BPR0C261, An Analogous of Microtubule Disrupting Agent D-24851 Enhances the Radiosensitivity of Human Non-Small Cell Lung Cancer Cells via p53-Dependent and p53-Independent Pathways
Abstract
1. Introduction
2. Results
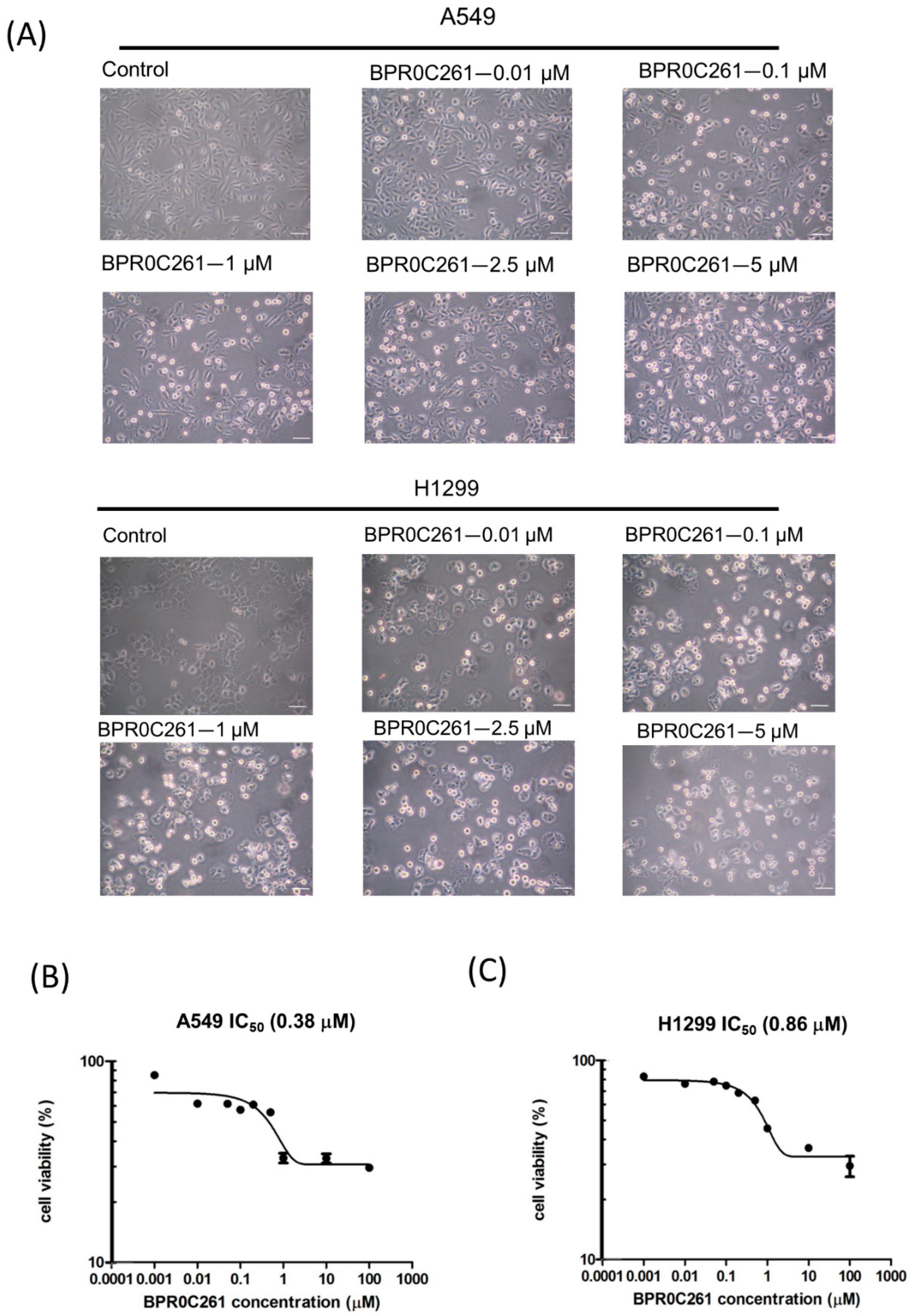
2.1. Effects of BPR0C261 on NSCLC Cells
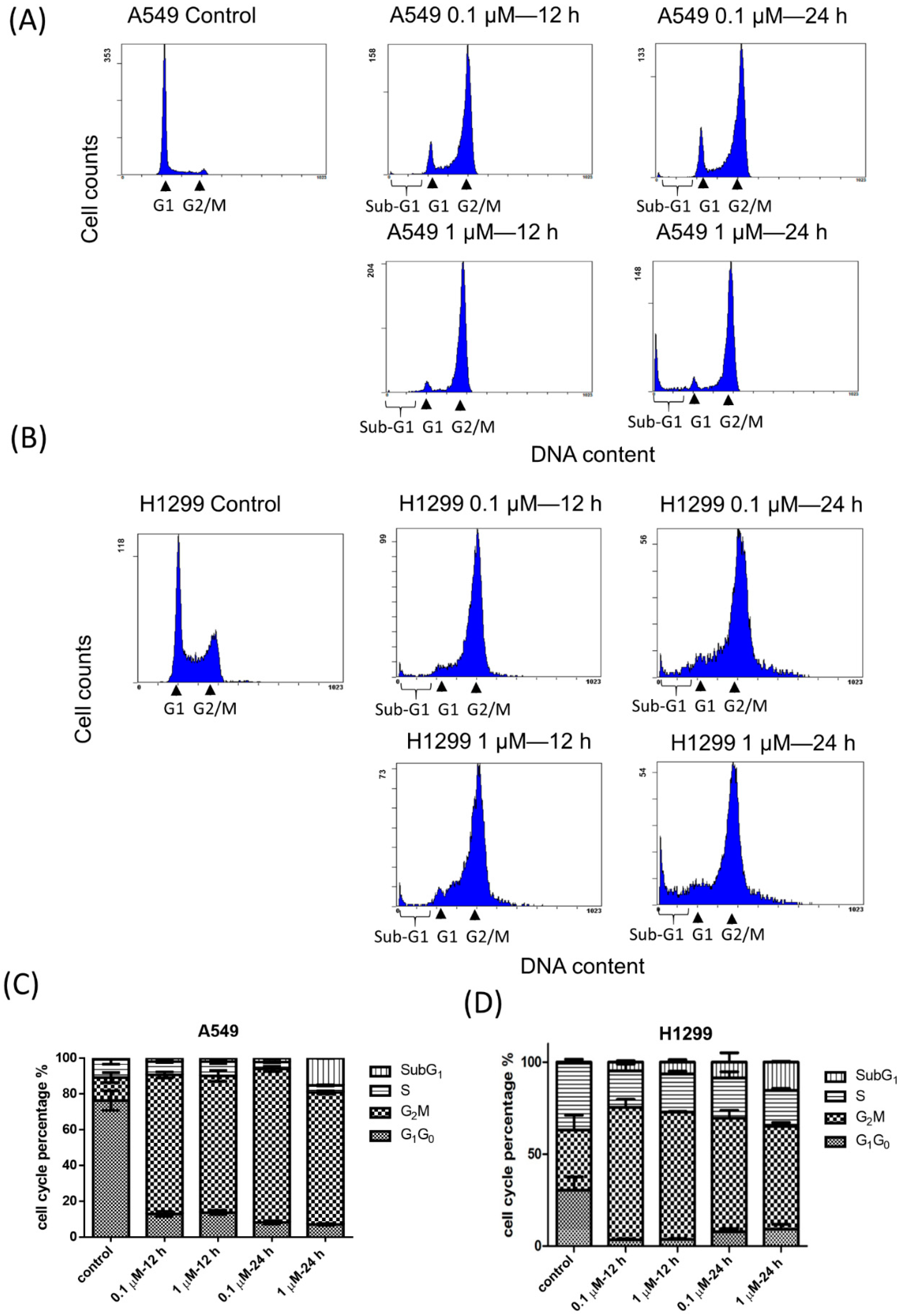
2.2. Effects of BPR0C261 on the Redistribution of Cell Cycle
2.3. Effects of High Concentration of BPR0C261 on Induction of Sub-G1 Population in NSCLC Cells
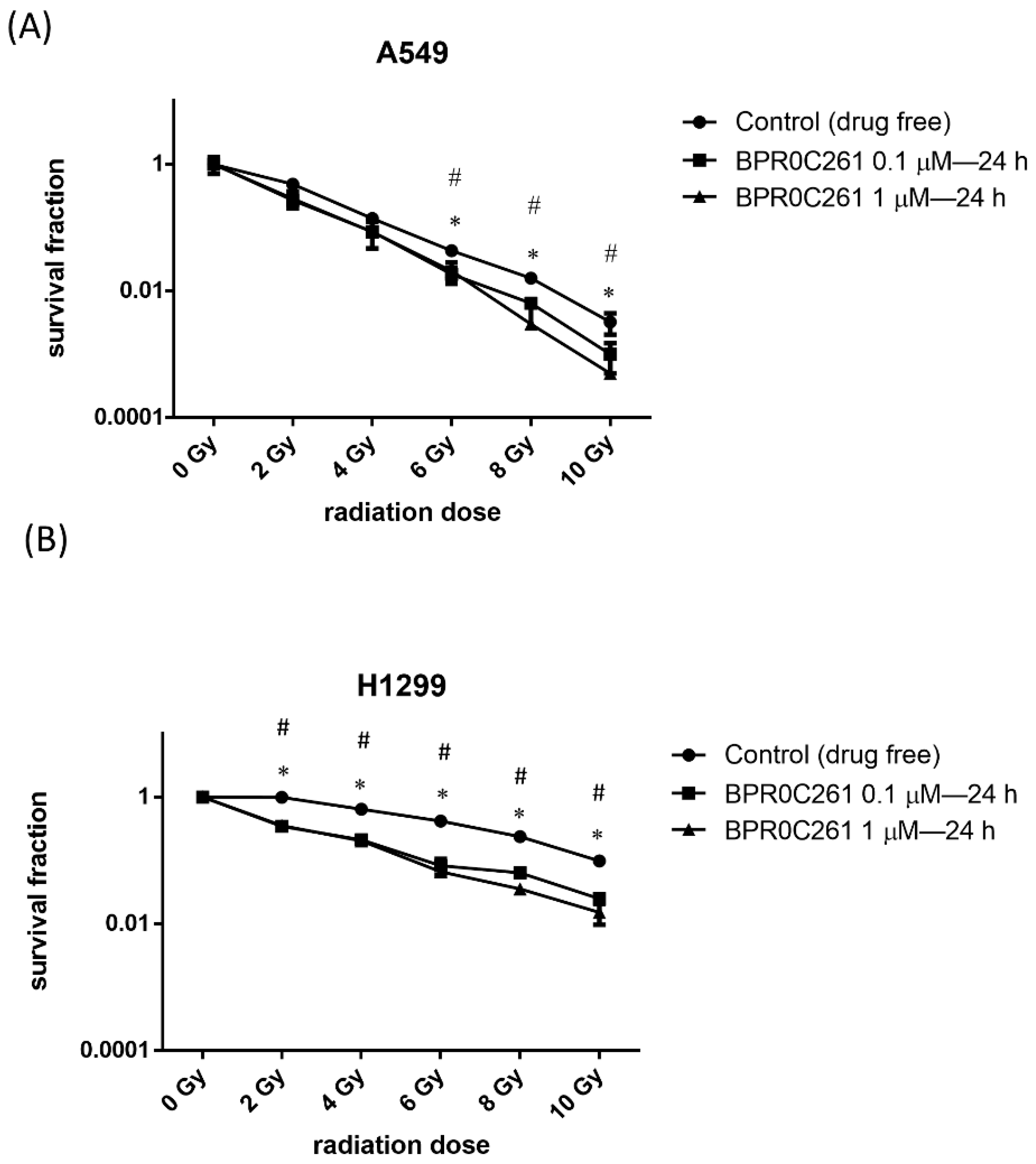
2.4. The Combination Treatment of Radiation and BPR0C261 Decreased the Survival Fraction in NSCLC Cells
2.5. Effects of BPR0C261 and Radiation on Induction of DNA Damage in NSCLC Cells
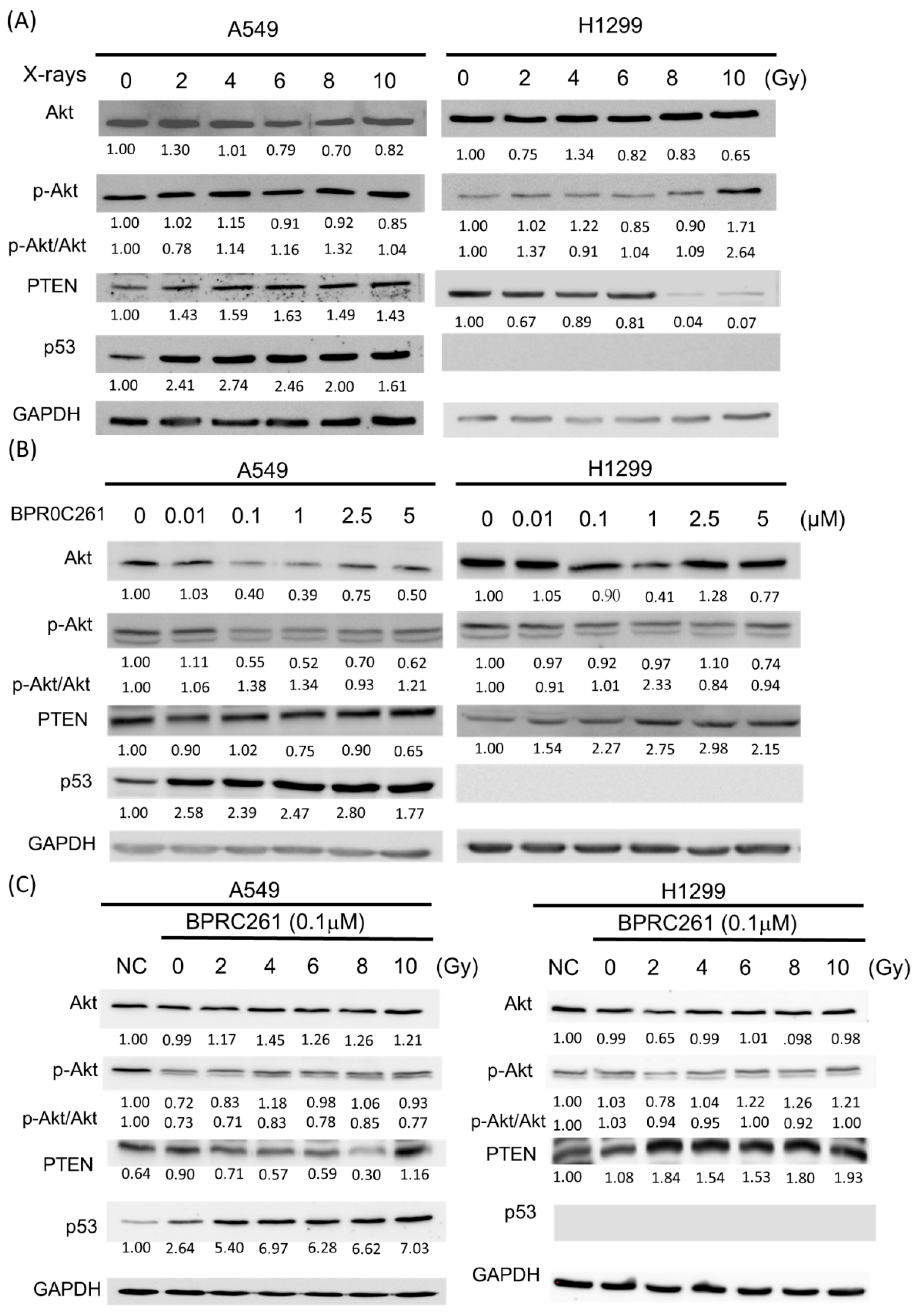
2.6. The Expression of p53 and PTEN in NSCLC Cells Treated with BPR0C261 and Radiation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagent
4.3. Radiation Source
4.4. Cell Viability Assay
4.5. Flow Cytometry Analysis
4.6. Colony Formation Assay
4.7. Single-Cell Gel Electrophoresis Assay (Comet Assay)
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.F.; Goggins, W.B.; Tse, S.L.A. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Conibear, J.; AstraZeneca, U.K.L. Rationale for concurrent chemoradiotherapy for patients with stage III non-small-cell lung cancer. Br. J. Cancer 2020, 123 (Suppl. S1), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ohe, Y. Chemoradiotherapy for lung cancer: Current status and perspectives. Int. J. Clin. Oncol. 2004, 9, 435–443. [Google Scholar] [CrossRef]
- Li, W.T.; Hwang, D.R.; Chen, C.P.; Shen, C.W.; Huang, C.L.; Chen, T.W.; Lin, C.H.; Chang, Y.L.; Chang, Y.Y.; Lo, Y.K.; et al. Synthesis and biological evaluation of N-heterocyclic indolyl glyoxylamides as orally active anticancer agents. J. Med. Chem. 2003, 46, 1706–1715. [Google Scholar] [CrossRef]
