Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye
Abstract
1. Introduction
2. Results and Discussion
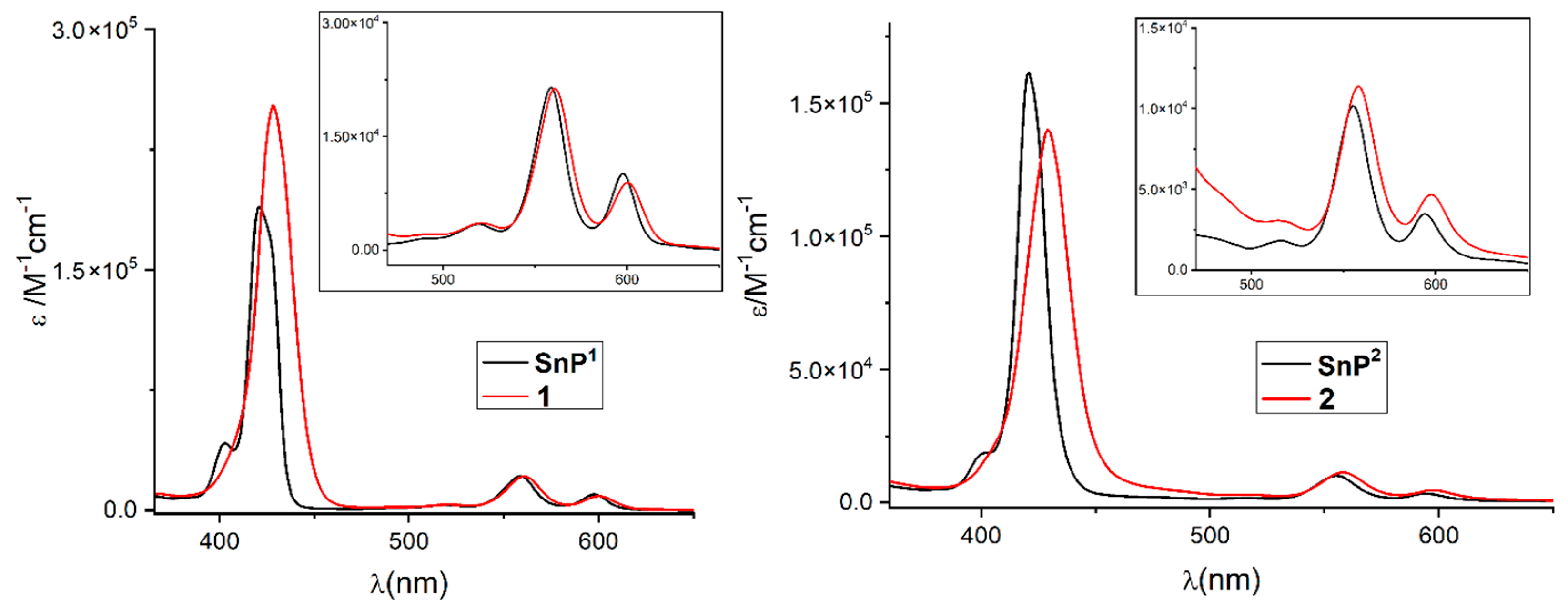
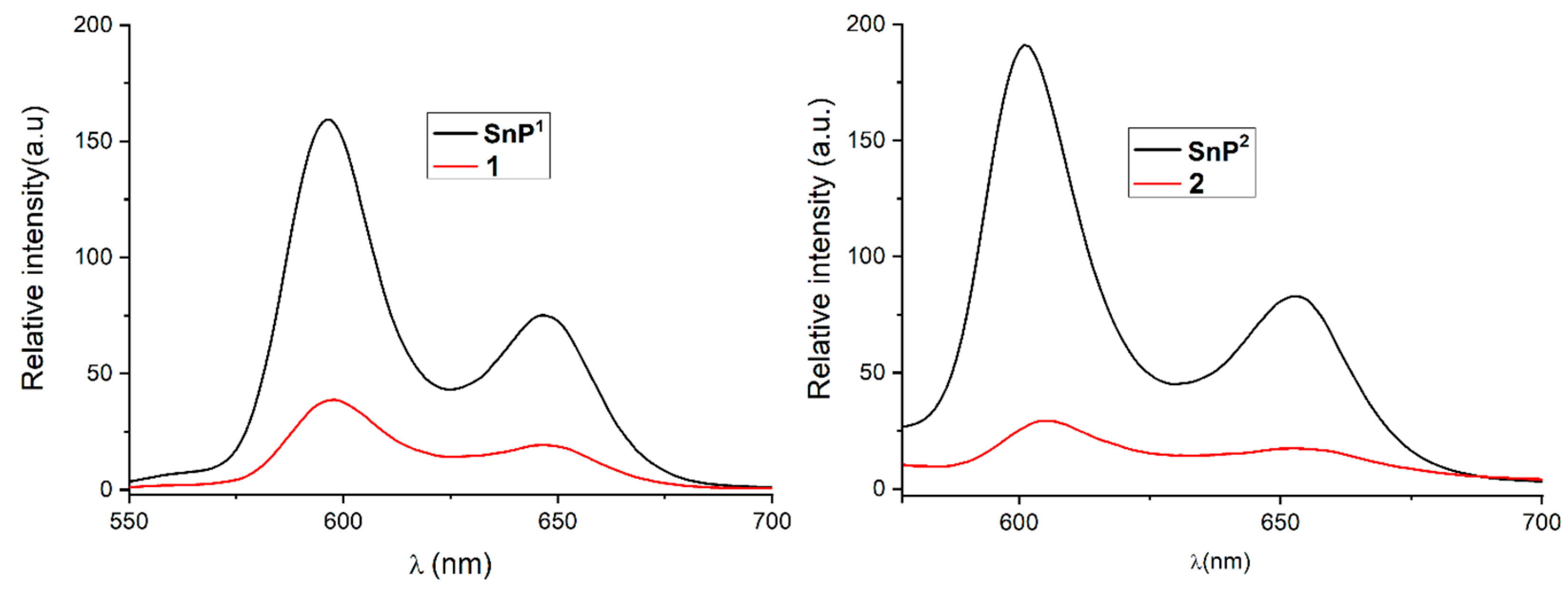
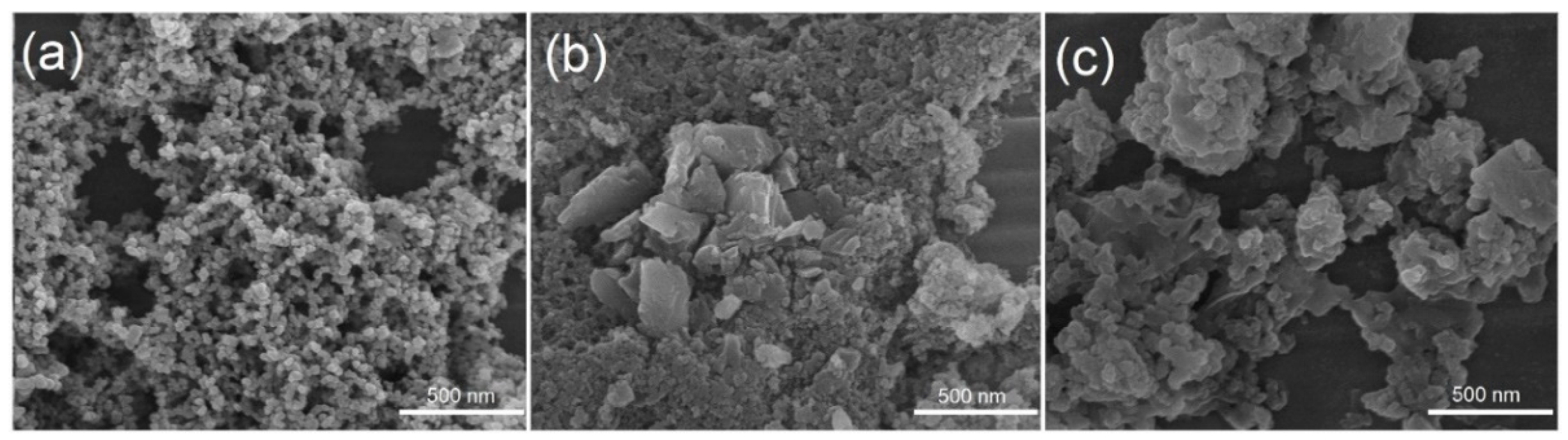
2.1. Synthesis and Spectroscopic Characterization
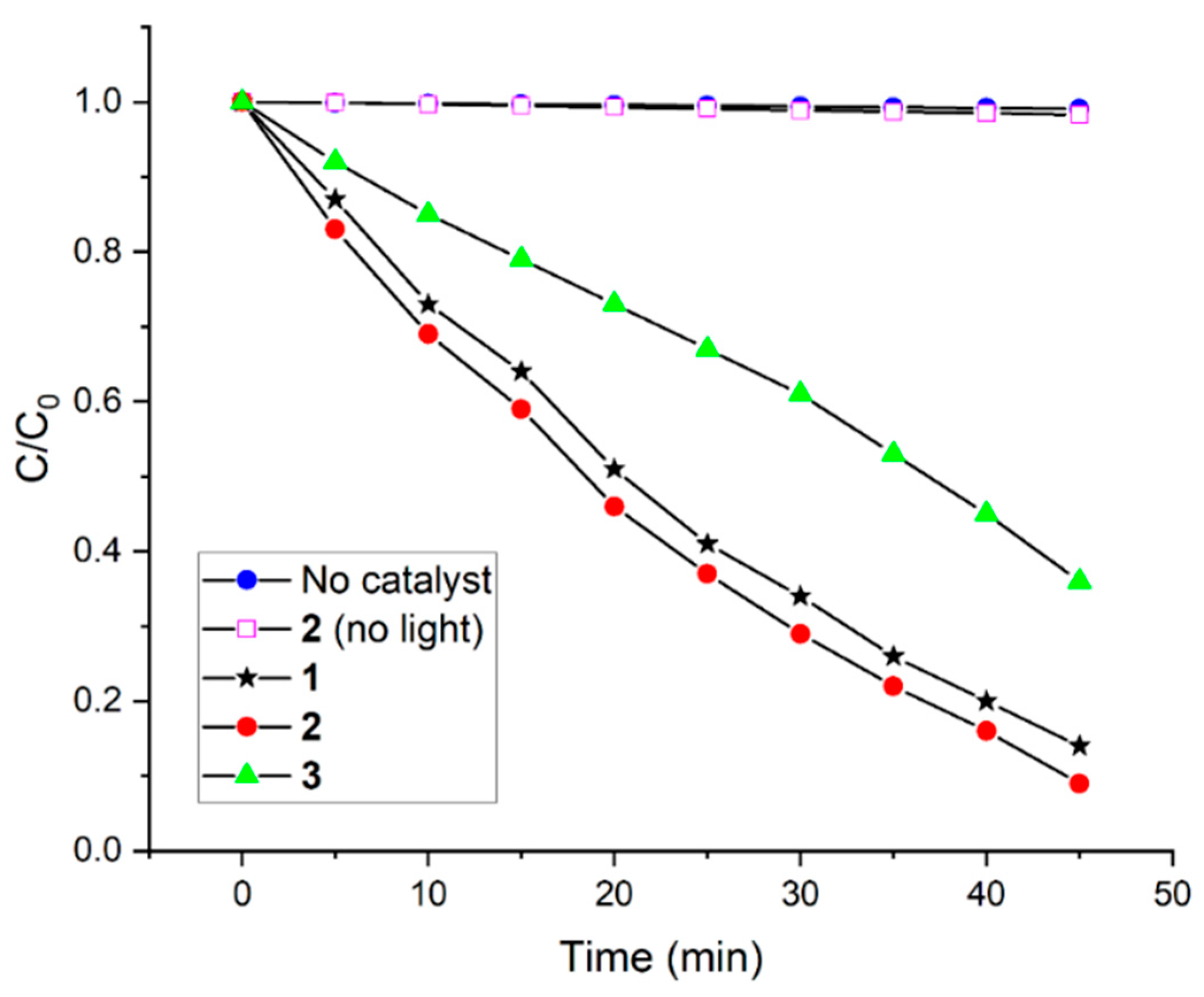
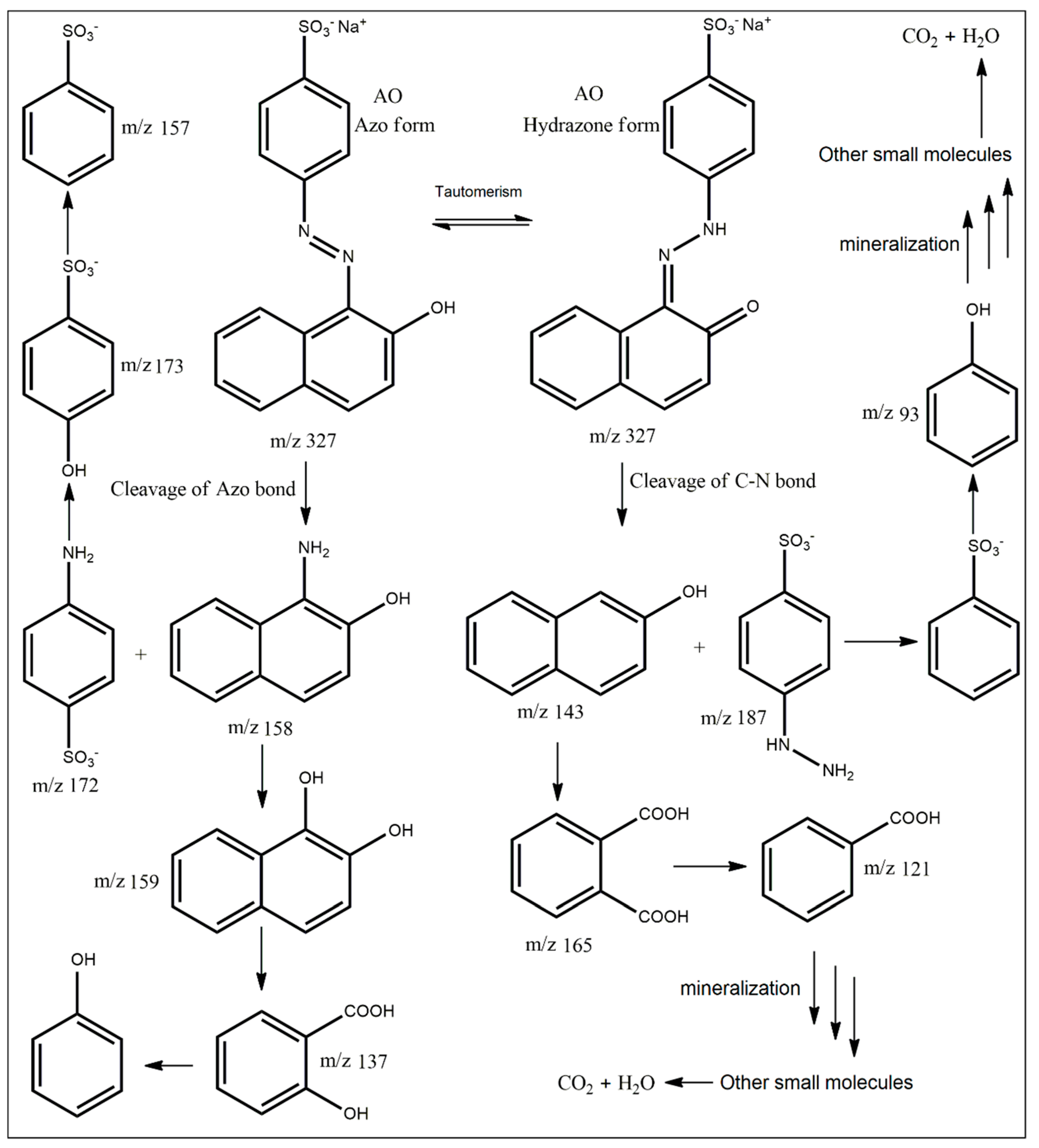
2.2. Photocatalytic Performance for the Degradation of AO
3. Materials and Methods
3.1. Material Synthesis
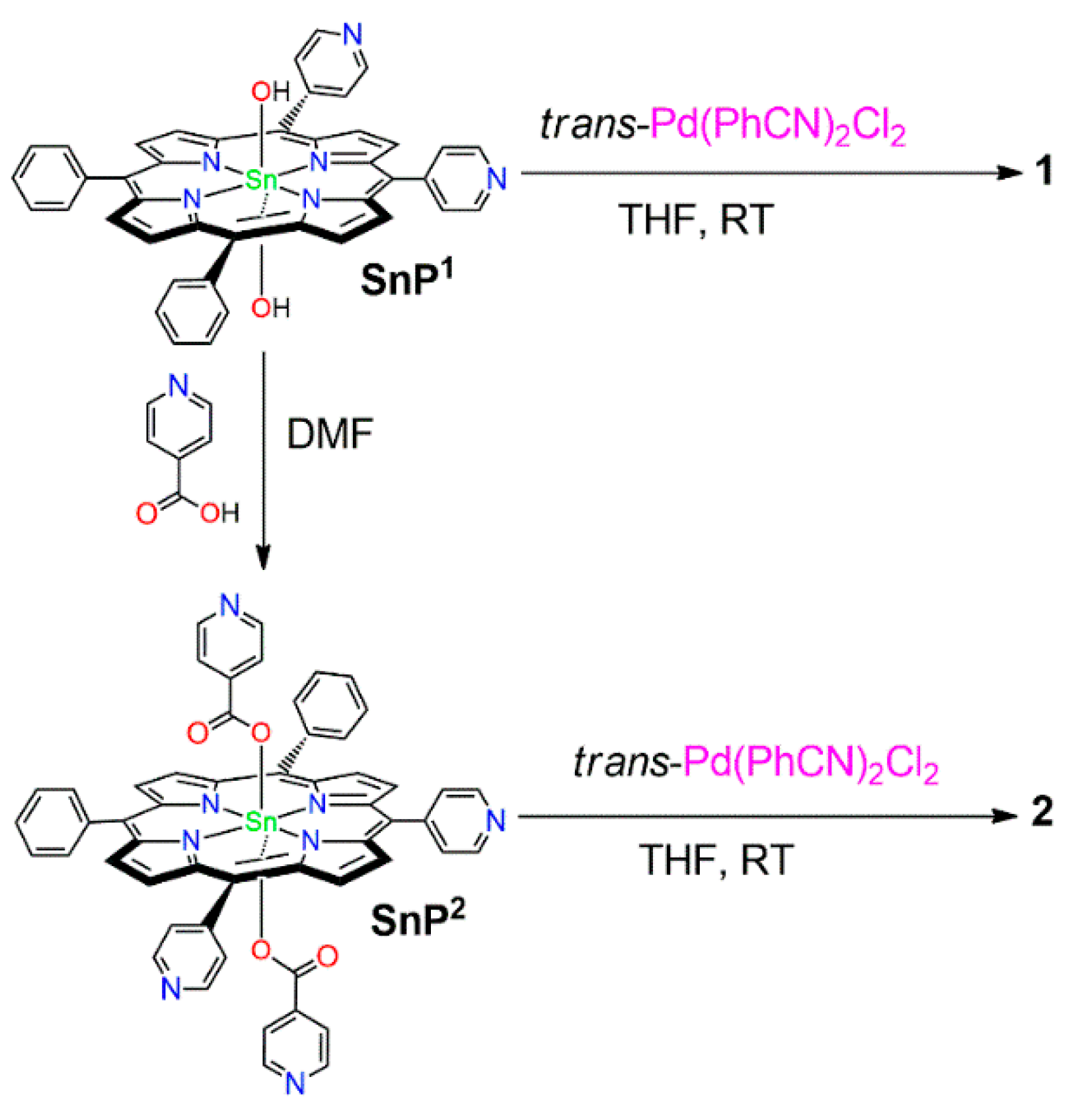
3.1.1. {(trans-Dihydroxo)[5,10-bis(4-Pyridyl)-15,20-bis(phenyl)porphyrinato]}tin(IV) SnP1
3.1.2. {(trans-Diisonicotinato)[5,10-bis(4-pyridyl)-15,20-bis(phenyl)porphyrinato]}tin(iv) SnP2
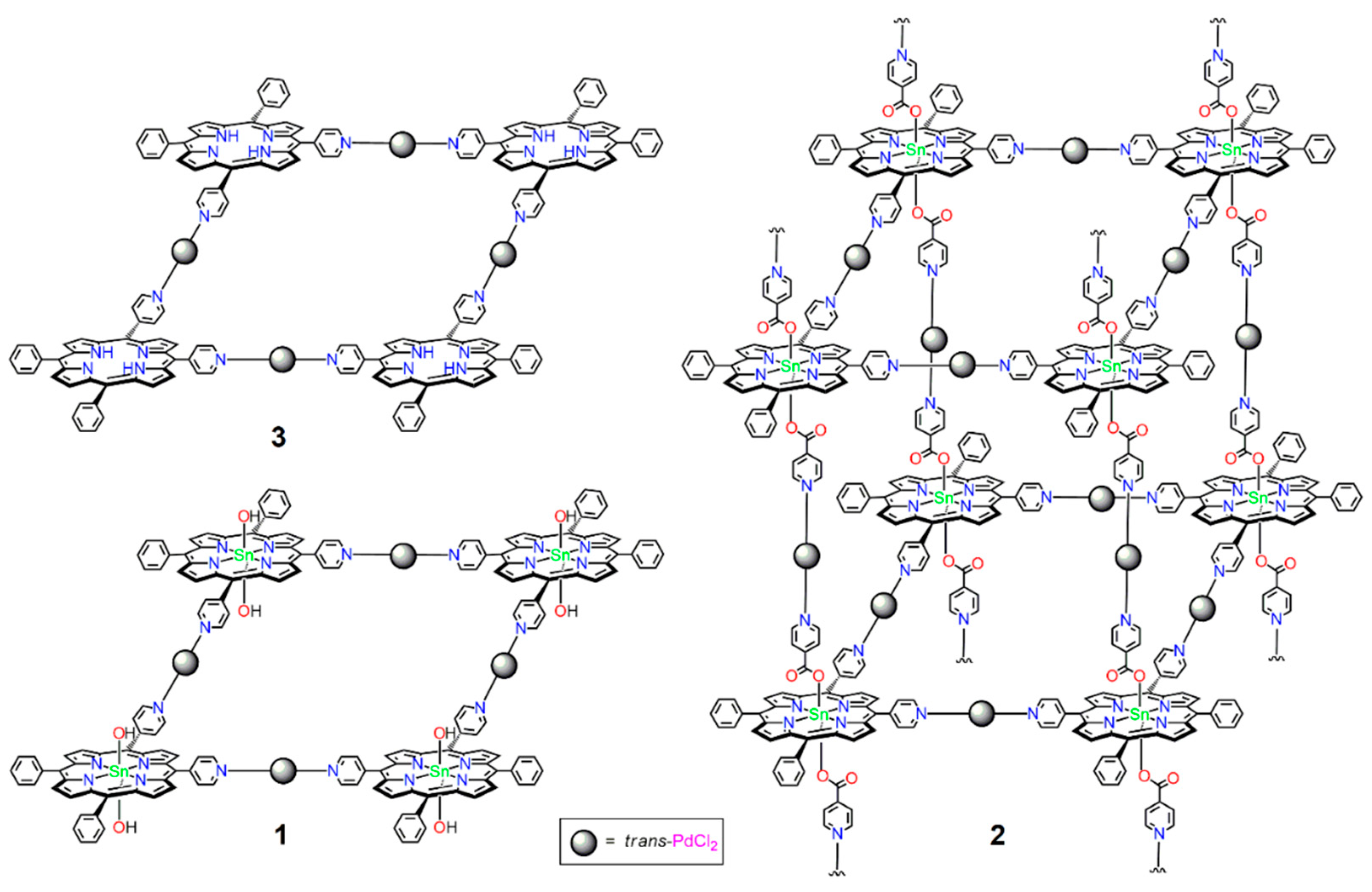
3.1.3. Supramolecular Array 1
3.1.4. Supramolecular Array 2
3.2. Photocatalytic Degradation Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Nagatani-Yoshida, K. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical Pollution: A Growing Peril and Potential Catastrophic Risk to Humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Boelee, E.; Geerling, G.; van der Zaan, B.; Blauw, A.; Vethaak, A.D. Water and health: From environmental pressures to integrated responses. Acta Trop. 2019, 193, 217–226. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent Progress and Prospects in Catalytic Water Treatment. Chem. Rev. 2022, 122, 2981–3121. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef]
- Glugoski, L.P.; de Jesus Cubas, P.; Fujiwara, S.T. Reactive Black 5 dye degradation using filters of smuggled cigarette modified with Fe3+. Environ. Sci. Pollut. Res. 2017, 24, 6143–6150. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Wastewater chemical contaminants: Remediation by advanced oxidation processes. Photochem. Photobiol. Sci. 2018, 17, 1573–1598. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Rana, A.G.; Tasbihi, M.; Schwarze, M.; Minceva, M. Efficient Advanced Oxidation Process (AOP) for Photocatalytic Contaminant Degradation Using Exfoliated Metal-Free Graphitic Carbon Nitride and Visible Light-Emitting Diodes. Catalysts 2021, 11, 662. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced Oxidation Processes Coupled with Nanomaterials for Water Treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn(IV) porphyrins with Ag(I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-based nanostructures for photocatalytic applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules 2021, 26, 3598. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Morphology-controlled self-assembled nanostructures of complementary metalloporphyrin triads through intermolecular coordination tuning and their photocatalytic degradation for Orange II. Inorg. Chem. Front. 2022, 9, 5538–5548. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Sn(IV) Porphyrin-Based Ionic Self-Assembled Nanostructures and Their Application in Visible Light Photo-Degradation of Malachite Green. Catalysts 2022, 12, 799. [Google Scholar] [CrossRef]
- La, D.D.; Ngo, H.H.; Nguyen, D.D.; Tran, N.T.; Vo, H.T.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, M.D. Advances and prospects of porphyrin-based nanomaterials via self-assembly for photocatalytic applications in environmental treatment. Coord. Chem. Rev. 2022, 463, 214543. [Google Scholar]
- Nikolaou, V.; Charalambidis, G.; Coutsolelos, A.G. Photocatalytic hydrogen production of porphyrin nanostructures: Spheres vs. fibrils, a case study. Chem. Commun. 2021, 57, 4055–4058. [Google Scholar] [CrossRef]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for Energy Conversion and Storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef]
- Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY- and Porphyrin-Based Sensors for Recognition of Amino Acids and Their Derivatives. Molecules 2020, 25, 4523. [Google Scholar] [CrossRef]
- Grigore, M.E.; Ion, R.-M.; Iancu, L. Tailored porphyrin–gold nanoparticles for biomedical applications. J. Porphyr. Phthalocyanines 2019, 23, 766–780. [Google Scholar] [CrossRef]
- Lehn, J.-M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef]
- Durot, S.; Taesch, J.; Heitz, V. Multiporphyrinic Cages: Architectures and Functions. Chem. Rev. 2014, 114, 8542–8578. [Google Scholar] [CrossRef]
