Low-Dose IL-2 Attenuated Depression-like Behaviors and Pathological Changes through Restoring the Balances between IL-6 and TGF-β and between Th17 and Treg in a Chronic Stress-Induced Mouse Model of Depression
Abstract
1. Introduction
2. Results
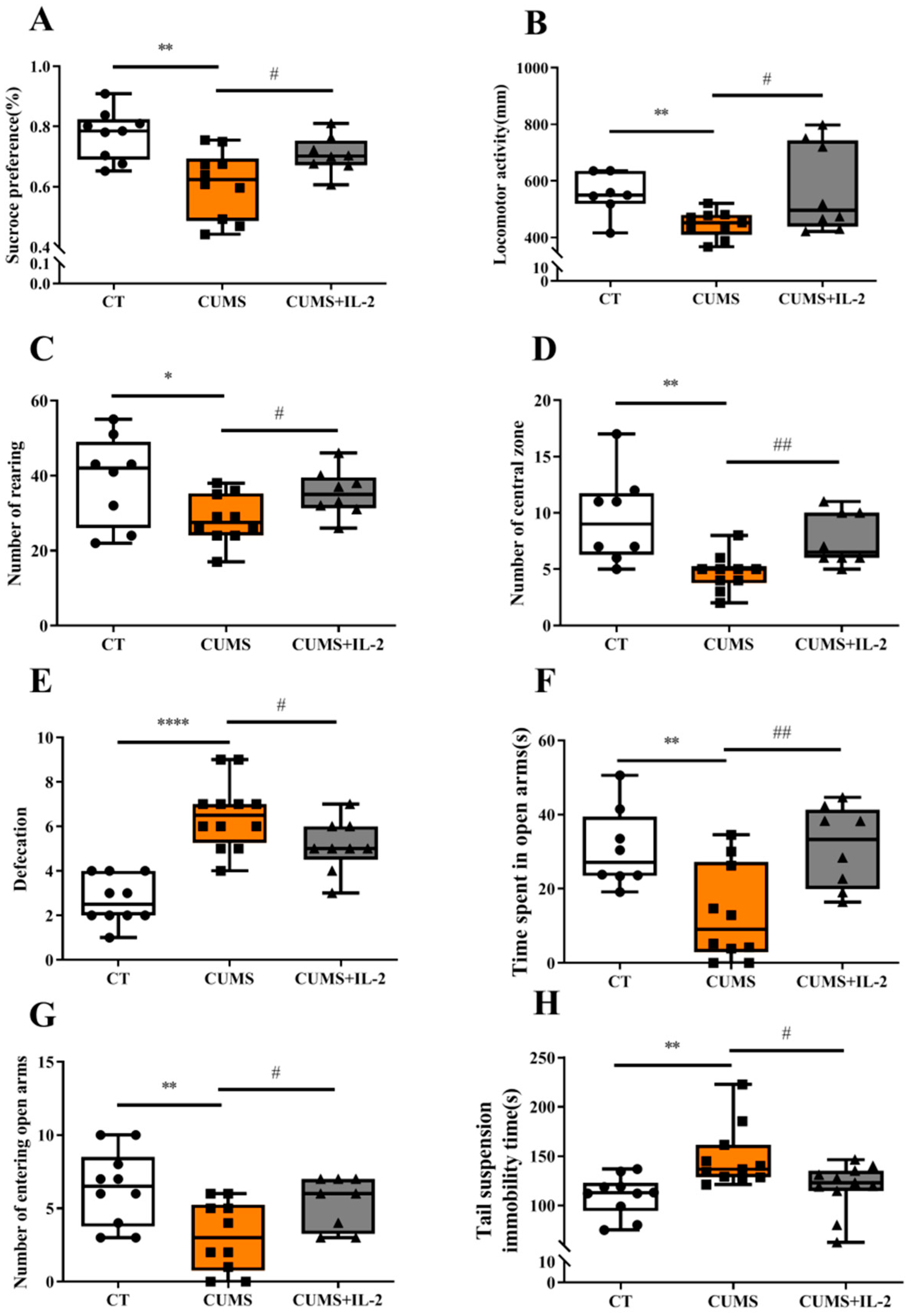
2.1. Low-Dose IL-2 Attenuated Anxiety- and Depression-Like Behaviors Induced by CUMS
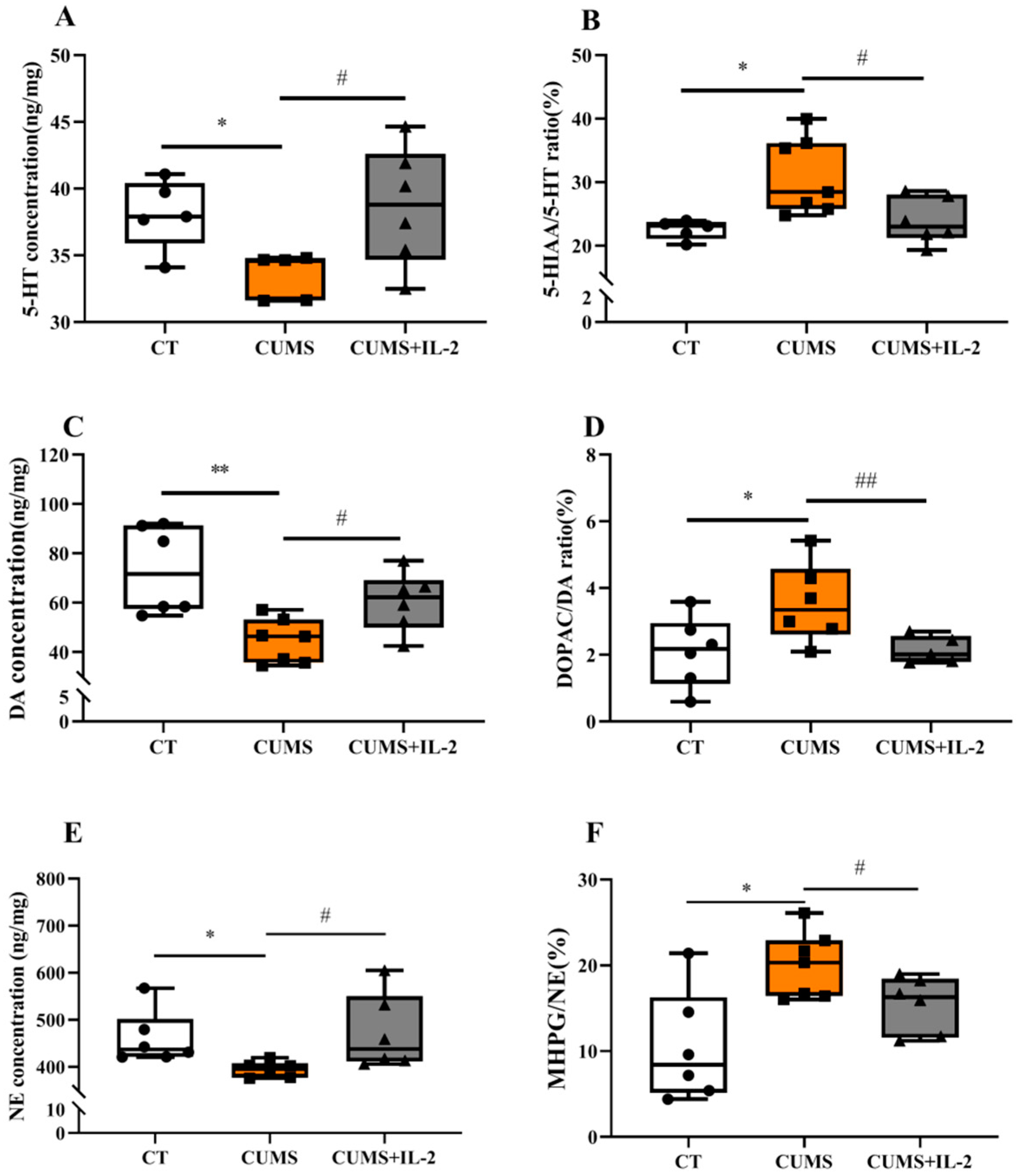
2.2. IL-2 Normalized the Concentration of Monoamine Neurotransmitters and Their Metabolites in the Hippocampus and Amygdala of CUMS Mice
2.3. IL-2 Reversed or Ameliorated CUMS-Induced Changes in Hippocampal Concentrations of Pro- and Anti-Inflammatory Cytokines and IL-17A Receptor Expression
2.4. IL-2 Restored the mRNA and Protein Expression of Th17 and Treg Cell Transcription Factors in the CUMS Group
2.5. IL-2 Inhibited the Activation of M1 and A1 Phenotypes and the IDO Enzyme Induced by CUMS
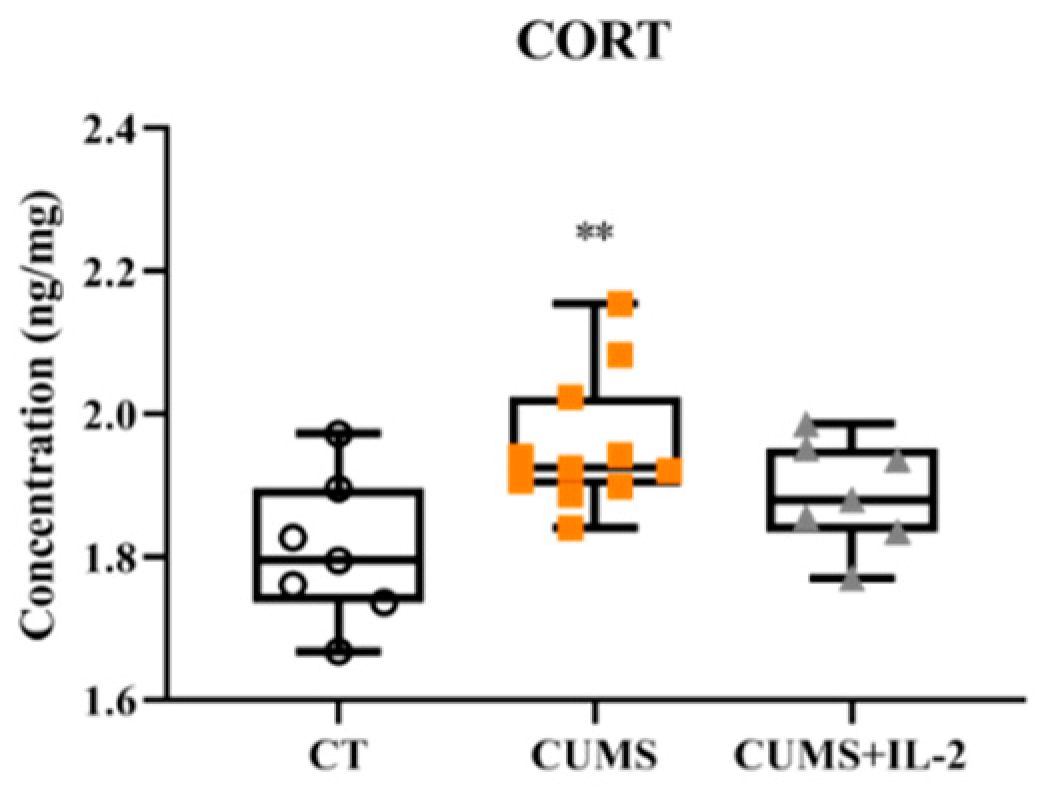
2.6. IL-2 Did Not Attenuate Increased CORT Concentration in the CUMS Group
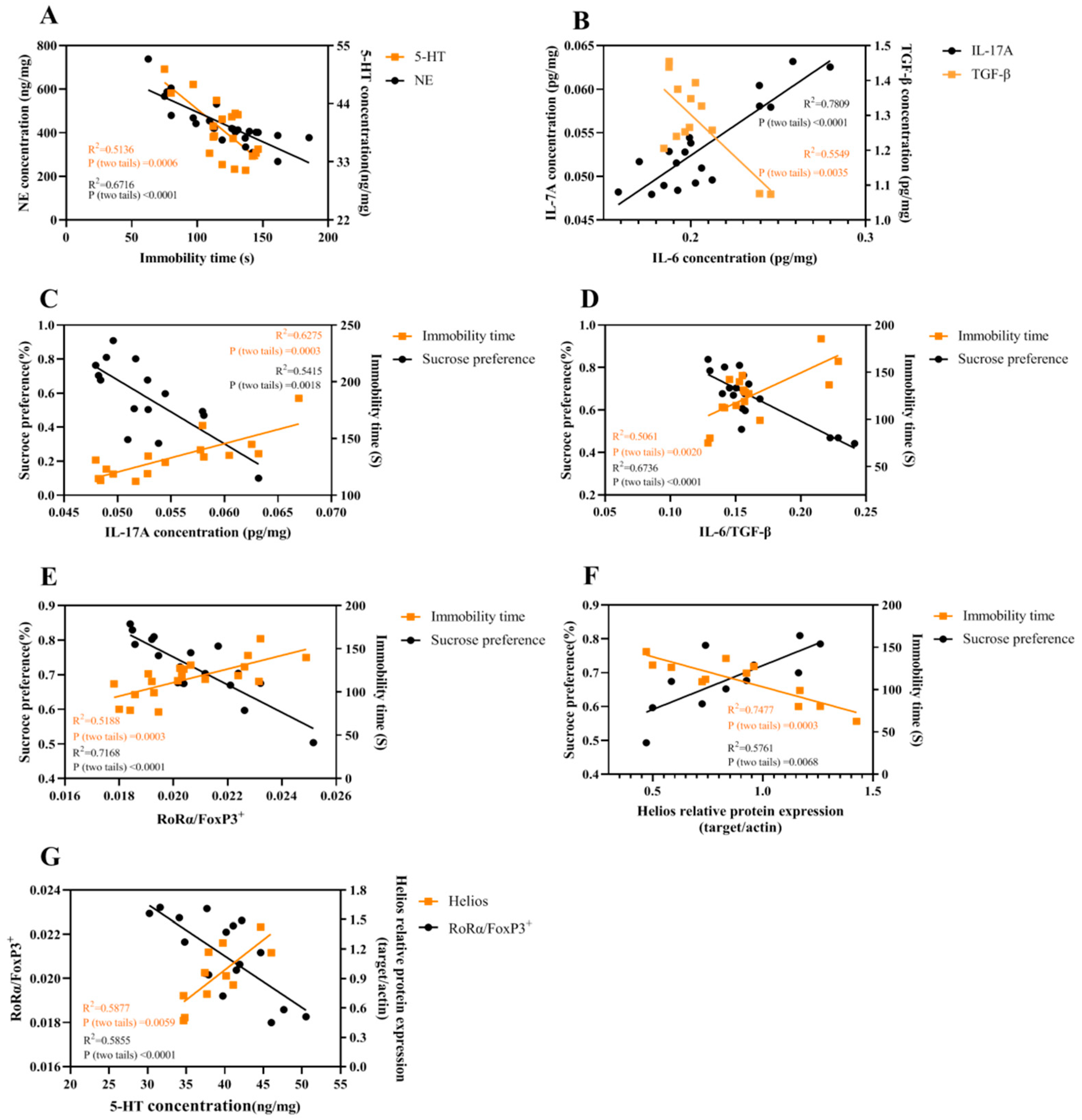
2.7. The Correlations among Different Parameters
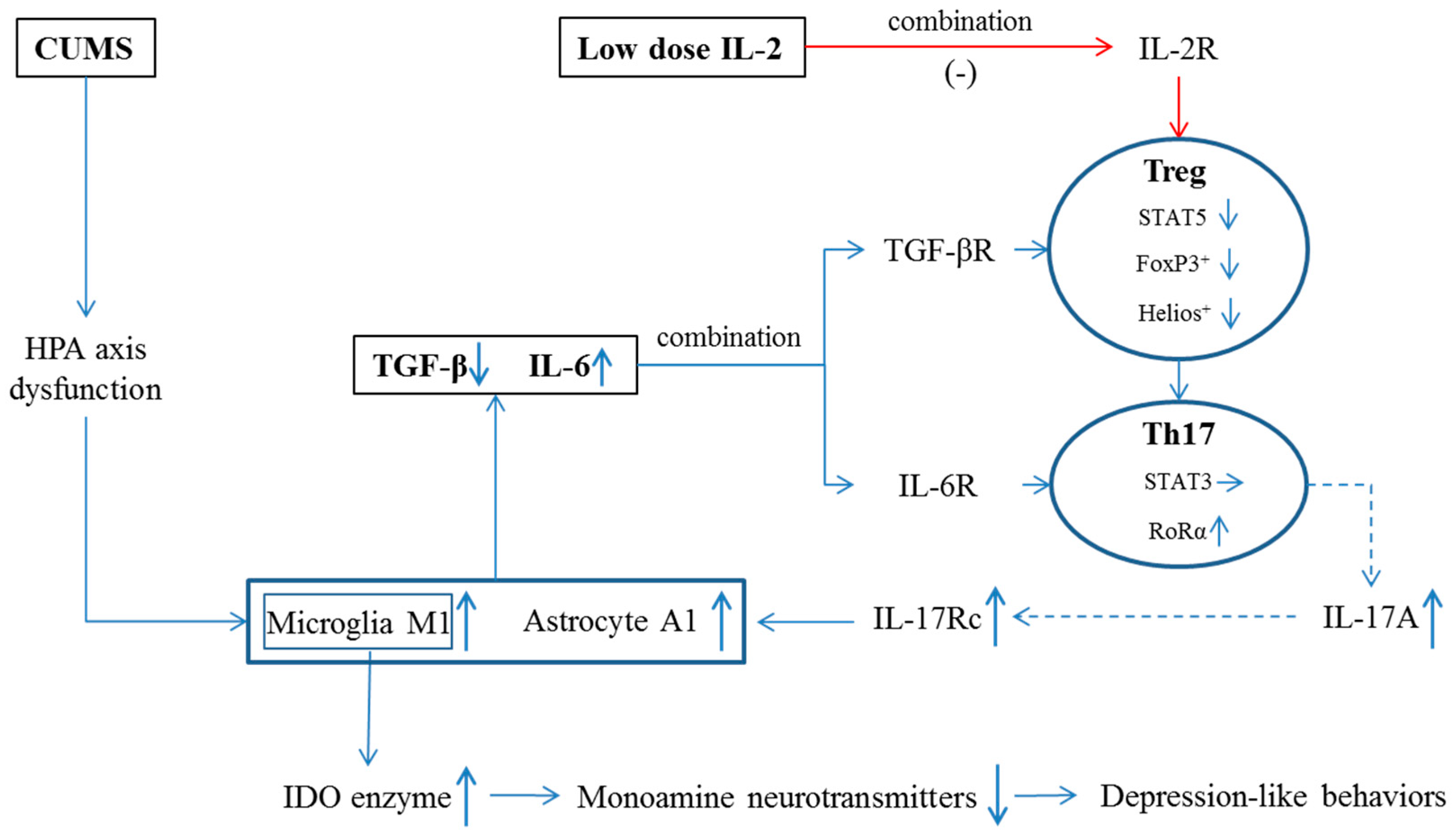
3. Discussion
3.1. The Imbalance between IL-6 and TGF-β in the Hippocampus of CUMS Mice
3.2. The Imbalance between Th17 and Treg Occurred in the Hippocampus of CUMS Mice
3.3. Low-Dose IL-2 Improved Depression-Like Behavior and Neuropathological Changes through Restoring the Balance between Two Pairs of Lymphocyte Subtypes
4. Materials and Methods
4.1. Materials
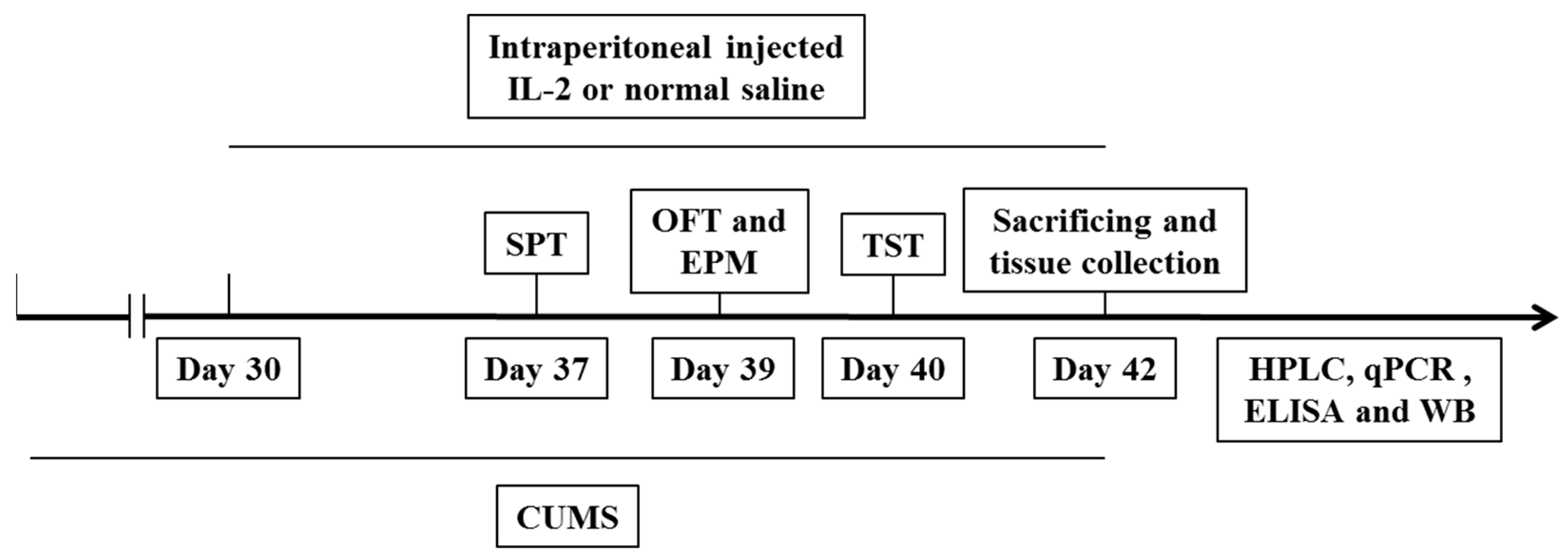
4.2. Experimental Procedure
4.3. Behavioral Tests
4.3.1. Sucrose Preference Test
4.3.2. Open Field Test
4.3.3. Elevated Plus-Maze Test
4.3.4. Tail Suspension Test
4.4. HPLC Analysis of Neurotransmitters and Metabolites in the Hippocampus and Amygdala
4.5. Quantitative Real-Time PCR
4.6. ELISA Assays for Cytokines, Their Receptor and Microglia Markers