- Bacher, G.; Nickel, B.; Emig, P.; Vanhoefer, U.; Seeber, S.; Shandra, A.; Klenner, T.; Beckers, T. D-24851, a novel synthetic microtubule inhibitor, exerts curative antitumoral activity in vivo, shows efficacy toward multidrug-resistant tumor cells, and lacks neurotoxicity. Cancer Res. 2001, 61, 392–399. [Google Scholar]
- Ito, H.; Kanzawa, T.; Kondo, S.; Kondo, Y. Microtubule inhibitor D-24851 induces p53-independent apoptotic cell death in malignant glioma cells through Bcl-2 phosphorylation and Bax translocation. Int. J. Oncol. 2005, 26, 589–596. [Google Scholar] [CrossRef]
- Huang, T.H.; Chiu, S.J.; Chiang, P.H.; Chiou, S.H.; Li, W.T.; Chen, C.T.; Chang, C.A.; Chen, J.C.; Lee, Y.J. Antiproliferative effects of N-heterocyclic indolyl glyoxylamide derivatives on human lung cancer cells. Anticancer Res. 2011, 31, 3407–3415. [Google Scholar]
- Hu, C.B.; Chen, C.P.; Yeh, T.K.; Song, J.S.; Chang, C.Y.; Chuu, J.J.; Tung, F.F.; Ho, P.Y.; Chen, T.W.; Lin, C.H.; et al. BPR0C261 is a novel orally active antitumor agent with antimitotic and anti-angiogenic activities. Cancer Sci. 2011, 102, 182–191. [Google Scholar] [CrossRef]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell. Death Differ. 2018, 25, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, 9th ed.; Sinauer Associates: Sunderland, MA, USA, 2010; 80p, ISBN 9780878933846. [Google Scholar]
- Ghosh, M.; Saha, S.; Bettke, J.; Nagar, R.; Parrales, A.; Iwakuma, T.; van der Velden, A.W.M.; Martinez, L.A. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell. 2021, 39, 494–508.e5. [Google Scholar] [CrossRef] [PubMed]
