Transcription Factor AceA. Front. Microbiol. 2017, 8, 912. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2015, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- CRC Concise Encyclopedia of Nanotechnology; Kharisov, B.I., Kharissova, O.V., Ortiz-Mendez, U., Eds.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429103391. [Google Scholar]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B. Microbial synthesis of gold nanoparticles: Current status and future prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef]
- Konvičková, Z.; Holišová, V.; Kolenčík, M.; Niide, T.; Kratošová, G.; Umetsu, M.; Seidlerová, J. Phytosynthesis of colloidal Ag-AgCl nanoparticles mediated by Tilia sp. leachate, evaluation of their behaviour in liquid phase and catalytic properties. Colloid Polym. Sci. 2018, 296, 677–687. [Google Scholar] [CrossRef]
- Principles of Colloid and Surface Chemistry, Revised and Expanded; Hiemenz, P.C., Rajagopalan, R., Eds.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781315274287. [Google Scholar]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized Silver Nanoparticles: A Step Forward for Cancer Theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Akbarzadeh, A.; Norouzian, D.; Amini, A.; Gholami-Shabani, Z.; Imani, A.; Chiani, M.; Riazi, G.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Antimicrobial Activity and Physical Characterization of Silver Nanoparticles Green Synthesized Using Nitrate Reductase from Fusarium oxysporum. Appl. Biochem. Biotechnol. 2014, 172, 4084–4098. [Google Scholar] [CrossRef]
- Kumari, R.; Barsainya, M.; Singh, D.P. Biogenic synthesis of silver nanoparticle by using secondary metabolites from Pseudomonas aeruginosa DM1 and its anti-algal effect on Chlorella vulgaris and Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2016, 24, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.T.; Khan, M.J.; Jameel, J.; Jameel, N.; Rheman, S.U.A. An Overview: Biological Organisms That Serves as Nanofactories for Metallic Nanoparticles Synthesis and Fungi Being the Most Appropriate. Bioceram. Dev. Appl. 2017, 7. [Google Scholar] [CrossRef]
- Seshadri, S.; Saranya, K.; Kowshik, M. Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast, Rhodosporidium diobovatum. Biotechnol. Prog. 2011, 27, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef]
- Gudikandula, K.; Vadapally, P.; Charya, M.S. Biogenic synthesis of silver nanoparticles from white rot fungi: Their characterization and antibacterial studies. OpenNano 2017, 2, 64–78. [Google Scholar] [CrossRef]
- Metuku, R.P.; Pabba, S.; Burra, S.; Hima Bindu, N.; SV SS, S.L.; Gudikandula, K.; Charya, S. Biosynthesis of silver nanoparticles from Schizophyllum radiatum HE 863742.1: Their characterization and antimicrobial activity. 3 Biotech 2013, 4, 227–234. [Google Scholar] [CrossRef]
- Rajput, S.; Werezuk, R.; Lange, R.M.; McDermott, M.T. Fungal Isolate Optimized for Biogenesis of Silver Nanoparticles with Enhanced Colloidal Stability. Langmuir 2016, 32, 8688–8697. [Google Scholar] [CrossRef]
- Kitching, M.; Choudhary, P.; Inguva, S.; Guo, Y.; Ramani, M.; Das, S.K.; Marsili, E. Fungal surface protein mediated one-pot synthesis of stable and hemocompatible gold nanoparticles. Enzym. Microb. Technol. 2016, 95, 76–84. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.; Derita, M.; Zachino, S.; Rai, M. Colletotrichum sp.- mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. LWT Food Sci. Technol. 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Simões, M.F.; Fernandes, S.; dos Santos, J.G.; da Silva, E.S.; de Souza, R.F.B.; Maiorano, A.E. Screening of filamentous fungi for antimicrobial silver nanoparticles synthesis. AMB Express 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Su, W.; Liu, J.-X.; Zeng, X.-X.; Huang, Z.; Li, W.; Liu, Z.-C.; Tang, J.-X. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater. Sci. Eng. C 2017, 77, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Azmath, P.; Baker, S.; Rakshith, D.; Satish, S. Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharm. J. 2016, 24, 140–146. [Google Scholar] [CrossRef] [PubMed]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.-Z.M.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Saeed, H.; Iqtedar, M.; Hussain, S.Z.; Kaleem, A.; Abdullah, R.; Sharif, S.; Naz, S.; Saleem, F.; Aihetasham, A.; et al. Size-Controlled Production of Silver Nanoparticles by Aspergillus fumigatus BTCB10: Likely Antibacterial and Cytotoxic Effects. J. Nanomater. 2019, 2019, 5168698. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid Synthesis of Silver Nanoparticles from Fusarium oxysporum by Optimizing Physicocultural Conditions. Sci. World J. 2013, 2013, 796018. [Google Scholar] [CrossRef]
- Sreedharan, S.M.; Gupta, S.; Saxena, A.K.; Singh, R. Macrophomina phaseolina: Microbased biorefinery for gold nanoparticle production. Ann. Microbiol. 2019, 69, 435–445. [Google Scholar] [CrossRef]
- Azam, Z.; Ayaz, A.; Younas, M.; Qureshi, Z.; Arshad, B.; Zaman, W.; Ullah, F.; Nasar, M.Q.; Bahadur, S.; Irfan, M.M.; et al. Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 2020, 144, 104188. [Google Scholar] [CrossRef]
- Borovaya, M.; Pirko, Y.; Krupodorova, T.; Naumenko, A.; Blume, Y.; Yemets, A. Biosynthesis of cadmium sulphide quantum dots by using Pleurotus ostreatus (Jacq.) P. Kumm. Biotechnol. Biotechnol. Equip. 2015, 29, 1156–1163. [Google Scholar] [CrossRef]
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Shanmugam, S.; Varukattu, N.B.; MubarakAli, D.; Kathiresan, K.; Wang, M.-H. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B Biol. 2019, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mahanty, S.; Das, P.; Chaudhuri, P.; Das, S. Biofabrication of iron oxide nanoparticles using manglicolous fungus Aspergillus niger BSC-1 and removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2019, 385, 123790. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Nascimento, C.A.O.; Corrêa, B. Nickel oxide nanoparticles film produced by dead biomass of filamentous fungus. Sci. Rep. 2014, 4, 6404. [Google Scholar] [CrossRef] [PubMed]
- Diko, C.S.; Qu, Y.; Henglin, Z.; Li, Z.; Nahyoon, N.A.; Fan, S. Biosynthesis and characterization of lead selenide semiconductor nanoparticles (PbSe NPs) and its antioxidant and photocatalytic activity. Arab. J. Chem. 2020, 13, 8411–8423. [Google Scholar] [CrossRef]
- Tarver, S.; Gray, D.; Loponov, K.; Das, D.B.; Sun, T.; Sotenko, M. Biomineralization of Pd nanoparticles using Phanerochaete chrysosporium as a sustainable approach to turn platinum group metals (PGMs) wastes into catalysts. Int. Biodeterior. Biodegrad. 2019, 143, 104724. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.R.; Pradhan, N.; Behera, D.; Pradhan, K.M.; Mishra, S.; Sukla, L.B.; Mishra, B.K. Green synthesis of silver nanoparticle by Penicillium purpurogenum NPMF: The process and optimization. J. Nanoparticle Res. 2011, 13, 3129–3137. [Google Scholar] [CrossRef]
- Du, L.; Xu, Q.; Huang, M.; Xian, L.; Feng, J.-X. Synthesis of small silver nanoparticles under light radiation by fungus Penicillium oxalicum and its application for the catalytic reduction of methylene blue. Mater. Chem. Phys. 2015, 160, 40–47. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surfaces B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Balakumaran, M.; Ramachandran, R.; Kalaichelvan, P. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.C.; Oliveira, J.P.; Keijok, W.J.; da Silva, A.R.; Aguiar, A.R.; Guimarães, M.C.C.; Ferraz, C.M.; Araújo, J.V.; Tobias, F.L.; Braga, F.R. Extracellular biosynthesis of silver nanoparticles using the cell-free filtrate of nematophagous fungus Duddingtonia flagrans. Int. J. Nanomed. 2017, 12, 6373–6381. [Google Scholar] [CrossRef] [PubMed]
- Saxena, J.; Sharma, P.K.; Sharma, M.M.; Singh, A. Process optimization for green synthesis of silver nanoparticles by Sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. SpringerPlus 2016, 5, 861. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.K.; Soni, R.; Rishi, P.; Soni, S.K. Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens. Green Process. Synth. 2019, 8, 144–156. [Google Scholar] [CrossRef]
