Choline Improves Neonatal Hypoxia-Ischemia Induced Changes in Male but Not Female Rats
Abstract
1. Introduction
2. Results
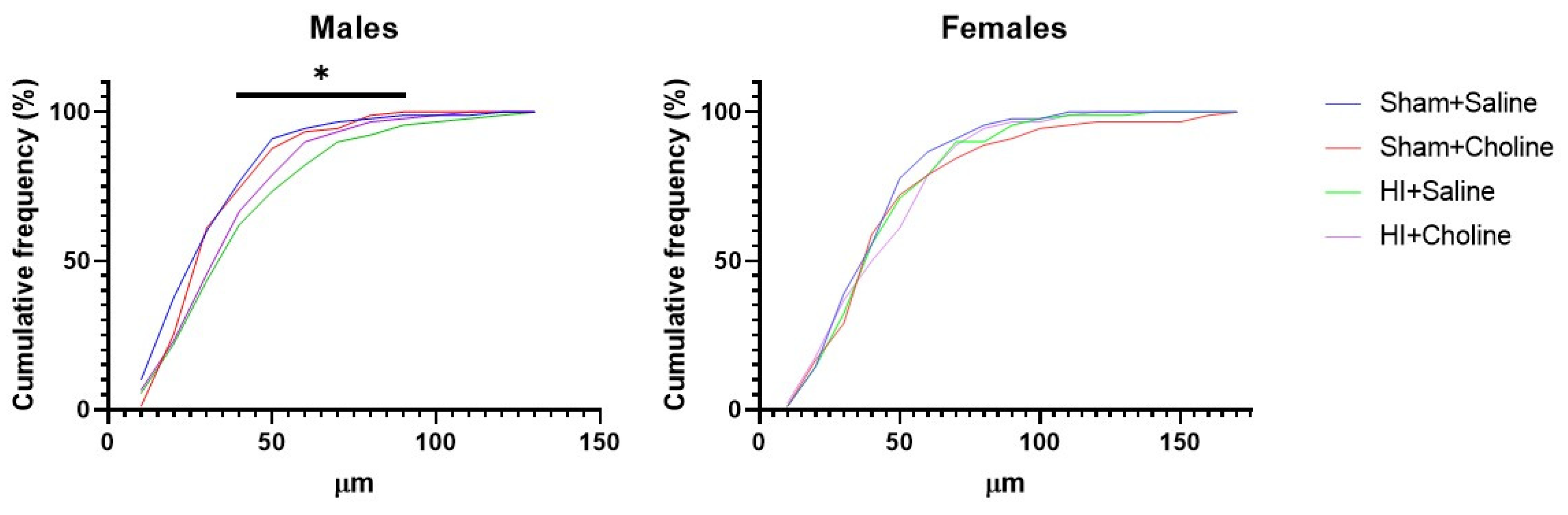
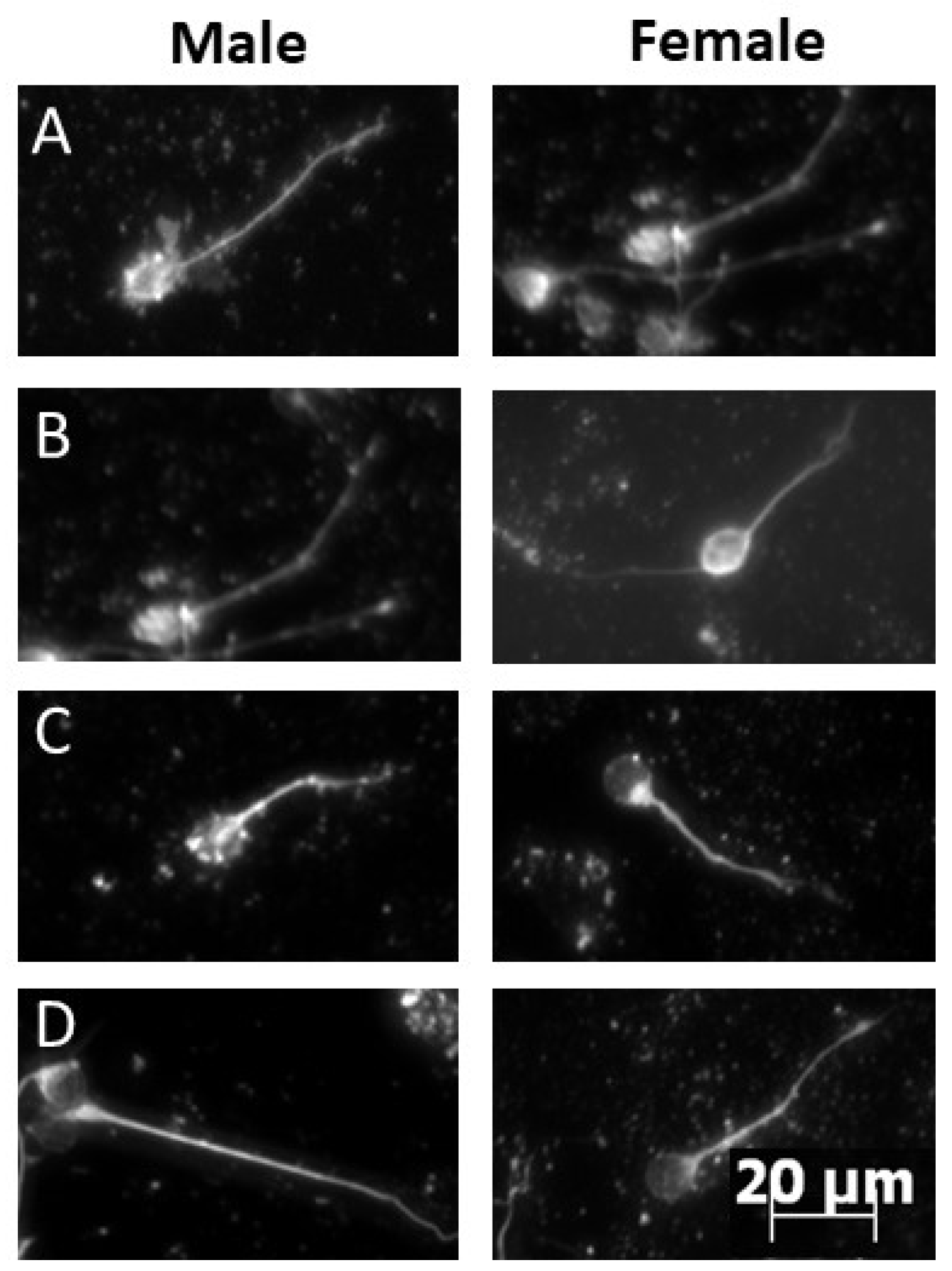
2.1. Neurite Outgrowth in Sex-Specific Cultured Cerebellar Granule Cells after HI
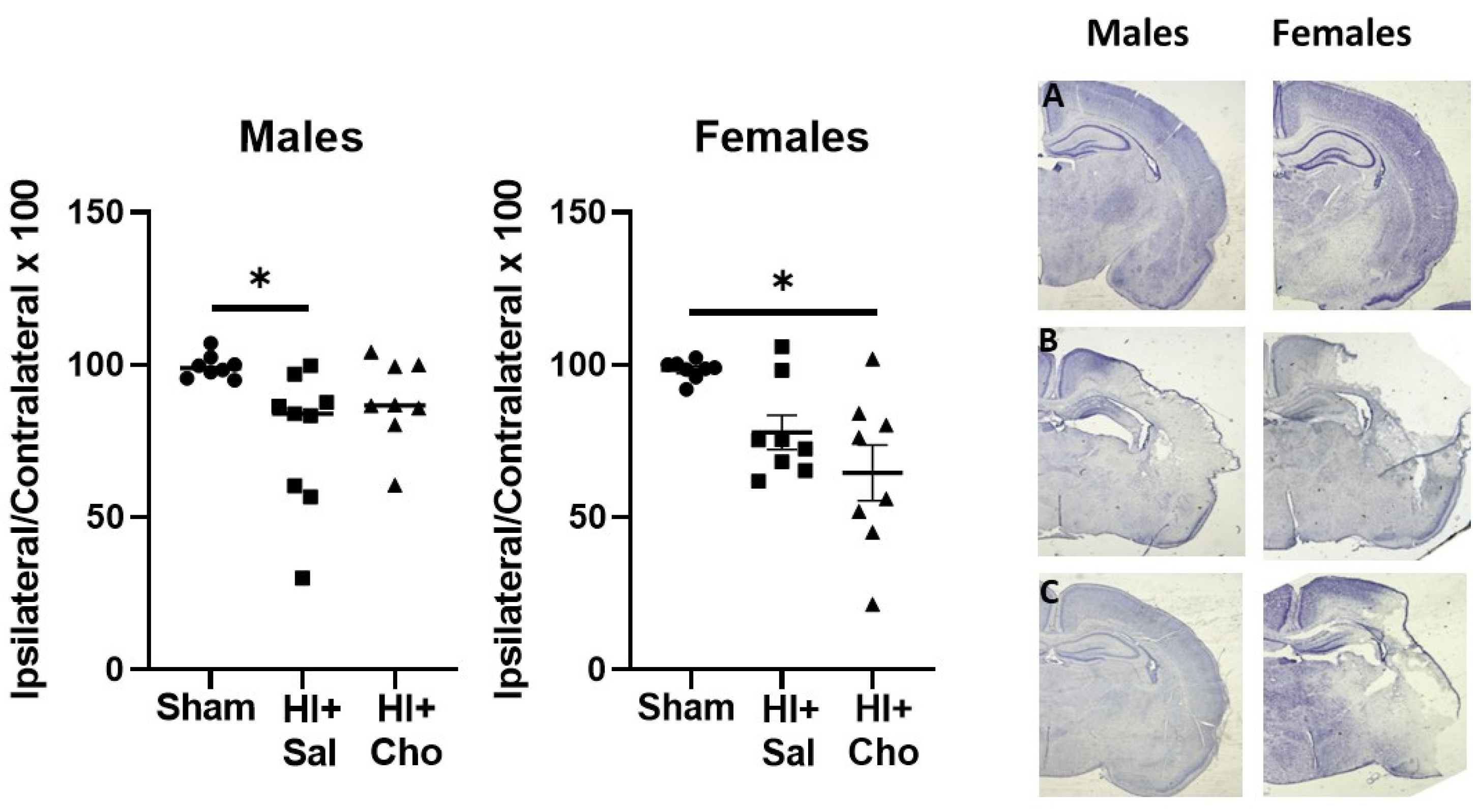
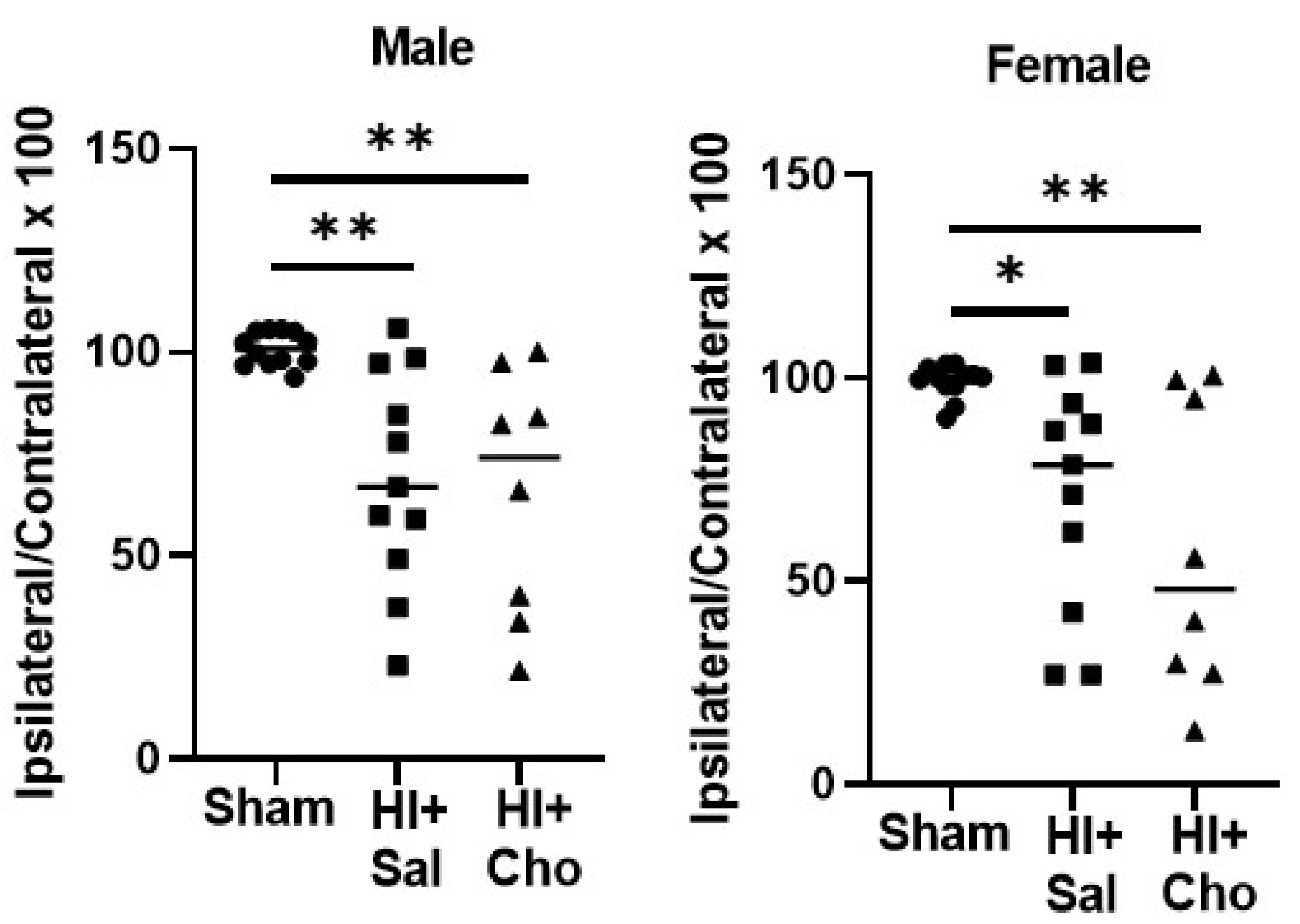
2.2. Magnitude of Injury as Determined by Area of Ipsilateral and Contralateral Hemispheres
2.3. Behavioral Outcomes following HI with or without Choline Administration
2.3.1. Dowel Crossing and Rotarod Tests
2.3.2. Eyeblink Conditioning
3. Discussion
4. Methods
4.1. Animals
4.2. Choline Administration
4.3. Hypoxia Ischemia Procedure
4.4. Neurite Outgrowth Culture and Quantification
4.5. Histology
4.6. Behavior
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambalavanan, N.; Shankaran, S.; Laptook, A.R.; Carper, B.A.; Das, A.; Carlo, W.A.; Cotten, C.M.; Duncan, A.F.; Higgins, R.D. Early Determination of Prognosis in Neonatal Moderate or Severe Hypoxic-Ischemic Encephalopathy. Pediatrics 2021, 147, e2020048678. [Google Scholar] [CrossRef] [PubMed]
- Bennet, L.; Dhillon, S.; Lear, C.A.; van den Heuij, L.; King, V.; Dean, J.M.; Wassink, G.; Davidson, J.O.; Gunn, A.J. Chronic Inflammation and Impaired Development of the Preterm Brain. J. Reprod. Immunol. 2018, 125, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-Ischemic Encephalopathy A Review for the Clinician. JAMA Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Laptook, A.R.; Robertson, N.J.; Barks, J.D.; Thoresen, M.; Wassink, G.; Bennet, L. Therapeutic Hypothermia Translates from Ancient History in to Practice. Pediatr. Res. 2017, 81, 202–209. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Lear, C.A.; Juul, S.E.; Northington, F.; Bennet, L.; Gunn, A.J. A Working Model for Hypothermic Neuroprotection. J. Physiol. 2018, 596, 5641–5654. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of Hypothermia for Perinatal Asphyxia on Childhood Outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef]
