Polydopamine Nanoparticles Functionalized Electrochemical DNA Aptasensor for Serum Glycated Albumin Detection
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of PDA Nanoparticles
2.2. Characterization of Electrochemical Aptasensor
2.3. Optimization of the Experimental Conditions
2.3.1. Optimization of GA Aptamer Immobilization
2.3.2. Optimization of Blocking and Reaction Time
2.4. Electrochemical Detection of GA
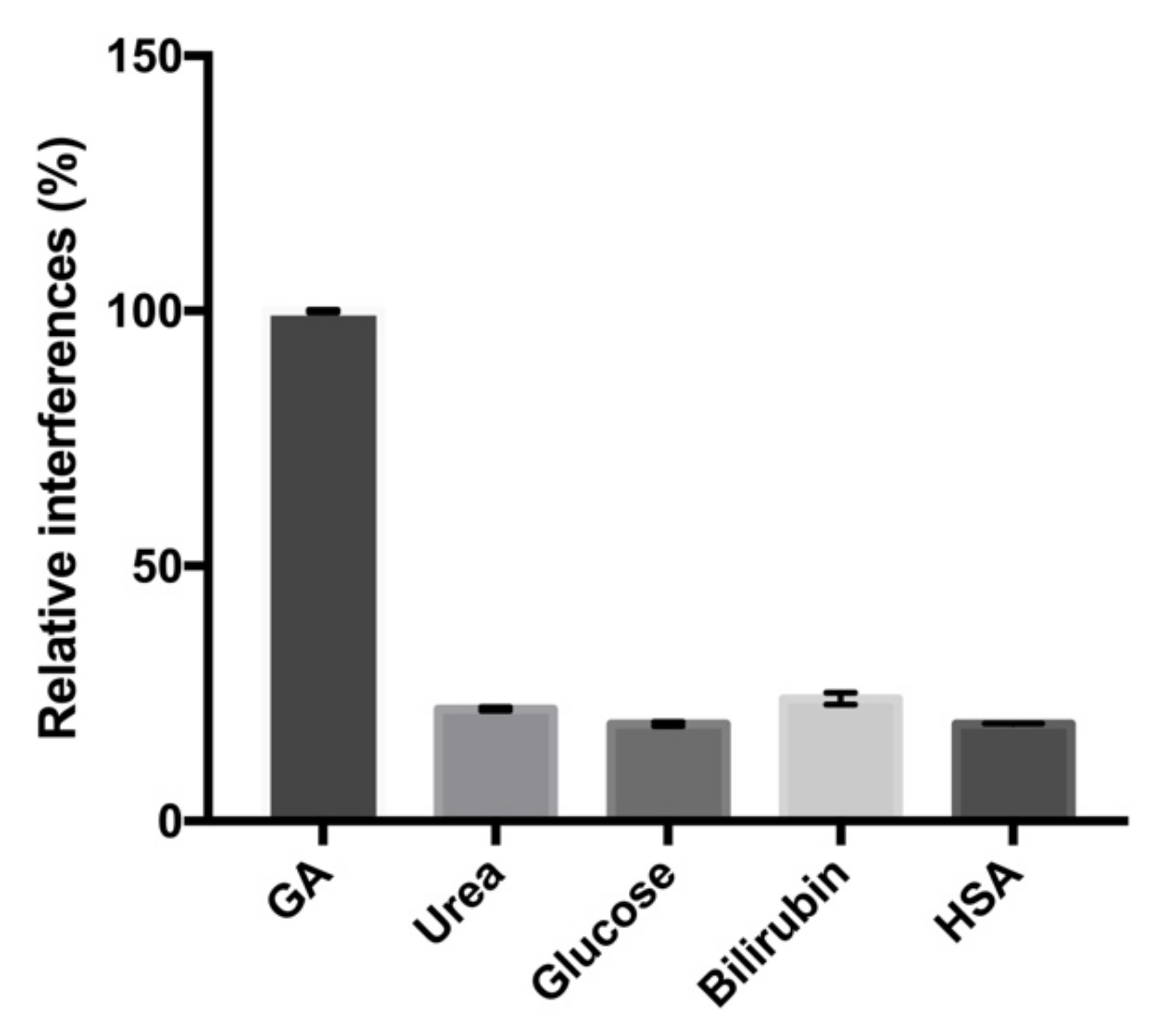
2.5. Selectivitiy and Reproducibility of the Electrochemical Aptasensor
2.6. Spike Recovery Assay
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instruments
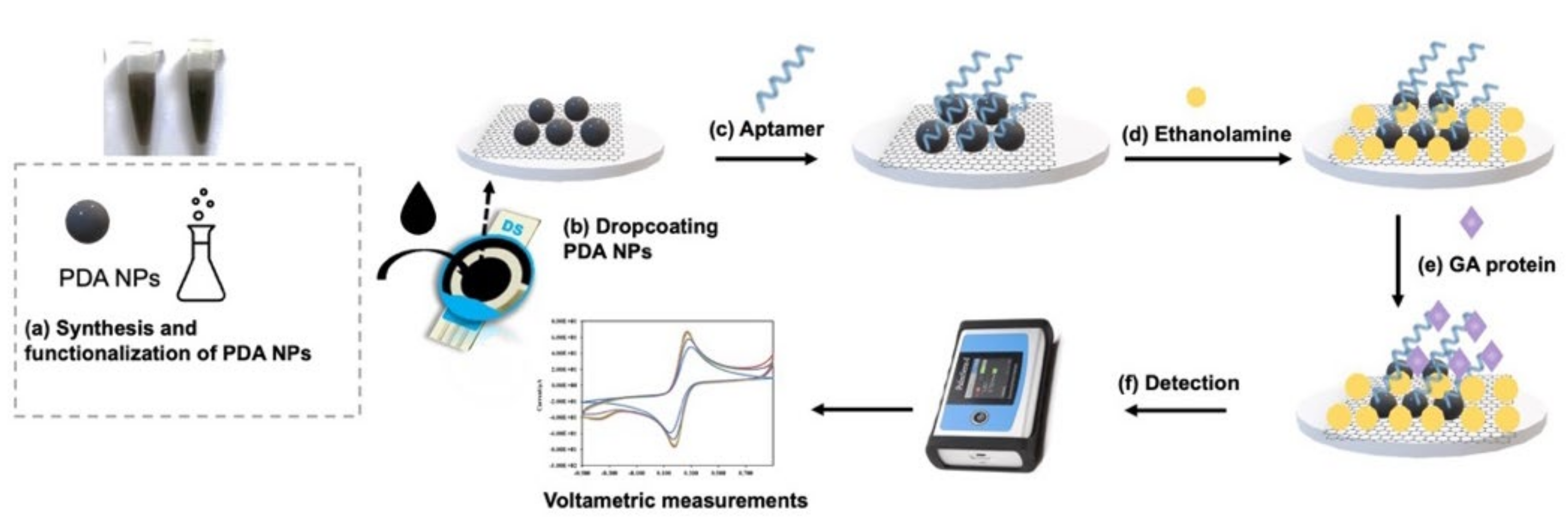
3.3. Synthesis of PDA-NPs and In Vitro Conjugation of GA Aptamer on PDA-NPs
3.4. Fabrication of the Electrochemical Aptasensor
3.5. Electrochemical Analysis
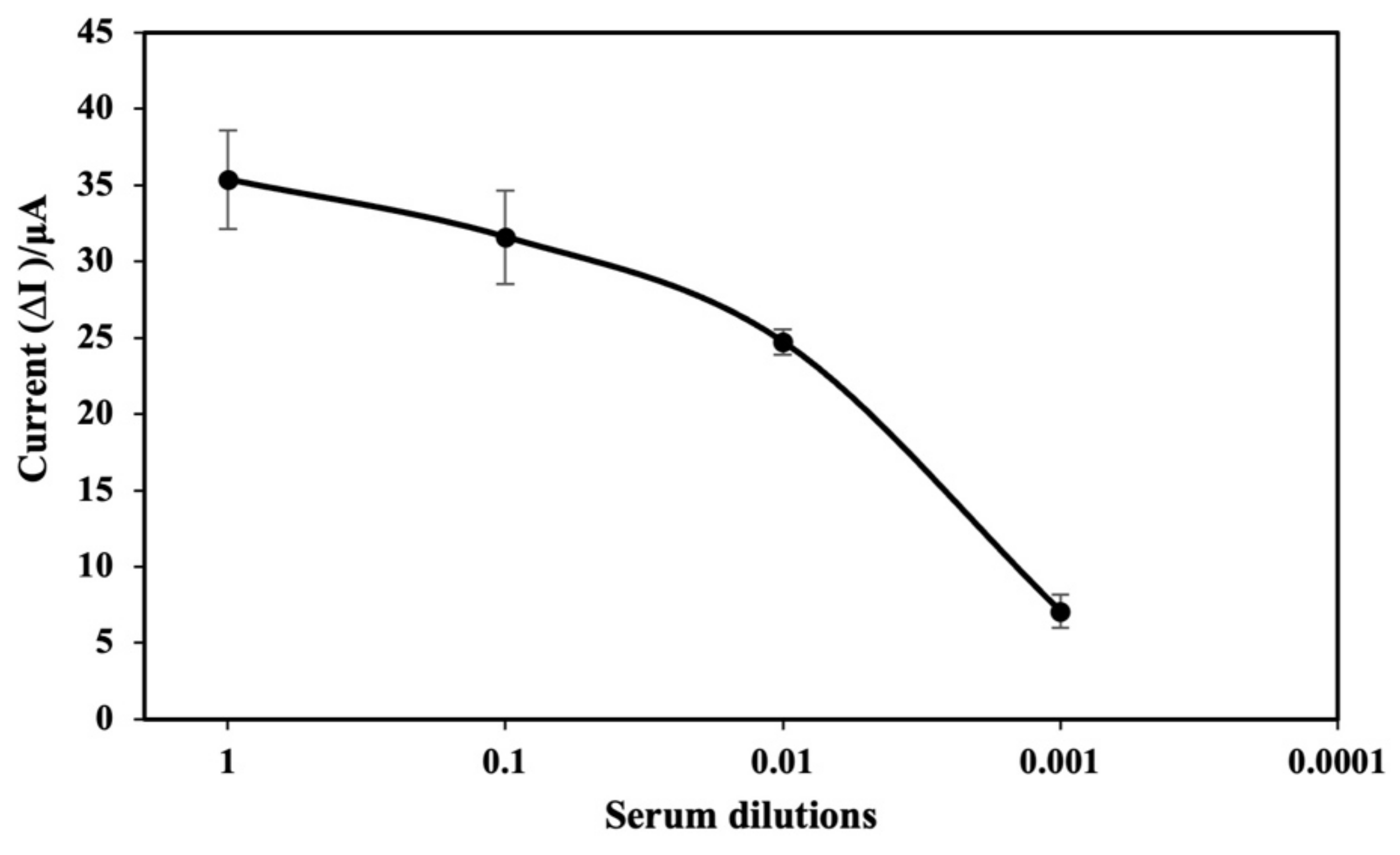
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Hashimoto, K.; Murai, J.; Saito, H.; Mukai, M.; Ikegame, K.; Ogawa, H.; Kasayama, S. Usefulness of Glycated Albumin as an Indicator of Glycemic Control Status in Patients with Hemolytic Anemia. Clin. Chim. Acta 2011, 412, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and Pitfalls of Fructosamine and Glycated Albumin in the Diagnosis and Treatment of Diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176. [Google Scholar] [CrossRef]
- Hinton, D.J.S.; Ames, J.M. Analysis of Glycated Protein by Capillary Electrophoresis. Int. Congr. Ser. 2002, 1245, 471–474. [Google Scholar] [CrossRef]
- Zhernovaya, O.S.; Tuchin, V.V.; Meglinski, I.V. Monitoring of Blood Proteins Glycation by Refractive Index and Spectral Measurements. Laser Phys. Lett. 2008, 5, 460. [Google Scholar] [CrossRef]
