Improving Reporter Gene Assay Methodology for Evaluating the Ability of Compounds to Restore P53 Activity
Abstract
1. Introduction
2. Results
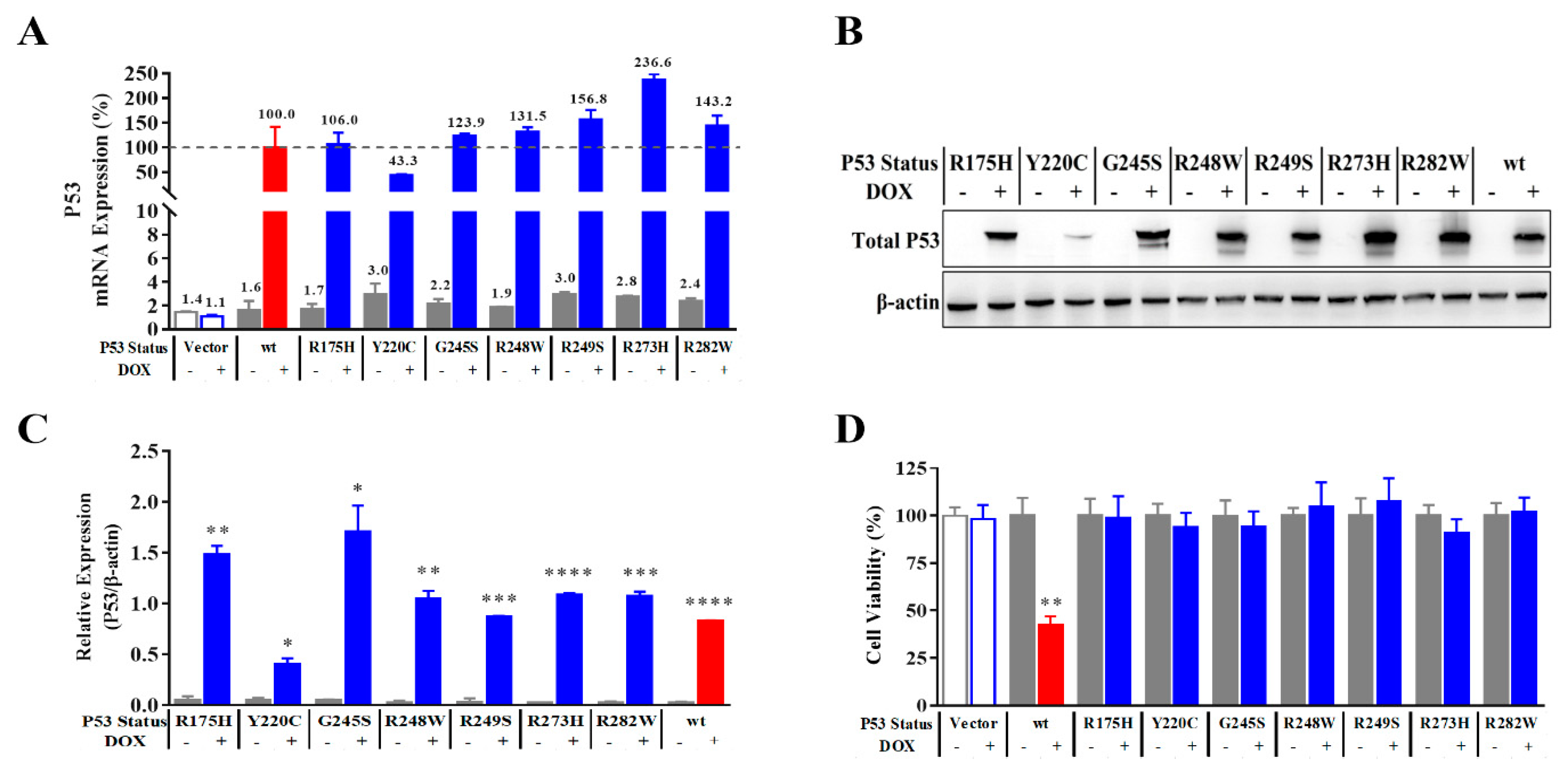
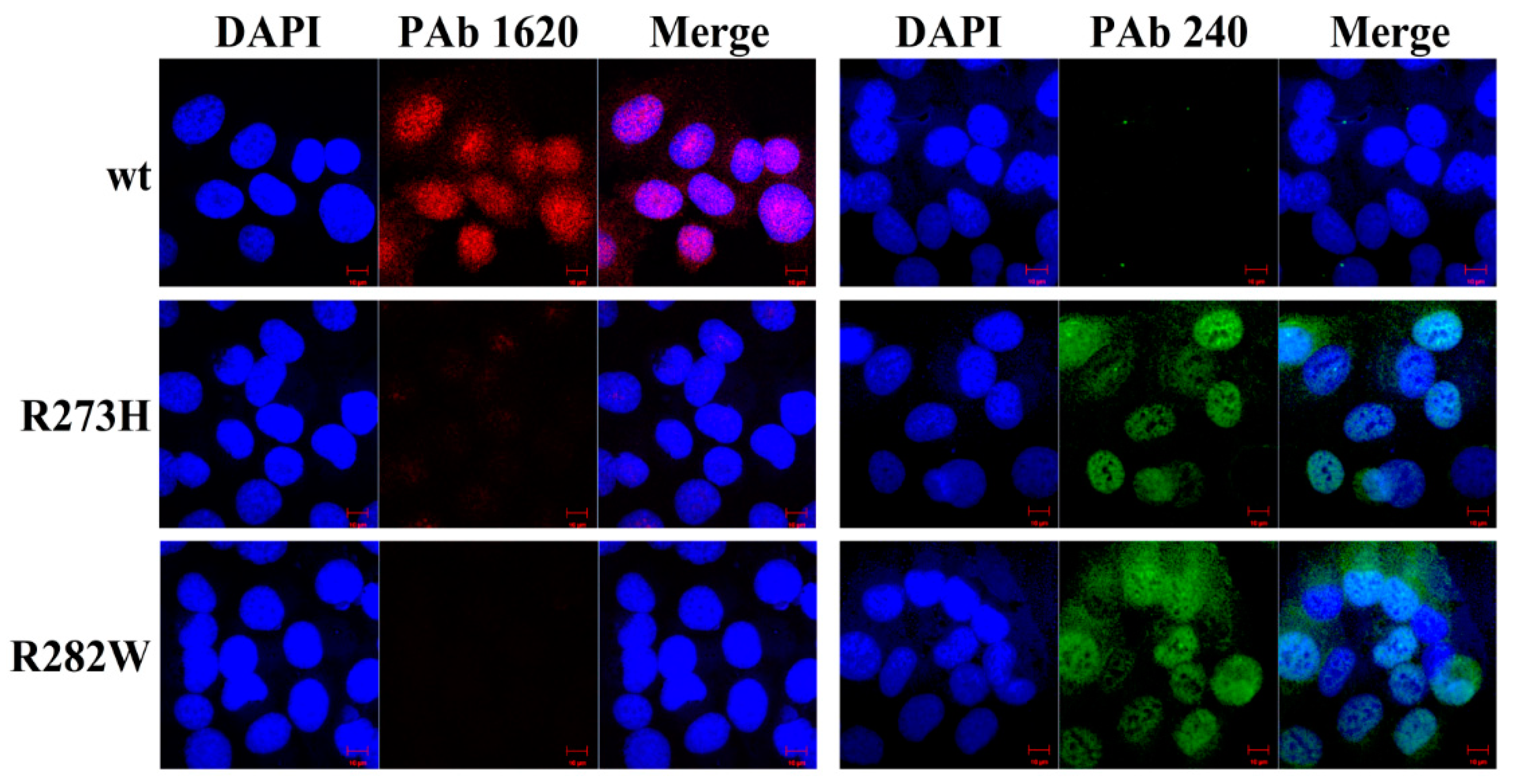
2.1. Tetracycline-Inducible Expression of Wild-Type P53 and Its Mutants
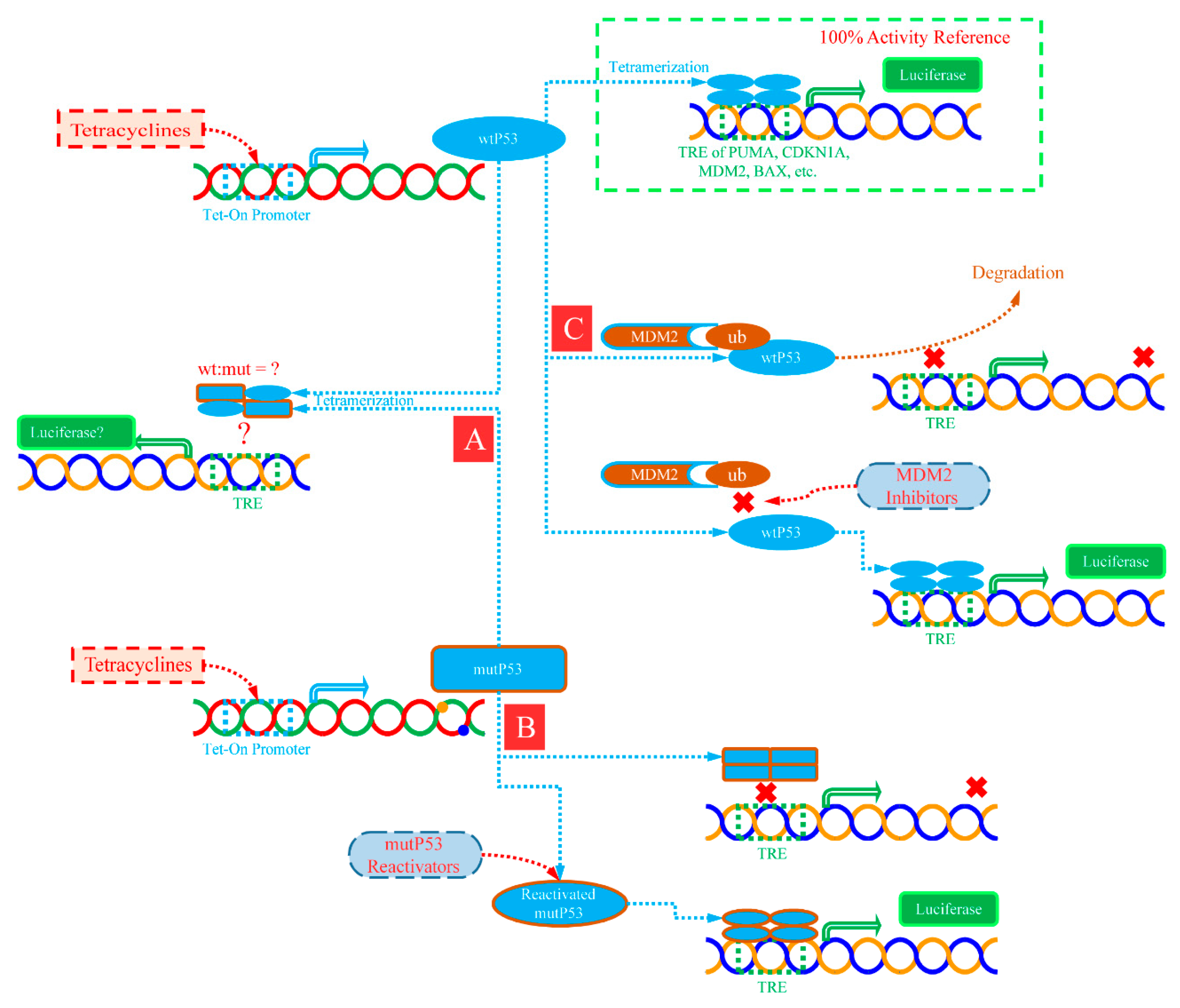
2.2. Improved Reporter Gene System can Correctly Reflect the Transcriptional Activity of P53
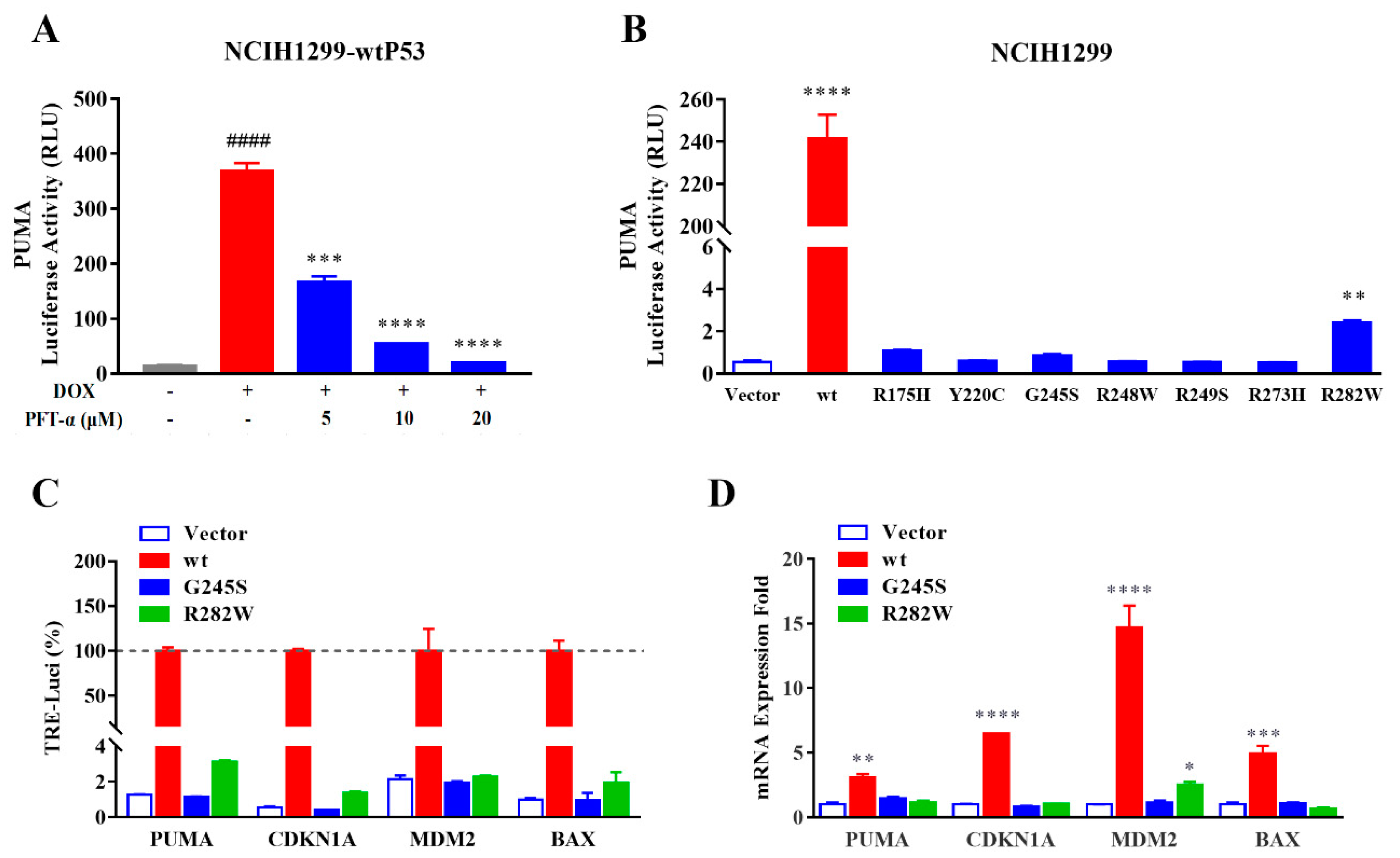
2.3. Different Response Modes of Typical Transcriptional Response Elements to Dominant Negative P53 Mutation Effect Have Been Observed
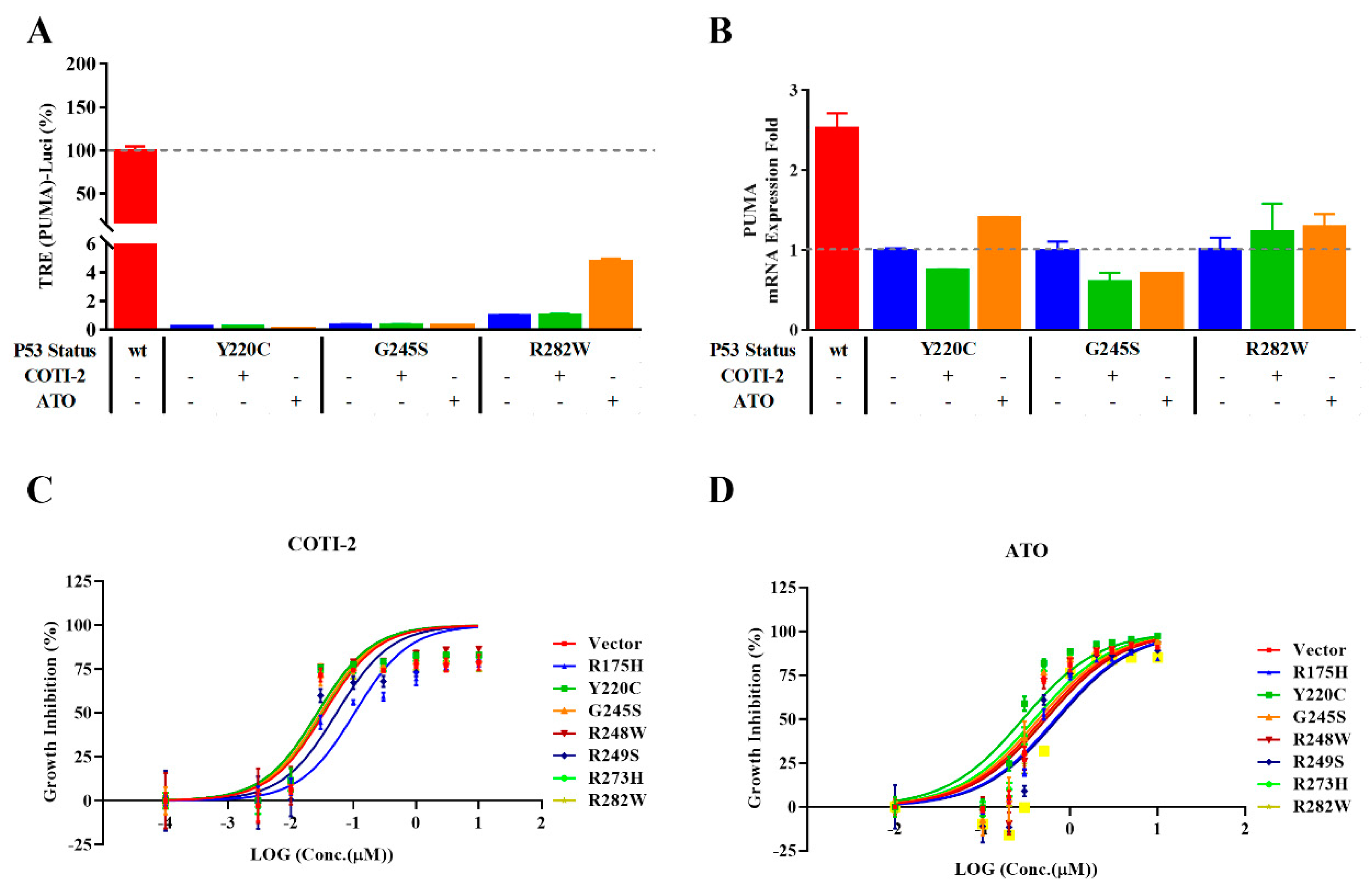
2.4. Improved Reporter Gene System Could Be Applied to Evaluate mutP53 Reactivators
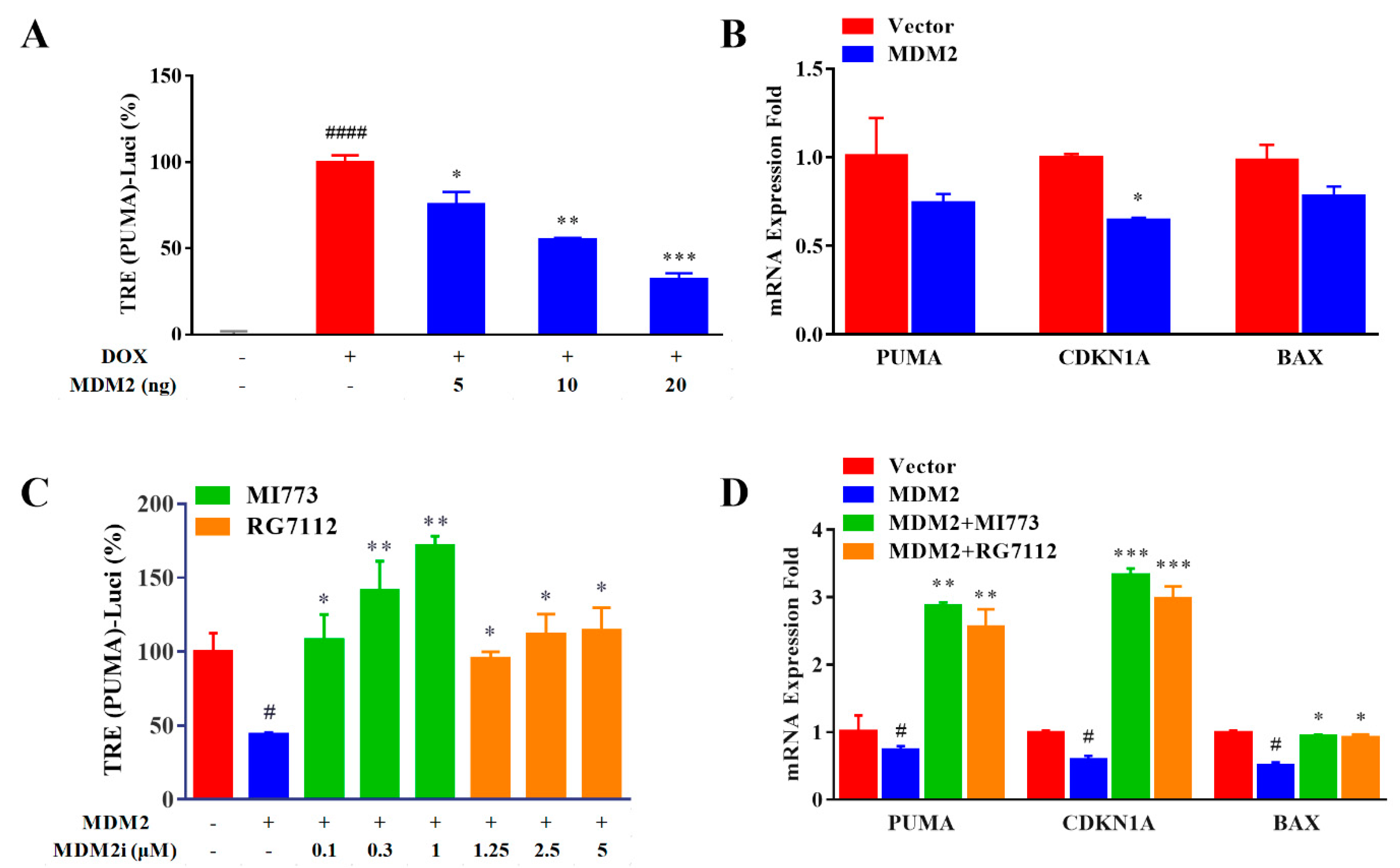
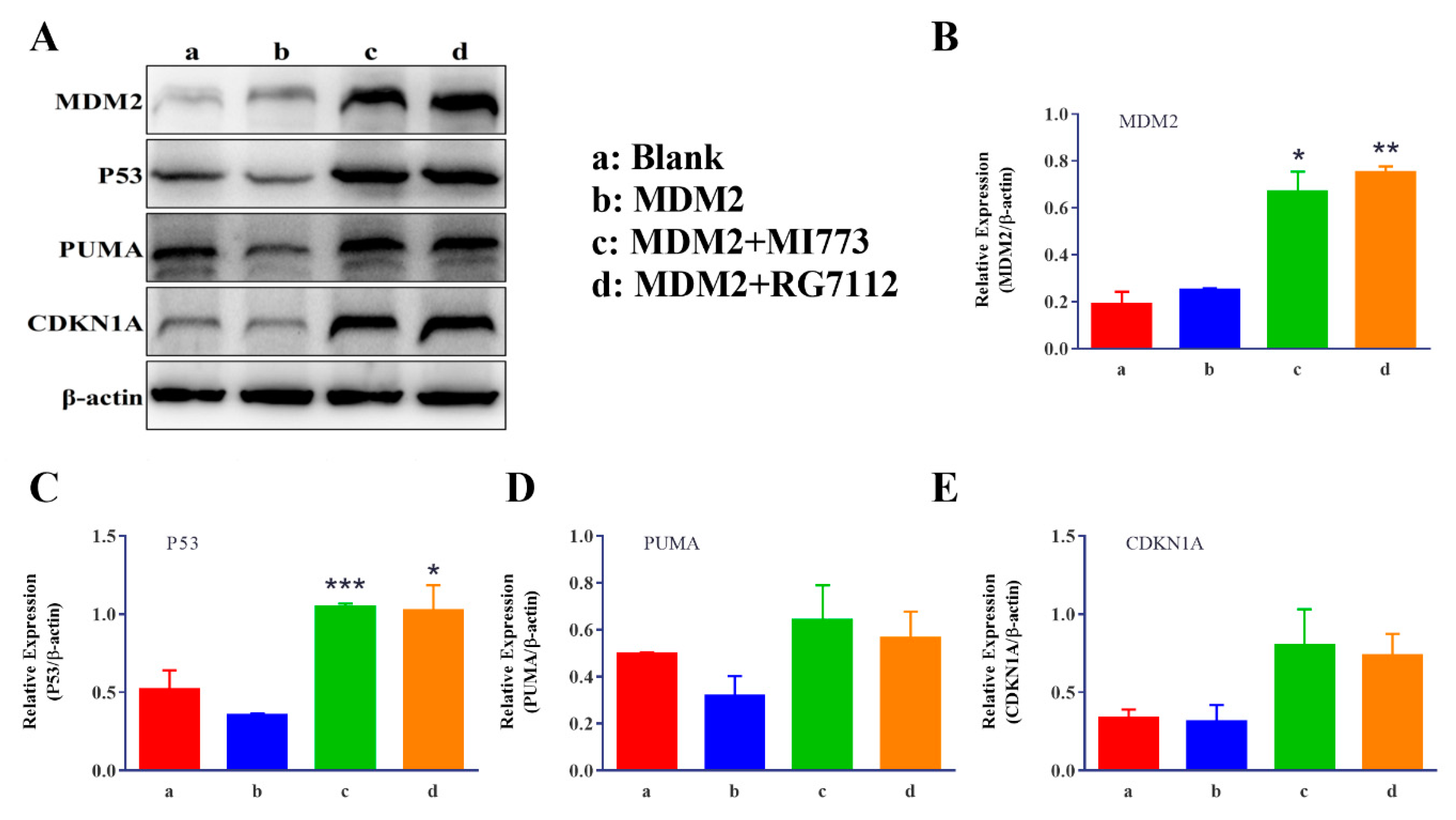
2.5. MDM2 Inhibitors could Be Evaluated Using the Improved Reporter Gene System
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Compounds
4.3. Reconstruction of Tetracycline-Inducible Overexpression Plasmids and Lentivirus Packaging
4.4. Real-Time Quantitative PCR
4.5. Western Blot Analysis
4.6. Immunofluorescence
4.7. Cell Viability Assay
4.8. Luciferase Reporter Gene Assay
4.9. The Response Modes of P53 Dominant Negative Effects Predicted by Mathematical Modeling
4.10. Data Analysis and Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, C.; Zhang, P.; Harper, J.W.; Elledge, S.J.; Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef]