- Shankaran, S.; Pappas, A.; McDonald, S.A.; Vohr, B.R.; Hintz, S.R.; Yolton, K.; Gustafson, K.E.; Leach, T.M.; Green, C.; Bara, R.; et al. Childhood Outcomes after Hypothermia for Neonatal Encephalopathy. Obstet. Gynecol. Surv. 2012, 67, 617–619. [Google Scholar] [CrossRef]
- Babb, S.M.; Ke, Y.; Lange, N.; Kaufman, M.J.; Renshaw, P.F.; Cohen, B.M. Oral Choline Increases Choline Metabolites in Human Brain. Psychiatry Res.-Neuroimaging 2004, 130, 1–9. [Google Scholar] [CrossRef]
- Mehedint, M.G.; Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Choline Deficiency Alters Global Histone Methylation and Epigenetic Marking at the Rel Site of the Calbindin 1 Gene. FASEB J. 2010, 24, 184–195. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary Choline Deficiency Alters Global and Gene-specific DNA Methylation in the Developing Hippocampus of Mouse Fetal Brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.L. Choline Supplementation during Prenatal Development Reduces Proactive Interference in Spatial Memory. Dev. Brain Res. 1999, 118, 51–59. [Google Scholar] [CrossRef]
- Meck, W.H.; Smith, R.A.; Williams, C.L. Organizational Changes in Cholinergic Activity and Enhanced Visuospatial Memory as a Function of Choline Administered Prenatally or Postnatally or Both. Behav. Neurosci. 1989, 103, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.K.; Meck, W.H. Prenatal Choline Supplementation Increases Sensitivity to Time by Reducing Non-Scalar Sources of Variance in Adult Temporal Processing. Brain Res. 2007, 1186, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Ferreira, E.; Knowles, S.; Fux, C.; Rodin, A.; Winslow, W.; Oddo, S. Lifelong Choline Supplementation Ameliorates Alzheimer’s Disease Pathology and Associated Cognitive Deficits by Attenuating Microglia Activation. Aging Cell 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Powers, B.E.; Velazquez, R.; Strawderman, M.S.