- Dingari, N.C.; Horowitz, G.L.; Kang, J.W.; Dasari, R.R.; Barman, I. Raman Spectroscopy Provides a Powerful Diagnostic Tool for Accurate Determination of Albumin Glycation. PLoS ONE 2012, 7, e32406. [Google Scholar] [CrossRef]
- Bunyarataphan, S.; Dharakul, T.; Fucharoen, S.; Paiboonsukwong, K.; Japrung, D. Glycated Albumin Measurement Using an Electrochemical Aptasensor for Screening and Monitoring of Diabetes Mellitus. Electroanalysis 2019, 31, 2254–2261. [Google Scholar] [CrossRef]
- Farzadfard, A.; Shayeh, J.S.; Habibi-Rezaei, M.; Omidi, M. Modification of Reduced Graphene/Au-Aptamer to Develop an Electrochemical Based Aptasensor for Measurement of Glycated Albumin. Talanta 2020, 211, 120722. [Google Scholar] [CrossRef]
- Waiwinya, W.; Putnin, T.; Pimalai, D.; Chawjiraphan, W.; Sathirapongsasuti, N.; Japrung, D. Immobilization-Free Electrochemical Sensor Coupled with a Graphene-Oxide-Based Aptasensor for Glycated Albumin Detection. Biosensors 2021, 11, 85. [Google Scholar] [CrossRef]
- Mikula, E.; Wyslouch-Cieszynska, A.; Zhukova, L.; Verwilst, P.; Dehaen, W.; Radecki, J.; Radecka, H. Electrochemical Biosensor for the Detection of Glycated Albumin. Curr. Alzheimer Res. 2017, 14, 345–351. [Google Scholar] [CrossRef]
- Sett, A. Aptamers: Magic Bullet for Theranostic Applications. In Theranostics—An Old Concept in New Clothing; IntechOpen: London, UK, 2020. [Google Scholar]
- Liu, Q.; Zhang, W.; Chen, S.; Zhuang, Z.; Zhang, Y.; Jiang, L.; LIN, J.S. SELEX Tool: A Novel and Convenient Gel-Based Diffusion Method for Monitoring of Aptamer-Target Binding. J. Biol. Eng. 2020, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Soo Oh, S.; Nie, J.; Stewart, R.; Eisenstein, M.; Chambers, J.; Marth, J.D.; Walker, F.; Thomson, J.A.; Soh, H.T. Quantitative Selection and Parallel Characterization of Aptamers. Proc. Natl. Acad. Sci. USA 2013, 110, 18460–18465. [Google Scholar] [CrossRef] [PubMed]