4.7. Western Blot
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.F.; Wang, X.Y.; Zhou, Y.; Zheng, Q.M.; Chen, Z.; Zhang, H.; Sun, Z.L.; Xu, G.H.; Hu, G. Hypothalamus-pituitary-adrenal axis imbalance and inflammation contribute to sex differences in separation- and restraint-induced depression. Horm. Behav. 2020, 122, 104741. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 7–11. [Google Scholar] [PubMed]
- Burrows, K.; Stewart, J.L.; Kuplicki, R.; Figueroa-Hall, L.; Spechler, P.A.; Zheng, H. Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain Behav. Immun. 2021, 93, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Song, C. The effect of thymectomy and IL-1 on memory: Implications for the relationship between immunity and depression. Brain Behav. Immun. 2002, 16, 557–568. [Google Scholar] [CrossRef]
- Mello, C.; Swain, M.G. Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression. Curr. Top. Behav. Neurosci. 2016, 31, 73–94. [Google Scholar]
- Wang, Q.; Jie, W.; Liu, J.H.; Yang, J.M.; Gao, T.M. An astroglial basis of major depressive disorder? An overview. Glia 2017, 65, 1227–1250. [Google Scholar] [CrossRef]
- Da Silva Dias, I.C.; Carabelli, B.; Ishii, D.K.; de Morais, H.; de Carvalho, M.C.; Rizzo de Souza, L.E.; Zanata, S.M.; Brandão, M.L.; Cunha, T.M.; Ferraz, A.C.; et al. Indoleamine-2, 3-Dioxygenase/Kynurenine Pathway as a Potential Pharmacological Target to Treat Depression Associated with Diabetes. Mol. Neurobiol. 2016, 53, 6997–7009. [Google Scholar] [CrossRef]
- Wang, D.; Huang, S.; Yuan, X.; Liang, J.; Xu, R.; Yao, G.; Sun, L. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell. Mol. Immunol. 2017, 14, 423–431. [Google Scholar] [CrossRef]