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal Choline Supplementation as a Potential Therapy for Down Syndrome: Assessment of Effects Throughout the Lifespan. Front. Aging Neurosci. 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Chin, E.W.M.; Lim, W.M.; Ma, D.; Rosales, F.J.; Goh, E.L.K. Choline Rescues Behavioural Deficits in a Mouse Model of Rett Syndrome by Modulating Neuronal Plasticity. Mol. Neurobiol. 2019, 56, 3882–3896. [Google Scholar] [CrossRef]
- Hunter, S.K.; Hoffman, M.C.; D’Alessandro, A.; Walker, V.K.; Balser, M.; Noonan, K.; Law, A.J.; Freedman, R. Maternal Prenatal Choline and Inflammation Effects on 4-Year-Olds’ Performance on the Wechsler Preschool and Primary Scale of Intelligence-IV. J. Psychiatr. Res. 2021, 141, 50–56. [Google Scholar] [CrossRef]
- Freedman, R.; Hunter, S.K.; Law, A.J.; Wagner, B.D.; D’Alessandro, A.; Christians, U.; Noonan, K.; Wyrwa, A.; Hoffman, M.C. Higher Gestational Choline Levels in Maternal Infection Are Protective for Infant Brain Development. J. Pediatr. 2019, 208, 198–206.e2. [Google Scholar] [CrossRef]
- Cermak, J.M.; Holler, T.; Jackson, D.A.; Blusztajn, J.K. Prenatal Availability of Choline Modifies Development of the Hippocampal Cholinergic System. FASEB J. 1998, 12, 349–357. [Google Scholar] [CrossRef]
- Thomas, J.D.; La Fiette, M.H.; Quinn, V.R.E.; Riley, E.P. Neonatal Choline Supplementation Ameliorates the Effects of Prenatal Alcohol Exposure on a Discrimination Learning Task in Rats. Neurotoxicol. Teratol. 2000, 22, 703–711. [Google Scholar] [CrossRef]
- Waddell, J.; Mooney, S.M. Choline and Working Memory Training Improve Cognitive Deficits Caused by Prenatal Exposure to Ethanol. Nutrients 2017, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Kroupina, M.G.; Miller, N.C.; Boys, C.J.; Brearley, A.M.; Fink, B.A.; Hoecker, H.L.; Zeisel, S.H.; et al. Choline Supplementation in Children with Fetal Alcohol Spectrum