- Ruscito, A.; DeRosa, M.C. Small-Molecule Binding Aptamers: Selection Strategies, Characterization, and Applications. Front. Chem. 2016, 4, 14. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as Therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Aye, N.N.S.; Maraming, P.; Tavichakorntrakool, R.; Chaibunruang, A.; Boonsiri, P.; Daduang, S.; Teawtrakul, N.; Prasongdee, P.; Amornkitbamrung, V.; Daduang, J. A Simple Graphene Functionalized Electrochemical Aptasensor for the Sensitive and Selective Detection of Glycated Albumin. Appl. Sci. 2021, 11, 10315. [Google Scholar] [CrossRef]
- Davidsen, M.B.; Teixeira, J.F.L.; Dehli, J.; Karlsson, C.; Kraft, D.; Souza, P.P.C.; Foss, M. Post-Treatments of Polydopamine Coatings Influence Cellular Response. Colloids Surf. B Biointerfaces 2021, 207, 111972. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Zmerli, I.; Michel, J.-P.; Makky, A. Multifunctional Polydopamine-Based Nanoparticles: Synthesis, Physico-Chemical Properties and Applications for Bimodal Photothermal/Photodynamic Therapy of Cancer. Multifunct. Mater. 2021, 4, 022001. [Google Scholar] [CrossRef]
- Bolat, G.; Vural, O.A.; Yaman, Y.T.; Abaci, S. Polydopamine Nanoparticles-Assisted Impedimetric Sensor towards Label-Free Lung Cancer Cell Detection. Mater. Sci. Eng. C 2021, 119, 111549. [Google Scholar] [CrossRef]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef]
- Cortés, M.T.; Vargas, C.; Blanco, D.A.; Quinchanegua, I.D.; Cortés, C.; Jaramillo, A.M. Bioinspired Polydopamine Synthesis and Its Electrochemical Characterization. J. Chem. Educ. 2019, 96, 1250–1255. [Google Scholar] [CrossRef]
