Ni Nanoparticles Stabilized by Hyperbranched Polymer: Does the Architecture of the Polymer Affect the Nanoparticle Characteristics and Their Performance in Catalysis?
Abstract
1. Introduction
2. Results and Discussion
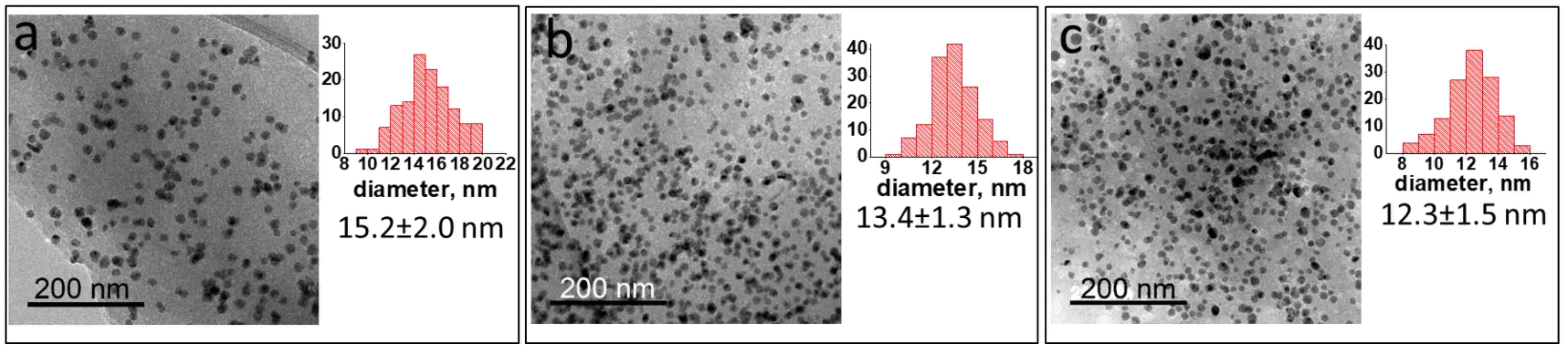
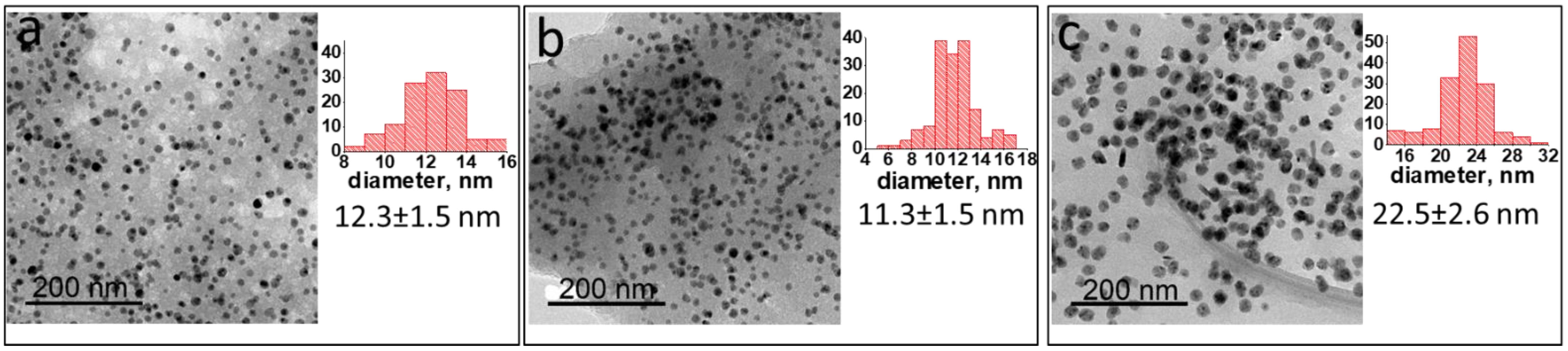
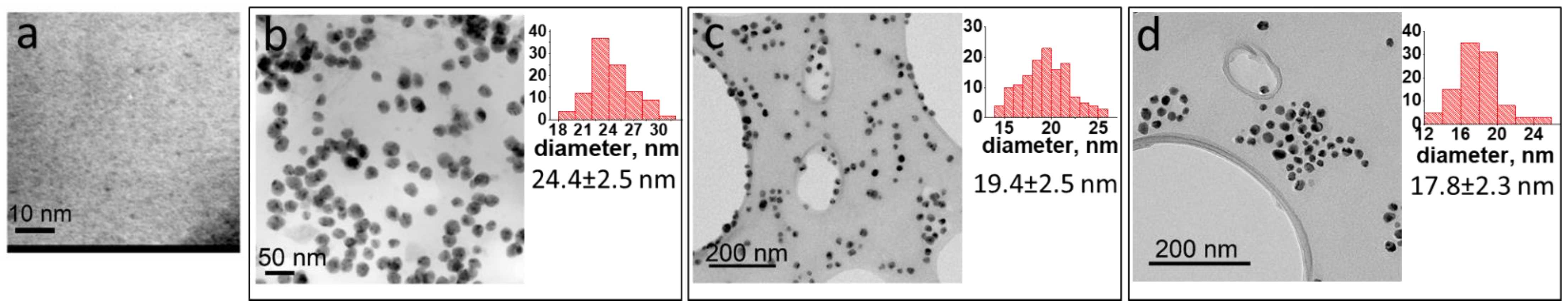
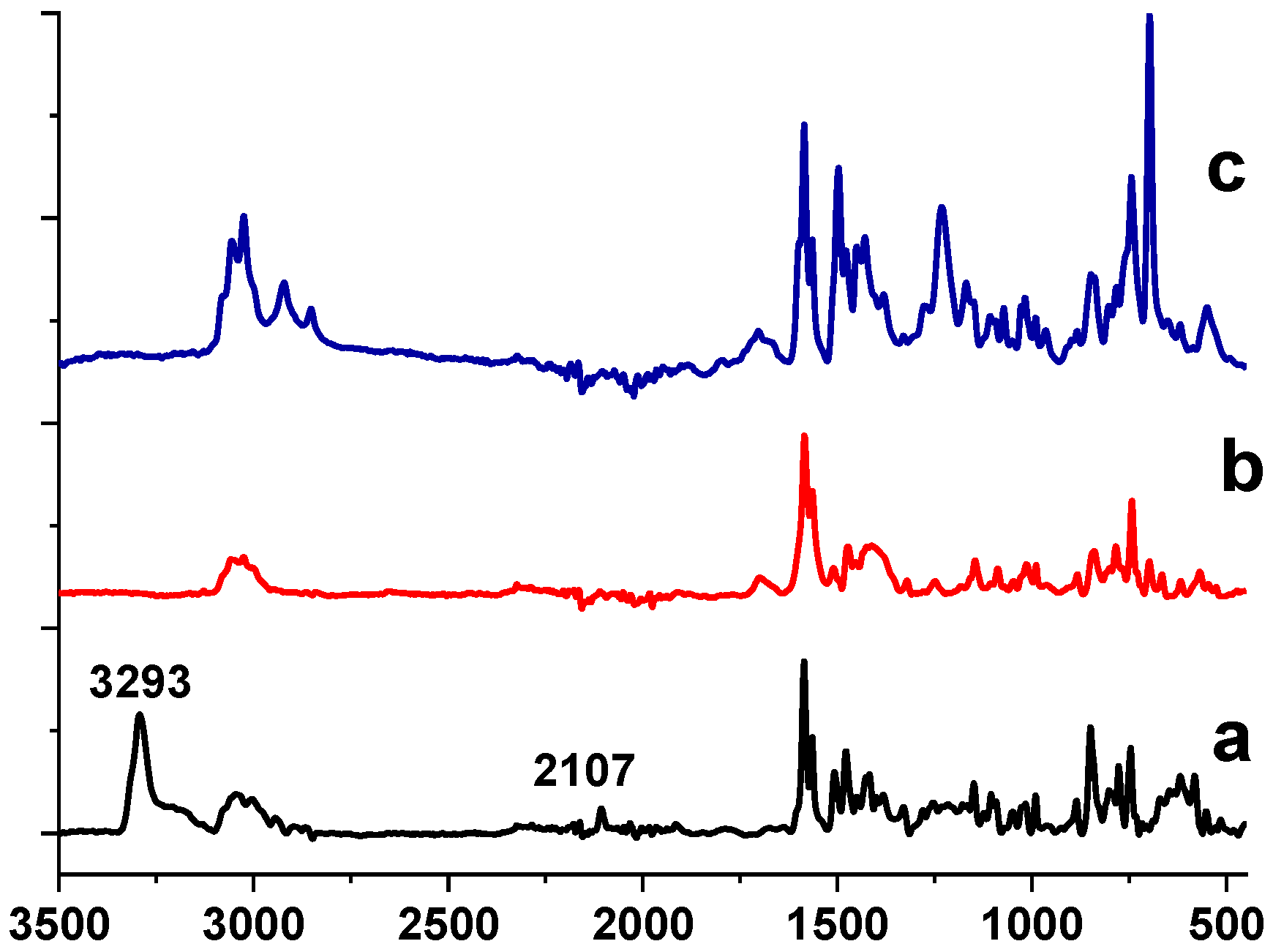
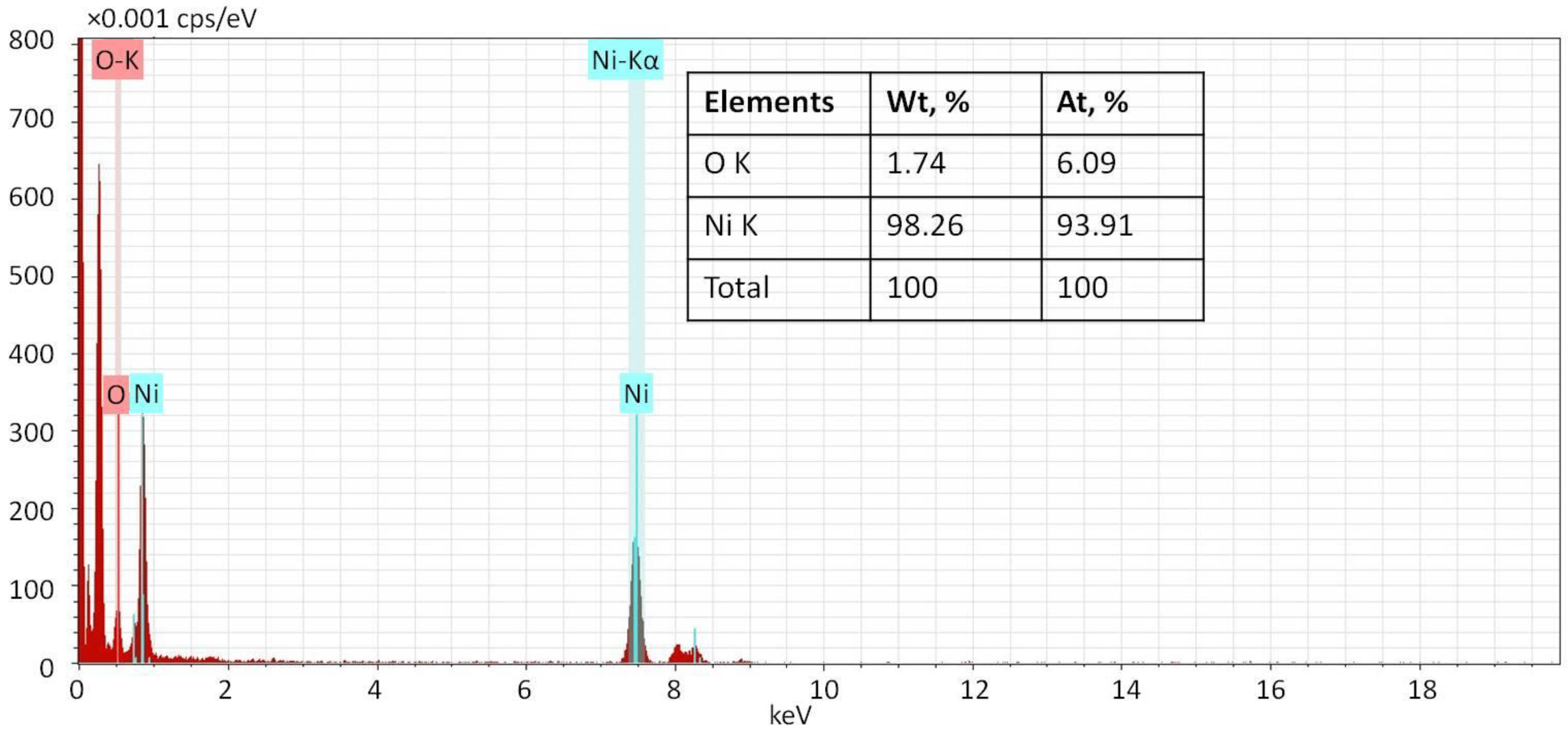
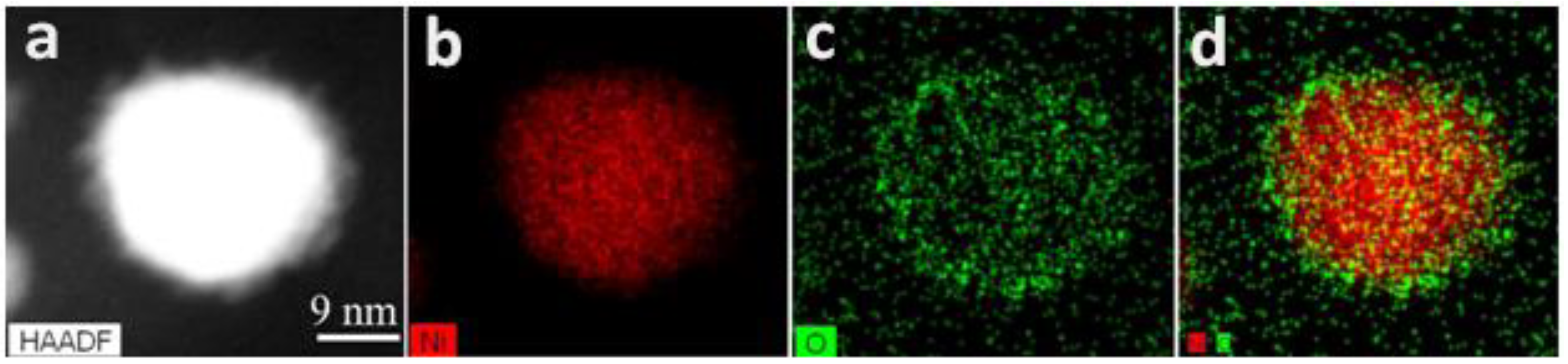
2.1. Ni NP Formation. Morphological and Structural Characteristics vs. a Preparation Method
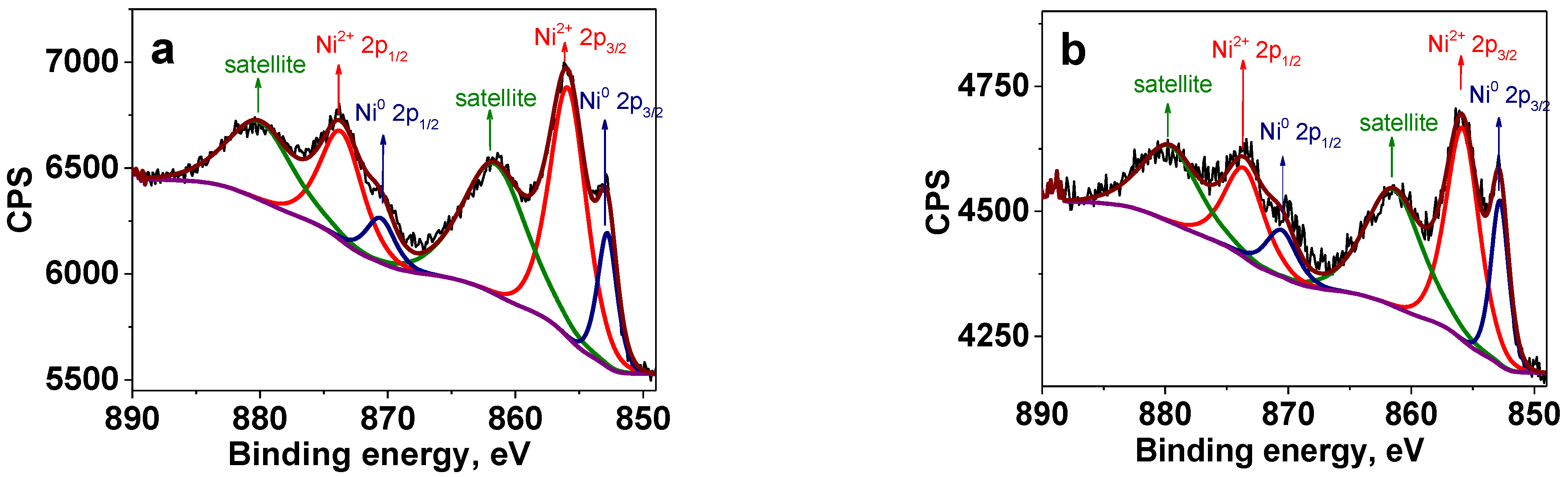
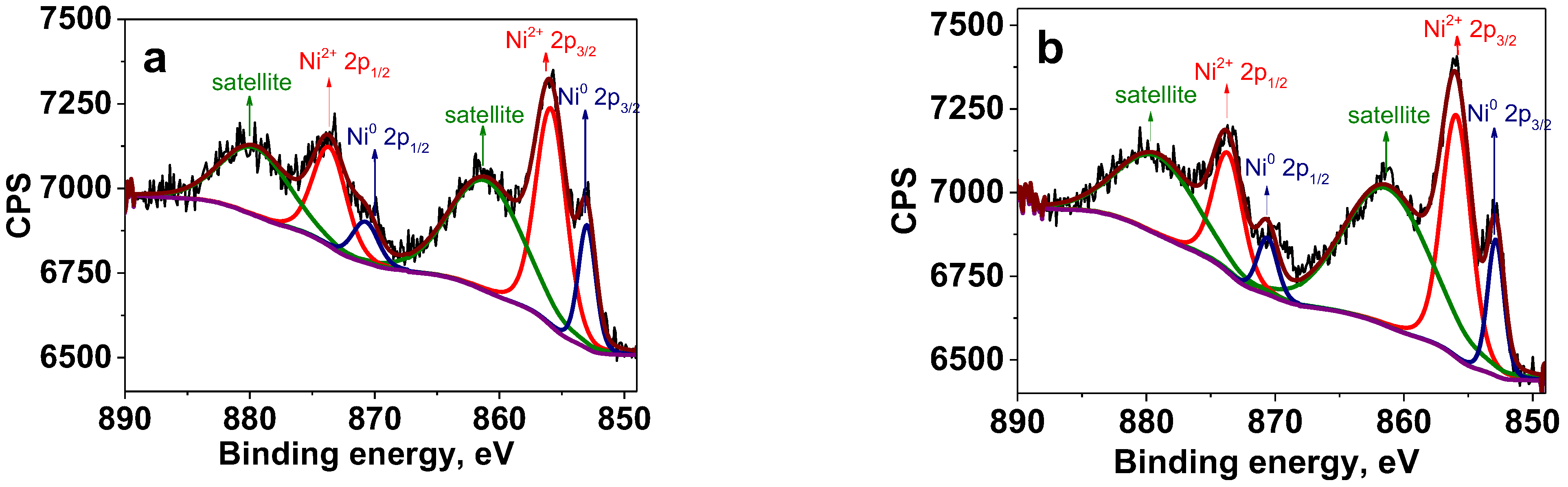
2.2. Oxidation State of Ni NPs
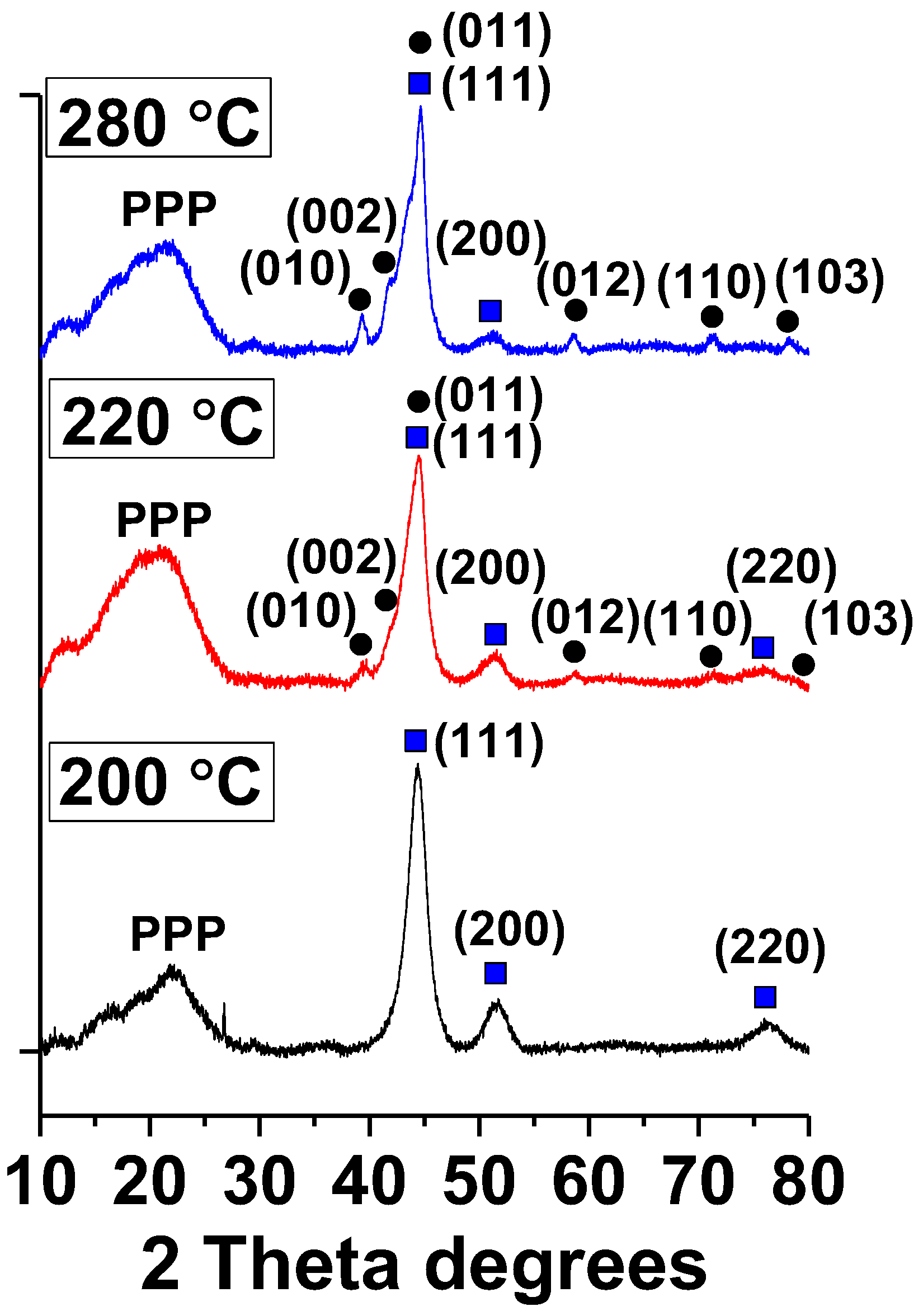
2.3. XRD Analysis
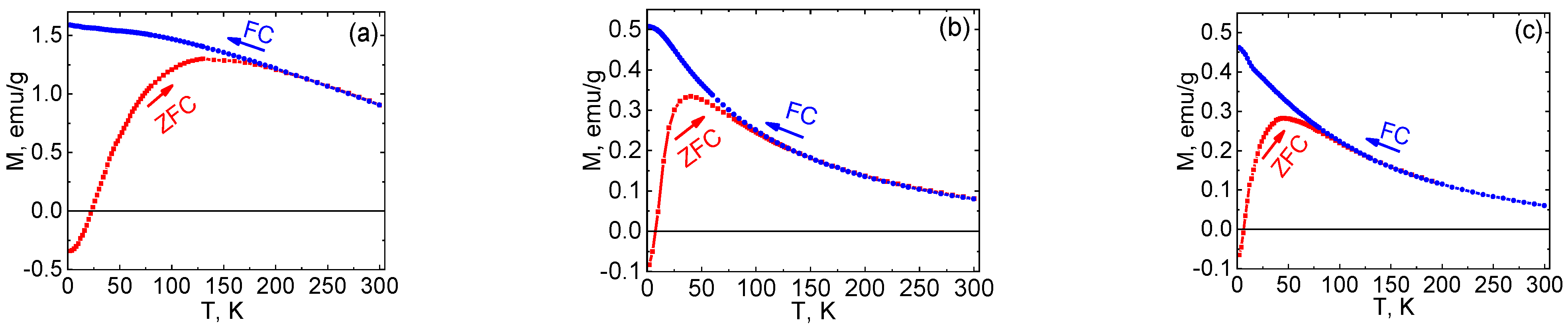
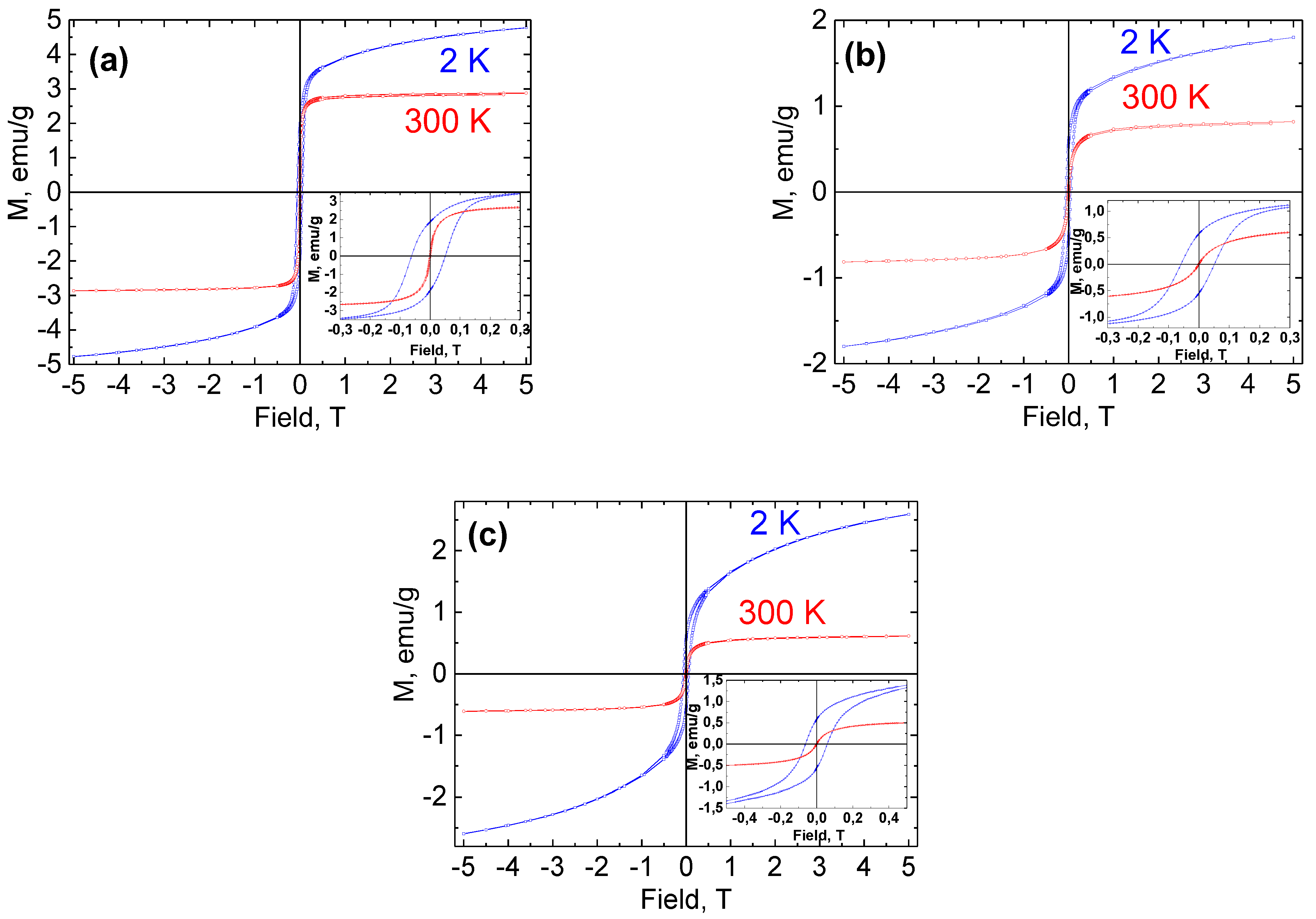
2.4. Magnetic Properties of Ni NPs@PPP
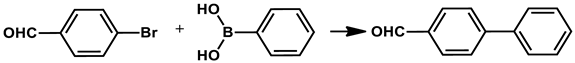
2.5. Catalyst Testing in Suzuki-Miyaura Cross-Coupling Reaction
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ni NPs
3.3. General Procedure for Suzuki-Miyaura Cross-Coupling Reaction
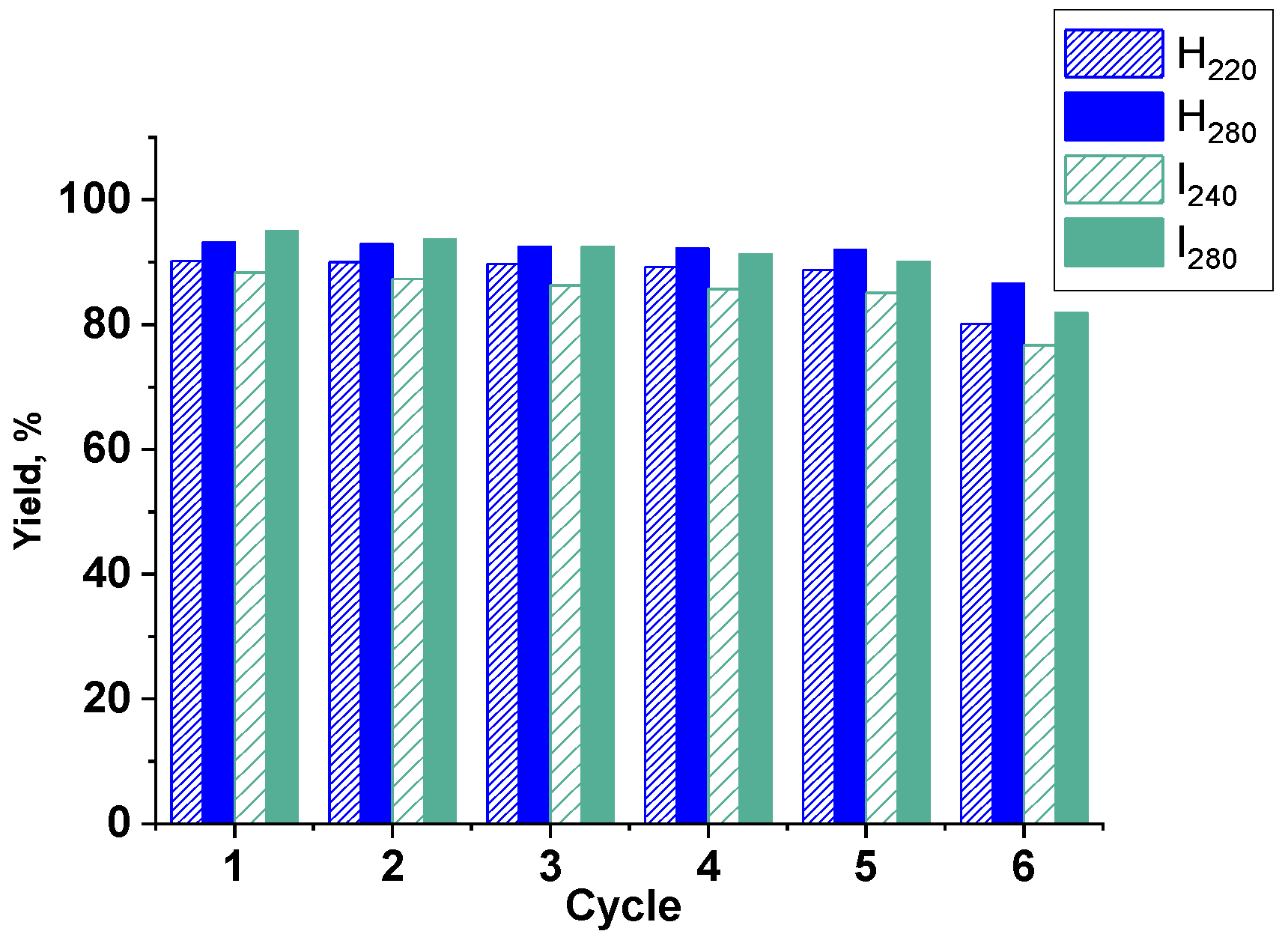
3.4. Catalyst Recycling Experiments
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narayanan, R.; El-Sayed, M.A. Catalysis with transition metal nanoparticles in colloidal solution: Nanoparticle shape dependence and stability. J. Phys. Chem. B 2005, 109, 12663–12676. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R. Recent Advances in Noble Metal Nanocatalysts for Suzuki and Heck Cross-Coupling Reactions. Molecules 2010, 15, 2124–2138. [Google Scholar] [CrossRef] [PubMed]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Palladium nanoparticles as efficient green homogeneous and heterogeneous carbon-carbon coupling precatalysts: A unifying view. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Astruc, D.; Wang, D.; Deraedt, C.; Liang, L.Y.; Ciganda, R.; Ruiz, J. Catalysis Inside Dendrimers. Synth.-Stuttg. 2015, 47, 2017–2031. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Shifrina, Z.B. Dendrimers as Encapsulating, Stabilizing, or Directing Agents for Inorganic Nanoparticles. Chem. Rev. 2011, 111, 5301–5344. [Google Scholar] [CrossRef]
- Alibegovic, K.; Kuchkina, N.; Serkova, E.; Morgan, D.G.; Losovyj, Y.; Salnikova, K.; Matveeva, V.; Shifrina, Z.; Sulman, E.; Bronstein, L. Structure determines function: Role of polymer in stabilization of magnetic catalyst for furfural hydrogenation. Abstr. Pap. Am. Chem. S. 2017, 254, 1155. [Google Scholar]
- Alibegovic, K.; Morgan, D.G.; Losovyj, Y.; Pink, M.; Stein, B.D.; Kuchkina, N.V.; Serkova, E.S.; Salnikova, K.E.; Shifrina, Z.B.; Matveeva, V.G.; et al. Efficient Furfuryl Alcohol Synthesis from Furfural over Magnetically Recoverable Catalysts: Does the Catalyst Stabilizing Medium Matter? Chemistryselect 2017, 2, 5485–5491. [Google Scholar] [CrossRef]
- Eskandari, A.; Jafarpour, M.; Rezaeifard, A.; Salimi, M. A dendritic TiO2-Co(II) nanocomposite based on the melamine catalyzed one-pot aerobic photocatalytic synthesis of benzimidazoles. New J. Chem. 2018, 42, 6449–6456. [Google Scholar] [CrossRef]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef]