Disorders Has High Feasibility and Tolerability. Nutr. Res. 2013, 33, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Fuglestad, A.J.; Eckerle, J.K.; Fink, B.A.; Hoecker, H.L.; Boys, C.J.; Radke, J.P.; Kroupina, M.G.; Miller, N.C.; Brearley, A.M.; et al. Choline Supplementation in Children with Fetal Alcohol Spectrum Disorders: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2015, 102, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W.; Raith, M.; Shunova, A.; Lorenz, S.; Böckmann, K.; Minarski, M.; Poets, C.F.; Franz, A.R. Choline Kinetics in Neonatal Liver, Brain and Lung—Lessons from a Rodent Model for Neonatal Care. Nutrients 2022, 14, 720. [Google Scholar] [CrossRef]
- Northington, F.J.; Kratimenos, P.; Turnbill, V.; Flock, D.L.; Asafu-Adjaye, D.; Chavez-Valdez, R.; Martin, L.J. Basal Forebrain Magnocellular Cholinergic Systems Are Damaged in Mice Following Neonatal Hypoxia-Ischemia. J. Comp. Neurol. 2022, 530, 1148–1163. [Google Scholar] [CrossRef]
- Pang, R.; Martinello, K.A.; Meehan, C.; Avdic-Belltheus, A.; Lingam, I.; Sokolska, M.; Mutshiya, T.; Bainbridge, A.; Golay, X.; Robertson, N.J. Proton Magnetic Resonance Spectroscopy Lactate/N-Acetylaspartate Within 48 h Predicts Cell Death Following Varied Neuroprotective Interventions in a Piglet Model of Hypoxia–Ischemia with and Without Inflammation-Sensitization. Front. Neurol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Pavlakis, S.G.; Kingsley, P.B.; Harper, R.; Buckwald, S.; Spinazzola, R.; Frank, Y.; Prohovnik, I. Correlation of Basal Ganglia Magnetic Resonance Spectroscopy with Apgar Score in Perinatal Asphyxia. Arch. Neurol. 1999, 56, 1476–1481. [Google Scholar] [CrossRef]
- Cady, E.B. Metabolite Concentrations and Relaxation in Perinatal Cerebral Hypoxic-Ischemic Injury. Neurochem. Res. 1996, 21, 1043–1052. [Google Scholar] [CrossRef]