- Erlacher, M.; Michalak, E.M.; Kelly, P.N.; Labi, V.; Niederegger, H.; Coultas, L.; Adams, J.M.; Strasser, A.; Villunger, A. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005, 106, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Michalak, E.M.; Villunger, A.; Adams, J.M.; Strasser, A. In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 2008, 15, 1019–1029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villunger, A.; Michalak, E.M.; Coultas, L.; Müllauer, F.; Böck, G.; Ausserlechner, M.J.; Adams, J.M.; Strasser, A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003, 302, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, W.; Li, X.; Han, X.; Zhang, X.; Wang, J.; Liu, C.; Huang, L.; Gao, W. p53: A double-edged sword in tumor ferroptosis. Pharmacol. Res. 2021, 177, 106013. [Google Scholar] [CrossRef]
- Shen, L.; Sun, X.; Fu, Z.; Yang, G.; Li, J.; Yao, L. The fundamental role of the p53 pathway in tumor metabolism and its implication in tumor therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1561–1567. [Google Scholar] [CrossRef]
- Mendiratta, G.; Ke, E.; Aziz, M.; Liarakos, D.; Tong, M.; Stites, E.C. Cancer gene mutation frequencies for the U.S. population. Nat. Commun. 2021, 12, 5961. [Google Scholar]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Bykov, V.J.N.; Eriksson, S.E.; Bianchi, J.; Wiman, K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 2018, 18, 89–102. [Google Scholar] [CrossRef]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Raimundo, L.; Pereira, N.A.; dos Santos, D.J.; Pérez, M.; Queiroz, G.; Leão, M.; Santos, M.M.; Saraiva, L. A tryptophanol-derived