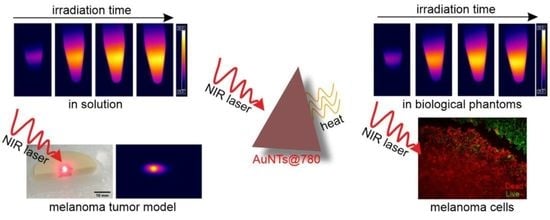
Assessing the Efficiency of Triangular Gold Nanoparticles as NIR Photothermal Agents In Vitro and Melanoma Tumor Model
Abstract
1. Introduction
2. Results and Discussion
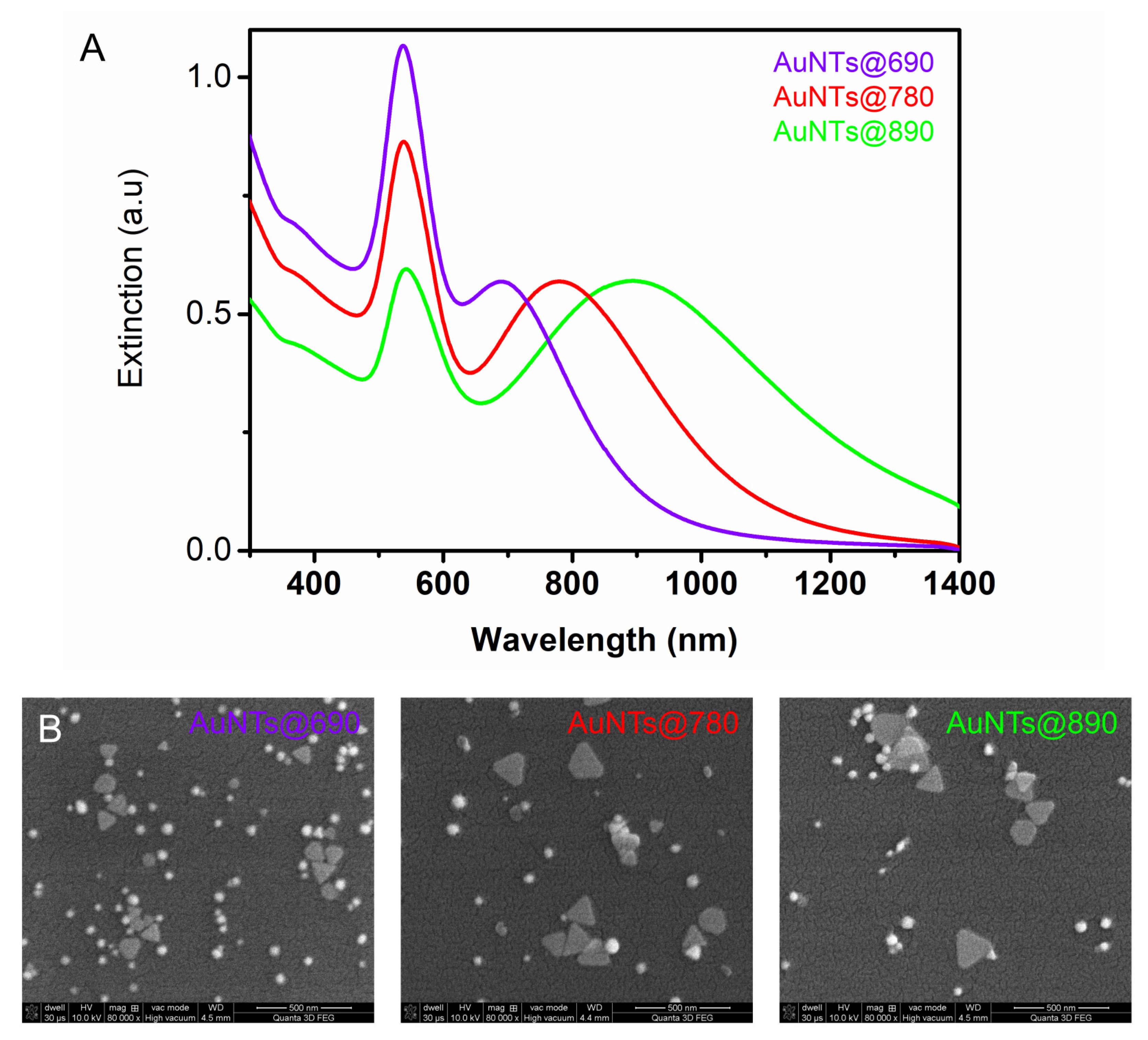
2.1. Gold Nanotriangles Characterization
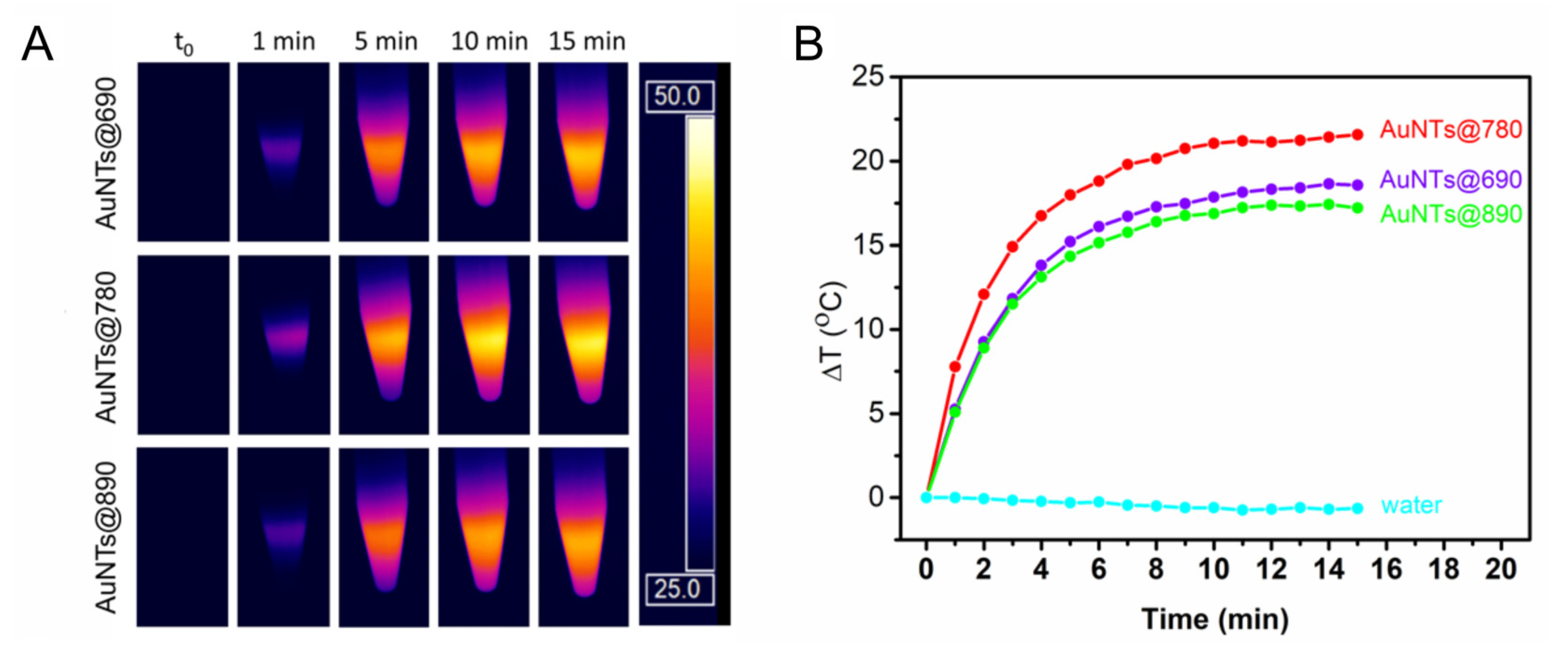
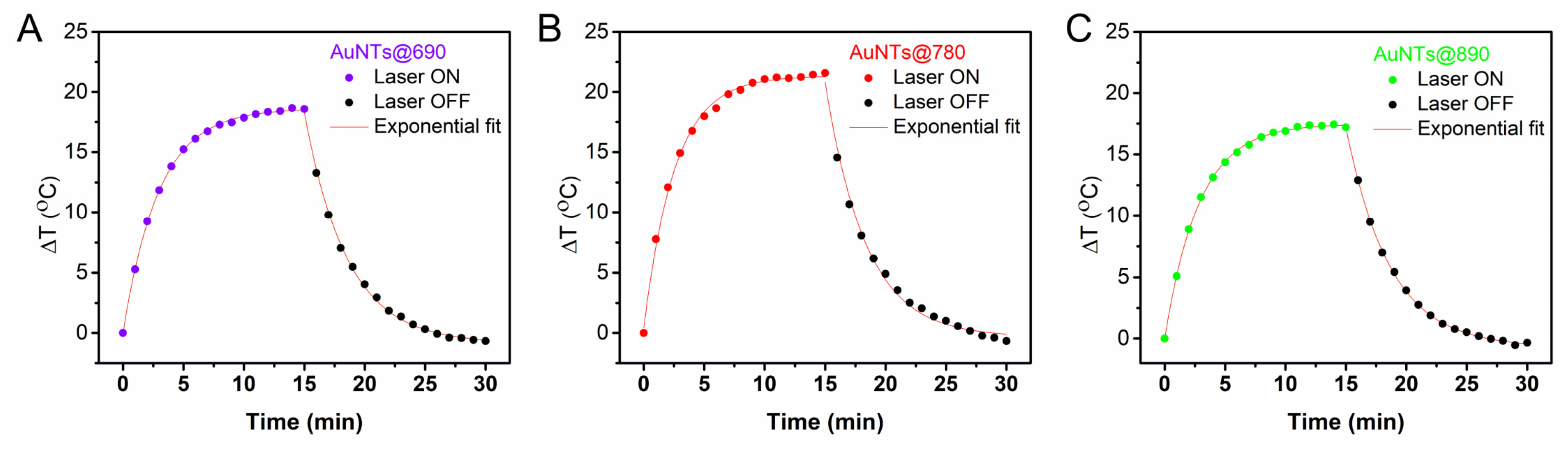
2.2. Investigation of the AuNTs Light-to-Heat Conversion Performances in Solution
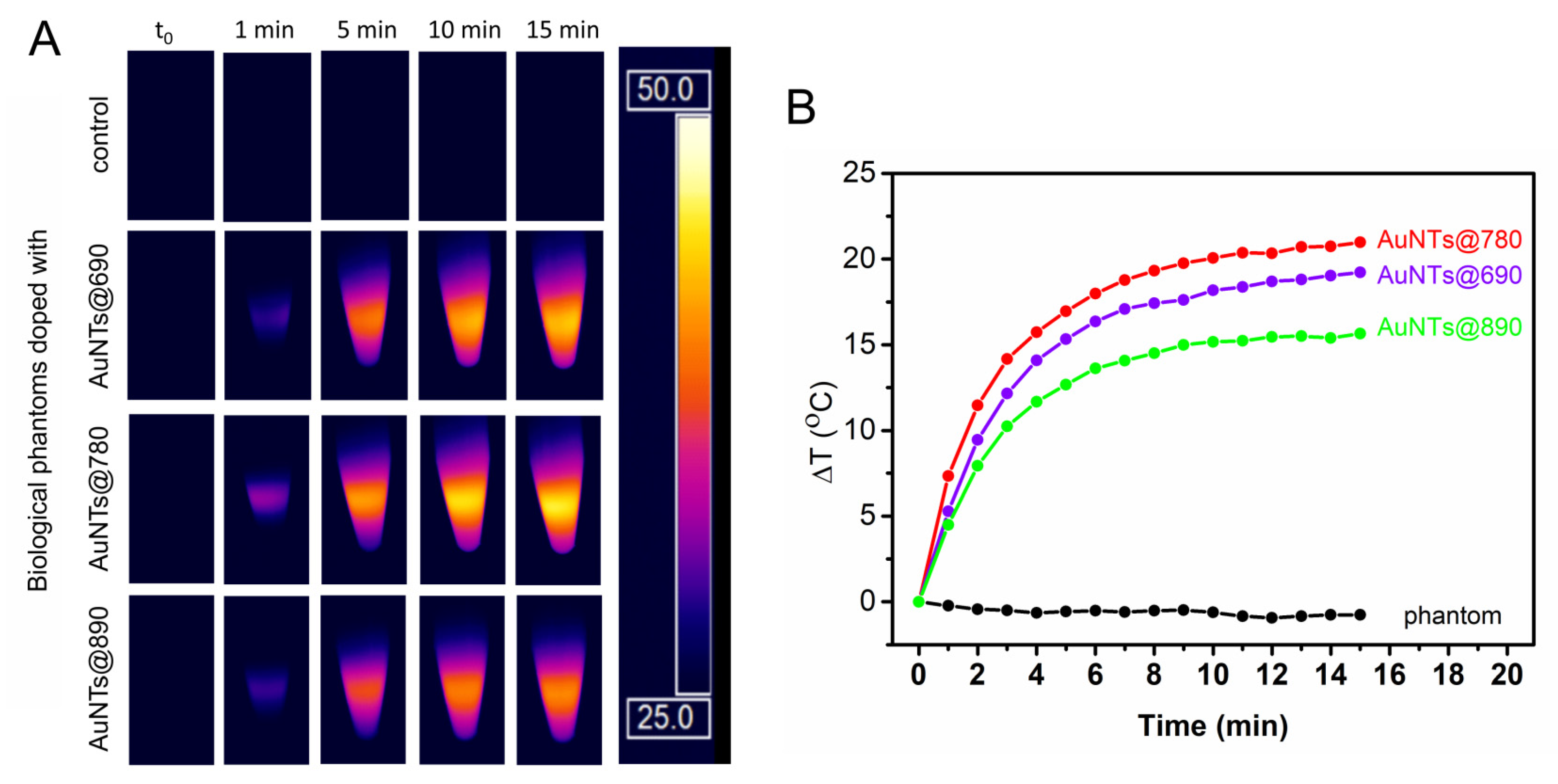
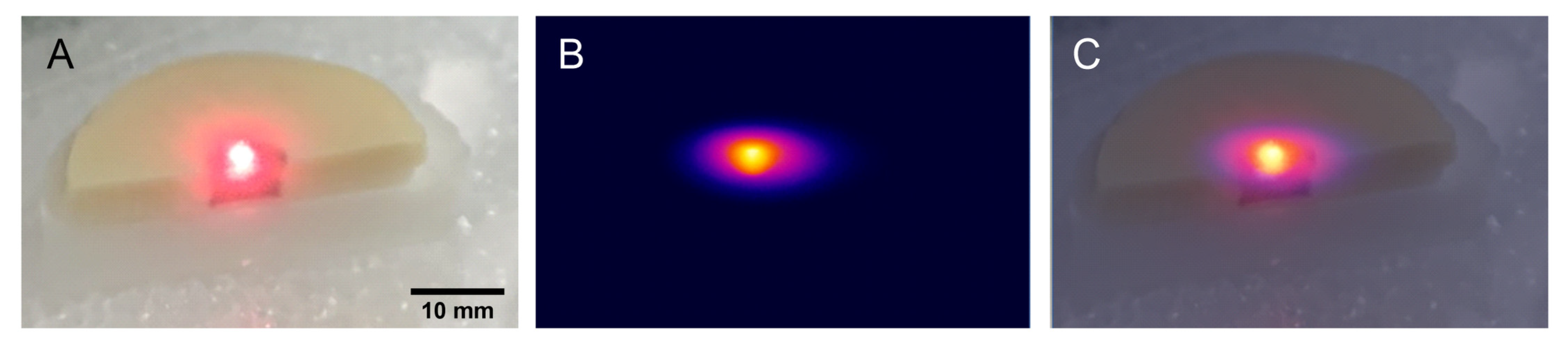
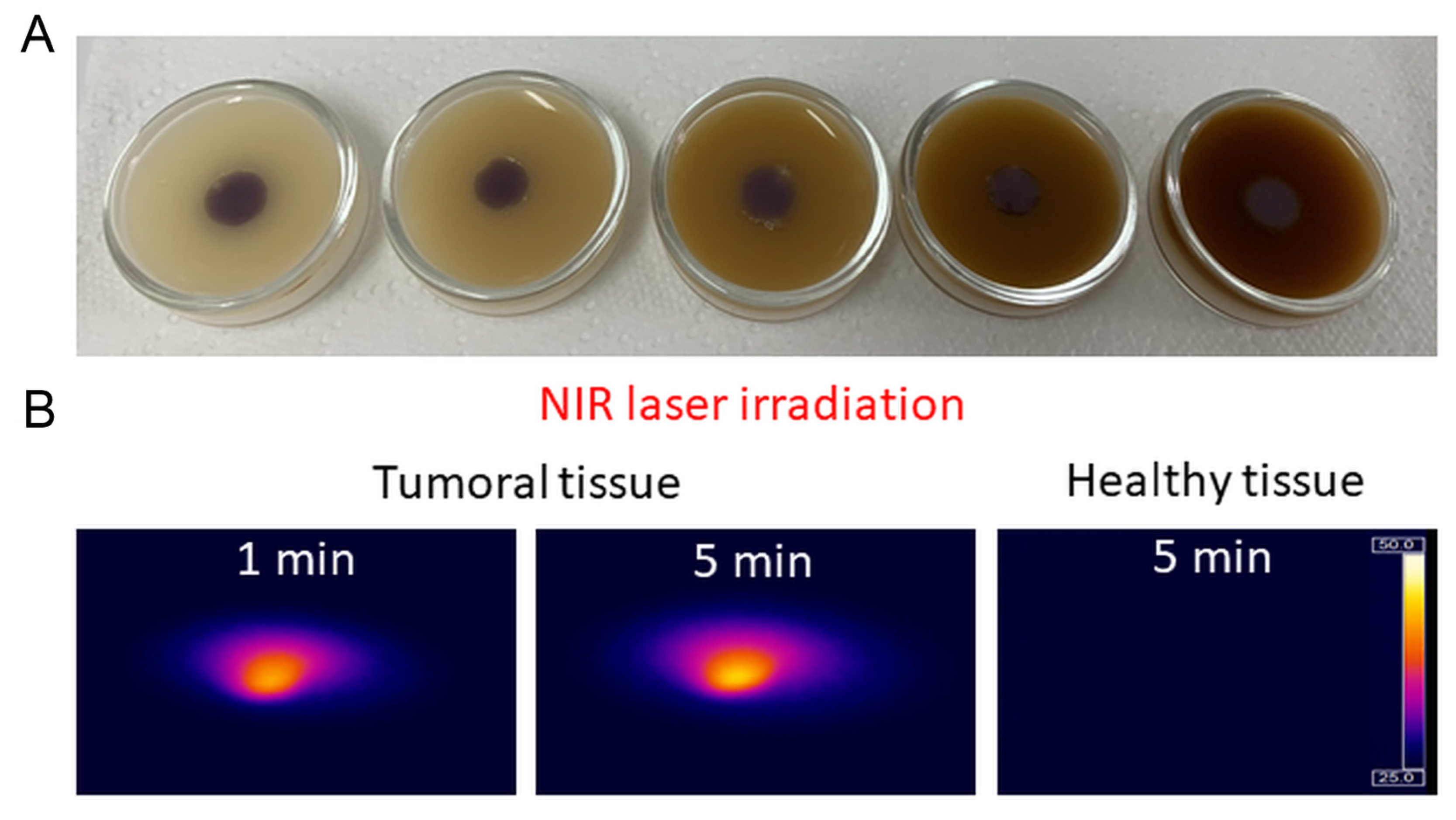
2.3. Investigation of the AuNTs Light-to-Heat Conversion Performances in Biological Phantoms
2.4. Bio- and Thermostability of AuNTs@gelatin
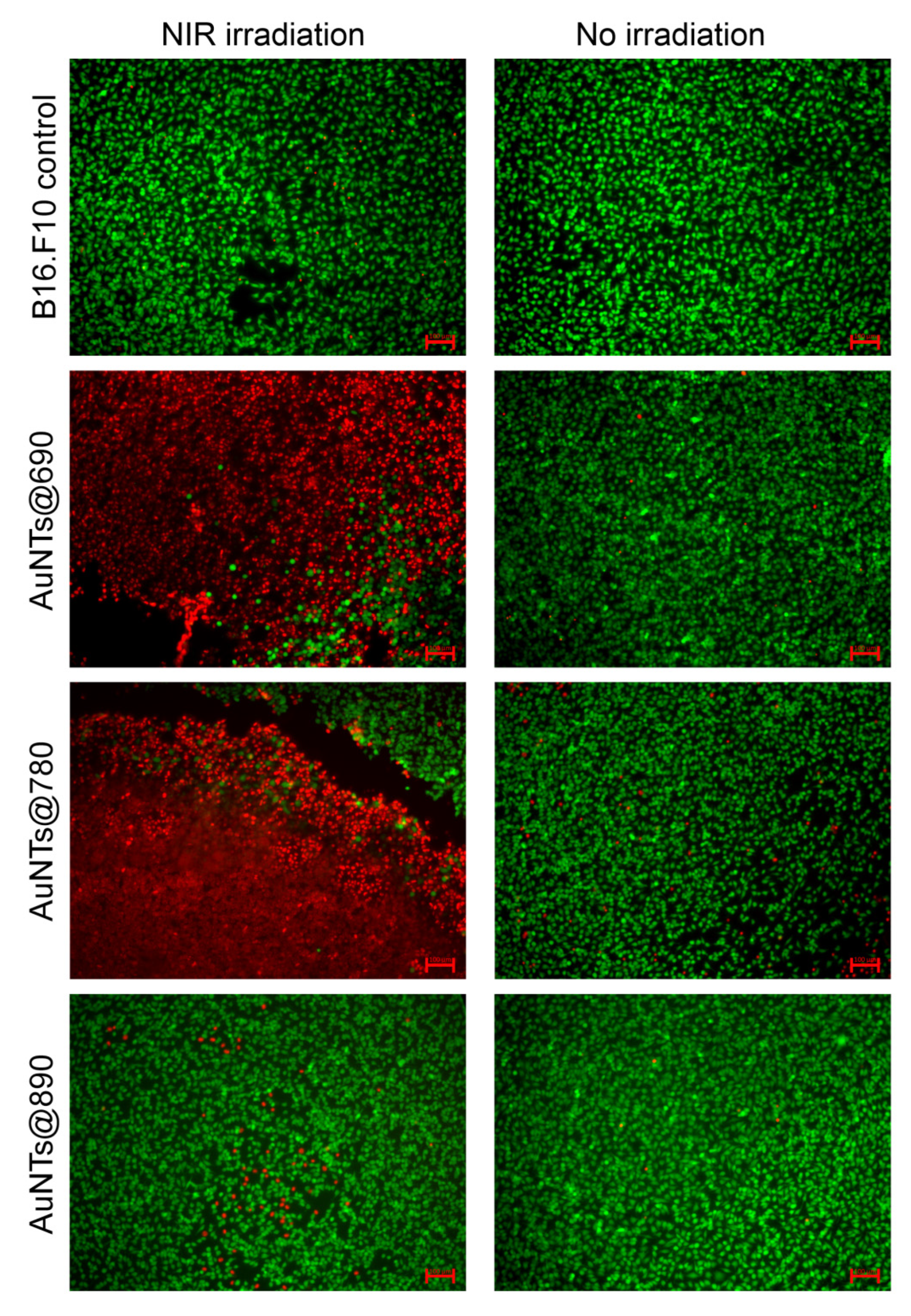
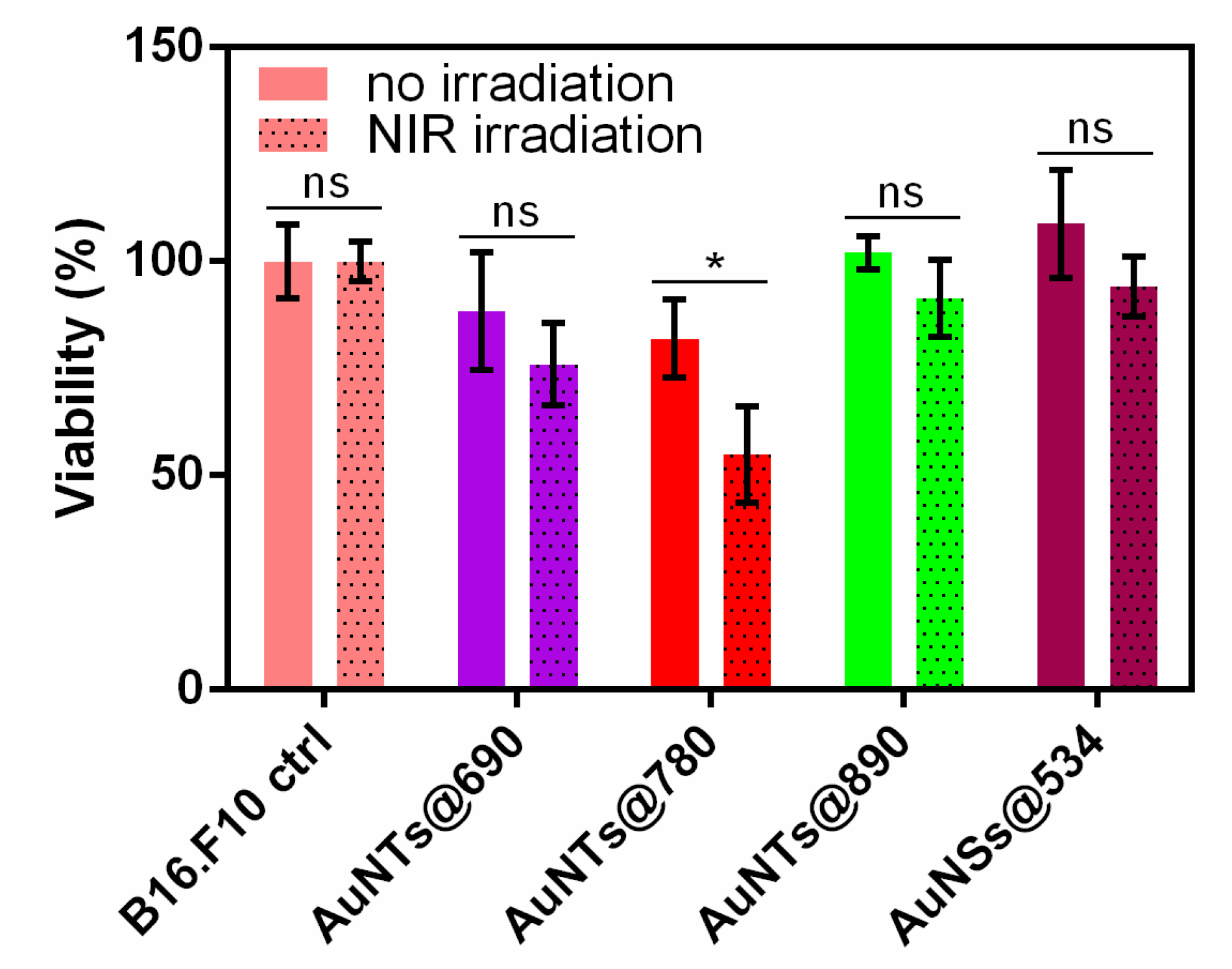
2.5. In Vitro NIR Phototherapy
3. Experimental Section
3.1. Materials
3.2. Sample Preparation
3.3. In Vitro Cellular Studies
3.4. Photothermal Efficiency in Solution and Biological Phantoms
3.5. Cellular Imaging Assay
3.6. In Vitro Cell Viability Study
3.7. Equipment and Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, S.; Prewett, S. Principles of chemotherapy and radiotherapy. Obstet. Gynaecol. Reprod. Med. 2020, 30, 72–78. [Google Scholar] [CrossRef]