- Lucke, A.M.; Shetty, A.N.; Hagan, J.L.; Walton, A.; Stafford, T.D.; Chu, Z.D.; Rhee, C.J.; Kaiser, J.R.; Sanz Cortes, M. Early Proton Magnetic Resonance Spectroscopy during and after Therapeutic Hypothermia in Perinatal Hypoxic–Ischemic Encephalopathy. Pediatr. Radiol. 2019, 49, 941–950. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Makarewicz, D.; Stańczak-Mrozek, K.; Grieb, P. CDP-Choline (Citicoline) Attenuates Brain Damage in a Rat Model of Birth Asphyxia. Acta Neurobiol. Exp. (Wars). 2008, 68, 389–397. [Google Scholar]
- Brandt, M.J.V.; Nijboer, C.H.; Nessel, I.; Mutshiya, T.R.; Michael-titus, A.T.; Counotte, D.S.; Schipper, L.; van der Aa, N.E.; Benders, M.J.N.L.; de Theije, C.G.M. Nutritional Supplementation Reduces Lesion Size and Neuroinflammation in a Sex-dependent Manner in a Mouse Model of Perinatal Hypoxic-ischemic Brain Injury. Nutrients 2022, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The Influence of Immaturity on Hypoxic-Ischemic Brain Damage in the Rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Pierce, L.; Ciardiello, A.; Hutton, A.; Paskewitz, S.; Aronowitz, E.; Voss, H.U.; Moore, H.; Vannucci, S.J. Therapeutic Hypothermia and Hypoxia-Ischemia in the Term-Equivalent Neonatal Rat: Characterization of a Translational Preclinical Model. Pediatr. Res. 2015, 78, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Christian, K.M.; Thompson, R.F. Neural Substrates of Eyeblink Conditioning: Acquisition and Retention. Learn. Mem. 2003, 11, 427–455. [Google Scholar] [CrossRef]
- Waddell, J.; Hill, E.; Tang, S.; Jiang, L.; Xu, S.; Mooney, S.M. Choline plus Working Memory Training Improves Prenatal Alcohol-Induced Deficits in Cognitive Flexibility and Functional Connectivity in Adulthood in Rats. Nutrients 2020, 12, 3513. [Google Scholar] [CrossRef]
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline Intake during Pregnancy and Child Cognition at Age 7 Years. Am. J. Epidemiol. 2013, 177, 1338–1347. [Google Scholar] [CrossRef]