oxazolopiperidone lactam is cytotoxic against tumors via inhibition of p53 interaction with murine double minute proteins. Pharmacol. Res. 2015, 95–96, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Molica, M.; Mazzone, C.; Niscola, P.; de Fabritiis, P. TP53 Mutations in Acute Myeloid Leukemia: Still a Daunting Challenge? Front. Oncol. 2020, 10, 610820. [Google Scholar] [CrossRef] [PubMed]
- Kogan, S.; Carpizo, D.R. Zinc Metallochaperones as Mutant p53 Reactivators: A New Paradigm in Cancer Therapeutics. Cancers 2018, 10, 166. [Google Scholar] [CrossRef]
- Megalathan, A.; Wijesinghe, K.M.; Dhakal, S. Single-Molecule FRET-Based Dynamic DNA Sensor. ACS Sens. 2021, 6, 1367–1374. [Google Scholar] [CrossRef]
- Bykov, V.J.; Issaeva, N.; Shilov, A.; Hultcrantz, M.; Pugacheva, E.; Chumakov, P.; Bergman, J.; Wiman, K.G.; Selivanova, G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat. Med. 2002, 8, 282–288. [Google Scholar] [CrossRef]
- Yap, D.B.; Hsieh, J.K.; Zhong, S.; Heath, V.; Gusterson, B.; Crook, T.; Lu, X. Ser392 phosphorylation regulates the oncogenic function of mutant p53. Cancer Res. 2004, 64, 4749–4754. [Google Scholar] [CrossRef]
- Rökaeus, N.; Shen, J.; Eckhardt, I.; Bykov, V.J.; Wiman, K.G.; Wilhelm, M.T. PRIMA-1(MET)/APR-246 targets mutant forms of p53 family members p63 and p73. Oncogene 2010, 29, 6442–6451. [Google Scholar] [CrossRef]
- Milner, J.; Medcalf, E.A. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell 1991, 65, 765–774. [Google Scholar] [CrossRef]
