Long Chain N3-PUFA Decreases ACE2 Protein Levels and Prevents SARS-CoV-2 Cell Entry
Abstract
1. Introduction
2. Results
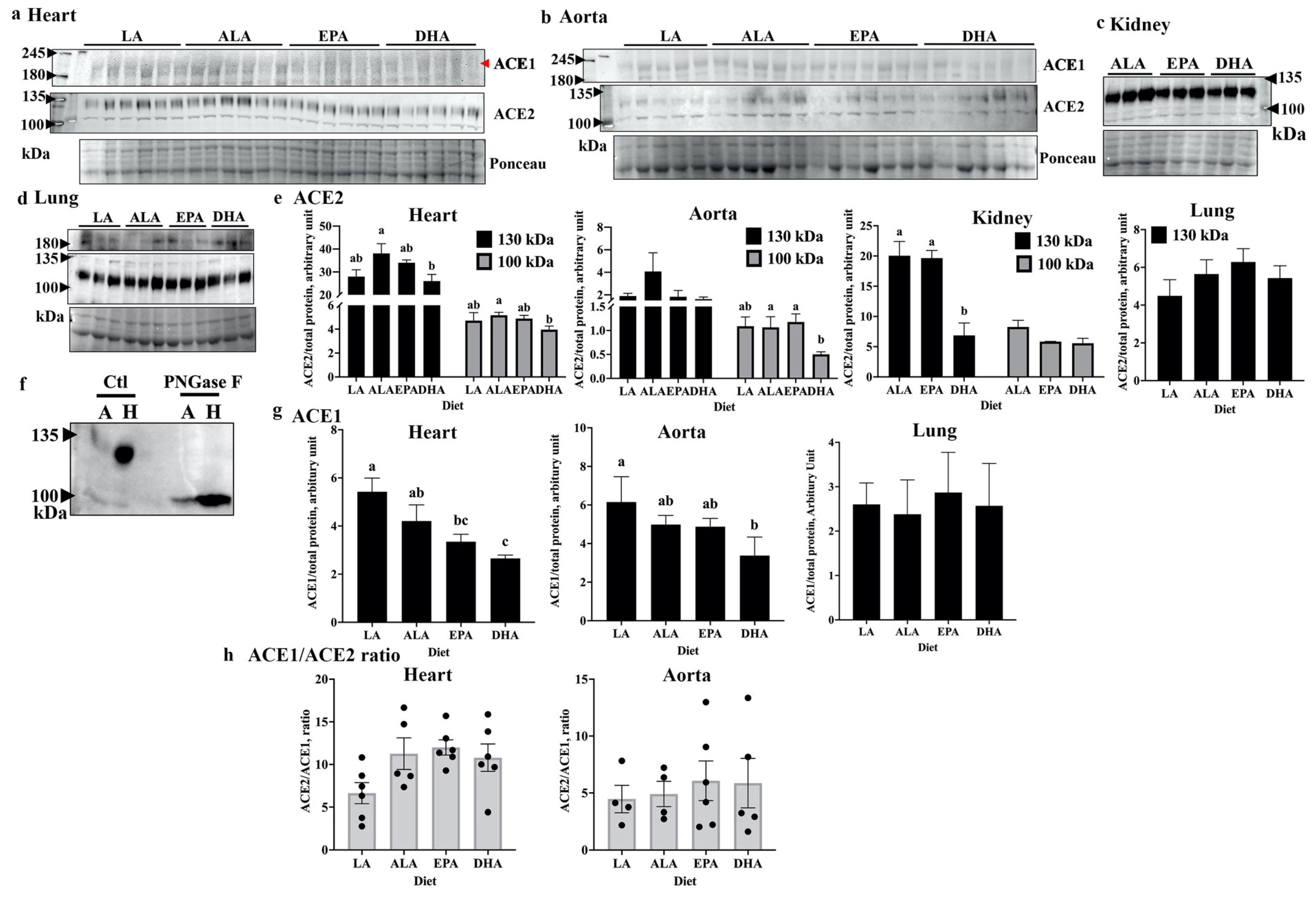
2.1. DHA Modulates Tissue Levels of ACE2 and ACE1 in Rats
2.2. DHA Reduces ACE2 Levels of Human Endothelial Cells
2.3. DHA Supplementation Does Not Alter Circulating ACE2 Levels
2.4. DHA Treatment Reduces Pseudovirus Entry into Cells
3. Discussion
4. Materials and Methods
4.1. Animal Tissue Protein Extraction
4.2. Cell Culture and Treatments
4.3. Sample Preparation and Western Blotting
4.4. Sample De-Glycosylation
4.5. Circulating ACE2 ELISA
4.6. Pseudo SARS-CoV-2 Cell Entry Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. EPI-Win & Infodemic. Clinical Long-Term Effects of COVID-19; World Health Organization: Geneva, Switzerland, 2021; pp. 1–15. [Google Scholar]
- Siddiqi, H.K.; Libby, P.; Ridker, P.M. COVID-19—A vascular disease. Trends Cardiovasc. Med. 2021, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Menez, S.; Parikh, C.R. Overview of acute kidney manifestations and management of patients with COVID-19. Am. J. Physiol.—Ren. Physiol. 2021, 321, F403–F410. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, M.; Lipka, S.; Mlynarska, E.; Franczyk, B.; Rysz, J. Acute Kidney Injury in COVID-19. Int. J. Mol. Sci. 2021, 22, 8081. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, X.; Wang, Y.; Zeng, X.; Luo, T.; Liu, Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0250602. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Narayan, R.K.; Kumari, C.; Faiq, M.A.; Kulandhasamy, M.; Kant, K.; Pareek, V. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med. Hypotheses 2020, 145, 110320. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Toe, Q.K.; Issitt, T.; Mahomed, A.S.; Panselinas, I.; Almaghlouth, F.; Burke-Gaffney, A.; Wort, S.J.; Quinlan, G.J. Human pulmonary artery endothelial cells upregulate ACE2 expression in response to iron-regulatory elements: Potential implications for SARS-CoV-2 infection of vascular endothelial cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, P.A. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 2020, 177, 4825–4844. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.K.A.A.; Shurbaji, S. Do changes in ace-2 expression affect sars-cov-2 virulence and related complications: A closer look into membrane-bound and soluble forms. Int. J. Mol. Sci. 2012, 22, 6703. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Kabbani, N. A comprehensive guide to the pharmacologic regulation of angiotensin converting enzyme 2 (ACE2), the SARS-CoV-2 entry receptor. Pharmacol. Ther. 2021, 221, 107750. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Taylor, C.G.; Aukema, H.M.; Zahradka, P. Regulation of docosahexaenoic acid-induced apoptosis of confluent endothelial cells: Contributions of MAPKs and caspases. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2021, 1866, 158902. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Taylor, C.G.; Zahradka, P. Modulation of endothelial cell responses and vascular function by dietary fatty acids. Nutr. Rev. 2019, 77, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Leão, M.D.C.; Santana, T.M.; Pimentel, M.V.D.M.; Carlini, G.C.; da Silveira, T.F.; Gonçalves, R.C.; Castro, I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020, 156, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, A.M.; Bassiouni, W.; Sosnowski, D.K.; Seubert, J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2021, 219, 107703. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.W.; Wang, P.H.; Lee, H.-S.; Liu, B.H.; Mersmann, H.J.; Lin, E.C.; Ding, S.-T. Regulation of the expression of angiotensin-converting enzyme 2 by polyunsaturated fatty acids in porcine adipocytes. J. Anim. Sci. 2010, 88, 3563–3567. [Google Scholar] [CrossRef] [PubMed]