- Ding, H.; Dai, Y.; Lei, Y.; Wang, Z.; Liu, D.; Li, R.; Hu, Y. Upregulation of CD81 in trophoblasts induces an imbalance of Treg/Th17 cells by promoting IL-6 expression in preeclampsia. Cell. Mol. Immunol. 2019, 16, 302–312. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Nichols, J.R.; Aldrich, A.L.; Mariani, M.M.; Vidlak, D.; Esen, N.; Kielian, T. TLR2 deficiency leads to increased Th17 infiltrates in experimental brain abscesses. J. Immunol. 2009, 182, 7119–7130. [Google Scholar] [CrossRef] [PubMed]
- Waisman, A.; Hauptmann, J.; Regen, T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015, 129, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Liblau, R.S. Endothelial cells and lymphatics at the interface between the immune and central nervous systems: Implications for multiple sclerosis. Curr. Opin. Neurol. 2017, 30, 222–230. [Google Scholar]
- Song, C.; Merali, Z.; Anisman, H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience 1999, 88, 823–836. [Google Scholar] [CrossRef]
- Fakhri, H.; Lang, F.; Lang, U.E.; Ricken, R. Faster speed of onset of the depressive episode is associated with lower cytokine serum levels (IL-2, -4, -6, -10, TNF-α and IFN-γ) in patients with major depression. J. Psychiatr. Res. 2021, 141, 287–292. [Google Scholar]
- Darko, D.F.; Gillin, J.C.; Risch, S.C.; Bulloch, K.; Golshan, S.; Tasevska, Z.; Hamburger, R.N. Mitogen-stimulated lymphocyte proliferation and pituitary hormones in major depression. Biol. Psychiatry 1989, 26, 145–155. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Rasmussen, J.P.; Gavin, M.A.; Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005, 6, 1142–1151. [Google Scholar] [CrossRef]
- Lee, H.; Son, Y.S.; Lee, M.O.; Ryu, J.W.; Park, K.; Kwon, O.; Son, M.Y. Low-dose interleukin-2 alleviates dextran sodium sulfate-induced colitis in mice by recovering intestinal integrity and inhibiting AKT-dependent pathways. Theranostics 2020, 10, 5048–5063. [Google Scholar] [CrossRef]
- Sharabi, A.; Li, H.; Kasper, I.R.; Pan, W.; Meidan, E.; Tsokos, M.G.; Tsokos, G.C. PP2A enables IL-2 signaling by preserving IL-2Rβ chain expression during Treg development. JCI Insight 2019, 5, e126294. [Google Scholar] [CrossRef]