- Shao, S.; Rajendiran, V.; Lovell, J.F. Metalloporphyrin nanoparticles: Coordinating diverse theranostic functions. Coord. Chem. Rev. 2019, 379, 99–120. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin Supramolecular 1D Structures via Surfactant-Assisted Self-Assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin Nanotubes by Ionic Self-Assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef]
- Hasobe, T.; Oki, H.; Sandanayakaa, A.S.D.; Murata, H. Sonication-assisted supramolecular nanorods of meso-diaryl-substituted porphyrins. Chem. Commun. 2008, 724–726. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials 2020, 10, 2314. [Google Scholar] [CrossRef]
- Gong, X.; Milic, T.; Xu, C.; Batteas, J.D.; Drain, C.M. Preparation and Characterization of Porphyrin Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14290–14291. [Google Scholar] [CrossRef]
- Anderson, H.L. Conjugated porphyrin ladders. Inorg. Chem. 1994, 33, 972–981. [Google Scholar] [CrossRef]
- Benkstein, K.D.; Stern, C.L.; Splan, K.E.; Johnson, R.C.; Walters, K.A.; Vanhelmont, F.W.M.; Hupp, J.T. Collapsed Molecular Rectangles Based on Rhenium(I) Coordination of Ethynylpyridyl Porphyrins—Synthesis, Structure, and Bending-Induced Charge-Transfer Behavior. Eur. J. Inorg. Chem. 2002, 2002, 2818–2822. [Google Scholar] [CrossRef]
- Meng, W.; Breiner, B.; Rissanen, K.; Thoburn, J.D.; Clegg, J.K.; Nitschke, J.R. A Self-Assembled M8L6 Cubic Cage That Selectively Encapsulates Large Aromatic Guests. Angew. Chem. Int. Ed. 2011, 50, 3479–3483. [Google Scholar] [CrossRef]
- O’Sullivan, M.C.; Sprafke, J.K.; Kondratuk, D.V.; Rinfray, C.; Claridge, T.D.W.; Saywell, A.; Blunt, M.O.; O’Shea, J.N.; Beton, P.H.; Malfois, M.; et al. Vernier templating and synthesis of a 12-porphyrin nano-ring. Nature 2011, 469, 72–75. [Google Scholar] [CrossRef]
- Zha, Q.; Rui, X.; Weia, T.; Xie, Y. Recent advances in the design strategies for porphyrin-based coordination polymers. CrystEngComm 2014, 16, 7371–7384. [Google Scholar] [CrossRef]
- Shee, N.K.; Seo, J.-W.; Kim, H.-J. Spectrophotometric Study of Bridging N-Donor Ligand-Induced Supramolecular Assembly of Conjugated Zn-Trisporphyrin with a Triphenylamine Core. Molecules 2021, 26, 4771. [Google Scholar] [CrossRef]