- Wang, W.J.; Chamkina, E.S.; Cal, E.G.; di Silvio, D.; Moro, M.M.; Moya, S.; Hamon, J.R.; Astruc, D.; Shifrina, Z.B. Ferrocenyl-terminated polyphenylene-type “click” dendrimers as supports for efficient gold and palladium nanocatalysis. Dalt. Trans. 2021, 50, 11852–11860. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Ornelas, C.; Diallo, A.K.; Deraedt, C.; Wang, Y.L.; Lu, F.; Gu, H.B.; Astruc, D. Ferrocene-based dendritic macromolecules as efficient supports in nanocatalysis. Polymer 2022, 246, 124714. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. Supramolecular nanoreactors for catalysis. Coord. Chem. Rev. 2016, 324, 106–122. [Google Scholar] [CrossRef]
- Liu, X.; Gregurec, D.; Irigoyen, J.; Martinez, A.; Moya, S.; Ciganda, R.; Hermange, P.; Ruiz, J.; Astruc, D. Precise localization of metal nanoparticles in dendrimer nanosnakes or inner periphery and consequences in catalysis. Nat. Commun. 2016, 7, 13152. [Google Scholar] [CrossRef]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Magnetic and Dendritic Catalysts. Acc. Chem. Res. 2015, 48, 1871–1880. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Bronstein, L.M. Magnetically Recoverable Catalysts: Beyond Magnetic Separation. Front. Chem. 2018, 6, 298. [Google Scholar] [CrossRef]
- Kim, D.; Lee, N.; Park, M.; Kim, B.H.; An, K.; Hyeon, T.J. Synthesis of uniform ferrimagnetic magnetite nanocubes. J. Am. Chem. Soc. 2009, 131, 454–455. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Kuchkina, N.V.; Lawson, B.P.; Krasnova, I.Y.; Nemygina, N.A.; Nikoshvili, L.Z.; Talanova, V.N.; Stein, B.D.; Pink, M.; Morgan, D.G.; et al. Pyridylphenylene dendrons immobilized on the surface of chemically modified magnetic silica as efficient stabilizing molecules of Pd species. Appl. Surf. Sci. 2019, 488, 865–873. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Sorokina, S.A.; Bykov, A.V.; Sulman, M.G.; Bronstein, L.M.; Shifrina, Z.B. Magnetically Recoverable Nanoparticulate Catalysts for Cross-Coupling Reactions: The Dendritic Support Influences the Catalytic Performance. Nanomaterials 2021, 11, 3345. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Haskell, A.K.; Sorokina, S.A.; Torozova, A.S.; Nikoshvili, L.Z.; Sulman, E.M.; Stein, B.D.; Morgan, D.G.; Bronstein, L.M.; Shifrina, Z.B. Pd Catalyst Based on Hyperbranched Polypyridylphenylene Formed In Situ on Magnetic Silica Allows for Excellent Performance in Suzuki-Miyaura Reaction. Appl. Mater. Inter. 2020, 12, 22170–22178. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, S.A.; Mikhailov, S.P.; Kuchkina, N.V.; Bykov, A.V.; Vasiliev, A.L.; Ezernitskaya, M.G.; Golovin, A.L.; Nikoshvili, L.Z.; Sulman, M.G.; Shifrina, Z.B. Ru@hyperbranched Polymer for Hydrogenation of Levulinic Acid to Gamma-Valerolactone: The Role of the Catalyst Support. Int. J. Mol. Sci. 2022, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions. Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C-C cross-coupling reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. “Homeopathic” Palladium Nanoparticle Catalysis of Cross Carbon-Carbon Coupling Reactions. Acc. Chem. Res. 2014, 47, 494–503. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Vicic, D.A.; Klein, A. Exploring Mechanisms in Ni Terpyridine Catalyzed C-C Cross-Coupling Reactions—A Review. Inorganics 2018, 6, 18. [Google Scholar] [CrossRef]