- Song, C.; Leonard, B.E. Interleukin-2-induced changes in behavioural, neurotransmitter, and immunological parameters in the olfactory bulbectomized rat. Neuroimmunomodulation 1995, 2, 263–273. [Google Scholar] [CrossRef]
- Smith, R.S. The macrophage theory of depression. Med. Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef]
- Lutter, M.; Sakata, I.; Osborne, L.S.; Rovinsky, S.A.; Anderson, J.G.; Jung, S.; Birnbaum, S.; Yanagisawa, M.; Elmquist, J.K.; Nestler, E.J.; et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008, 11, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.A.; Quartermain, D. Alpha-1-noradrenergic neurotransmission, corticosterone, and behavioral depression. Biol. Psychiatry 1999, 46, 1287–1300. [Google Scholar] [CrossRef]
- Kim, H.J.; Barnitz, R.A.; Kreslavsky, T.; Brown, F.D.; Moffett, H.; Lemieux, M.E.; Kaygusuz, Y.; Meissner, T.; Holderried, T.A.; Chan, S.; et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015, 350, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Marsh, S.E.; Stevens, B. Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol. 2020, 41, 820–835. [Google Scholar] [CrossRef] [PubMed]
- West, P.K.; Viengkhou, B.; Campbell, I.L.; Hofer, M.J. Microglia responses to interleukin-6 and type I interferons in neuroinflammatory disease. Glia 2019, 67, 1821–1841. [Google Scholar] [CrossRef]
- Roberts, A.J.; Khom, S.; Bajo, M.; Vlkolinsky, R.; Polis, I.; Cates-Gatto, C.; Gruol, D.L. Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain Behav. Immun. 2019, 82, 188–202. [Google Scholar] [CrossRef]
