Advances in Targeted Immunotherapy for Hepatobiliary Cancers
Abstract
1. Introduction
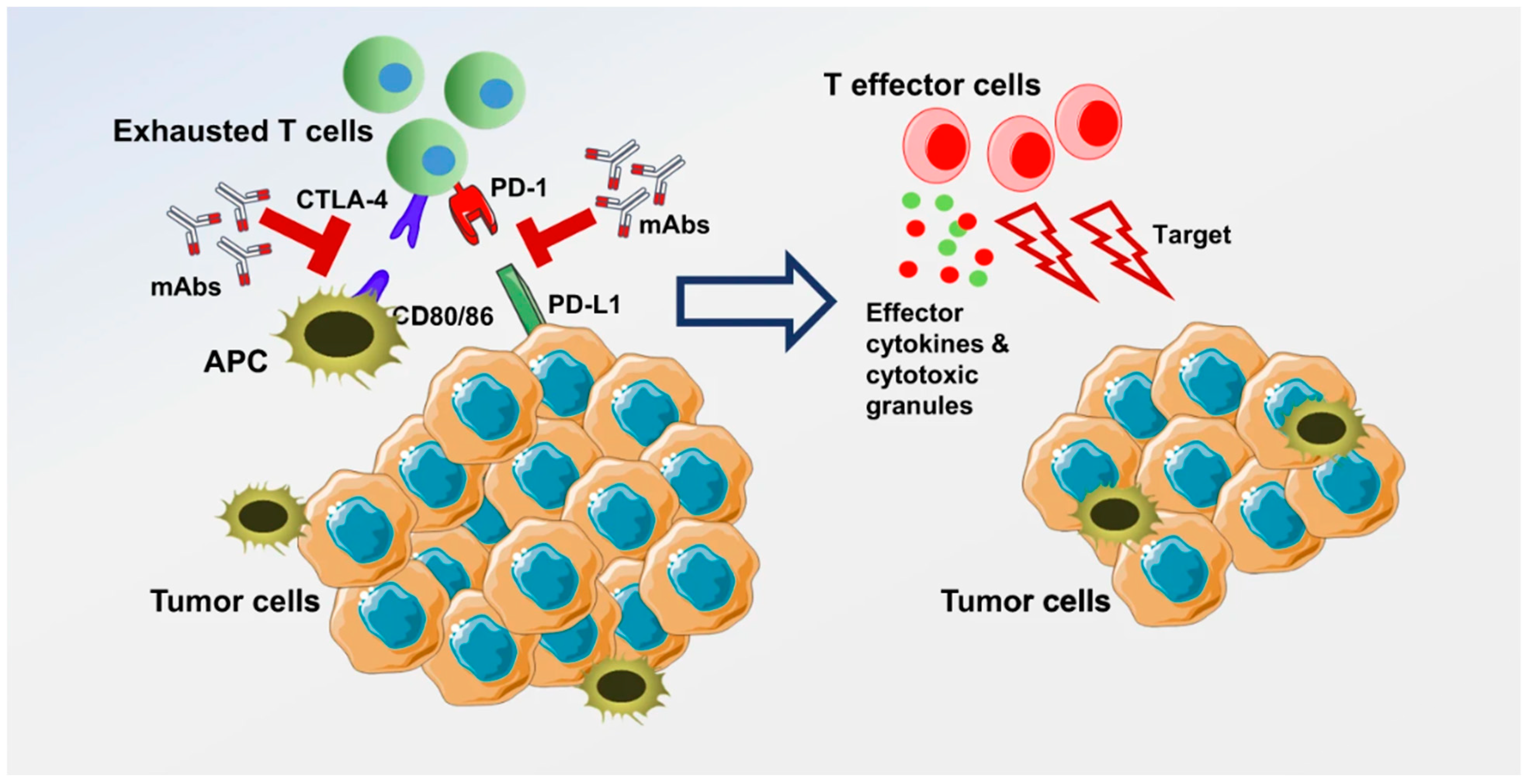
2. Immune Checkpoint Inhibitors
2.1. Tremelimumab and Durvalumab in HCC
2.2. Tremelimumab and Durvalumab in BTC
2.3. Nivolumab in HCC
2.4. Nivolumab in BTC
2.5. Pembrolizumab in HCC
2.6. Pembrolizumab in BTC
2.7. Atezolizumab in HCC
2.8. Atezolizumab in BTC
2.9. Other ICIs under Investigation for HCC
3. Adoptive Cell Therapy
3.1. Tumor Infiltrating Lymphocytes (TIL) in HCC
3.2. Tumor-Infiltrating Lymphocytes in BTC
3.3. Chimeric Antigen Receptor T Cell (CAR-T Cell) in HCC
3.4. CAR-T Cell in BTC
4. Vaccine Therapy
4.1. Vaccine Therapy in HCC
4.2. Vaccine Therapy in BTC
5. Challenges of Immunotherapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD-L1 | programmed death ligand 1 |
| PD-1 | programmed death 1 |
| VEGF | vascular endothelial growth factor |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| MMR | mismatch repair |
| IL-2 | interleukin 2 |
| TIL | tumor infiltrating lymphocytes |
| BTC | biliary tract cancers |
| HCC | hepatocellular carcinoma |
| CCA | cholangiocarcinoma |
| GBC | gallbladder cancer |
| AFP | alpha fetoprotein |
| GPC-3 | glypican-3 |
| MAGE | melanoma associated antigen |
| NY-ESO-1 | New York esophageal squamous cell carcinoma 1 |
| hTERT | human telomerase reverse transcriptase |
| NKG2DL | natural killer group 2, member D ligand |
| EpCAM | epithelial cellular adhesion molecule |
| CD133 | prominin-1 |
| CD147 | cluster of differentiation 147 |
| MUC1 | mucin 1 |
| EGFR | epidermal growth factor receptor |
| Her2 | human epidermal growth factor receptor |
| SSX-2 | synovial sarcoma X |
| APC | antigen presenting cell |
| NK | natural killer |
| CAR-T | chimeric antigen receptor T cell |
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Trends in Liver Cancer Mortality Among Adults Aged 25 and Over in the United States, 2000–2016. NCHS Data Brief 2018; pp. 1–8. Available online: https://pubmed.ncbi.nlm.nih.gov/30044212/ (accessed on 13 October 2022).
- Rizvi, S.; Gores, G.J. Pathogenesis, Diagnosis, and Management of Cholangiocarcinoma. Gastroenterology 2013, 145, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, T.; Kayahara, M.; Ikeda, S.; Futakawa, S.; Kakita, A.; Kawarada, H.; Matsuno, M.; Takada, T.; Takasaki, K.; Tanimura, H.; et al. Biliary tract cancer treatment: Results from the Biliary Tract Cancer Statistics Registry in Japan. J. Hepato-Biliary-Pancreatic Surg. 2002, 9, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Wasan, H.; Johnson, P.; Jones, E.; Dixon, L.; Swindell, R.; Baka, S.; Maraveyas, A.; Corrie, P.; Falk, S.; et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: A multicentre randomised phase II study—The UK ABC-01 Study. Br. J. Cancer 2009, 101, 621–627. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Glimelius, B.; Hoffman, K.; Sjödén, P.-O.; Jacobsson, G.; Sellström, H.; Enander, L.-K.; Linné, T.; Svensson, C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996, 7, 593–600. [Google Scholar] [CrossRef]