- Ulu, A.; Harris, T.R.; Morisseau, C.; Miyabe, C.; Inoue, H.; Schuster, G.; Dong, H.; Iosif, A.-M.; Liu, J.-Y.; Weiss, R.H.; et al. Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J. Cardiovasc. Pharmacol. 2013, 62, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids Health Dis. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hughes, T.A.; Kelkar, A.; Yu, X.; Cheng, K.; Park, S.; Huang, W.-C.; Lovell, J.F.; Neelamegham, S. Inhibition of SARS-CoV-2 viral entry upon blocking N-and O-glycan elaboration. Elife 2020, 9, e61552. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, A.R.; Hummer, G. Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. Proc. Natl. Acad. Sci. USA 2021, 118, e2100425118. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Taylor, C.G.; Aukema, H.M.; Zahradka, P. Importance of extracellular matrix and growth state for the EA.hy926 endothelial cell response to polyunsaturated fatty acids. PLoS ONE 2018, 13, e0197613. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.P.; Zhou, R.; Chen, K.-H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2021, 184, 2212–2228.e12. [Google Scholar] [CrossRef]
- Ramchand, J.; Burrell, L.M. Circulating ACE2: A novel biomarker of cardiovascular risk. Lancet 2020, 396, 937–939. [Google Scholar] [CrossRef]
- Patel, S.K.; Juno, J.A.; Lee, W.S.; Wragg, K.M.; Hogarth, P.M.; Kent, S.J.; Burrell, L.M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: Implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 2021, 57, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Lundström, A.; Ziegler, L.; Havervall, S.; Rudberg, A.; von Meijenfeldt, F.; Lisman, T.; Mackman, N.; Sandén, P.; Thålin, C. Soluble angiotensin-converting enzyme 2 is transiently elevated in COVID-19 and correlates with specific inflammatory and endothelial markers. J. Med. Virol. 2021, 93, 5908–5916. [Google Scholar] [CrossRef] [PubMed]
- Fagyas, M.; Fejes, Z.; Sütő, R.; Nagy, Z.; Székely, B.; Pócsi, M.; Ivády, G.; Bíró, E.; Bekő, G.; Nagy, A.; et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int. J. Infect. Dis. 2022, 115, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Zahradka, P.; Taylor, C.G.; Aukema, H.M. Time Course and Sex Effects of α-Linolenic Acid-Rich and DHA-Rich Supplements on Human Plasma Oxylipins: A Randomized Double-Blind Crossover Trial. J. Nutr. 2021, 151, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Rodway, L.A.; Pauls, S.D.; Aukema, H.M.; Zahradka, P.; Taylor, C.G. Rationale and design of a randomized controlled trial examining the effects of marine- and plant-sourced omega-3 fatty acid supplements on octadecanoid profiles and inflammation in females with obesity (OXBIO trial). Prostaglandins Leukot. Essent. Fat. Acids 2021, 170, 102284. [Google Scholar] [CrossRef]