- Yazbeck, V.; Alesi, E.; Myers, J.; Hackney, M.H.; Cuttino, L.; Gewirtz, D.A. An overview of chemotoxicity and radiation toxicity in cancer therapy. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Kumar, A.V.P.; Dubey, S.K.; Tiwari, S.; Puri, A.; Hejmady, S.; Gorain, B.; Kesharwani, P. Recent advances in nanoparticles mediated photothermal therapy induced tumor regression. Int. J. Pharm. 2021, 606, 120848. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, J.; Tarighatnia, A.; Barar, J.; Aghanejad, A.; Davaran, S. Recent advances and trends in nanoparticles based photothermal and photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 37, 102697. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Taylor, M.L.; Wilson, R.E.; Amrhein, K.D.; Huang, X. Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies. Bioengineering 2022, 9, 200. [Google Scholar] [CrossRef]
- Yi, X.; Duan, Q.-Y.; Wu, F.-G. Low-Temperature Photothermal Therapy: Strategies and Applications. Research 2021, 2021, 38. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, X.; Liu, Y.; Liu, H.; Zhao, K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 2016, 11, 349–364. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Rau, L.-R.; Huang, W.-Y.; Liaw, J.-W.; Tsai, S.-W. Photothermal effects of laser-activated surface plasmonic gold nanoparticles on the apoptosis and osteogenesis of osteoblast-like cells. Int. J. Nanomed. 2016, 11, 3461–3473. [Google Scholar] [CrossRef]