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’Alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers 2020, 12, 641. [Google Scholar] [CrossRef]
- Robinson, M.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021, 7, 651. [Google Scholar] [CrossRef]
- Keenan, B.P.; Fong, L.; Kelley, R.K. Immunotherapy in hepatocellular carcinoma: The complex inter-face between inflammation, fibrosis, and the immune response. J. Immunother. Cancer 2019, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.-K.; Qin, S.; Tai, D.W.-M.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients with Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Évid. 2022, 1. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Évid. 2022, 1. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2021, 23, 77–90. [Google Scholar] [CrossRef]
- Onuma, A.E.; Zhang, H.; Huang, H.; Williams, T.M.; Noonan, A.; Tsung, A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020, 20, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kassel, R.; Cruise, M.W.; Iezzoni, J.C.; Taylor, N.A.; Pruett, T.L.; Hahn, Y.S. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology 2009, 50, 1625–1637. [Google Scholar] [CrossRef]
- Wang, J.-Z. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J. Gastroenterol. 2011, 17, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Zhu, Q.; Durham, J.; Popovic, A.; Xavier, S.; Leatherman, J.; Mohan, A.; Mo, G.; Zhang, S.; Gross, N.; et al. Neoadjuvant cabozantinib and nivolumab convert locally advanced hepatocellular carcinoma into resectable disease with enhanced antitumor immunity. Nat. Cancer 2021, 2, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O.; Hasanov, E.; Cao, H.S.T.; Xiao, L.; Vauthey, J.-N.; Lee, S.S.; Yavuz, B.G.; I Mohamed, Y.; Qayyum, A.; Jindal, S.; et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: A randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 208–218. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef]
- Chiang, N.-J.; Tan, K.T.; Bai, L.-Y.; Hsiao, C.-F.; Huang, C.-Y.; Hung, Y.-P.; Huang, C.-J.; Chen, S.-C.; Shan, Y.-S.; Chao, Y.; et al. Impaired Chromatin Remodeling Predicts Better Survival to Modified Gemcitabine and S-1 plus Nivolumab in Advanced Biliary Tract Cancer: A Phase II T1219 Study. Clin. Cancer Res. 2022, 28, 4248–4257. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients with Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Zalupski, M.M. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer 2022, 128, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; et al. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Zhang, Y.; Liu, T.; Si, H.; Wang, Z.; Yan, H.; Qian, N.; Dai, G. PD-1 Inhibitors Could Improve the Efficacy of Chemotherapy as First-Line Treatment in Biliary Tract Cancers: A Propensity Score Matching Based Analysis. Front. Oncol. 2021, 11, 648068. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Monge, C.; Pehrsson, E.C.; Xie, C.; Duffy, A.G.; Mabry, D.; Wood, B.J.; E Kleiner, D.; Steinberg, S.M.; Figg, W.D.; Redd, B.; et al. A Phase II Study of Pembrolizumab in Combination with Capecitabine and Oxaliplatin with Molecular Profiling in Patients with Advanced Biliary Tract Carcinoma. Oncol. 2022, 27, e273–e285. [Google Scholar] [CrossRef]
