Genome-Wide Identification of the Aconitase Gene Family in Tomato (Solanum lycopersicum) and CRISPR-Based Functional Characterization of SlACO2 on Male-Sterility
Abstract
1. Introduction
2. Results
2.1. In Silico Functional and Expressional Characterization of Tomato SlACO Genes and Determination of the SlACO2 Expression Level by RT-qPCR
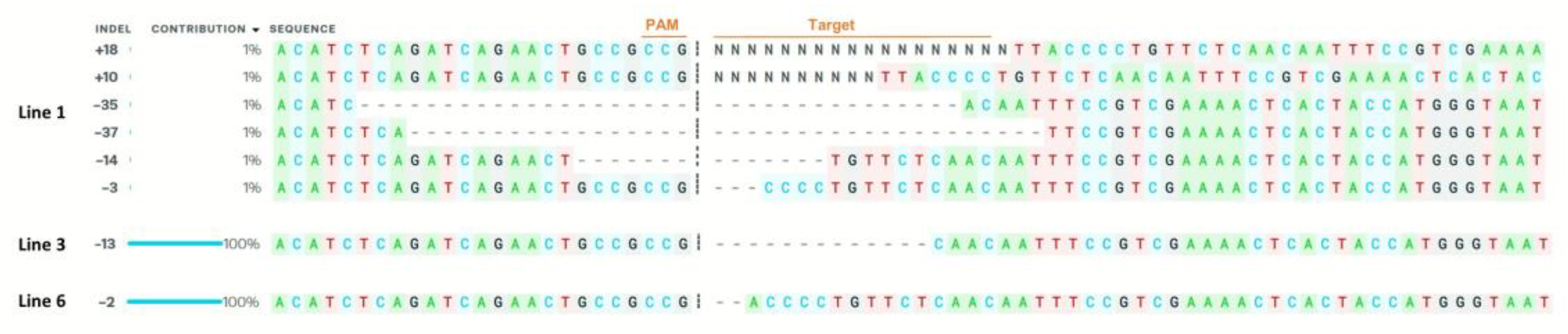
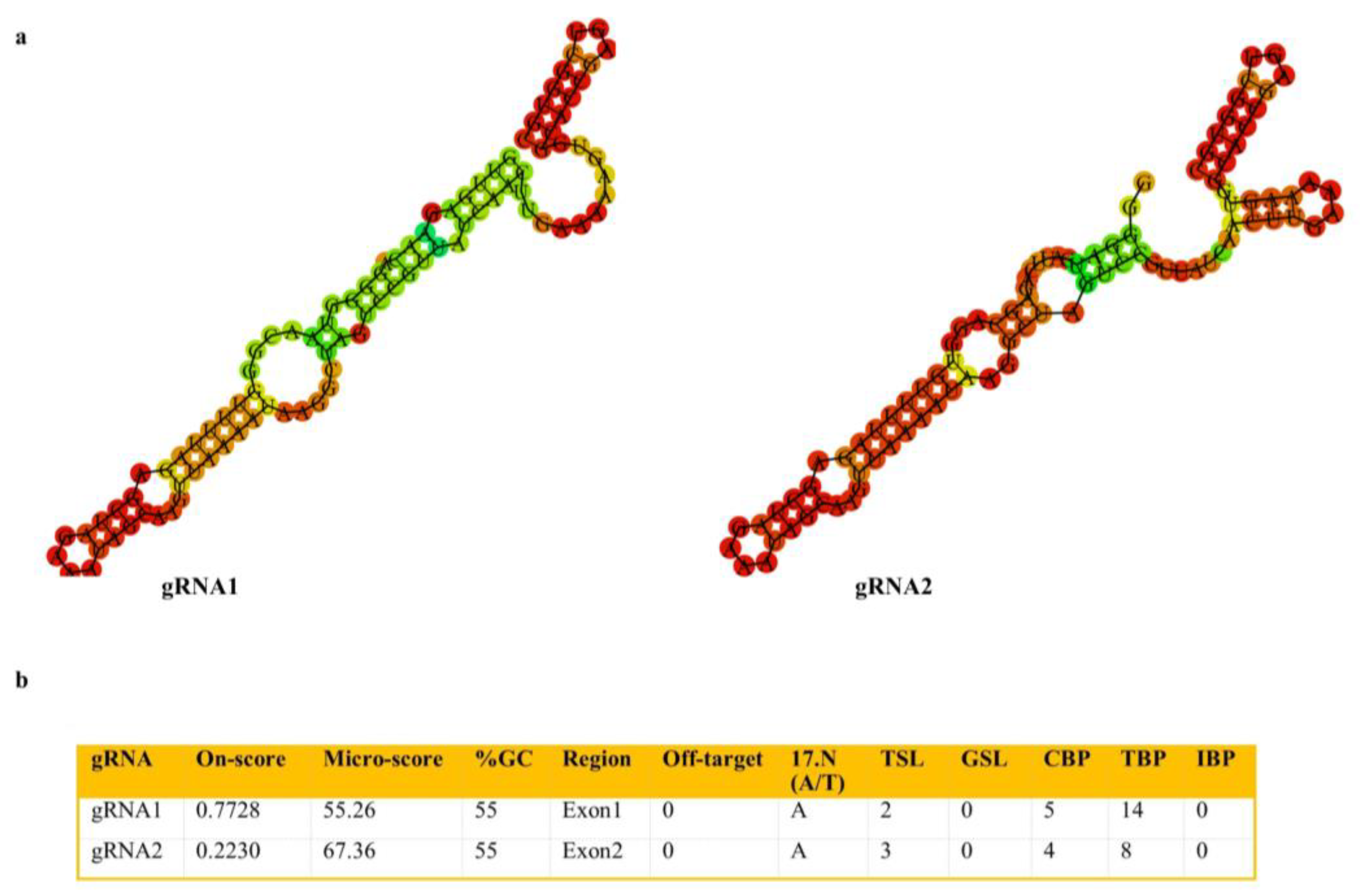
2.2. Generation of SlACO2 Mutants Using the CRISPR/Cas9 Gene-Editing System
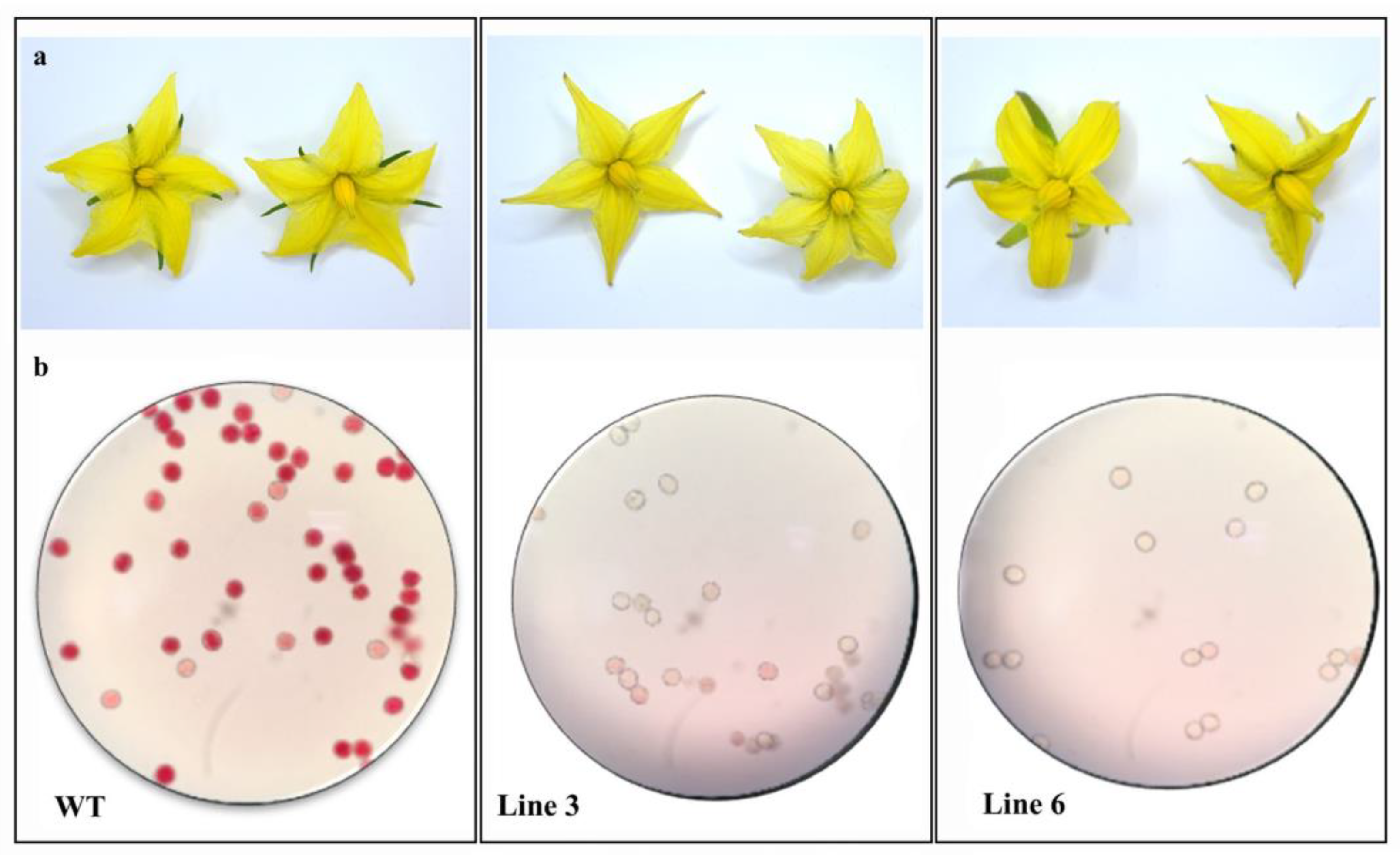
2.3. Control of Pollen Viability and Determination of Seed Formation in SlACO2 Mutant Lines
2.4. Expression Analysis of SlACO2
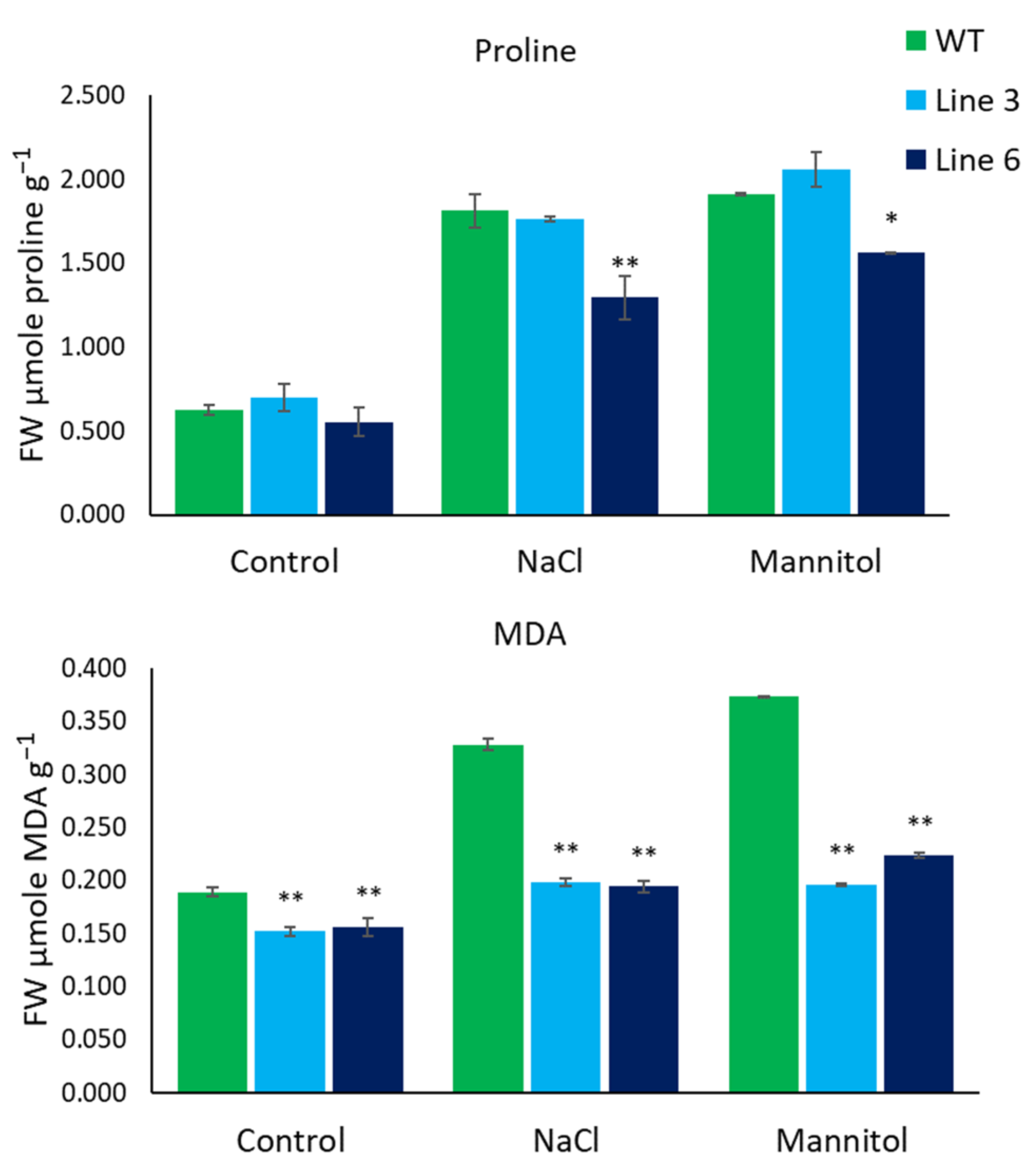
2.5. Evaluation of the Effect of Salt and Drought Stress on Genome-Edited Lines
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification and Analysis of SlACO Genes
4.2. Phylogenetic Analysis and Multiple Sequence Alignment of SlACO Genes
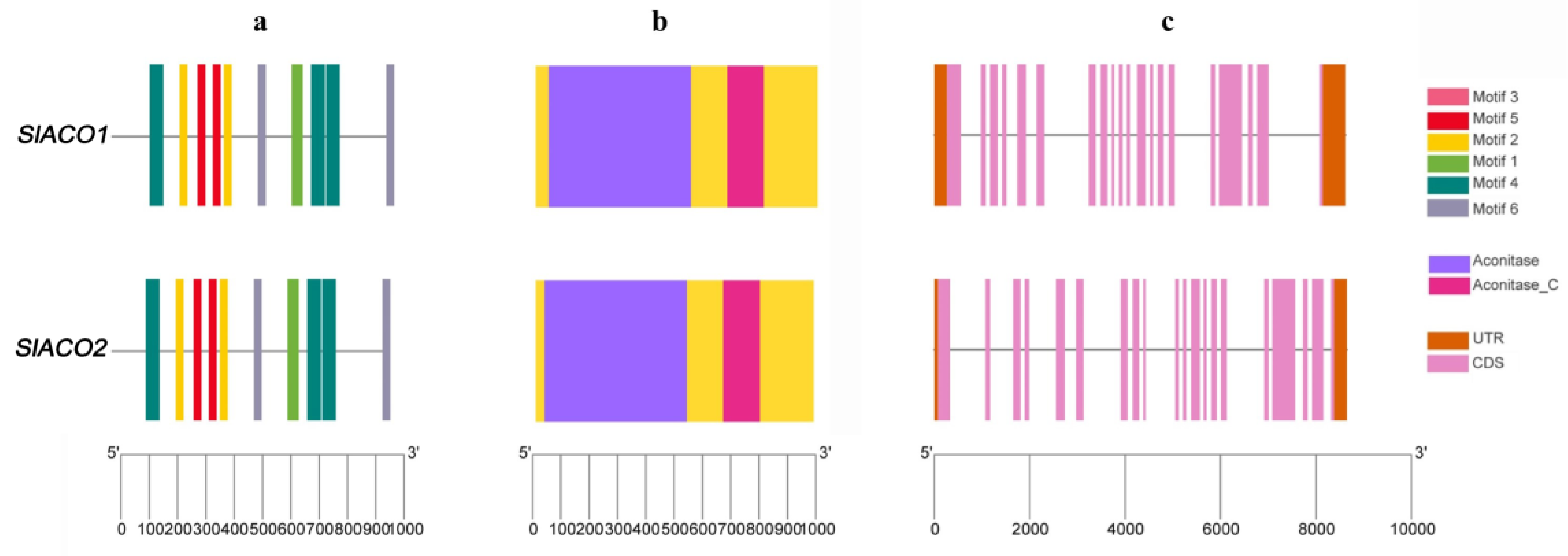
4.3. Exon/intron Structure and Conserved Motif Analysis of SlACO Genes
4.4. Chromosome Location and Determination of Gene Duplications of SlACO
4.5. In Silico Expression Analysis of Tomato SlACO Genes
4.6. Construction of Cas9/gRNA-Expressing Vectors
4.7. Generation of Transgenic CRISPR/Cas9 Mutant Lines in Tomato
4.8. Identification of Transgenes and CRISPR/Cas9 Mutation
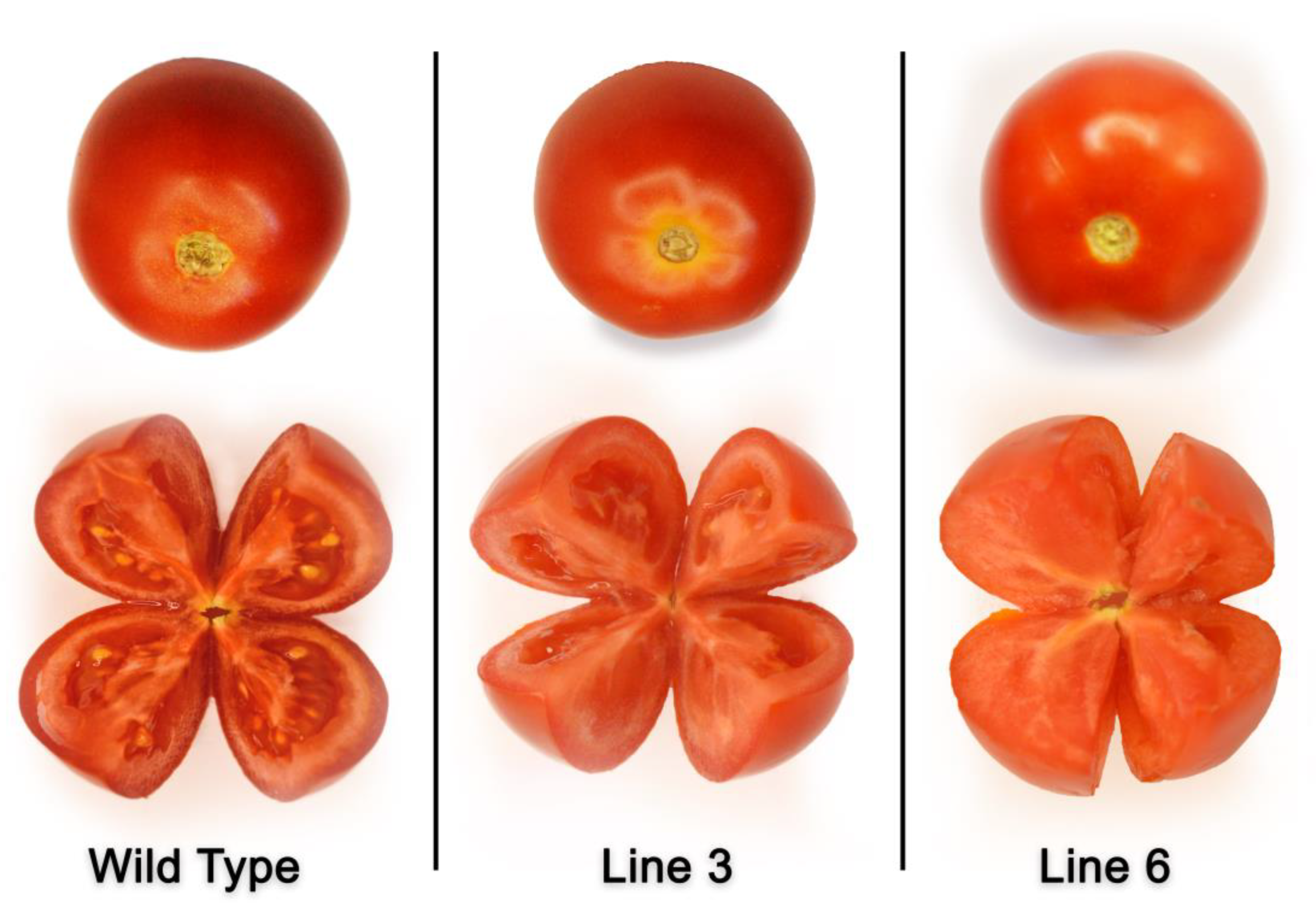
4.9. Characterizing the Morphology of Mutant Plants
4.10. Physiological Analysis of Genome-Edited Plants
4.11. Total RNA Isolation and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, R.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. CRISPR/Cas9-mediated mutagenesis of lncRNA1459 alters tomato fruit ripening. Plant J. 2018, 94, 513–524. [Google Scholar] [CrossRef]