- Krajnik, B.; Golacki, L.W.; Fiedorczyk, E.; Bański, M.; Noculak, A.; Holodnik, K.M.; Podhorodecki, A. Quantitative comparison of luminescence probes for biomedical applications. Methods Appl. Fluoresc. 2021, 9, 045001. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, Y.; Huang, Y.; Tian, Y.; Wang, S.; Chen, Y. PEGylated gold nanoprisms for photothermal therapy at low laser power density. RSC Adv. 2015, 5, 81682–81688. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.-M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape effects of gold nanoparticles in photothermal cancer therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Maestro, L.M.; Haro-González, P.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; García Solé, J.; Jaque, D. Quantum Dot Thermometry Evaluation of Geometry Dependent Heating Efficiency in Gold Nanoparticles. Langmuir 2014, 30, 1650–1658. [Google Scholar] [CrossRef]
- Wang, Y.; Black, K.C.L.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.-Y.; Li, M.; Kim, P.; et al. Comparison Study of Gold Nanohexapods, Nanorods, and Nanocages for Photothermal Cancer Treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Wang, Y.; Chen, Y.; Gu, J.; Zhao, W.; Ding, J.; Shi, J. Controlled Synthesis of Multilayered Gold Nanoshells for Enhanced Photothermal Therapy and SERS Detection. Small 2015, 11, 77–83. [Google Scholar] [CrossRef]