- Assadian, S.; El-Assaad, W.; Wang, X.Q.; Gannon, P.O.; Barres, V.; Latour, M.; Mes-Masson, A.M.; Saad, F.; Sado, Y.; Dostie, J.; et al. p53 inhibits angiogenesis by inducing the production of Arresten. Cancer Res. 2012, 72, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D.; Hu, M.; Xiong, S.; Lang, A.; Ellis, L.M.; Pollock, R.E. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res. 2000, 60, 3655–3661. [Google Scholar]
- Lanni, J.S.; Lowe, S.W.; Licitra, E.J.; Liu, J.O.; Jacks, T. p53-independent apoptosis induced by paclitaxel through an indirect mechanism. Proc. Natl. Acad. Sci. USA 1997, 94, 9679–9683. [Google Scholar] [CrossRef]
- Miyake, N.; Chikumi, H.; Takata, M.; Nakamoto, M.; Igishi, T.; Shimizu, E. Rapamycin induces p53-independent apoptosis through the mitochondrial pathway in non-small cell lung cancer cells. Oncol. Rep. 2012, 28, 848–854. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Tan, C.Y.; Rakotondraibe, L.H.; Carcache de Blanco, E.C. Tumor suppressor p53 independent apoptosis in HT-29 cells by auransterol from Penicillium aurantiacobrunneum. Biomed Pharmacother. 2020, 127, 110124. [Google Scholar] [CrossRef]
- Ma, X.; Choudhury, S.N.; Hua, X.; Dai, Z.; Li, Y. Interaction of the oncogenic miR-21 microRNA and the p53 tumor suppressor pathway. Carcinogenesis 2013, 34, 1216–1223. [Google Scholar] [CrossRef]
- Yehia, L.; Ngeow, J.; Eng, C. PTEN-opathies: From biological insights to evidence-based precision medicine. J. Clin. Investig. 2019, 129, 452–464. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. The PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2015, 7, a026609. [Google Scholar] [CrossRef]
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T.W. Regulation of PTEN transcription by p53. Mol. Cell. 2001, 8, 317–325. [Google Scholar] [CrossRef]