- Gencel-Augusto, J.; Lozano, G. p53 tetramerization: At the center of the dominant-negative effect of mutant p53. Genes Dev. 2020, 34, 1128–1146. [Google Scholar] [CrossRef]
- Willis, A.; Jung, E.J.; Wakefield, T.; Chen, X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene 2004, 23, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant p53 as a target for cancer treatment. Eur. J. Cancer 2017, 83, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Salim, K.Y.; Maleki Vareki, S.; Danter, W.R.; Koropatnick, J. COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget 2016, 7, 41363–41379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Yan, X.J.; Zhou, Z.R.; Yang, F.F.; Wu, Z.Y.; Sun, H.B.; Liang, W.X.; Song, A.X.; Lallemand-Breitenbach, V.; Jeanne, M.; et al. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 2010, 328, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Vuaroqueaux, V.; Hendriks, H.R.; Al-Hasani, H.; Peille, A.L.; Das, S.; Fiebig, H.H. Pharmacogenomics characterization of the MDM2 inhibitor MI-773 reveals candidate tumours and predictive biomarkers. NPJ Precis. Oncol. 2021, 5, 96. [Google Scholar] [CrossRef]
- Wang, H.Q.; Mulford, I.J.; Sharp, F.; Liang, J.; Kurtulus, S.; Trabucco, G.; Quinn, D.S.; Longmire, T.A.; Patel, N.; Patil, R.; et al. Inhibition of MDM2 Promotes Antitumor Responses in p53 Wild-Type Cancer Cells through Their Interaction with the Immune and Stromal Microenvironment. Cancer Res. 2021, 81, 3079–3091. [Google Scholar] [CrossRef]