- Knights, O.; Freear, S.; McLaughlan, J.R. Improving Plasmonic Photothermal Therapy of Lung Cancer Cells with Anti-EGFR Targeted Gold Nanorods. Nanomaterials 2020, 10, 1307. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Q.; Qi, H.; Ruan, L. Experimental Comparison of Photothermal Conversion Efficiency of Gold Nanotriangle and Nanorod in Laser Induced Thermal Therapy. Nanomaterials 2017, 7, 416. [Google Scholar] [CrossRef]
- Boca, S.C.; Potara, M.; Gabudean, A.-M.; Juhem, A.; Baldeck, P.L.; Astilean, S. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011, 311, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Maturi, M.; Locatelli, E.; Sambri, L.; Tortorella, S.; Šturm, S.; Kostevšek, N.; Comes Franchini, M. Synthesis of Ultrasmall Single-Crystal Gold-Silver Alloy Nanotriangles and Their Application in Photothermal Therapy. Nanomater. Basel Switz. 2021, 11, 912. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Soritau, O.; Maniu, D.; Astilean, S. One-pot, green synthesis of gold nanoparticles by gelatin and investigation of their biological effects on Osteoblast cells. Colloids Surf. B Biointerfaces 2015, 132, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F.; Wong, W.-T. Property-Tuneable Microgels Fabricated by Using Flow-Focusing Microfluidic Geometry for Bioactive Agent Delivery. Pharmaceutics 2021, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ardjmand, M.; Rad, A.S.; Esfahani, M.R. Gelatin-coated gold nanoparticles as an effective pH-sensitive methotrexate drug delivery system for breast cancer treatment. Mater. Today Chem. 2021, 20, 100474. [Google Scholar] [CrossRef]
- Lai, W.-F. Development of Hydrogels with Self-Healing Properties for Delivery of Bioactive Agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.-F. Multi-Component Hydrogel Beads Incorporated with Reduced Graphene Oxide for pH-Responsive and Controlled Co-Delivery of Multiple Agents. Pharmaceutics 2021, 13, 313. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Fernandes, D.D.; Malik, A.; Gomes, G.-N.W.; Appak-Baskoy, S.; Berndl, E.; Gradinaru, C.C.; Kolios, M.C. Multifunctional nanoparticles as theranostic agents for therapy and imaging of breast cancer. J. Photochem. Photobiol. B 2021, 218, 112110. [Google Scholar] [CrossRef]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef]
- Barani, M.; Rahdar, A.; Mukhtar, M.; Razzaq, S.; Qindeel, M.; Hosseini Olam, S.A.; Paiva-Santos, A.C.; Ajalli, N.; Sargazi, S.; Balakrishnan, D.; et al. Recent application of cobalt ferrite nanoparticles as a theranostic agent. Mater. Today Chem. 2022, 26, 101131. [Google Scholar] [CrossRef]