- Gu, M.; Li, X.; Yan, L.; Zhang, Y.; Yang, L.; Li, S.; Song, C. Endogenous ω-3 fatty acids in Fat-1 mice attenuated depression-like behaviors, spatial memory impairment and relevant changes induced by olfactory bulbectomy. Prostaglandins Leukot. Essent. Fat. Acids 2021, 171, 102313. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.L.; Liu, S.; Liu, Y.; Yang, X.; Liu, G.; Ma, J. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca-dependent PKC/p38MAPK/NF-κB pathway. J. Neuroinflammation 2019, 16, 10. [Google Scholar] [CrossRef]
- Li, C.; Wu, H.; Sen Ta Na, H.; Wang, L.; Zhong, C.; Deng, B.; Liu, C.; Bao, H.; Sang, H.; Hou, L. Neuronal-Microglial Liver X receptor β Activating Decrease Neuroinflammation and Chronic Stress-induced Depression-Related Behavior in Mice. Brain Res. 2022, 7, 148112. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, G.; Wang, H.; Wang, X. Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci. Lett. 2011, 503, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.M.; Zhang, J.R.; Wan, T.F.; Wang, Y.; Chen, H.S.; Liu, L. SRT2104 attenuates chronic unpredictable mild stress-induced depressive-like behaviors and imbalance between microglial M1 and M2 phenotypes in the mice. Behav. Brain Res. 2020, 378, 112296. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Castro, O.; Ortega, E.M.; Maldonado-García, C.; Cruz, J.S.; Pérez-Montesinos, G.; Bonifaz, L.C. Association of Pathogenic Th17 Cells with the Disease Severity and Its Potential Implication for Biological Treatment Selection in Psoriasis Patients. Mediators Inflamm. 2020, 2020, 8065147. [Google Scholar]
- Tateishi, N.; Shimoda, T.; Yada, N.; Shinagawa, R.; Kagamiishi, Y. S100B: Astrocyte specific protein. Nihon Shinkei Seishin Yakurigaku Zasshi 2006, 26, 11–16. (In Japanese) [Google Scholar]
- Horiguchi, K.; Higuchi, M.; Yoshida, S.; Nakakura, T.; Tateno, K.; Hasegawa, R.; Kato, Y. Proton receptor GPR68 expression in dendritic-cell-like S100β-positive cells of rat anterior pituitary gland: GPR68 induces interleukin-6 gene expression in extracellular acidification. Cell Tissue Res. 2014, 358, 515–525. [Google Scholar] [CrossRef]