- Wu, B.T.F.; Dyer, R.A.; King, D.J.; Richardson, K.J.; Innis, S.M. Early Second Trimester Maternal Plasma Choline and Betaine Are Related to Measures of Early Cognitive Development in Term Infants. PLoS ONE 2012, 7, e43448. [Google Scholar] [CrossRef]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal Choline Supplementation during the Third Trimester of Pregnancy Improves Infant Information Processing Speed: A Randomized, Double-Blind, Controlled Feeding Study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef]
- Merz, E.C.; Monk, C.; Bansal, R.; Sawardekar, S.; Lee, S.; Feng, T.; Spann, M.; Foss, S.; McDonough, L.; Werner, E.; et al. Neonatal Brain Metabolite Concentrations: Associations with Age, Sex, and Developmental Outcomes. PLoS ONE 2020, 15, e0243255. [Google Scholar] [CrossRef]
- Shibasaki, J.; Aida, N.; Morisaki, N.; Tomiyasu, M.; Nishi, Y.; Toyoshima, K. Changes in Brain Metabolite Concentrations after Neonatal Hypoxic-Ischemic Encephalopathy. Radiology 2018, 288, 840–848. [Google Scholar] [CrossRef]
- Kreis, R.; Hofmann, L.; Kuhlmann, B.; Boesch, C.; Bossi, E.; Hüppi, P.S. Brain Metabolite Composition during Early Human Brain Development as Measured by Quantitative in Vivo 1H Magnetic Resonance Spectroscopy. Magn. Reson. Med. 2002, 48, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Etchell, A.; Adhikari, A.; Weinberg, L.S.; Choo, A.L.; Garnett, E.O.; Chow, H.M.; Chang, S.E. A Systematic Literature Review of Sex Differences in Childhood Language and Brain Development. Neuropsychologia 2018, 114, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Demarest, T.G.; Waite, E.L.; Kristian, T.; Puche, A.C.; Waddell, J.; McKenna, M.C.; Fiskum, G. Sex-Dependent Mitophagy and Neuronal Death Following Rat Neonatal Hypoxia–Ischemia. Neuroscience 2016, 335, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xu, S.; Lu, X.; Gullapalli, R.P.; McKenna, M.C.; Waddell, J. Neuroprotective Effects of Acetyl- L -Carnitine on Neonatal Hypoxia Ischemia-Induced Brain Injury in Rats. Dev. Neurosci. 2017, 38, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Reeb-Sutherland, B.C.; Fox, N.A. Eyeblink Conditioning: A Non-Invasive Biomarker for Neurodevelopmental Disorders. J. Autism Dev. Disord. 2015, 45, 376–394. [Google Scholar] [CrossRef]