- Trino, S.; De Luca, L.; Laurenzana, I.; Caivano, A.; Del Vecchio, L.; Martinelli, G.; Musto, P. P53-MDM2 Pathway: Evidences for A New Targeted Therapeutic Approach in B-Acute Lymphoblastic Leukemia. Front. Pharmacol. 2016, 7, 491. [Google Scholar] [CrossRef]
- Vu, B.; Wovkulich, P.; Pizzolato, G.; Lovey, A.; Ding, Q.; Jiang, N.; Liu, J.J.; Zhao, C.; Glenn, K.; Wen, Y.; et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med. Chem. Lett. 2013, 4, 466–469. [Google Scholar] [CrossRef]
- Warner, K.A.; Nör, F.; Acasigua, G.A.; Martins, M.D.; Zhang, Z.; McLean, S.A.; Spector, M.E.; Chepeha, D.B.; Helman, J.; Wick, M.J.; et al. Targeting MDM2 for Treatment of Adenoid Cystic Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 3550–3559. [Google Scholar] [CrossRef]
- Bykov, V.J.; Zache, N.; Stridh, H.; Westman, J.; Bergman, J.; Selivanova, G.; Wiman, K.G. PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene 2005, 24, 3484–3491. [Google Scholar] [CrossRef]
- Bauer, M.R.; Joerger, A.C.; Fersht, A.R. 2-Sulfonylpyrimidines: Mild alkylating agents with anticancer activity toward p53-compromised cells. Proc. Natl. Acad. Sci. USA 2016, 113, E5271-80. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Vazquez, A.; Levine, A.J.; Carpizo, D.R. Allele-specific p53 mutant reactivation. Cancer Cell 2012, 21, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, J.L.; Liang, Y.; Tang, Y.G.; Song, H.X.; Wu, L.L.; Xing, Y.F.; Yan, N.; Li, Y.T.; Wang, Z.Y.; et al. Arsenic Trioxide Rescues Structural p53 Mutations through a Cryptic Allosteric Site. Cancer Cell 2021, 39, 225–239.e8. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Chung, K.J.; Hwang, I.H.; Gwak, J.; Park, S.; Ju, B.G.; Yun, E.; Kim, D.E.; Chung, Y.H.; Na, M.; et al. Activation of p53 with ilimaquinone and ethylsmenoquinone, marine sponge metabolites, induces apoptosis and autophagy in colon cancer cells. Mar. Drugs 2015, 13, 543–557. [Google Scholar] [CrossRef]
- Tishler, R.B.; Lamppu, D.M.; Park, S.; Price, B.D. Microtubule-active drugs taxol, vinblastine, and nocodazole increase the levels of transcriptionally active p53. Cancer Res. 1995, 55, 6021–6025. [Google Scholar]
- Leu, J.I.; Dumont, P.; Hafey, M.; Murphy, M.E.; George, D.L. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 2004, 6, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Kamada, H.; Nito, C.; Nishi, T.; Chan, P.H. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 7974–7983. [Google Scholar] [CrossRef]
- Faccion, R.S.; Bernardo, P.S.; de Lopes, G.P.F.; Bastos, L.S.; Teixeira, C.L.; de Oliveira, J.A.; Fernandes, P.V.; Dubois, L.G.; Chimelli, L.; Maia, R.C. p53 expression and subcellular survivin localization improve the diagnosis and prognosis of patients with diffuse astrocytic tumors. Cell. Oncol. 2018, 41, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.P.; Gu, W. MSL2 promotes Mdm2-independent cytoplasmic localization of p53. J. Biol. Chem. 2009, 284, 3250–3263. [Google Scholar] [CrossRef]
- Filtz, T.M.; Vogel, W.K.; Leid, M. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol. Sci. 2014, 35, 76–85. [Google Scholar] [CrossRef]