- Refik, B. Commonalities of Molecular Response in Tomato Plants against Parasitic Nematodes. Biol. Bull. 2021, 48, S12–S21. [Google Scholar] [CrossRef]
- Bozbuga, R. Molecular analysis of nematode-responsive defence genes CRF1, WRKY45, and PR7 in Solanum lycopersicum tissues during the infection of plant-parasitic nematode species of the genus Meloidogyne. Genome 2022, 65, 265–275. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; An, X.; Xie, K.; Dong, Z.; Zhou, Y.; Xu, L.; Fang, W.; Liu, S.; Liu, S.; et al. Construction of a multicontrol sterility system for a maize male-sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD-finger transcription factor. Plant Biotechnol. J. 2018, 16, 459–471. [Google Scholar] [CrossRef]
- Bao, H.; Ding, Y.; Yang, F.; Zhang, J.; Xie, J.; Zhao, C.; Du, K.; Zeng, Y.; Zhao, K.; Li, Z.; et al. Gene silencing, knockout and over-expression of a transcription factor ABORTED MICROSPORES (SlAMS) strongly affects pollen viability in tomato (Solanum lycopersicum). BMC Genom. 2022, 23, 346. [Google Scholar] [CrossRef]
- Carroll, D. Genome Engineering With Zinc-Finger Nucleases. Genetics 2011, 188, 773–782. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Wang, D.; Samsulrizal, N.H.; Yan, C.; Allcock, N.S.; Craigon, J.; Blanco-Ulate, B.; Ortega-Salazar, I.; Marcus, S.E.; Bagheri, H.M.; Fons, L.P.; et al. Characterization of CRISPR Mutants Targeting Genes Modulating Pectin Degradation in Ripening Tomato. Plant Physiol. 2018, 179, 544–557. [Google Scholar]
- Chen, G.; Fillebeen, C.; Wang, J.; Pantopoulos, K. Overexpression of iron regulatory protein 1 suppresses growth of tumor xenografts. Carcinogenesis 2007, 28, 785–791. [Google Scholar] [CrossRef][Green Version]