- Yin, C.; Armstrong, S.A.; Agarwal, S.; Wang, H.; Noel, M.S.; Weinberg, B.A.; Marshall, J.; He, A.R. Phase II study of combination pembrolizumab and olaparib in patients with advanced cholangiocarcinoma: Interim results. J. Clin. Oncol. 2022, 40, 452. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2021, 76, 862–873. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, X.; Ma, H.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut 2015, 64, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Xu, Y.; Wang, Z.; Wang, T.; Du, X.; Song, X.; Guo, X.; Peng, J.; Zhang, J.; Liang, Y.; et al. Tim-3 hampers tumor surveillance of liver resident and conventional NK cells by disrupting PI3K signaling. Cancer Res. 2020, 80, 1130–1142. [Google Scholar] [CrossRef]
- Guo, M.; Qi, F.; Rao, Q.; Sun, J.; Du, X.; Qi, Z.; Yang, B.; Xia, J. Serum LAG-3 Predicts Outcome and Treatment Response in Hepatocellular Carcinoma Patients with Transarterial Chemoembolization. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Yan, Q.; Lin, H.-M.; Zhu, K.; Cao, Y.; Xu, X.-L.; Zhou, Z.-Y.; Xu, L.-B.; Liu, C.; Zhang, R. Immune Checkpoint FGL1 Expression of Circulating Tumor Cells Is Associated with Poor Survival in Curatively Resected Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 810269. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, Z.-L.; He, M.; Gao, Z.; Kuang, D.-M. BTLA identifies dysfunctional PD-1-expressing CD4+ T cells in human hepatocellular carcinoma. OncoImmunology 2016, 5, e1254855. [Google Scholar] [CrossRef]
- Guo, M.; Yuan, F.; Qi, F.; Sun, J.; Rao, Q.; Zhao, Z.; Huang, P.; Fang, T.; Yang, B.; Xia, J. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+T cells in hepatocellular carcinoma using multiplex quantitative analysis. J. Transl. Med. 2020, 18, 306. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Seah, Y.H.J.; Fang, J.; Orpilla, N.; Lee, J.N.L.W.; Toh, H.C.; Choo, S.P.; Lim, K.H.; Tai, W.M.D.; Yeong, J. 89 The immune marker LAG-3 increases the predictive value of CD38+ immune cells for survival outcome in immunotherapy-treated hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, A97–A98. [Google Scholar] [CrossRef]
- Jiang, S.-S.; Tang, Y.; Zhang, Y.-J.; Weng, D.-S.; Zhou, Z.-G.; Pan, K.; Pan, Q.-Z.; Wang, Q.-J.; Liu, Q.; He, J.; et al. A phase I clinical trial utilizing autologous tumor-infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Oncotarget 2015, 6, 41339–41349. [Google Scholar] [CrossRef]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 1–18. [Google Scholar] [CrossRef]