- Du, J.; Huang, C.; Zhou, B.; Ziegler, S.F. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J. Immunol. 2008, 180, 4785–4792. [Google Scholar] [CrossRef]
- Ihle, J.N.; Kerr, I.M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995, 11, 69–74. [Google Scholar] [CrossRef]
- Zhou, L.; Lopes, J.E.; Chong, M.M.W.; Ivanov, I.I.; Min, R.; Victora, G.D.; Littman, D.R. TGF-beta-induced Foxp3 inhibits T (H) 17 cell differentiation by antagonizing RORgammat function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef]
- Freudenberg, K.; Lindner, N.; Dohnke, S.; Garbe, A.I.; Schallenberg, S.; Kretschmer, K. Critical Role of TGF-β and IL-2 Receptor Signaling in Foxp3 Induction by an Inhibitor of DNA Methylation. Front. Immunol. 2018, 9, 125. [Google Scholar] [CrossRef]
- Yu, W.B.; Wang, Q.; Chen, S. The therapeutic potential of ginkgolide K in experimental autoimmune encephalomyelitis via peripheral immunomodulation. Int. Immunopharmacol. 2019, 70, 284–294. [Google Scholar] [CrossRef]
- Klatzmann, D.; Abbas, A.K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 2015, 15, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Sudom, K.; Turrin, N.P.; Hayley, S.; Anisman, H. Influence of chronic interleukin-2 infusion and stressors on sickness behaviors and neurochemical change in mice. Neuroimmunomodulation 2004, 11, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, M.Q.; Xue, Y.; Yang, R.; Tang, M.M. Dietary of n-3 polyunsaturated fatty acids influence neurotransmitter systems of rats exposed to unpredictable chronic mild stress. Behav. Brain Res. 2019, 376, 112172. [Google Scholar] [CrossRef]
- Levin-Arama, M.; Abraham, L.; Waner, T.; Harmelin, A.; Steinberg, D.M.; Lahav, T.; Harlev, M. Subcutaneous Compared with Intraperitoneal KetamineXylazine for Anesthesia of Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 794–800. [Google Scholar] [PubMed]