- Moeder, W.; del Pozo, O.; Navarre, D.A.; Martin, G.B.; Klessig, D.F. Aconitase plays a role in regulating resistance to oxidative stress and cell death in Arabidopsis and Nicotiana benthamiana. Plant Mol. Biol. 2007, 63, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Heazlewood, J.; Tonti-Filippini, J.; Gout, A.M.; Day, D.; Whelan, J.; Millar, A.H. Experimental Analysis of the Arabidopsis Mitochondrial Proteome Highlights Signaling and Regulatory Components, Provides Assessment of Targeting Prediction Programs, and Indicates Plant-Specific Mitochondrial Proteins. Plant Cell 2004, 16, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Hooks, M.A.; Allwood, J.W.; Harrison, J.K.D.; Kopka, J.; Erban, A.; Goodacre, R.; Balk, J. Selective induction and subcellular distribution of ACONITASE 3 reveal the importance of cytosolic citrate metabolism during lipid mobilization in Arabidopsis. Biochem. J. 2014, 463, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, R.-Y.; Li, H.; Xu, R.-F.; Qiu, C.-H.; Sun, Y.-C.; Ma, H.; Yang, Y.-C.; Ni, D.-H.; Li, L.; et al. Identification and analysis of the mechanism underlying heat-inducible expression of rice aconitase 1. Plant Sci. 2015, 233, 22–31. [Google Scholar] [CrossRef]
- Du, K.; Liu, Q.; Wu, X.; Jiang, J.; Wu, J.; Fang, Y.; Li, A.; Wang, Y. Morphological Structure and Transcriptome Comparison of the Cytoplasmic Male Sterility Line in Brassica napus (SaNa-1A) Derived from Somatic Hybridization and Its Maintainer Line SaNa-1B. Front. Plant Sci. 2016, 7, 1313. [Google Scholar] [CrossRef] [PubMed]