- Kitayner, M.; Rozenberg, H.; Kessler, N.; Rabinovich, D.; Shaulov, L.; Haran, T.E.; Shakked, Z. Structural basis of DNA recognition by p53 tetramers. Mol. Cell 2006, 22, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Natan, E.; Hirschberg, D.; Morgner, N.; Robinson, C.V.; Fersht, A.R. Ultraslow oligomerization equilibria of p53 and its implications. Proc. Natl. Acad. Sci. USA 2009, 106, 14327–14332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, Z.; Myers, J.N. TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J. Cell Biochem. 2016, 117, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Stiewe, T.; Haran, T.E. How mutations shape p53 interactions with the genome to promote tumorigenesis and drug resistance. Drug Resist. Updat. 2018, 38, 27–43. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Hwang, P.M.; Kinzler, K.W.; Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 2001, 7, 673–682. [Google Scholar] [CrossRef]
- Veprintsev, D.B.; Fersht, A.R. Algorithm for prediction of tumour suppressor p53 affinity for binding sites in DNA. Nucleic Acids Res. 2008, 36, 1589–1598. [Google Scholar] [CrossRef]
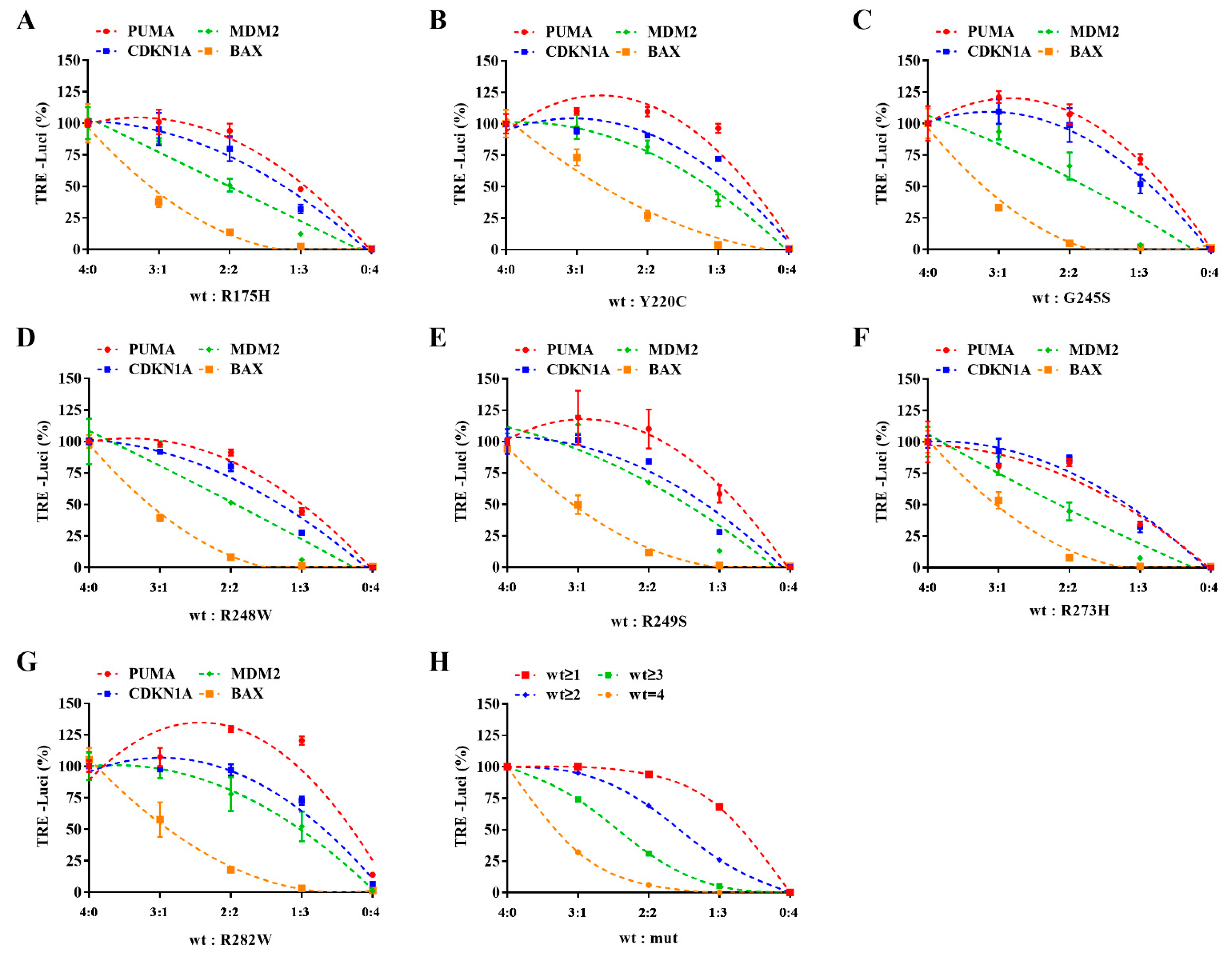
| Proportion | Deduced Probability Distribution | Deduced Activation Percentage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monomer | Dimer | Tetramer | TRE Response Mode | ||||||||||
| wt | mut | wt/wt | wt/mut | mut/mut | 4 wt | 3 wt/1 mut | 2 wt/2 mut | 1 wt/3 mut | 4 mut | wt = 4 | wt ≥ 3 | wt ≥ 2 | wt ≥ 1 |
| 4 | 0 | 100% | 0% | 0% | 100% | 0% | 0% | 0% | 0% | 100% | 100% | 100% | 100% |
| 3 | 1 | 56% | 38% | 6% | 32% | 42% | 21% | 5% | 0% | 32% | 74% | 95% | 100% |