- Slone, R.V.; Hupp, J.T. Synthesis, Characterization, and Preliminary Host–Guest Binding Studies of Porphyrinic Molecular Squares Featuring fac-Tricarbonylrhenium(I) Chloro Corners. Inorg. Chem. 1997, 36, 5422–5423. [Google Scholar] [CrossRef]
- Scandola, F.; Chiorboli, C.; Prodi, A.; Iengo, E.; Alessio, E. Photophysical properties of metal-mediated assemblies of porphyrins. Coord. Chem. Rev. 2006, 250, 1471–1496. [Google Scholar] [CrossRef]
- Huh, S.; Kim, S.J.; Kim, Y. Porphyrinic metal-organic frameworks from custom-designed porphyrins. Cryst. Eng. Comm. 2016, 18, 345–368. [Google Scholar] [CrossRef]
- Da Silveira, C.H.; Vieceli, V.; Clerici, D.J.; Santos, R.C.V.; Iglesias, B.A. Investigation of isomeric tetra-cationic porphyrin activity with peripheral Pd(bpy)Cl+ units by antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2020, 31, 101920. [Google Scholar] [CrossRef]
- Plutino, M.R.; Romeo, A.; Castriciano, M.A.; Scolaro, L.M. 1,1′-Bis(diphenylphosphino)ferrocene Platinum(II) Complexes as a Route to Functionalized Multiporphyrin Systems. Nanomaterials 2021, 11, 178. [Google Scholar] [CrossRef]
- Kim, H.J.; Shee, N.K.; Park, K.M.; Kim, H.-J. Assembly and X-ray crystal structures of heterometallic multiporphyrins with complementary coordination between ruthenium (II) and tin (IV) porphyrins. Inorg. Chim. Acta 2019, 488, 1–7. [Google Scholar] [CrossRef]
- Liu, S.; Wang, G.-L.; Jin, G.-X. Multinuclear self-assembly via half-sandwich complexes Cp*M[S2C2(B10H10)] and pyridine-based ligands. Dalton Trans. 2008, 425–432. [Google Scholar] [CrossRef]
- Lee, S.J.; Hupp, J.T. Porphyrin-containing molecular squares: Design and applications. Coord. Chem. Rev. 2006, 250, 1710–1723. [Google Scholar] [CrossRef]
- Koposova, E.A.; Offenhäusser, A.; Ermolenko, Y.E.; Mourzina, Y.G. Photoresponsive Porphyrin Nanotubes of Meso-tetra(4-Sulfonatophenyl)Porphyrin and Sn(IV) meso-tetra(4-pyridyl)porphyrin. Front. Chem. 2019, 7, 351. [Google Scholar] [CrossRef]