- Peyret, P.; Perez, P.; Alric, M. Structure, Genomic Organization, and Expression of the Arabidopsis thaliana Aconitase Gene: Plant aconitase show significant homology with mammalian iron-responsive element-binding protein. J. Biol. Chem. 1995, 270, 8131–8137. [Google Scholar] [CrossRef]
- Arnaud, N.; Ravet, K.; Borlotti, A.; Touraine, B.; Boucherez, J.; Fizames, C.; Briat, J.; Cellier, F.; Gaymard, F. The iron-responsive element (IRE)/iron-regulatory protein 1 (IRP1)–cytosolic aconitase iron-regulatory switch does not operate in plants. Biochem. J. 2007, 405, 523–531. [Google Scholar] [CrossRef]
- Li, M.; Sun, L.; Gu, H.; Cheng, D.; Guo, X.; Chen, R.; Wu, Z.; Jiang, J.; Fan, X.; Chen, J. Genome-wide characterization and analysis of bHLH transcription factors related to anthocyanin biosynthesis in spine grapes (Vitis davidii). Sci. Rep. 2021, 11, 6863. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Y.; Wei, L.; Yu, H.; Cao, Y.; Li, Y.; Yang, N.; Song, Y.; Liang, C.; Wang, T. Transcriptomics analyses reveal the molecular roadmap and long non-coding RNA landscape of sperm cell lineage development. Plant J. 2018, 96, 421–437. [Google Scholar] [CrossRef]
- Gökdemir, G.; Seçgin, Z.; Uluisik, S.; Kavas, M. CRISPR/Cas9 knock-out of SlPHD_MS1 (Solyc04g008420) gene results in complete male sterility in tomato. Plant Growth Regul. 2022, 98, 329–341. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, D.H.; Lee, H.J.; Nam, K.H.; Bae, S.; Nou, I.S.; Cho, Y.-G.; Kim, M.K.; Kang, K.K. Knockout of SlMS10 Gene (Solyc02g079810) Encoding bHLH Transcription Factor Using CRISPR/Cas9 System Confers Male Sterility Phenotype in Tomato. Plants 2020, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhou, K.; Liu, Y.; Deng, L.; Zhang, X.; Lin, L.; Zhou, M.; Zhao, W.; Wen, C.; Xing, J.; et al. A