- Du, X.D.; Yin, M.; Yuan, L. Reduction of depression-like behavior in rat model induced by ShRNA targeting norepinephrine transporter in locus coeruleus. Transl. Psychiatry 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Scheinert, R.B.; Haeri, M.H.; Lehmann, M.L.; Herkenham, M. Therapeutic effects of stress-programmed lymphocytes transferred to chronically stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 70, 1–7. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Yan, L.; Zhang, Y.; Yang, Z.; Wang, J.; Song, C. EPA is More Effective than DHA to Improve Depression-Like Behavior, Glia Cell Dysfunction and Hippcampal Apoptosis Signaling in a Chronic Stress-Induced Rat Model of Depression. Int. J. Mol. Sci. 2020, 21, 1769. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.P.; Li, Y.Y.; Liu, B.P.; Wang, H.Y.; Li, K.W.; Zhao, S.; Song, C. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav. Brain Res. 2019, 356, 348–357. [Google Scholar] [CrossRef]



| Stressor | Days |
|---|---|
| Shaker for 1.5 h | D4,D8,D11,D17,D23,D30,D36,D41 |
| 4 °C environment for 1 h | D1,D5,D9,D14,D18,D24,D29,D35,D40 |
| Swimming at 18 °C for 10 min | D2,D6,D13,D20,D26,D33,D38 |
| Crowded environment for 3 h | D3,D7,D10,D15,D21,D27,D32,D36,D41 |
| Tilt the cage 45° for 12 h | D3,D7,D13,D16,D22,D26,D31,D37 |
| Illumination for 24 h | D1,D9,D12,D18,D22,D27,D31,D34,D37,D40 |
| Strobe lamp | D10,D16,D20,D25,D30,D33,D42 |
| Peculiar smell | D12,D21,D29,D35,D42 |
| Lack of water | D2,D6,D11,D15,D19,D23,D28,D34,D39 |
| Lack of feed | D5,D14,D19,D24,D28,D39 |
| Wet bedding | D4,D8,D17,D25,D32,D38 |
| Mouse Genes | Primer Sequences |
|---|---|
| IL-6 F | 5′-GAGGATACCACTCCCACAGACC-3′ 5′-AAGTGCATCATCGTTGTTCATACA-3′ |
| IL-6 R | |
| IL-17A F | 5′-CCCTCAGACTACCTCAACCGTTC-3′ 5′-TTCATGTGGTGGTCCAGCTTTC-3′ |
| IL-17A R | |
| IL-17RA F | 5′-AGTTCCAGTTTCTGTCCATGC-3′ 5′-TGGATTTGTGGTTTGGGTC-3′ |
| IL-17RA R | |
| IL-17RC F | 5′-GCAGAGCCTGAAGAAGCTG-3′ 5′-CCCAAGACTAGCCTCGAAAC-3′ |