- Jang, J.H.; Jeon, K.-S.; Oh, S.; Kim, H.-J.; Asahi, T.; Masuhara, H.; Yoon, M. Synthesis of Sn-Porphyrin-Intercalated Trititanate Nanofibers: Optoelectronic Properties and Photocatalytic Activities. Chem. Mater. 2007, 19, 1984–1991. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Three Isomeric Zn(II)-Sn(IV)-Zn(II) Porphyrin-Triad-Based Supramolecular Nanoarchitectures for the Morphology-Dependent Photocatalytic Degradation of Methyl Orange. ACS Omega 2022, 7, 9775–9784. [Google Scholar] [CrossRef]
- Tian, Y.; Busani, T.; Uyeda, G.H.; Martin, K.E.; van Swol, F.; Medforth, C.J.; Montaño, G.A.; Shelnutt, J.A. Hierarchical cooperative binary ionic porphyrin nanocomposites. Chem. Commun. 2012, 48, 4863–4865. [Google Scholar] [CrossRef] [PubMed]
- Zargari, S.; Rahimi, R.; Yousefi, A. An efficient visible light photocatalyst based on tin porphyrin intercalated between TiO2–graphene nanosheets for inactivation of E. coli and investigation of charge transfer mechanism. RSC Adv. 2016, 6, 24218–24228. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.-M.; Ahn, T.K.; Kim, S.K.; Kim, K.S.; Kim, D.; Kim, H.-J. Novel fullerene–porphyrin–fullerene triad linked by metal axial coordination: Synthesis, X-ray crystal structure, and spectroscopic characterizations of trans-bis([60]fullerenoacetato)tin(iv) porphyrin. Chem. Commun. 2004, 2594–2595. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jo, H.J.; Kim, J.; Kim, S.-Y.; Kim, D.; Kim, K. Supramolecular self-assembly of tin(IV) porphyrin channels stabilizing single-file chains of water molecules. CrystEngComm 2005, 7, 417–420. [Google Scholar] [CrossRef]
- Li, C.; Park, K.-M.; Kim, H.-J. Ionic assembled hybrid nanoparticle consisting of tin(IV) porphyrin cations and polyoxomolybdate anions, and photocatalytic hydrogen production by its visible light sensitization. Inorg. Chem. Commun. 2015, 60, 8–11. [Google Scholar] [CrossRef]
- Kim, H.-J. Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion·Anion and Anion·π System. Molbank 2022, 2022, M1454. [Google Scholar] [CrossRef]
- Shee, N.K.; Lee, C.-J.; Kim, H.-J. Hexacoordinated Sn(IV) porphyrin-based square-grid frameworks exhibiting selective uptake of CO2 over N2. Bull. Korean Chem. Soc. 2022, 43, 103–109. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, H.-J. Photocatalytic Hydrogen Production by the Sensitization of Sn(IV)-Porphyrin Embedded in a Nafion Matrix Coated on TiO2. Molecules 2022, 27, 3770. [Google Scholar] [CrossRef]