biotechnology-based male-sterility system for hybrid seed production in tomato. Plant J. 2020, 102, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, C.; Zhu, J.; Liu, C.; Huang, C.; Li, X.; Xie, C. Genome Editing Enables Next-Generation Hybrid Seed Production Technology. Mol. Plant 2020, 13, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Terol, J.; Soler, G.; Talon, M.; Cercos, M. The aconitate hydratase family from Citrus. BMC Plant Biol 2010, 10, 222. [Google Scholar] [CrossRef]
- Paul, P.; Chaturvedi, P.; Selymesi, M.; Ghatak, A.; Mesihovic, A.; Scharf, K.-D.; Weckwerth, W.; Simm, S.; Schleiff, E. The membrane proteome of male gametophyte in Solanum lycopersicum. J. Proteom. 2016, 131, 48–60. [Google Scholar] [CrossRef]
- Roschzttardtz, H.; Fuentes, I.; Vásquez, M.; Corvalán, C.; León, G.; Gómez, I.; Araya, A.; Holuigue, L.; Vicente-Carbajosa, J.; Jordana, X. A Nuclear Gene Encoding the Iron-Sulfur Subunit of Mitochondrial Complex II Is Regulated by B3 Domain Transcription Factors during Seed Development in Arabidopsis. Plant Physiol. 2009, 150, 84–95. [Google Scholar] [CrossRef]
- van der Merwe, M.J.; Osorio, S.; Araújo, W.L.; Balbo, I.; Nunes-Nesi, A.; Maximova, E.; Carrari, F.; Bunik, V.I.; Persson, S.; Fernie, A.R. Tricarboxylic Acid Cycle Activity Regulates Tomato Root Growth via Effects on Secondary Cell Wall Production. Plant Physiol. 2010, 153, 611–621. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, G.; Sang, L.; Deng, Y.; Gao, L.; Yu, Y.; Liu, J. Mitochondrial citrate synthase plays important roles in anthocyanin synthesis in petunia. Plant Sci. 2021, 305, 110835. [Google Scholar] [CrossRef]