| IL-17RC R | |
| STAT3 F | 5′-CTACAGTGACAGCTTCCCAAT-3′ 5′-TTGGCTTCTCAAGATACCTGC-3′ |
| STAT3 R | |
| RoRa F | 5′-TCACCTCTCTGCTTGTTCTG-3′ 5′-GCTTCTTCCCCTACTGTTCCTT-3′ |
| RoRa R | |
| STAT5 F | 5′-CAAGCAAGTGGTCCCTGAGT-3′ 5′-CATTGGTCGGCGTAAGAGTT-3′ |
| STAT5 R | |
| FoxP3+ F | 5′-GGCCCTTCTCCAGGACAGA-3′ 5′-GCTGATCATGGCTGGGTTGT-3′ |
| FoxP3+ R | |
| GAPDH F | 5′-GAAGGTGAAGGTCGGAGTC-3′ 5′-GAAGATGGTGATGGGATTTC-3′ |
| GAPDH R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhang, F.; Li, P.; Song, C. Low-Dose IL-2 Attenuated Depression-like Behaviors and Pathological Changes through Restoring the Balances between IL-6 and TGF-β and between Th17 and Treg in a Chronic Stress-Induced Mouse Model of Depression. Int. J. Mol. Sci. 2022, 23, 13856. https://doi.org/10.3390/ijms232213856
Huang C, Zhang F, Li P, Song C. Low-Dose IL-2 Attenuated Depression-like Behaviors and Pathological Changes through Restoring the Balances between IL-6 and TGF-β and between Th17 and Treg in a Chronic Stress-Induced Mouse Model of Depression. International Journal of Molecular Sciences. 2022; 23(22):13856. https://doi.org/10.3390/ijms232213856
Chicago/Turabian StyleHuang, Chengyi, Fucheng Zhang, Peng Li, and Cai Song. 2022. "Low-Dose IL-2 Attenuated Depression-like Behaviors and Pathological Changes through Restoring the Balances between IL-6 and TGF-β and between Th17 and Treg in a Chronic Stress-Induced Mouse Model of Depression" International Journal of Molecular Sciences 23, no. 22: 13856. https://doi.org/10.3390/ijms232213856
APA StyleHuang, C., Zhang, F., Li, P., & Song, C. (2022). Low-Dose IL-2 Attenuated Depression-like Behaviors and Pathological Changes through Restoring the Balances between IL-6 and TGF-β and between Th17 and Treg in a Chronic Stress-Induced Mouse Model of Depression. International Journal of Molecular Sciences, 23(22), 13856. https://doi.org/10.3390/ijms232213856