- Molazemhosseini, A.; Magagnin, L.; Vena, P.; Liu, C.-C. Single-Use Disposable Electrochemical Label-Free Immunosensor for Detection of Glycated Hemoglobin (HbA1c) Using Differential Pulse Voltammetry (DPV). Sensors 2016, 16, 1024. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. Aptamer-Based Label-Free Electrochemical Biosensor Array for the Detection of Total and Glycated Hemoglobin in Human Whole Blood. Sci. Rep. 2017, 7, 1016. [Google Scholar] [CrossRef] [PubMed]
- Kausaite-Minkstimiene, A.; Mazeiko, V.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of Amperometric Glucose Biosensors Based on Glucose Oxidase Encapsulated within Enzymatically Synthesized Polyaniline and Polypyrrole. Sens. Actuators B Chem. 2011, 158, 278–285. [Google Scholar] [CrossRef]
- Sukeri, A.; Arjunan, A.; Bertotti, M. New Strategy to Fabricate a Polydopamine Functionalized Self-Supported Nanoporous Gold Film Electrode for Electrochemical Sensing Applications. Electrochem. Commun. 2020, 110, 106622. [Google Scholar] [CrossRef]
- Khan, Z.; Shanker, R.; Um, D.; Jaiswal, A.; Ko, H. Bioinspired Polydopamine and Composites for Biomedical Applications. In Electrically Conductive Polymer and Polymer Composites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–29. ISBN 978-3-527-80791-8. [Google Scholar]
- Yang, Y.; Song, C.; Wang, P.; Fan, X.; Xu, Y.; Dong, G.; Liu, Z.; Pan, Z.; Song, Y.; Song, C. Insights into the Impact of Polydopamine Modification on Permeability and Anti-Fouling Performance of Forward Osmosis Membrane. Chemosphere 2022, 291, 132744. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Li, S.; Hou, X.; Lu, S.; Xu, W.; Tao, J.; Zhao, Z.; Hu, G.; Gao, F. Fabrication and Simulation of a Layered Ultrahigh Thermal Conductive Material Made of Self-Assembled Graphene and Polydopamine on a Copper Substrate. RSC Adv. 2021, 11, 34676–34687. [Google Scholar] [CrossRef]