- Kim, M.K.; Shee, N.K.; Lee, J.; Yoon, M.; Kim, H.-J. Photoinduced Electron Transfer upon Supramolecular Complexation of (Porphyrinato)Sn-Viologen with Cucurbit[7]uril. Photochem. Photobiol. Sci. 2019, 18, 1996–2002. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Fluorescent chemosensing for aromatic compounds by supramolecular complex composed of tin(IV) porphyrin, viologen, and cucurbit[8]uril. Chem. Commun. 2019, 55, 10575–10578. [Google Scholar] [CrossRef]
- Fleischer, E.B.; Shachter, A.M. Coordination oligomers and a coordination polymer of zinc tetraarylporphyrins. Inorg. Chem. 1991, 30, 3763–3769. [Google Scholar] [CrossRef]
- Anderson, G.K.; Lin, M.; Sen, A.; Gretz, E. Bis(benzonitrile)dichloro Complexes of Palladium and Platinum. Inorg. Synth. 2007, 28, 60–63. [Google Scholar]
- Drain, C.M.; Lehn, J.M. Self-assembly of Square Multiporphyrin Arrays by Metal Ion Coordination. J. Chem. Soc. Chem. Commun. 1994, 19, 2313–2315. [Google Scholar] [CrossRef]
- Drain, C.M.; Nifiatis, F.; Vasenko, A.; Batteas, J.D. Porphyrin Tessellation by Design: Metal-Mediated Self-Assembly of Large Arrays and Tapes. Angew. Chem., Int. Ed. 1998, 37, 2344–2347. [Google Scholar] [CrossRef]
- Milic, T.; Garno, J.C.; Smeureanu, G.; Batteas, J.D.; Drain, C.M. Organization of Self-Assembled Tetrameric Porphyrin Arrays on Surfaces. Langmuir 2004, 20, 3974–3983. [Google Scholar] [CrossRef] [PubMed]
- Divya, N.; Bansal, A.; Jana, A.K. Photocatalytic degradation of azo dye Orange II in aqueous solutions using copper-impregnated titania. Int. J. Environ. Sci. Technol. 2013, 10, 1265–1274. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Lu, F.; Wei, F.; Wang, X.; Wang, S. Magnetic Recoverable MnFe2O4 and MnFe2O4-Graphene Hybrid as Heterogeneous Catalysts of Peroxymonosulfate Activation for Efficient Degradation of Aqueous Organic Pollutants. J. Hazard. Mater. 2014, 270, 61–70. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Lu, F.; Qin, J.; Wei, F.; Xu, C.; Wang, S. Magnetic ZnFe2O4-C3N4 hybrid for photocatalytic degradation of aqueous organic pollutants by visible light. Ind. Eng. Chem. Res. 2014, 53, 17294–17302. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.; Dharmarajan, R.; Liu, Y.; Naidu, R. Photocatalytic degradation of azo dye acid orange 7 using different light sources over Fe3+-doped TiO2 nanocatalysts. Environ. Technol. Innov. 2018, 12, 27–42. [Google Scholar] [CrossRef]