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.S.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2022, 29, 217–231.e8. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Kim, J.H.; Lee, J.H.; Son, Y.H.; Lee, M.W.; Kim, H.J.; Noh, S.A.; Kim, K.P.; Kim, I.G.; Lee, M.J. Docosahexaenoic acid-mediated protein aggregates may reduce proteasome activity and delay myotube degradation during muscle atrophy in vitro. Exp. Mol. Med. 2017, 49, e287. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The Concept of Classical Herd Immunity May Not Apply to COVID-19. J. Infect. Dis. 2022, 226, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, D.; Verma, V.; Yadav, R.; Kumar, R. Angiotensin-converting enzyme 2 as a potential therapeutic target for COVID-19: A review. J. Pharm. Anal. 2012, 12, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Zhang, Y.; Wang, Y.; Zhang, Z.; Wei, H.; Yu, J.; Wang, Q.; Wang, G.; Zhang, B.; Wang, C. ACE2-Targeting antibody suppresses SARS-CoV-2 Omicron and Delta variants. Signal Transduct. Target. Ther. 2022, 7, 43. [Google Scholar] [CrossRef]
- Ulu, A.; Lee, K.S.S.; Miyabe, C.; Yang, J.; Hammock, B.G.; Dong, H. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J. Cardiovasc. Pharmacol. 2014, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Praissman, J.L.; Grant, O.C.; Cai, Y.; Xiao, T.; Rosenbalm, K.E.; Aoki, K.; Kellman, B.P.; Bridger, R.; Barouch, D.H.; et al. Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. Cell Host Microbe 2020, 28, 586–601.e6. [Google Scholar] [CrossRef]
- Stupin, A.; Cvetko, A.; Kralik, G.; Mihalj, M.; Šušnjara, P.; Kolobarić, N.; Ćurić, Ž.B.; Lukinac, A.M.; Kibel, A.; Selthofer-Relatić, K.; et al. The effect of n-3 polyunsaturated fatty acids-enriched hen eggs consumption on IgG and total plasma protein N-glycosylation in healthy individuals and cardiovascular patients. Glycobiology 2021, 31, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Niedzwiecki, A.; Rath, M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci. Rep. 2021, 11, 5207. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W. Comprehensive profiling of plasma fatty acid concentrations in young healthy canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef]
- Chiang, E.I.; Syu, J.N.; Hung, H.C.; Rodriguez, R.L.; Wang, W.J.; Chiang, E.R.; Chiu, S.C.; Chao, C.Y.; Tang, F.Y. N-3 polyunsaturated fatty acids block the trimethylamine-N-oxide- ACE2- TMPRSS2 cascade to inhibit the infection of human endothelial progenitor cells by SARS-CoV-2. J. Nutr. Biochem. 2022, 109, 109102. [Google Scholar] [CrossRef] [PubMed]
- Farías, J.G.; Carrasco-Pozo, C.; Carrasco Loza, R.; Sepúlveda, N.; Álvarez, P.; Quezada, M.; Quiñones, J.; Molina, V.; Castillo, R.L. Polyunsaturated fatty acid induces cardioprotection against ischemia-reperfusion through the inhibition of NF-kappaB and induction of Nrf2. Exp. Biol. Med. 2017, 242, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Chen, C.J.; Lee, T.S.; Chao, H.Y.; Wu, W.H.; Hsieh, S.C.; Sheu, H.H.; Chiang, A.N. Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells. J. Nutr. Biochem. 2011, 22, 187–194. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, S.; Wang, Q.; Yao, S.; Qian, L.; Jiang, S.; Tang, Y.; Han, T. DHA-enriched phosphatidylserine ameliorates high-fat diet-induced kidney injury in mice possibly by regulating TLR4/NF-κB and AMPK pathways. J. Food Sci. 2022, 87, 4233–4249. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef]