- Ivkovich, D.; Paczkowski, C.M.; Stanton, M.E. Ontogeny of Delay versus Trace Eyeblink Conditioning in the Rat. Dev. Psychobiol. 2000, 36, 148–160. [Google Scholar] [CrossRef]
- Freeman, J.H. Cerebellar Learning Mechanisms. Brain Res. 2015, 1621, 260–269. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Stanton, M.E.; Dodge, N.C.; Pienaar, M.; Fuller, D.S.; Molteno, C.D.; Meintjes, E.M.; Hoyme, H.E.; Robinson, L.K.; Khaole, N.; et al. Impaired Delay and Trace Eyeblink Conditioning in School-Age Children with Fetal Alcohol Syndrome. Alcohol. Clin. Exp. Res. 2011, 35, 250–264. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Risbud, R.D.; Mattson, S.N.; Chambers, C.D.; Thomas, J.D. Choline Supplementation in School-Aged Children with Fetal Alcohol Spectrum Disorders. Am. J. Clin. Nutr. 2016, 104, 1683–1692. [Google Scholar] [CrossRef]
- Thomas, J.D.; Tran, T.D. Choline Supplementation Mitigates Trace, but Not Delay, Eyeblink Conditioning Deficits in Rats Exposed to Alcohol during Development. Hippocampus 2012, 22, 619–630. [Google Scholar] [CrossRef]
- Maeng, L.Y.; Waddell, J.; Shors, T.J. The Prefrontal Cortex Communicates with the Amygdala to Impair Learning after Acute Stress in Females but Not in Males. J. Neurosci. 2010, 30, 16188–16196. [Google Scholar] [CrossRef] [PubMed]
- Waddell, J.; Mallimo, E.; Shors, T. D-Cycloserine Reverses the Detrimental Effects of Stress on Learning in Females and Enhances Retention in Males. Neurobiol. Learn. Mem. 2010, 93, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Waddell, J.; Bangasser, D.A.; Shors, T.J. The Basolateral Nucleus of the Amygdala Is Necessary to Induce the Opposing Effects of Stressful Experience on Learning in Males and Females. J. Neurosci. 