- Hodi, F.S.; Dranoff, G. The biologic importance of tumor-infiltrating lymphocytes. J. Cutan. Pathol. 2010, 37, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-R.; Corrales, L.; Gajewski, T.F. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015, 36, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Diggs, L.P.; Ruf, B.; Ma, C.; Heinrich, B.; Cui, L.; Zhang, Q.; McVey, J.C.; Wabitsch, S.; Heinrich, S.; Rosato, U.; et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 74, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-R.; Wu, C.-E.; Chen, M.-H.; Huang, W.-K.; Shih, H.-J.; Lan, K.-L.; Yeh, C.-N. Comprehensive Evaluation of Immune-Checkpoint DNA Cancer Vaccines in a Rat Cholangiocarcinoma Model. Vaccines 2020, 8, 703. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, T.; Umebayashi, M.; Kiyota, A.; Koya, N.; Tanaka, H.; Onishi, H.; Katano, M. Combining cetuximab with killer lymphocytes synergistically inhibits human cholangiocarcinoma cells in vitro. Anticancer Res. 2012, 32, 2249–2256. [Google Scholar] [PubMed]
- Sawasdee, N.; Thepmalee, C.; Sujjitjoon, J.; Yongpitakwattana, P.; Junking, M.; Poungvarin, N.; Yenchitsomanus, P.-T.; Panya, A. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int. Immunopharmacol. 2019, 78, 106006. [Google Scholar] [CrossRef] [PubMed]
- Rochigneux, P.; Chanez, B.; De Rauglaudre, B.; Mitry, E.; Chabannon, C.; Gilabert, M. Adoptive Cell Therapy in Hepatocellular Carcinoma: Biological Rationale and First Results in Early Phase Clinical Trials. Cancers 2021, 13, 271. [Google Scholar] [CrossRef]
- Shi, D.; Shi, Y.; Kaseb, A.O.; Qi, X.; Zhang, Y.; Chi, J.; Lu, Q.; Gao, H.; Jiang, H.; Wang, H.; et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. 2020, 26, 3979–3989. [Google Scholar] [CrossRef]
- McKiver, B.; Damaj, M.I.; Sarkar, D. Assessment of Current Gene Therapy Practices in Hepatocellular Carcinoma. Gastrointest. Disord. 2020, 2, 469–480. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. OncoImmunology 2018, 7, e1440169. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Tong, C.; Shi, D.; Chen, M.; Guo, Y.; Chen, D.; Han, X.; Wang, H.; Wang, Y.; Shen, P. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: A single-arm, open-label, phase II trial. OncoImmunology 2020, 9, 1846926. [Google Scholar] [CrossRef] [PubMed]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.-T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Phanthaphol, N.; Somboonpatarakun, C.; Suwanchiwasiri, K.; Chieochansin, T.; Sujjitjoon, J.; Wongkham, S.; Maher, J.; Junking, M.; Yenchitsomanus, P.-T. Chimeric Antigen Receptor T Cells Targeting Integrin αvβ6 Expressed on Cholangiocarcinoma Cells. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.-C.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.-M.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor–Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- E Kam, A.; Masood, A.; Shroff, R.T. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroenterol. Hepatol. 2021, 6, 956–969. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2017, 9, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.Y.; Williams, G.R.; Paluri, R.K. CAR T Cell Therapy in Pancreaticobiliary Cancers: A Focused Review of Clinical Data. J. Gastrointest. Cancer 2020, 52, 1–10. [Google Scholar] [CrossRef]