- Campu, A.; Craciun, A.-M.; Focsan, M.; Astilean, S. Assessment of the photothermal conversion efficiencies of tunable gold bipyramids under irradiation by two laser lines in a NIR biological window. Nanotechnology 2019, 30, 405701. [Google Scholar] [CrossRef] [PubMed]
- Vardaki, M.Z.; Kourkoumelis, N. Tissue Phantoms for Biomedical Applications in Raman Spectroscopy: A Review. Biomed. Eng. Comput. Biol. 2020, 11, 1179597220948100. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, G.; Kim, D.; Yoo, J.; Kim, D.-K.; Kim, H. Numerical Study on Effective Conditions for the Induction of Apoptotic Temperatures for Various Tumor Aspect Ratios Using a Single Continuous-Wave Laser in Photothermal Therapy Using Gold Nanorods. Cancers 2019, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta BBA–Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef] [PubMed]
- Campu, A.; Focsan, M.; Lerouge, F.; Borlan, R.; Tie, L.; Rugina, D.; Astilean, S. ICG-loaded gold nano-bipyramids with NIR activatable dual PTT-PDT therapeutic potential in melanoma cells. Colloids Surf. B Biointerfaces 2020, 194, 111213. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.-W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef]
- Nagy-Simon, T.; Potara, M.; Craciun, A.-M.; Licarete, E.; Astilean, S. IR780-dye loaded gold nanoparticles as new near infrared activatable nanotheranostic agents for simultaneous photodynamic and photothermal therapy and intracellular tracking by surface enhanced resonant Raman scattering imaging. J. Colloid Interface Sci. 2018, 517, 239–250. [Google Scholar] [CrossRef]
- Suarasan, S.; Craciun, A.-M.; Licarete, E.; Focsan, M.; Magyari, K.; Astilean, S. Intracellular Dynamic Disentangling of Doxorubicin Release from Luminescent Nanogold Carriers by Fluorescence Lifetime Imaging Microscopy (FLIM) under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2019, 11, 7812–7822. [Google Scholar] [CrossRef]
- Mustari, A.; Nishidate, I.; Wares, M.A.; Maeda, T.; Kawauchi, S.; Sato, S.; Sato, M.; Aizu, Y. Agarose-based Tissue Mimicking Optical Phantoms for Diffuse Reflectance Spectroscopy. J. Vis. Exp. JoVE 2018, 138, e57578. [Google Scholar] [CrossRef]
- Nishidate, I.; Tanaka, N.; Kawase, T.; Maeda, T.; Yuasa, T.; Aizu, Y.; Yuasa, T.; Niizeki, K. Noninvasive imaging of human skin hemodynamics using a digital red-green-blue camera. J. Biomed. Opt. 2011, 16, 086012. [Google Scholar] [CrossRef]