2008, 28, 5290–5294. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Klatt, K.C.; McDougall, M.Q.; Malysheva, O.V.; Taesuwan, S.; Loinard-González, A.P.; Nevins, J.E.H.; Beckman, K.; Bhawal, R.; Anderson, E.; Zhang, S.; et al. Prenatal Choline Supplementation Improves Biomarkers of Maternal Docosahexaenoic Acid Status among Pregnant Participants Consuming Supplemental DHA: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2022, 116, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bamford, P.; Jones, J.; He, M.; Kane, M.A.; Mooney, S.M.; Bearer, C.F. Choline Partially Prevents the Impact of Ethanol on the Lipid Raft Dependent Functions of L1 Cell Adhesion Molecule. Alcohol. Clin. Exp. Res. 2014, 38, 2722–2730. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemo, T.; Jaiyesimi, A.; Bumgardner, J.G.; Lohr, C.; Banerjee, A.; McKenna, M.C.; Waddell, J. Choline Improves Neonatal Hypoxia-Ischemia Induced Changes in Male but Not Female Rats. Int. J. Mol. Sci. 2022, 23, 13983. https://doi.org/10.3390/ijms232213983
Adeyemo T, Jaiyesimi A, Bumgardner JG, Lohr C, Banerjee A, McKenna MC, Waddell J. Choline Improves Neonatal Hypoxia-Ischemia Induced Changes in Male but Not Female Rats. International Journal of Molecular Sciences. 2022; 23(22):13983. https://doi.org/10.3390/ijms232213983
Chicago/Turabian StyleAdeyemo, Tayo, Ayodele Jaiyesimi, Jill G. Bumgardner, Charity Lohr, Aditi Banerjee, Mary C. McKenna, and Jaylyn Waddell. 2022. "Choline Improves Neonatal Hypoxia-Ischemia Induced Changes in Male but Not Female Rats" International Journal of Molecular Sciences 23, no. 22: 13983. https://doi.org/10.3390/ijms232213983
APA StyleAdeyemo, T., Jaiyesimi, A., Bumgardner, J. G., Lohr, C., Banerjee, A., McKenna, M. C., & Waddell, J. (2022). Choline Improves Neonatal Hypoxia-Ischemia Induced Changes in Male but Not Female Rats. International Journal of Molecular Sciences, 23(22), 13983. https://doi.org/10.3390/ijms232213983