- Nandiyanto, A.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Batul, R.; Bhave, M.; Mahon, P.J.; Yu, A. Polydopamine Nanosphere with In-Situ Loaded Gentamicin and Its Antimicrobial Activity. Molecules 2020, 25, 2090. [Google Scholar] [CrossRef]
- Samanta, A.; Ojha, K.; Mandal, A. Interactions between Acidic Crude Oil and Alkali and Their Effects on Enhanced Oil Recovery. Energy Fuels 2011, 25, 1642–1649. [Google Scholar] [CrossRef]
- Ho, C.-C.; Ding, S.-J. The PH-Controlled Nanoparticles Size of Polydopamine for Anti-Cancer Drug Delivery. J. Mater. Sci. Mater. Med. 2013, 24, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Kanyong, P.; Krampa, F.D.; Aniweh, Y.; Awandare, G.A. Polydopamine-Functionalized Graphene Nanoplatelet Smart Conducting Electrode for Bio-Sensing Applications. Arab. J. Chem. 2020, 13, 1669–1677. [Google Scholar] [CrossRef]
- Beck, F. Cyclic Voltammetry—Simulation and Analysis of Reaction Mechanisms. Electroanalysis 1995, 7, 298. [Google Scholar] [CrossRef]
- Dijksma, M.; Kamp, B.; Hoogvliet, J.C.; Van Bennekom, W.P. Development of an Electrochemical Immunosensor for Direct Detection of Interferon-γ at the Attomolar Level. Anal. Chem. 2001, 73, 901–907. [Google Scholar] [CrossRef]
- Bardea, A.; Katz, E.; Willner, I. Biosensors with Amperometric Detection of Enzymatically Controlled PH-Changes. Electroanalysis 2000, 12, 731–735. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of Electrochemical Impedance Spectroscopy in Protein Biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Chen, Z.; Mu, X.; Guo, L. Aptamer Biosensor for Label-Free Square-Wave Voltammetry Detection of Angiogenin. Biosens. Bioelectron. 2011, 30, 261–266. [Google Scholar] [CrossRef]