- Xue, B.; He, D.; Gao, S.; Wang, D.; Yokoyama, K.; Wang, L. Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016, 11, 1899–1906. [Google Scholar] [CrossRef]
- Phanjom, P.; Ahmed, G. Effect of different physicochemical conditions on the synthesis of silver nanoparticles using fungal cell filtrate of Aspergillus oryzae(MTCC No. 1846) and their antibacterial effect. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045016. [Google Scholar] [CrossRef]
- Nasr, M. Nanotechnology Application in Agricultural Sector. In Nanobiotechnology in Bioformulations; Prasad, R., Kumar, V., Kumar, M., Choudhary, D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 317–329. ISBN 978-3-030-17061-5. [Google Scholar]
- Wu, Z.; Yang, S.; Wu, W. Shape control of inorganic nanoparticles from solution. Nanoscale 2015, 8, 1237–1259. [Google Scholar] [CrossRef]
- Gahukar, R.T.; Das, R.K. Plant-derived nanopesticides for agricultural pest control: Challenges and prospects. Nanotechnol. Environ. Eng. 2020, 5, 3. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for Synthesis of Silver Nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Jian, W.; Zhang, L.; Siu, K.-C.; Song, A.; Wu, J.-Y. Formation and Physiochemical Properties of Silver Nanoparticles with Various Exopolysaccharides of a Medicinal Fungus in Aqueous Solution. Molecules 2016, 22, 50. [Google Scholar] [CrossRef]
- Bharde, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sarkar, I.; Yusuf, S.M.; Sanyal, M.; Sastry, M. Extracellular Biosynthesis of Magnetite using Fungi. Small 2006, 2, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Ansary, A.A.; Ahmad, A.; Khan, M.I. Extracellular Biosynthesis of CdSe Quantum Dots by the Fungus, Fusarium Oxysporum. J. Biomed. Nanotechnol. 2007, 3, 190–194. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P.; Puri, S. Enzymatic Formation of Gold Nanoparticles Using Phanerochaete Chrysosporium. Adv. Chem. Eng. Sci. 2011, 1, 154–162. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef]
- Polavarapu, L.; Xu, Q.-H. A single-step synthesis of gold nanochains using an amino acid as a capping agent and characterization of their optical properties. Nanotechnology 2008, 19, 075601. [Google Scholar] [CrossRef]
- Liu, F.-K.; Ko, F.-H.; Huang, P.-W.; Wu, C.-H.; Chu, T.-C. Studying the size/shape separation and optical properties of silver nanoparticles by capillary electrophoresis. J. Chromatogr. A 2004, 1062, 139–145. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Boury, B.; Plumejeau, S. Metal oxides and polysaccharides: An efficient hybrid association for materials chemistry. Green Chem. 2014, 17, 72–88. [Google Scholar] [CrossRef]
- Pochanavanich, P.; Suntornsuk, W. Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 2002, 35, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Schierz, G.; Basche, S.; Petto, C.; Boening, K.; Wieckiewicz, M. Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material. Molecules 2020, 25, 5899. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2019, 147, 104459. [Google Scholar] [CrossRef]
- Virkutyte, J.; Varma, R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Cheng, F.; Betts, J.W.; Kelly, S.M.; Schaller, J.; Heinze, T. Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using aminocellulose as a combined reducing and capping reagent. Green Chem. 2013, 15, 989–998. [Google Scholar] [CrossRef]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surfaces B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Shaligram, N.S.; Bule, M.; Bhambure, R.; Singhal, R.S.; Singh, S.K.; Szakacs, G.; Pandey, A. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009, 44, 939–943. [Google Scholar] [CrossRef]