- Guerinot, A.; Cossy, J. Cobalt-Catalyzed Cross-Couplings between Alkyl Halides and Grignard Reagents. Acc. Chem. Res. 2020, 53, 1351–1363. [Google Scholar] [CrossRef]
- Satpute, D.P.; Vaidya, G.N.; Lokhande, S.K.; Shinde, S.D.; Bhujbal, S.M.; Chatterjee, D.R.; Rana, P.; Venkatesh, A.; Nagpure, M.; Kumar, D. Organic reactions in aqueous media catalyzed by nickel. Green Chem. 2021, 23, 6273–6300. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Hashimoto, S.; Ishizuka, K.; Nakamura, M. Highly Selective Biaryl Cross-Coupling Reactions between Aryl Halides and Aryl Grignard Reagents: A New Catalyst Combination of N-Heterocyclic Carbenes and Iron, Cobalt, and Nickel Fluorides. J. Am. Chem. Soc. 2009, 131, 11949–11963. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, G.Y. Recent developments in low-cost TM-catalyzed Heck-type reactions (TM = transition metal, Ni, Co, Cu, and Fe). Cat. Sci. Tech. 2016, 6, 2862–2876. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Ananikov, V.P. Nickel and Palladium Catalysis: Stronger Demand than Ever. ACS Catal. 2022, 12, 1180–1200. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Perez-Juste, J.; Labarta, A.; Batlle, X.; Liz-Marzan, L.M. Heating rate influence on the synthesis of iron oxide nanoparticles: The case of decanoic acid. Chem. Commun. 2010, 46, 6108–6110. [Google Scholar] [CrossRef]