- Kohzuma, T.; Yamamoto, T.; Uematsu, Y.; Shihabi, Z.K.; Freedman, B.I. Basic Performance of an Enzymatic Method for Glycated Albumin and Reference Range Determination. J. Diabetes Sci. Technol. 2011, 5, 1455–1462. [Google Scholar] [CrossRef]
- Choi, H.; Son, S.E.; Hur, W.; Tran, V.-K.; Lee, H.B.; Park, Y.; Han, D.K.; Seong, G.H. Electrochemical Immunoassay for Determination of Glycated Albumin Using Nanozymes. Sci. Rep. 2020, 10, 9513. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Dai, G.; Luo, F.; Chu, Z.; Geng, X.; He, P.; Zhang, F.; Wang, Q. A Ratiometric Electrochemical Biosensor for Glycated Albumin Detection Based on Enhanced Nanozyme Catalysis of Cuprous Oxide-Modified Reduced Graphene Oxide Nanocomposites. J. Mater. Chem. B 2021, 9, 9324–9332. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Tran, V.-K.; Geng, Y.; Kim, M.K.; Jin, G.H.; Son, S.E.; Hur, W.; Seong, G.H. Determination of Glycated Albumin Using Boronic Acid-Derived Agarose Beads on Paper-Based Devices. Biomicrofluidics 2018, 12, 014111. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Gupta, P.K.; Hur, W.; Choi, H.; Lee, H.B.; Park, Y.; Seong, G.H. Determination of Glycated Albumin Using a Prussian Blue Nanozyme-Based Boronate Affinity Sandwich Assay. Anal. Chim. Acta 2020, 1134, 41–49. [Google Scholar] [CrossRef]
- Kim, A.-R.; Choi, Y.; Kim, S.-H.; Moon, H.-S.; Ko, J.-H.; Yoon, M.-Y. Development of a Novel SsDNA Sequence for a Glycated Human Serum Albumin and Construction of a Simple Aptasensor System Based on Reduced Graphene Oxide (RGO). Biosensors 2020, 10, 141. [Google Scholar] [CrossRef]
- Karnes, H.T.; March, C. Precision, Accuracy, and Data Acceptance Criteria in Biopharmaceutical Analysis. Pharm. Res. 1993, 10, 1420–1426. [Google Scholar] [CrossRef]