- Balaji, D.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surfaces B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanopart. Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Cameron, S.J.; Sheng, J.; Hosseinian, F.; Willmore, W.G. Nanoparticle Effects on Stress Response Pathways and Nanoparticle–Protein Interactions. Int. J. Mol. Sci. 2022, 23, 7962. [Google Scholar] [CrossRef] [PubMed]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; A Alzohairy, M.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria acuta and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, MBI.S10957-16. [Google Scholar] [CrossRef]
- Banerjee, A.; Halder, U.; Bandopadhyay, R. Preparations and Applications of Polysaccharide Based Green Synthesized Metal Nanoparticles: A State-of-the-Art. J. Clust. Sci. 2017, 28, 1803–1813. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B. Polysaccharides templates for assembly of nanosilver. Carbohydr. Polym. 2016, 135, 300–307. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Dhanjal, D.S.; Mehra, P.; Bhardwaj, S.; Singh, R.; Sharma, P.; Nepovimova, E.; Chopra, C.; Kuca, K. Mycology-Nanotechnology Interface: Applications in Medicine and Cosmetology. Int. J. Nanomed. 2022, 17, 2505–2533. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A.; Kursky, V.F.; Nikitina, V.E. Nanoparticles synthesis by Agaricus soil basidiomycetes. J. Biosci. Bioeng. 2018, 126, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef] [PubMed]



| Species of Fungus | NP Type | Size (nm) | Biomolecule | Biomolecule’s Role | Source |
|---|---|---|---|---|---|
| Aspergillus flavus | TiO2 | 62 to 74 | Fungal proteins, amino acids | surface capping | [192] |
| Aspergillus niger | Ag | 20 | nitrate reductase and anthraquinones | precursor reduction and NP formation | [205] |
| fungal proteins | surface capping | ||||
| Aspergillus terreus | Co3O4 CuO Fe3O4 NiO ZnO | 5 to 15 10 to 30 20 to 40 20 to 60 20 to 50 | fungal proteins | precursor reduction and NP formation surface capping | [28] |
| Cs-HK1 fungus | Ag | 10 to 30 | exopolysaccharides | precursor reduction and NP formation | [206] |
| surface capping | |||||
| Fusarium oxysporum | Fe3O4 | 20 to 50 | 20–30 kDa fungal proteins | hydrolysis of NP precursors | [207] |
| CdSe | 9 to 15 | nitrate reductase protein/peptide | precursor reduction surface capping | [208] | |
| Phanerochaete chrysosporium | Au | 10 to 100 | laccase | extracellular formation | [209] |
| ligninase | intracellular formation | ||||
| fungal proteins | surface capping | ||||
| Pd | 10 to 14 | chitin, fungal proteins | precursor reduction, NP formation, and surface capping | [191] | |
| Rhodosporidium diobovatum | PbS | 2 to 5 | peptides like phytochelatin | surface capping | [168] |
| phytochelatinspurine biosynthesis pathway enzymes | intracellular formation | ||||
| Trichoderma sp. | PbSe | 10 to 30 | fungal proteinsreductase | Se reductionsurface cappingPbSe formation | [190] |
| Volvariella volvacea | Ag | 15 | fungal proteins | reducing agents | [210] |
| Au | 20 to 150 | surface capping | |||
| Ag-Au | 5 to 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolenčík, M. Mycosynthesis of Metal-Containing Nanoparticles—Fungal Metal Resistance and Mechanisms of Synthesis. Int. J. Mol. Sci. 2022, 23, 14084. https://doi.org/10.3390/ijms232214084
Šebesta M, Vojtková H, Cyprichová V, Ingle AP, Urík M, Kolenčík M. Mycosynthesis of Metal-Containing Nanoparticles—Fungal Metal Resistance and Mechanisms of Synthesis. International Journal of Molecular Sciences. 2022; 23(22):14084. https://doi.org/10.3390/ijms232214084
Chicago/Turabian StyleŠebesta, Martin, Hana Vojtková, Veronika Cyprichová, Avinash P. Ingle, Martin Urík, and Marek Kolenčík. 2022. "Mycosynthesis of Metal-Containing Nanoparticles—Fungal Metal Resistance and Mechanisms of Synthesis" International Journal of Molecular Sciences 23, no. 22: 14084. https://doi.org/10.3390/ijms232214084
APA StyleŠebesta, M., Vojtková, H., Cyprichová, V., Ingle, A. P., Urík, M., & Kolenčík, M. (2022). Mycosynthesis of Metal-Containing Nanoparticles—Fungal Metal Resistance and Mechanisms of Synthesis. International Journal of Molecular Sciences, 23(22), 14084. https://doi.org/10.3390/ijms232214084