- Araújo, W.L.; Tohge, T.; Osorio, S.; Lohse, M.; Balbo, I.; Krahnert, I.; Sienkiewicz-Porzucek, A.; Usadel, B.; Nunes-Nesi, A.; Fernie, A.R. Antisense Inhibition of the 2-Oxoglutarate Dehydrogenase Complex in Tomato Demonstrates Its Importance for Plant Respiration and during Leaf Senescence and Fruit Maturation. Plant Cell 2012, 24, 2328–2351. [Google Scholar] [CrossRef]
- Carrari, F.; Nunes-Nesi, A.; Gibon, Y.; Lytovchenko, A.; Loureiro, M.E.; Fernie, A.R. Reduced Expression of Aconitase Results in an Enhanced Rate of Photosynthesis and Marked Shifts in Carbon Partitioning in Illuminated Leaves of Wild Species Tomato. Plant Physiol. 2003, 133, 1322–1335. [Google Scholar] [CrossRef]
- Robinson, J.M.; Bunce, J.A. Influence of Drought-Induced Water Stress on Soybean and Spinach Leaf Ascorbate-Dehydroascorbate Level and Redox Status. Int. J. Plant Sci. 2000, 161, 271–279. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Yuan, G.-F.; Jia, C.-G.; Li, Z.; Sun, B.; Zhang, L.-P.; Liu, N.; Wang, Q.-M. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci. Hortic. 2010, 126, 103–108. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Wang, L.; Chen, L.; Zhao, R.; Sheng, J.; Shen, L. Reduction of Tomato-Plant Chilling Tolerance by CRISPR–Cas9-Mediated SlCBF1 Mutagenesis. J. Agric. Food Chem. 2018, 66, 9042–9051. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Li, R.; Zhao, R.; Yang, M.; Sheng, J.; Shen, L. Reduced Drought Tolerance by CRISPR/Cas9-Mediated SlMAPK3 Mutagenesis in Tomato Plants. J. Agric. Food Chem. 2017, 65, 8674–8682. [Google Scholar] [CrossRef] [PubMed]