- Waheed, A.; Shi, Q.; Maeda, N.; Meier, D.M.; Qin, Z.; Li, G.; Baiker, A. Strong Activity Enhancement of the Photocatalytic Degradation of an Azo Dye on Au/TiO2 Doped with FeOx. Catalysts 2020, 10, 933. [Google Scholar] [CrossRef]
- Lee, G.; Manivel, A.; Batalova, V.; Mokrousov, G.; Masten, S.; Wu, J. Mesoporous Microsphere of ZnS Photocatalysts Loaded with CuO or Mn3O4 for the Visible-Light-Assisted Photocatalytic Degradation of Orange II Dye. Ind. Eng. Chem. Res. 2013, 52, 11904–11912. [Google Scholar] [CrossRef]
- Jiang, T.; Poyraz, A.S.; Iyer, A.; Zhang, Y.; Luo, Z.; Zhong, W.; Miao, R.; El-Sawy, A.M.; Guild, C.J.; Sun, Y.; et al. Synthesis of Mesoporous Iron Oxides by an Inverse Micelle Method and Their Application in the Degradation of Orange II under Visible Light at Neutral pH. J. Phys. Chem. C 2015, 119, 10454–10468. [Google Scholar] [CrossRef]
- Zhu, K.; Jin, C.; Klencsár, Z.; Ganeshraja, A.S.; Wang, J. Cobalt-iron Oxide, Alloy and Nitride: Synthesis, Characterization and Application in Catalytic Peroxymonosulfate Activation for Orange II Degradation. Catalysts 2017, 7, 138. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, M. Degradation of Orange II by the Fe0 /H2O2 system. IOP Conf. Ser. Earth Environ. Sci. 2020, 526, 012062. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Fernández, L.; Bava, Y.B.; Buceta, D.; Vázquez-Vázquez, C.; López-Quintela, M.A.; Feijoo, G.; Moreira, M.T. Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light. Catalysts 2020, 10, 31. [Google Scholar] [CrossRef]
- Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, E.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO2 Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production. Nanomaterials 2020, 10, 1387. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Tsai, C.-J.; Lin, K.; Wu, J.-Y. Anion-Dominated Copper Salicyaldimine Complexes—Structures, Coordination Mode of Nitrate and Decolorization Properties toward Acid Orange 7 Dye. Polymers 2020, 12, 1910. [Google Scholar] [CrossRef]