- Guardia, P.; di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Ho, C.-H.; Tsai, C.-P.; Chung, C.-C.; Tsai, C.-Y.; Chen, F.-R.; Lin, H.-J.; Lai, C.-H. Shape-Controlled Growth and Shape-Dependent Cation Site Occupancy of Monodisperse Fe3O4 Nanoparticles. Chem. Mater. 2011, 23, 1753–1760. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Zinatullina, M.S.; Serkova, E.S.; Vlasov, P.S.; Peregudov, A.S.; Shifrina, Z.B. Hyperbranched pyridylphenylene polymers based on the first-generation dendrimer as a multifunctional monomer. RSC Adv. 2015, 5, 99510–99516. [Google Scholar] [CrossRef]
- Ikeda, S.-i.; Kondo, H.; Mori, N. Effects of monodentate oxazoline ligands in Ni/Al-catalyzed regioselective cyclotrimerization of enones and alkynes. Chem. Com. 2000, 10, 815–816. [Google Scholar] [CrossRef]
- Ikeda, S.-i.; Mori, N.; Sato, Y. Regioselective Cyclic Cotrimerization of α,β-Enones and Alkynes by a Nickel−Aluminum Catalyst System. J. Am. Chem. Soc. 1997, 119, 4779–4780. [Google Scholar] [CrossRef]
- Mori, N.; Ikeda, S.-i.; Sato, Y. Selective Cyclotrimerization of Enones and Alkynes by a Nickel and Aluminum Catalytic System. J. Am. Chem. Soc. 1999, 121, 2722–2727. [Google Scholar] [CrossRef]
- Schubert, J.S.; Popovic, J.; Haselmann, G.M.; Nandan, S.P.; Wang, J.; Giesriegl, A.; Cherevan, A.S.; Eder, D. Immobilization of Co, Mn, Ni and Fe oxide co-catalysts on TiO2 for photocatalytic water splitting reactions. J. Mater. Chem. A 2019, 7, 18568–18579. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interf. Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Das, S.; Jangam, A.; Du, Y.; Hidajat, K.; Kawi, S. Highly dispersed nickel catalysts via a facile pyrolysis generated protective carbon layer. Chem. Commun. 2019, 55, 6074–6077. [Google Scholar] [CrossRef]