- Masson, G.R.; Williams, R.L. Structural Mechanisms of PTEN Regulation. Cold Spring Harb Perspect Med 2020, 10, a036152. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, D.; Longy, M. Mutations of the human PTEN gene. Hum. Mutat. 2000, 16, 109–122. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; He, J.; Cai, S.; Weng, Y.; Huang, S.; Ma, S. PTEN inhibits non-small cell lung cancer cell growth by promoting G0/G1 arrest and cell apoptosis. Oncol. Lett. 2019, 17, 1333–1340. [Google Scholar] [CrossRef]
- Li, G.; Zhao, J.; Peng, X.; Liang, J.; Deng, X.; Chen, Y. Radiation/paclitaxel treatment of p53-abnormal non-small cell lung cancer xenograft tumor and associated mechanism. Cancer Biother. Radiopharm. 2012, 27, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Degraff, W.G.; Gamson, J.; Russo, D.; Gazdar, A.F.; Levitt, M.L.; Minna, J.D.; Mitchell, J.B. Radiation sensitivity of human lung cancer cell lines. Eur. J. Cancer Clin. Oncol. 1989, 25, 527–534. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo. Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Zdrowowicz, M.; Datta, M.; Rychlowski, M.; Rak, J. Radiosensitization of PC3 Prostate Cancer Cells by 5-Thiocyanato-2′-deoxyuridine. Cancers 2022, 14, 2035. [Google Scholar] [CrossRef]
- Cheng, G.; Kong, D.; Hou, X.; Liang, B.; He, M.; Liang, N.; Ma, S.; Liu, X. The tumor suppressor, p53, contributes to radiosensitivity of lung cancer cells by regulating autophagy and apoptosis. Cancer Biother. Radiopharm. 2013, 28, 153–159. [Google Scholar] [CrossRef]
- Liu, C.; Nie, J.; Wang, R.; Mao, W. The Cell Cycle G2/M Block Is an Indicator of Cellular Radiosensitivity. Dose Response 2019, 17, 1559325819891008. [Google Scholar] [CrossRef]
- Di Leonardo, A.; Linke, S.P.; Clarkin, K.; Wahl, G.M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes. Dev. 1994, 8, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- el-Deiry, W.S.; Harper, J.W.; O’Connor, P.M.; Velculescu, V.E.; Canman, C.E.; Jackman, J.; Pietenpol, J.A.; Burrell, M.; Hill, D.E.; Wang, Y.; et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994, 54, 1169–1174. [Google Scholar]