- Peleyeju, G.M.; Umukoro, E.H.; Babalola, J.O.; Arotiba, O.A. Solar-light Responsive Titanium-sheet-based Carbon Nanoparticles/B-BiVO4/WO3 Photoanode for the Photoelectrocatalytic Degradation of Orange II Dye Water Pollutant. ACS Omega 2020, 5, 4743–4750. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Chouchene, B.; Gries, T.; Desmarets, C.; Balan, L.; Gaumet, J.-J.; Medjahdi, G.; Bayo, K.; Schneider, R. Bismuth oxybromide/reduced graphene oxide heterostructure sensitized with Zn-tetracarboxyphthalocyanine as a highly efficient photocatalyst for the degradation of Orange II and phenol. J. Environ. Chem. Eng. 2022, 10, 107332. [Google Scholar] [CrossRef]
| Photocatalysts | Rate Constant (min−1) | Reference |
|---|---|---|
| TiO2 P-25/Cu | 0.041 | [66] |
| TiO2 P-25 | 0.045 | [66] |
| MnFe2O4-γGO | 0.019 | [67] |
| ZnFe2O4-C3N4(2:3) | 0.012 | [68] |
| Fe3+-doped TiO2 | 0.011 | [69] |
| Au-Fe(1:1)/TiO2 | 0.014 | [70] |
| ZnS-Mn3O4 (1.5%wt) | 0.054 | [71] |
| 2-line ferrihydrite | 0.026 | [72] |
| Co-Fe nitride | 0.024 | [73] |
| Fe0/H2O2 | 0.054 | [74] |
| ZnO | 0.021 | [75] |
| ZnO-Ag | 0.051 | [75] |
| g-CN/TiO2 | 0.016 | [76] |
| [Cu(HLsalpyca)(NO3)]n/H2O2 | 0.015 | [77] |
| CNP/B-BiVO4/WO3 | 0.014 | [78] |
| BiOBr/rGO/ZnPc(CO2H)4(0.25) | 0.023 | [79] |
| 1 | 0.043 | this study |
| 2 | 0.047 | this study |
| 3 | 0.021 | this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. Int. J. Mol. Sci. 2022, 23, 13702. https://doi.org/10.3390/ijms232213702
Shee NK, Kim H-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. International Journal of Molecular Sciences. 2022; 23(22):13702. https://doi.org/10.3390/ijms232213702
Chicago/Turabian StyleShee, Nirmal Kumar, and Hee-Joon Kim. 2022. "Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye" International Journal of Molecular Sciences 23, no. 22: 13702. https://doi.org/10.3390/ijms232213702
APA StyleShee, N. K., & Kim, H.-J. (2022). Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. International Journal of Molecular Sciences, 23(22), 13702. https://doi.org/10.3390/ijms232213702