- Tsukakoshi, K.; Abe, K.; Sode, K.; Ikebukuro, K. Selection of DNA Aptamers That Recognize α-Synuclein Oligomers Using a Competitive Screening Method. Anal. Chem. 2012, 84, 5542–5547. [Google Scholar] [CrossRef]

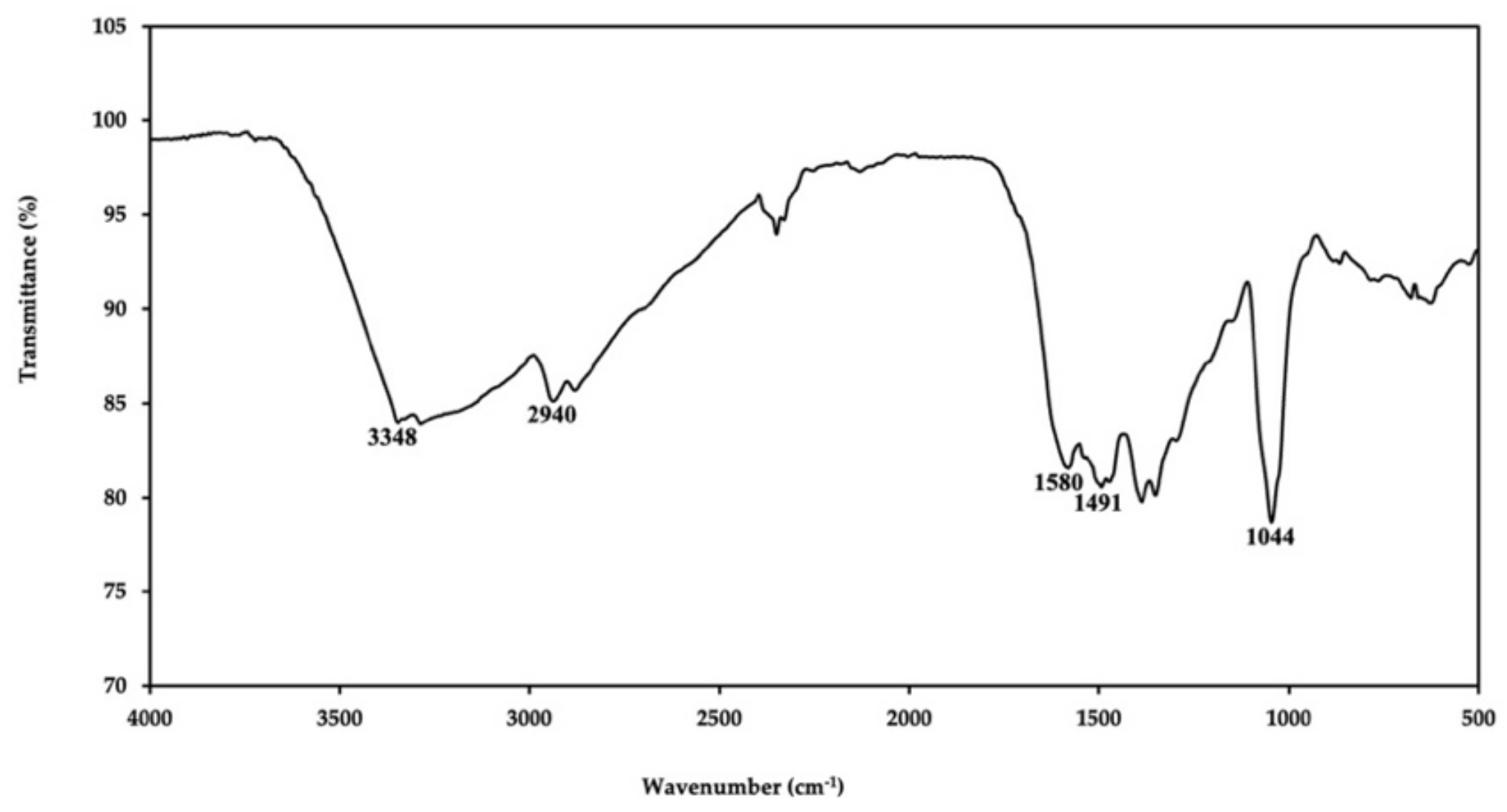



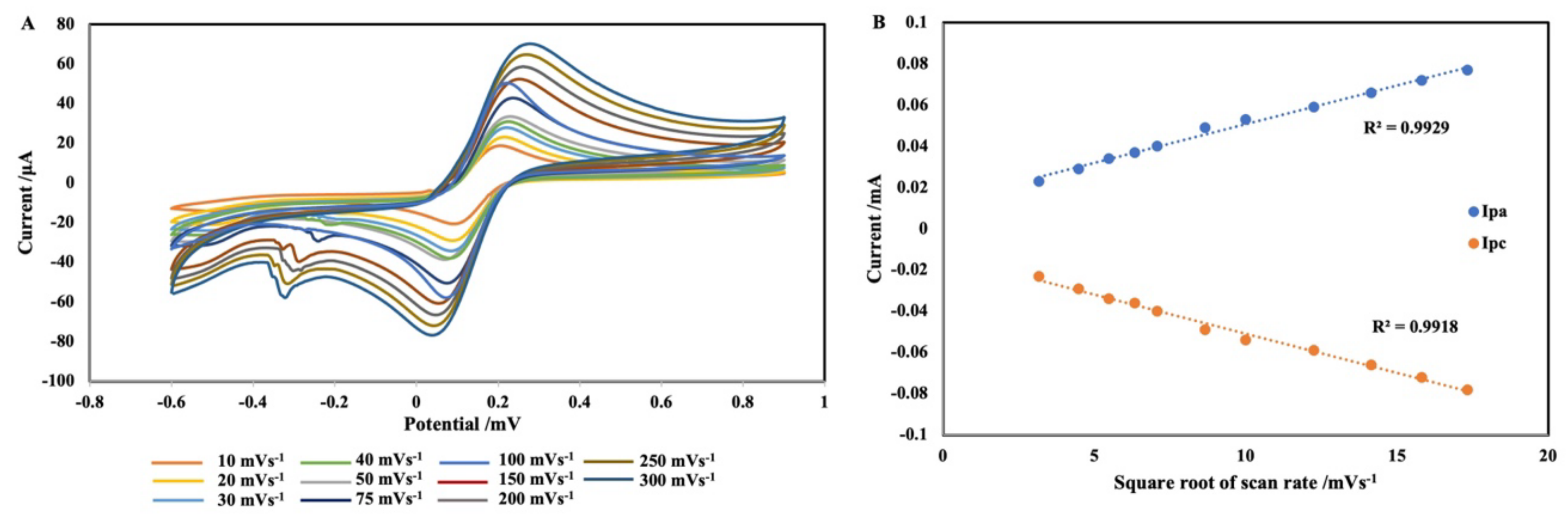
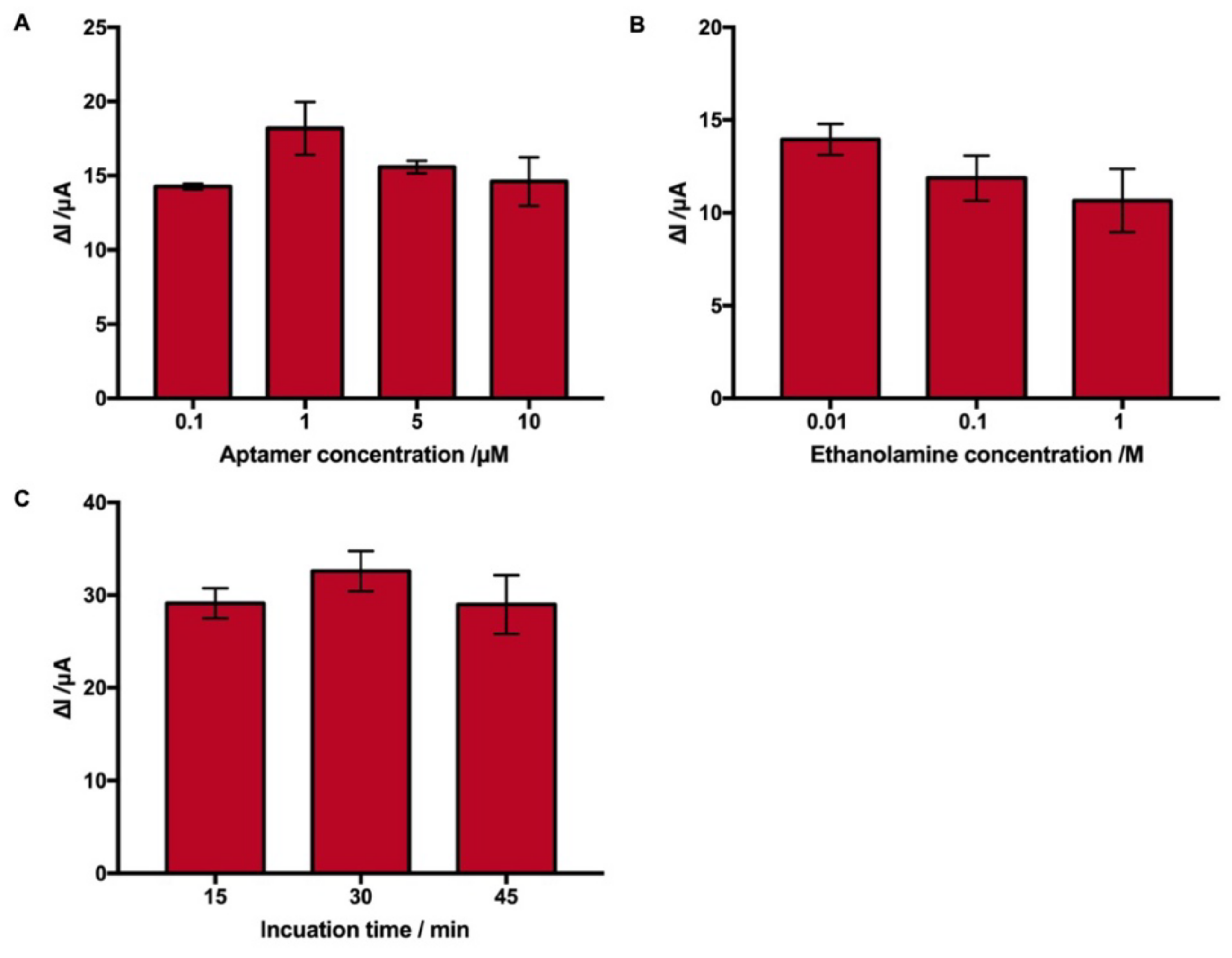
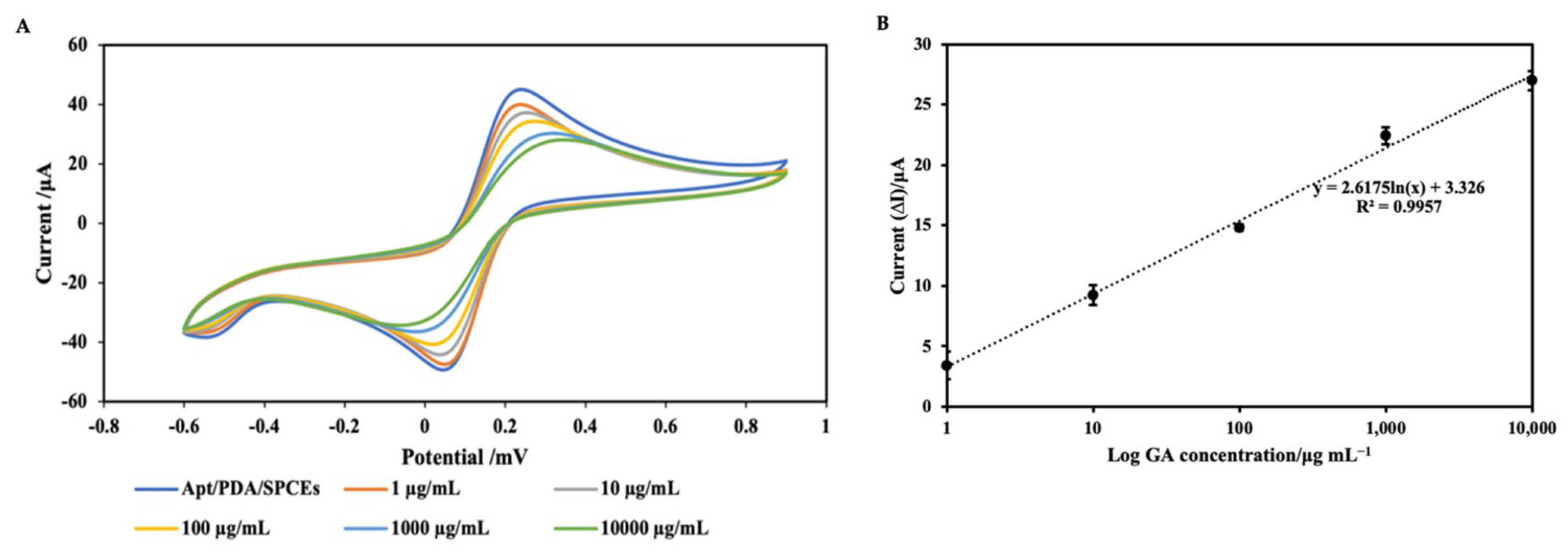
| No. | Techniques | Limit of Detection (LOD) | Linear Response Range | References |
|---|---|---|---|---|
| 1. | Nanozyme-based electrochemical immunoassay | 3.8 µg mL−1 | 5 µg mL−1 to 10 mg mL−1 | [43] |
| 2. | rGO/AuNPs based electrochemical aptasensor | 0.07 μg mL−1 | 2–10 μg mL−1 | [8] |
| 3 | Electrochemical-based aptasensor | 0.003 μg mL−1 | 0.002–16 mg mL−1 | [7] |
| 4. | Cu2O–rGO nanozyme-based electrochemical sensor | 0.007 μg mL−1 | 0.02–1500 μg mL−1 | [44] |
| 5. | Paper-based device with boronic acid-derived agarose beads | 7.1 μg mL−1 | 10 μg mL−1 to 10 mg mL−1 | [45] |
| 6. | Colorimetric immunoassay using Prussian blue nanoparticles | 7.32 μg mL−1 | 0.01–2.0 mg mL−1 | [46] |
| 7. | Enzymatic assay-based sensor | 0.36 µg mL−1 | 0–0.6 mg mL−1 | [42] |
| 8. | rGO-based aptasensor | 16.40 µg mL−1 | 0–125 µg mL−1 | [47] |
| 9. | GO-fucntionalized electrochemical aptasensor | 0.031 µg mL−1 | 0.001–10 mg mL−1 | [16] |
| 10. | PDA-NPs functionalized electrochemical aptasensor | 0.40 µg mL−1 | 0.001–10 mg mL−1 | This study |
| GA Concentration (mg mL−1) | Peak Current (µA) | Relative Standard Deviation (%) (n = 5) |
|---|---|---|
| 0.00 | 3.91 ± 0.32 | 8.28% |
| 0.10 | 14.83 ± 0.26 | 1.77% |
| 10.00 | 26.98 ± 0.76 | 2.82% |
| No. | Biosensors | Relative Interferences with HSA (%) | Reproducibility (CV%) | References |
|---|---|---|---|---|