- Repáraz, D.; Aparicio, B.; Llopiz, D.; Hervás-Stubbs, S.; Sarobe, P. Therapeutic Vaccines against Hepatocellular Carcinoma in the Immune Checkpoint Inhibitor Era: Time for Neoantigens? Int. J. Mol. Sci. 2022, 23, 2022. [Google Scholar] [CrossRef]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.-C.; Lee, W.-B.; Lee, H.-S.; Bae, Y.-S.; et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef]
- Löffler, M.W.; Gori, S.; Izzo, F.; Mayer-Mokler, A.; Ascierto, P.A.; Königsrainer, A.; Ma, Y.T.; Sangro, B.; Francque, S.; Vonghia, L.; et al. Phase I/II Multicenter Trial of a Novel Therapeutic Cancer Vaccine, HepaVac-101, for Hepatocellular Carcinoma. Clin. Cancer Res. 2022, 28, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, T.; Zhang, G.; Liang, T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol. Cancer 2021, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kaida, M.; Morita-Hoshi, Y.; Soeda, A.; Wakeda, T.; Yamaki, Y.; Kojima, Y.; Ueno, H.; Kondo, S.; Morizane, C.; Ikeda, M.; et al. Phase 1 Trial of Wilms Tumor 1 (WT1) Peptide Vaccine and Gemcitabine Combination Therapy in Patients with Advanced Pancreatic or Biliary Tract Cancer. J. Immunother. 2011, 34, 92–99. [Google Scholar] [CrossRef]
- Okusaka, T.; Ueno, M.; Sato, T.; Heike, Y. Possibility of immunotherapy for biliary tract cancer: How do we prove efficacy? Introduction to a current ongoing phase I and randomized phase II study to evaluate the efficacy and safety of adding Wilms tumor 1 peptide vaccine to gemcitabine and cisplatin for the treatment of advanced bili-ary tract cancer (WT-BT trial). J. Hepato-Biliary-Pancreatic Sci. 2012, 19, 314–318. [Google Scholar] [CrossRef]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef]
- Murahashi, M.; Tsuruta, T.; Yamada, K.; Hijikata, Y.; Ogata, H.; Kishimoto, J.; Yoshimura, S.; Hikichi, T.; Nakanishi, Y.; Tani, K. Clinical Trial of a Cancer Vaccine Targeting VEGF and KIF20A in Advanced Biliary Tract Cancer. Anticancer Res. 2021, 41, 1485–1496. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, D.; Li, Q.; Xu, S.; Ye, C.; Jiang, Q.; Yan, F.; Jia, Y.; Zhang, X.; Ruan, J. Immunotherapy of cholangiocarcinoma: Therapeutic strategies and predictive biomarkers. Cancer Lett. 2022. [Google Scholar] [CrossRef]
- Guo, J.; Tang, Q. Recent updates on chimeric antigen receptor T cell therapy for hepatocellular carci-noma. Cancer Gene Ther. 2021, 28, 1075–1087. [Google Scholar] [CrossRef]
- Su, Z.; Wang, X.; Zheng, L.; Lyu, T.; Figini, M.; Wang, B.; Procissi, D.; Shangguan, J.; Sun, C.; Pan, L.; et al. MRI-guided interventional natural killer cell delivery for liver tumor treatment. Cancer Med. 2018, 7, 1860–1869. [Google Scholar] [CrossRef]