- Molinari, H.B.C.; Marur, C.J.; Filho, J.C.B.; Kobayashi, A.K.; Pileggi, M.; Júnior, R.P.L.; Pereira, L.F.P.; Vieira, L.G.E. Osmotic adjustment in transgenic citrus rootstock Carrizo citrange (Citrus sinensis Osb. x Poncirus trifoliata L. Raf.) overproducing proline. Plant Sci. 2004, 167, 1375–1381. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Seçgin, Z.; Kavas, M.; Yildirim, K. Optimization of Agrobacterium-mediated transformation and regeneration for CRISPR/Cas9 genome editing of commercial tomato cultivars. Turk. J. Agric. For. 2021, 45, 704–716. [Google Scholar] [CrossRef]
- Conant, D.; Hsiau, T.; Rossi, N.; Oki, J.; Maures, T.; Waite, K.; Yang, J.; Joshi, S.; Kelso, R.; Holden, K.; et al. Inference of CRISPR Edits from Sanger Trace Data. bioRxiv 2019, 251082. Available online: https://ice.synthego.com/#/ (accessed on 10 October 2022). [CrossRef] [PubMed]
- Liu, W.; Xie, X.; Ma, X.; Li, J.; Chen, J.; Liu, Y.-G. DSDecode: A web-based tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol. Plant 2015, 8, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Chromosome | Length (bp) | CDS (pb) | Protein Length (A.A) | Accession Number | pI | Molecular Weight (Kda) | Instability Index (II) | Stability | GRAVY | Solubility/Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SlACO1 | ch07 | 8621 | 2988 | 996 | XP_004243472.1 | 7.29 | 108.20 | 32.74 | stable | −0.178 | Soluble/0.552 |
| SlACO2 | ch12 | 8650 | 2946 | 982 | XP_004251517.2 | 6.52 | 107.15 | 35.57 | stable | −0.223 | Soluble/0.568 |
| Primer Name | Sequence 5′-3′ | PCR Product |
|---|---|---|
| sgRNA1-F | ATTGGTTGAGAACAGGGGTAACGG | |
| sgRNA1-R | AAACCCGTTACCCCTGTTCTCAAC | |
| sgRNA2-F | ATTGGGGGATCATTCAGAGCAGGT | |
| sgRNA2-R | AAACACCTGCTCTGAATGATCCCC | |
| Acos Tomato Seq F | CACTTTCCGATCGCTGAGGT | |
| Acos Tomato Seq R | AAGCAGTGGAGCAAGTCATTT | |
| hptII-F | CGAAAAGTTCGACAGCGTC | 450 bp |
| hptII-R | GGTGTCGTCCATCACAGTTTG | |