- Lin, Z.; Han, D.; Li, S. Study on thermal decomposition of copper(II) acetate monohydrate in air. J. Therm. Anal. Cal. 2012, 107, 471–475. [Google Scholar] [CrossRef]
- Zeng, H.; Rice, P.M.; Wang, S.X.; Sun, S. Shape-controlled synthesis and shape-induced texture of MnFe2O4 nanoparticles. J. Am. Chem. Soc. 2004, 126, 11458–11459. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Kuvshinova, T.B.; Volodin, V.D.; Ellert, O.G.; Efimov, N.N.; Skorikov, V.M.; Baranchikov, A.E.; Novotortsev, V.M. Synthesis of high-purity nanocrystalline BiFeO3. Inorg. Mater. 2013, 49, 310–314. [Google Scholar] [CrossRef]
- Tsopoe, S.P.; Borgohain, C.; Fopase, R.; Pandey, L.M.; Borah, J.P. A comparative investigation of normal and inverted exchange bias effect for magnetic fluid hyperthermia applications. Sci. Rep. 2020, 10, 18666. [Google Scholar] [CrossRef]
- Sonogashira, K. Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley-VCH: New York, NY, USA, 2002. [Google Scholar]
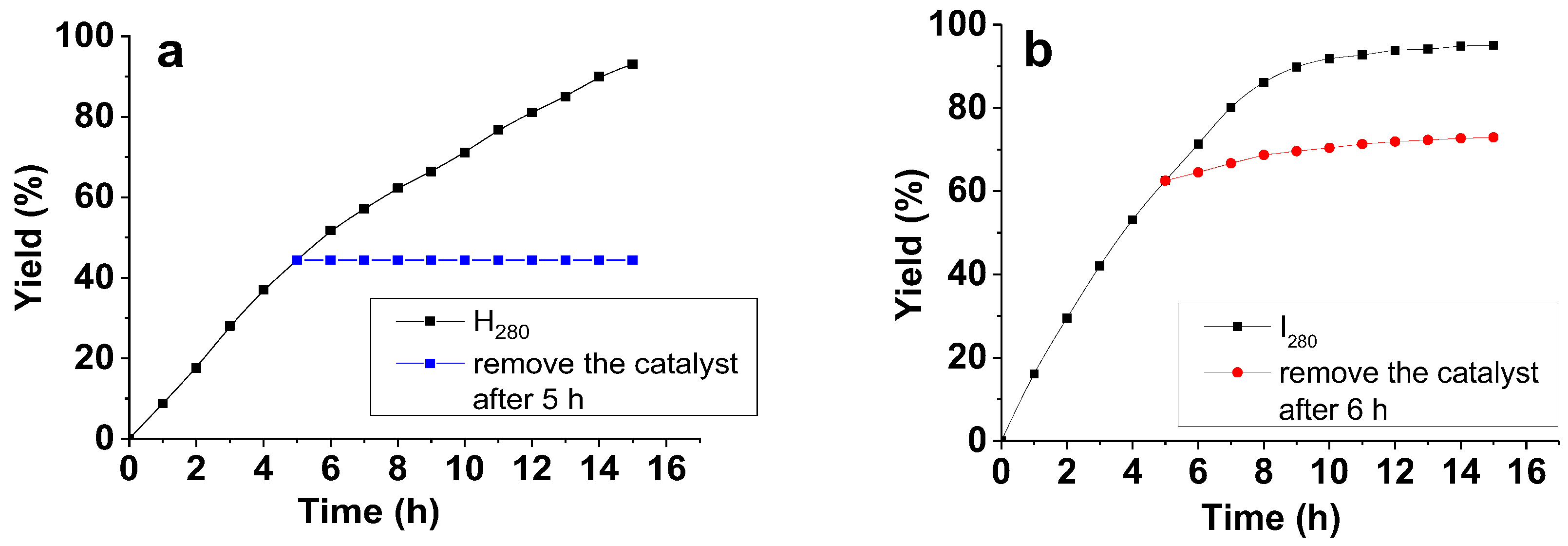
 | |||||||
| # | Sample Name | Time of Reaction, h | Conversion, % | Selectivity, % | Yield b, % | Yield c, % after 5th Cycle | Yield c, % after 6th Cycle |
| 1 | H220 | 6 | 53 | 78 | 41.3 | 88.8 | 80.1 |
| 2 | 15 | 93 | 97 | 90.2 | |||
| 3 | H280 | 6 | 69 | 75 | 51.8 | 92.0 | 86.6 |
| 4 | 15 | 99 | 94 | 93.1 | |||
| 5 | I240 | 6 | 71 | 83 | 58.9 | 85.1 | 76.7 |
| 6 | 15 | 92 | 96 | 88.3 | |||
| 7 | I280 | 6 | 81 | 88 | 71.3 | 90.1 | 81.9 |
| 8 | 15 | 100 | 95 | 95.0 | |||
| 9 * | H280 | 15 | 54.5 | 81.3 | 44.3 | 87.1 | 82.7 |
| 24 | 97.3 | 93.7 | 91.2 | ||||
| 10 * | I280 | 15 | 65.2 | 85.3 | 55.6 | 86.3 | 77.1 |