- Borghi, C.; Cicero, A.F.G. Omega-3 polyunsaturated fatty acids: Their potential role in blood pressure prevention and management. Heart Int. 2006, 2, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Narula, S.; Yusuf, S.; Chong, M.; Ramasundarahettige, C.; Rangarajan, S.; Bangdiwala, S.I.; van Eikels, M.; Leineweber, K.; Wu, A.; Pigeyre, M.; et al. Plasma ACE2 and risk of death or cardiometabolic diseases: A case-cohort analysis. Lancet 2020, 396, 968–976. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Singh, H.S.; Grundberg, I.; Nielsen, A.L.-L.; Rivellese, F.; Mehta, A.; Goldberg, M.B.; Filbin, M.R.; Qvist, P.; Bibby, B.M. Plasma ACE2 predicts outcome of COVID-19 in hospitalized patients. PLoS ONE 2021, 16, e0252799. [Google Scholar] [CrossRef] [PubMed]
- Gealekman, O.; Brodsky, S.V.; Zhang, F.; Chander, P.N.; Friedli, C.; Nasjletti, A.; Goligorsky, M.S. Endothelial dysfunction as a modifier of angiogenic response in Zucker diabetic fat rat: Amelioration with Ebselen. Kidney Int. 2004, 66, 2337–2347. [Google Scholar] [CrossRef]
- Martínez-Salazar, B.; Holwerda, M.; Stüdle, C.; Piragyte, I.; Mercader, N.; Engelhardt, B.; Rieben, R.; Döring, Y. COVID-19 and the Vasculature: Current Aspects and Long-Term Consequences. Front. Cell Dev. Biol. 2022, 10, 824851. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post COVID-19 Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69 (Suppl. 1), 7–21. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zahradka, P.; Cordero-Monroy, L.; Wright, B.; Taylor, C.G. Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) Operate by Different Mechanisms to Modulate Hepatic Steatosis and Hyperinsulemia in fa/fa Zucker Rats. Nutrients 2019, 11, 917. [Google Scholar] [CrossRef]
- Sabbir, M.G.; Taylor, C.G.; Zahradka, P. Hypomorphic CAMKK2 in EA.hy926 endothelial cells causes abnormal transferrin trafficking, iron homeostasis and glucose metabolism. Biochim. Biophys. Acta—Mol. Cell Res. 2020, 1867, 118763. [Google Scholar] [CrossRef]




| Overall | RS | p Value |
|---|---|---|
| BMI vs. sACE2 | 0.361 | 0.064 |
| SBP vs. sACE2 | 0.402 | 0.038 |
| HDL-C vs. sACE2 | −0.503 | 0.006 |
| Glc vs. sACE2 | 0.471 | 0.049 |
| Obese subgroup 1 | Rs | p value |
| TC vs. sACE2 | −0.439 | 0.069 |
| HDL-C vs. sACE2 | −0.393 | 0.096 |
| Glc vs. sACE2 | 0.403 | 0.087 |
| Healthy subgroup 2 | Rs | p value |
| SBP vs. sACE2 | 0.669 | 0.049 |
| HDL-C vs. sACE2 | −0.693 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Taylor, C.G.; Zahradka, P. Long Chain N3-PUFA Decreases ACE2 Protein Levels and Prevents SARS-CoV-2 Cell Entry. Int. J. Mol. Sci. 2022, 23, 13825. https://doi.org/10.3390/ijms232213825
Huang S, Taylor CG, Zahradka P. Long Chain N3-PUFA Decreases ACE2 Protein Levels and Prevents SARS-CoV-2 Cell Entry. International Journal of Molecular Sciences. 2022; 23(22):13825. https://doi.org/10.3390/ijms232213825
Chicago/Turabian StyleHuang, Shiqi, Carla G. Taylor, and Peter Zahradka. 2022. "Long Chain N3-PUFA Decreases ACE2 Protein Levels and Prevents SARS-CoV-2 Cell Entry" International Journal of Molecular Sciences 23, no. 22: 13825. https://doi.org/10.3390/ijms232213825
APA StyleHuang, S., Taylor, C. G., & Zahradka, P. (2022). Long Chain N3-PUFA Decreases ACE2 Protein Levels and Prevents SARS-CoV-2 Cell Entry. International Journal of Molecular Sciences, 23(22), 13825. https://doi.org/10.3390/ijms232213825