- Bae, W.K.; Lee, B.C.; Kim, H.-J.; Lee, J.-J.; Chung, I.-J.; Cho, S.B.; Koh, Y.S. A Phase I Study of Lo-coregional High-Dose Autologous Natural Killer Cell Therapy with Hepatic Arterial Infusion Chemo-therapy in Patients with Locally Advanced Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 879452. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, J.; Bao, X.; Xiong, F.; Ma, Y.; Tan, B.; Yu, L.; Zhao, Y.; Lu, J. Adoptive Transfer of Autologous Invariant Natural Killer T Cells as Immunotherapy for Advanced Hepatocellular Carcinoma: A Phase I Clinical Trial. Oncologist 2021, 26, e1919–e1930. [Google Scholar] [CrossRef] [PubMed]

| Identifier | Study Arm | Phase | Enrollment | Primary Endpoint | Stage | Study Start Date |
|---|---|---|---|---|---|---|
| NCT0304182 | Oral therapeutic vaccine V3-X | 1/2 | 20 | Changes in CA19.9 | Unknown | 20 February 2017 |
| NCT03633773 | MUC-1 CART cell immunotherapy cytokine induced killer cells | 1/2 | 9 | Disease control rate | Recruiting | 1 July 2018 |
| NCT01868490 | Cytokine induced killer cells | 1/2 | 13 | MRI scan for monitoring of tumor size and CIK cell-homing; Fluorescence activated cell sorting | Unknown | 17 April 2009 |
| NCT04951141 | Anti-GPC3 CAR T | 1 | 10 | Adverse events | Recruiting | 1 January 2019 |
| NCT03801083 | Tumor infiltrating lymphocytes | 2 | 59 | Objective Response Rate | Recruiting | 19 February 2019 |
| NCT03942328 | External beam radiation therapy, autologous dendritic cells, and Prevnar | 1 | 26 | Incidence of significant toxicity | Recruiting | 17 May 2019 |
| NCT03907852 | Gavo-cel, fludarabine, cyclophosphamide; Gavo-cel, fludarabine, cyclophosphamide, anti-PD1 | 1/2 | 70 | Safety | Recruiting | 15 April 2019 |
| NCT4853017 | ELI-002 2P Amph-CpG-7909 admixed with Amph modified KRAS peptides | 1 | 18 | Safety | Recruiting | 4 October 2021 |
| NCT05194735 | TCR-T Cell Drug Product; TCR-T Cell Drug Product with Aldesleukin (IL-2) | 1/2 | 180 | Safety | Recruiting | February 2022 |
| NCT04660929 | CT-0508 | 1 | 18 | Safety | Recruiting | 2 February 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruff, S.M.; Shannon, A.H.; Pawlik, T.M. Advances in Targeted Immunotherapy for Hepatobiliary Cancers. Int. J. Mol. Sci. 2022, 23, 13961. https://doi.org/10.3390/ijms232213961
Ruff SM, Shannon AH, Pawlik TM. Advances in Targeted Immunotherapy for Hepatobiliary Cancers. International Journal of Molecular Sciences. 2022; 23(22):13961. https://doi.org/10.3390/ijms232213961
Chicago/Turabian StyleRuff, Samantha M., Alexander H. Shannon, and Timothy M. Pawlik. 2022. "Advances in Targeted Immunotherapy for Hepatobiliary Cancers" International Journal of Molecular Sciences 23, no. 22: 13961. https://doi.org/10.3390/ijms232213961
APA StyleRuff, S. M., Shannon, A. H., & Pawlik, T. M. (2022). Advances in Targeted Immunotherapy for Hepatobiliary Cancers. International Journal of Molecular Sciences, 23(22), 13961. https://doi.org/10.3390/ijms232213961