- Agarwal, M.L.; Agarwal, A.; Taylor, W.R.; Stark, G.R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc. Natl. Acad. Sci. USA 1995, 92, 8493–8497. [Google Scholar] [CrossRef] [PubMed]
- Umansky, S.R.; Korol, B.A.; Nelipovich, P.A. In vivo DNA degradation in thymocytes of gamma-irradiated or hydrocortisone-treated rats. Biochim. Biophys. Acta 1981, 655, 9–17. [Google Scholar] [CrossRef]
- Gong, J.; Traganos, F.; Darzynkiewicz, Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal. Biochem. 1994, 218, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.F.; Chen, T.M.; Chen, Y.H. Apoptosis and necrosis are involved in the toxicity of Sauropus androgynus in an in vitro study. J. Formos. Med. Assoc. 2007, 106, 537–547. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Juan, G.; Li, X.; Gorczyca, W.; Murakami, T.; Traganos, F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis). Cytometry 1997, 27, 1–20. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Bedner, E.; Traganos, F. Difficulties and pitfalls in analysis of apoptosis. Methods Cell Biol 2001, 63, 527–546. [Google Scholar]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell. Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Zhang, P.; Jin, X.; Zheng, X.; Ye, F.; Chen, W.; Li, Q. The synergistic radiosensitizing effect of tirapazamine-conjugated gold nanoparticles on human hepatoma HepG2 cells under X-ray irradiation. Int. J. Nanomed. 2016, 11, 3517–3531. [Google Scholar] [CrossRef]
- Nosrati, H.; Charmi, J.; Abhari, F.; Attari, E.; Bochani, S.; Johari, B.; Rezaeejam, H.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. Improved synergic therapeutic effects of chemoradiation therapy with the aid of a co-drug-loaded nano-radiosensitizer under conventional-dose X-ray irradiation. Biomater Sci. 2020, 8, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, e1901223. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold Nanoparticles in Cancer Theranostics. Front Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef] [PubMed]