| SlActin-F | AGGCACACAGGTGTTATGGT | 186 bp |
| SlActin-R | AGCAACTCGAAGCTCATTGT | |
| SlACO2-qPCR-F | TCGCTGAGGTGGAGATACGGTGT | 84 bp |
| SlACO2-qPCR-R | AACAGGGGTAACGGCGGCAG | |
| M13 F seq primer (−20) | GTAAAACGACGGCCAGT | |
| M13 R seq primer (−20) | GTTTTCCCAGTCACGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secgin, Z.; Uluisik, S.; Yıldırım, K.; Abdulla, M.F.; Mostafa, K.; Kavas, M. Genome-Wide Identification of the Aconitase Gene Family in Tomato (Solanum lycopersicum) and CRISPR-Based Functional Characterization of SlACO2 on Male-Sterility. Int. J. Mol. Sci. 2022, 23, 13963. https://doi.org/10.3390/ijms232213963
Secgin Z, Uluisik S, Yıldırım K, Abdulla MF, Mostafa K, Kavas M. Genome-Wide Identification of the Aconitase Gene Family in Tomato (Solanum lycopersicum) and CRISPR-Based Functional Characterization of SlACO2 on Male-Sterility. International Journal of Molecular Sciences. 2022; 23(22):13963. https://doi.org/10.3390/ijms232213963
Chicago/Turabian StyleSecgin, Zafer, Selman Uluisik, Kubilay Yıldırım, Mohamed Farah Abdulla, Karam Mostafa, and Musa Kavas. 2022. "Genome-Wide Identification of the Aconitase Gene Family in Tomato (Solanum lycopersicum) and CRISPR-Based Functional Characterization of SlACO2 on Male-Sterility" International Journal of Molecular Sciences 23, no. 22: 13963. https://doi.org/10.3390/ijms232213963
APA StyleSecgin, Z., Uluisik, S., Yıldırım, K., Abdulla, M. F., Mostafa, K., & Kavas, M. (2022). Genome-Wide Identification of the Aconitase Gene Family in Tomato (Solanum lycopersicum) and CRISPR-Based Functional Characterization of SlACO2 on Male-Sterility. International Journal of Molecular Sciences, 23(22), 13963. https://doi.org/10.3390/ijms232213963