| 24 | 98.0 | 96.0 | 94.1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokina, S.A.; Kuchkina, N.V.; Ezernitskaya, M.G.; Bykov, A.V.; Vasiliev, A.L.; Efimov, N.N.; Shifrina, Z.B. Ni Nanoparticles Stabilized by Hyperbranched Polymer: Does the Architecture of the Polymer Affect the Nanoparticle Characteristics and Their Performance in Catalysis? Int. J. Mol. Sci. 2022, 23, 13874. https://doi.org/10.3390/ijms232213874
Sorokina SA, Kuchkina NV, Ezernitskaya MG, Bykov AV, Vasiliev AL, Efimov NN, Shifrina ZB. Ni Nanoparticles Stabilized by Hyperbranched Polymer: Does the Architecture of the Polymer Affect the Nanoparticle Characteristics and Their Performance in Catalysis? International Journal of Molecular Sciences. 2022; 23(22):13874. https://doi.org/10.3390/ijms232213874
Chicago/Turabian StyleSorokina, Svetlana A., Nina V. Kuchkina, Mariam G. Ezernitskaya, Alexey V. Bykov, Alexander L. Vasiliev, Nikolay N. Efimov, and Zinaida B. Shifrina. 2022. "Ni Nanoparticles Stabilized by Hyperbranched Polymer: Does the Architecture of the Polymer Affect the Nanoparticle Characteristics and Their Performance in Catalysis?" International Journal of Molecular Sciences 23, no. 22: 13874. https://doi.org/10.3390/ijms232213874
APA StyleSorokina, S. A., Kuchkina, N. V., Ezernitskaya, M. G., Bykov, A. V., Vasiliev, A. L., Efimov, N. N., & Shifrina, Z. B. (2022). Ni Nanoparticles Stabilized by Hyperbranched Polymer: Does the Architecture of the Polymer Affect the Nanoparticle Characteristics and Their Performance in Catalysis? International Journal of Molecular Sciences, 23(22), 13874. https://doi.org/10.3390/ijms232213874