| 1. | Electrochemical-based aptasensor | 29 ± 3% | 6.50% | [7] |
| 2. | Immobilization free electrochemical sensor | 52.91 ± 1.46% | 9.88% | [9] |
| 3. | GO-functionalized electrochemical aptasensor | 26.2 ± 0.2% | 2.50% | [16] |
| 4. | PDA-NPs functionalized electrochemical aptasensor | 19 ± 0.09% | 2.82% | This study |
| GA concentration Spiked in Serum (µg mL−1) | Measured Concentration (µg mL −1) | Recovery (%) | RSD a (%) (n = 3) |
|---|---|---|---|
| 47.60 | 53.1 ± 0.79 | 104 | 6.10 |
| 238.10 | 217 ± 0.56 | 90 | 2.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maraming, P.; Aye, N.N.S.; Boonsiri, P.; Daduang, S.; Buhome, O.; Daduang, J. Polydopamine Nanoparticles Functionalized Electrochemical DNA Aptasensor for Serum Glycated Albumin Detection. Int. J. Mol. Sci. 2022, 23, 13699. https://doi.org/10.3390/ijms232213699
Maraming P, Aye NNS, Boonsiri P, Daduang S, Buhome O, Daduang J. Polydopamine Nanoparticles Functionalized Electrochemical DNA Aptasensor for Serum Glycated Albumin Detection. International Journal of Molecular Sciences. 2022; 23(22):13699. https://doi.org/10.3390/ijms232213699
Chicago/Turabian StyleMaraming, Pornsuda, Nang Noon Shean Aye, Patcharee Boonsiri, Sakda Daduang, Onanong Buhome, and Jureerut Daduang. 2022. "Polydopamine Nanoparticles Functionalized Electrochemical DNA Aptasensor for Serum Glycated Albumin Detection" International Journal of Molecular Sciences 23, no. 22: 13699. https://doi.org/10.3390/ijms232213699
APA StyleMaraming, P., Aye, N. N. S., Boonsiri, P., Daduang, S., Buhome, O., & Daduang, J. (2022). Polydopamine Nanoparticles Functionalized Electrochemical DNA Aptasensor for Serum Glycated Albumin Detection. International Journal of Molecular Sciences, 23(22), 13699. https://doi.org/10.3390/ijms232213699