- Valeriote, F.; Lin, H. Synergistic interaction of anticancer agents: A cellular perspective. Cancer Chemother. Rep. 1975, 59, 895–900. [Google Scholar] [PubMed]
- Carpentier, Y.; Demange, L.; Loirette, M.; Hivet, J.; Desoize, B. Chronology of combined chemotherapy (5FU) and radiotherapy. I. In vitro study. Anticancer Res. 1993, 13, 2177–2180. [Google Scholar]
- Fischer, T.; Hartmann, O.; Reissland, M.; Prieto-Garcia, C.; Klann, K.; Pahor, N.; Schulein-Volk, C.; Baluapuri, A.; Polat, B.; Abazari, A.; et al. PTEN mutant non-small cell lung cancer require ATM to suppress pro-apoptotic signalling and evade radiotherapy. Cell. Biosci. 2022, 12, 50. [Google Scholar] [CrossRef]
- Branham, M.T.; Nadin, S.B.; Vargas-Roig, L.M.; Ciocca, D.R. DNA damage induced by paclitaxel and DNA repair capability of peripheral blood lymphocytes as evaluated by the alkaline comet assay. Mutat. Res. 2004, 560, 11–17. [Google Scholar] [CrossRef]
- Appella, E.; Anderson, C.W. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 2001, 268, 2764–2772. [Google Scholar] [CrossRef]
- Jin, G.; Kim, M.J.; Jeon, H.S.; Choi, J.E.; Kim, D.S.; Lee, E.B.; Cha, S.I.; Yoon, G.S.; Kim, C.H.; Jung, T.H.; et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer 2010, 69, 279–283. [Google Scholar] [CrossRef]
- Gkountakos, A.; Sartori, G.; Falcone, I.; Piro, G.; Ciuffreda, L.; Carbone, C.; Tortora, G.; Scarpa, A.; Bria, E.; Milella, M.; et al. PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around. Cancers 2019, 11, 1141. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.H.; Shim, S.; Kim, A.; Jang, H.; Lee, S.J.; Park, S.; Seo, S.; Jang, W.I.; Lee, S.B.; et al. Radiation-Activated PI3K/AKT Pathway Promotes the Induction of Cancer Stem-Like Cells via the Upregulation of SOX2 in Colorectal Cancer. Cells 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Kim, J.S.; Waldman, T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat. Oncol. 2009, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chang, C.Y.; Wang, C.Y.; Liu, K.; Kang, C.Y.; Lee, Y.J.; Chen, W.R. N-Dihydrogalactochitosan Potentiates the Radiosensitivity of Liver Metastatic Tumor Cells Originated from Murine Breast Tumors. Int. J. Mol. Sci. 2019, 20, 5581. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Sheu, T.J.; Keng, P.C. Enhancement of radiosensitivity in H1299 cancer cells by actin-associated protein cofilin. Biochem Biophys. Res. Commun. 2005, 335, 286–291. [Google Scholar] [CrossRef] [PubMed]


| The SFD of BPR0C261 0.1 μM = 0.368 | |||
|---|---|---|---|
| Radiation (Gy) | SFR | SFR+D | SFR × SFD |
| 2 Gy | 0.4824 | 0.0822 (synergism) | 0.1774 |
| 4 Gy | 0.1394 | 0.0264 (synergism) | 0.0513 |
| 6 Gy | 0.0433 | 0.0056 (synergism) | 0.0159 |
| 8 Gy | 0.0159 | 0.0020 (synergism) | 0.0058 |
| 10 Gy | 0.0032 | 0.0003 (synergism) | 0.0012 |
| The SFD of BPR0C261 1 μM = 0.305 | |||
| Radiation (Gy) | SFR | SFR+D | SFR × SFD |
| 2 Gy | 0.4824 | 0.1057 (synergism) | 0.1471 |
| 4 Gy | 0.1394 | 0.0313 (synergism) | 0.0425 |
| 6 Gy | 0.0433 | 0.0076 (synergism) | 0.0132 |
| 8 Gy | 0.0159 | 0.0011 (synergism) | 0.0048 |
| 10 Gy | 0.0032 | 0.0002 (synergism) | 0.0010 |
| The SFD of BPR0C261 0.1 μM = 0.504 | |||
|---|---|---|---|
| Radiation (Gy) | SFR | SFR+D | SFR × SFD |
| 2 Gy | 0.9917 | 0.3326 (synergism) | 0.4998 |
| 4 Gy | 0.6482 | 0.2123 (synergism) | 0.3267 |
| 6 Gy | 0.4202 | 0.0826 (synergism) | 0.2118 |
| 8 Gy | 0.2375 | 0.0640 (synergism) | 0.1197 |