- Câmpu, A.-M. Implementation of Gold Nano-bipyramids as Efficient Biosensing Enhancers and Thermo-plasmonic Generators. Ph.D. Thesis, Babes-Bolyai University, Cluj-Napoca, Romania, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarasan, S.; Campu, A.; Vulpoi, A.; Banciu, M.; Astilean, S. Assessing the Efficiency of Triangular Gold Nanoparticles as NIR Photothermal Agents In Vitro and Melanoma Tumor Model. Int. J. Mol. Sci. 2022, 23, 13724. https://doi.org/10.3390/ijms232213724
Suarasan S, Campu A, Vulpoi A, Banciu M, Astilean S. Assessing the Efficiency of Triangular Gold Nanoparticles as NIR Photothermal Agents In Vitro and Melanoma Tumor Model. International Journal of Molecular Sciences. 2022; 23(22):13724. https://doi.org/10.3390/ijms232213724
Chicago/Turabian StyleSuarasan, Sorina, Andreea Campu, Adriana Vulpoi, Manuela Banciu, and Simion Astilean. 2022. "Assessing the Efficiency of Triangular Gold Nanoparticles as NIR Photothermal Agents In Vitro and Melanoma Tumor Model" International Journal of Molecular Sciences 23, no. 22: 13724. https://doi.org/10.3390/ijms232213724
APA StyleSuarasan, S., Campu, A., Vulpoi, A., Banciu, M., & Astilean, S. (2022). Assessing the Efficiency of Triangular Gold Nanoparticles as NIR Photothermal Agents In Vitro and Melanoma Tumor Model. International Journal of Molecular Sciences, 23(22), 13724. https://doi.org/10.3390/ijms232213724