| 2 | 2 | 25% | 50% | 25% | 6% | 25% | 38% | 25% | 6% | 6% | 31% | 69% | 94% |
| 1 | 3 | 6% | 38% | 56% | 0% | 5% | 21% | 42% | 32% | 0% | 5% | 26% | 68% |
| 0 | 4 | 0% | 0% | 100% | 0% | 0% | 0% | 0% | 100% | 0% | 0% | 0% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Du, J.; Shi, D.; Li, L.; Li, D.; Zhang, K.; Lin, S.; Zhu, J.; Huang, Z.; Zhou, Y.; et al. Improving Reporter Gene Assay Methodology for Evaluating the Ability of Compounds to Restore P53 Activity. Int. J. Mol. Sci. 2022, 23, 13867. https://doi.org/10.3390/ijms232213867
Han X, Du J, Shi D, Li L, Li D, Zhang K, Lin S, Zhu J, Huang Z, Zhou Y, et al. Improving Reporter Gene Assay Methodology for Evaluating the Ability of Compounds to Restore P53 Activity. International Journal of Molecular Sciences. 2022; 23(22):13867. https://doi.org/10.3390/ijms232213867
Chicago/Turabian StyleHan, Xinle, Jun Du, Dandan Shi, Lingjie Li, Dandan Li, Kun Zhang, Suwen Lin, Jingzhong Zhu, Zoufang Huang, You Zhou, and et al. 2022. "Improving Reporter Gene Assay Methodology for Evaluating the Ability of Compounds to Restore P53 Activity" International Journal of Molecular Sciences 23, no. 22: 13867. https://doi.org/10.3390/ijms232213867
APA StyleHan, X., Du, J., Shi, D., Li, L., Li, D., Zhang, K., Lin, S., Zhu, J., Huang, Z., Zhou, Y., & Fang, Z. (2022). Improving Reporter Gene Assay Methodology for Evaluating the Ability of Compounds to Restore P53 Activity. International Journal of Molecular Sciences, 23(22), 13867. https://doi.org/10.3390/ijms232213867