| 10 Gy | 0.0983 | 0.0249 (synergism) | 0.0495 |
| The SFD of BPR0C261 1 μM = 0.412 | |||
| Radiation (Gy) | SFR | SFR+D | SFR × SFD |
| 2 Gy | 0.9917 | 0.3555 (synergism) | 0.4085 |
| 4 Gy | 0.6482 | 0.2038 (synergism) | 0.2670 |
| 6 Gy | 0.4202 | 0.0665 (synergism) | 0.1731 |
| 8 Gy | 0.2375 | 0.0358 (synergism) | 0.0978 |
| 10 Gy | 0.0983 | 0.0152 (synergism) | 0.0405 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, J.-D.; Lin, S.-T.; Chen, C.-T.; Chang, C.-A.; Lee, Y.-J. BPR0C261, An Analogous of Microtubule Disrupting Agent D-24851 Enhances the Radiosensitivity of Human Non-Small Cell Lung Cancer Cells via p53-Dependent and p53-Independent Pathways. Int. J. Mol. Sci. 2022, 23, 14083. https://doi.org/10.3390/ijms232214083
Leu J-D, Lin S-T, Chen C-T, Chang C-A, Lee Y-J. BPR0C261, An Analogous of Microtubule Disrupting Agent D-24851 Enhances the Radiosensitivity of Human Non-Small Cell Lung Cancer Cells via p53-Dependent and p53-Independent Pathways. International Journal of Molecular Sciences. 2022; 23(22):14083. https://doi.org/10.3390/ijms232214083
Chicago/Turabian StyleLeu, Jyh-Der, Shih-Ting Lin, Chiung-Tong Chen, C.-Allen Chang, and Yi-Jang Lee. 2022. "BPR0C261, An Analogous of Microtubule Disrupting Agent D-24851 Enhances the Radiosensitivity of Human Non-Small Cell Lung Cancer Cells via p53-Dependent and p53-Independent Pathways" International Journal of Molecular Sciences 23, no. 22: 14083. https://doi.org/10.3390/ijms232214083
APA StyleLeu, J.-D., Lin, S.-T., Chen, C.-T., Chang, C.-A., & Lee, Y.-J. (2022). BPR0C261, An Analogous of Microtubule Disrupting Agent D-24851 Enhances the Radiosensitivity of Human Non-Small Cell Lung Cancer Cells via p53-Dependent and p53-Independent Pathways. International Journal of Molecular Sciences, 23(22), 14083. https://doi.org/10.3390/ijms232214083






