Iron- and Neuromelanin-Weighted Neuroimaging to Study Mitochondrial Dysfunction in Patients with Parkinson’s Disease
Abstract
1. Introduction
The Scope of This Review
2. Main Body
2.1. Iron Dyshomeostasis as a Disease Mechanism in PD
2.2. The Complex Role of NM in PD Pathophysiology
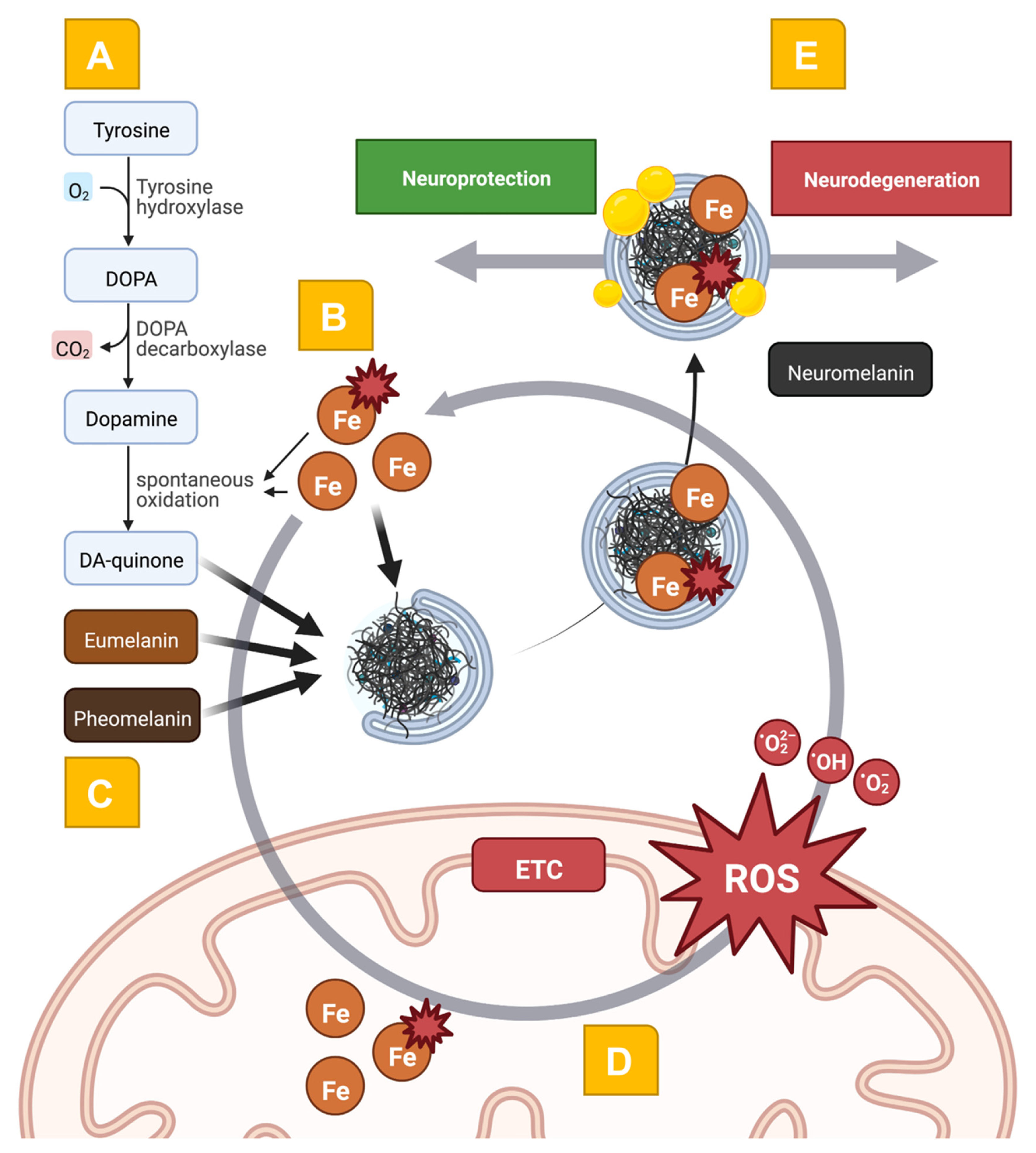
2.3. Mitochondrial Dysfunction at the Intersection of Iron- and NM-Related Pathways
2.4. Pathophysiology-Orientated Neuroimaging
2.4.1. Mapping Iron Dyshomeostasis
2.4.2. Iron-Weighted Neuroimaging in PwPD
2.4.3. Mapping NM Dyshomeostasis
2.4.4. NM-Weighted Neuroimaging in PwPD
2.4.5. Multimodal Iron- and NM-Weighted Neuroimaging and Their Implications for Pathophysiology-Orientated Studies
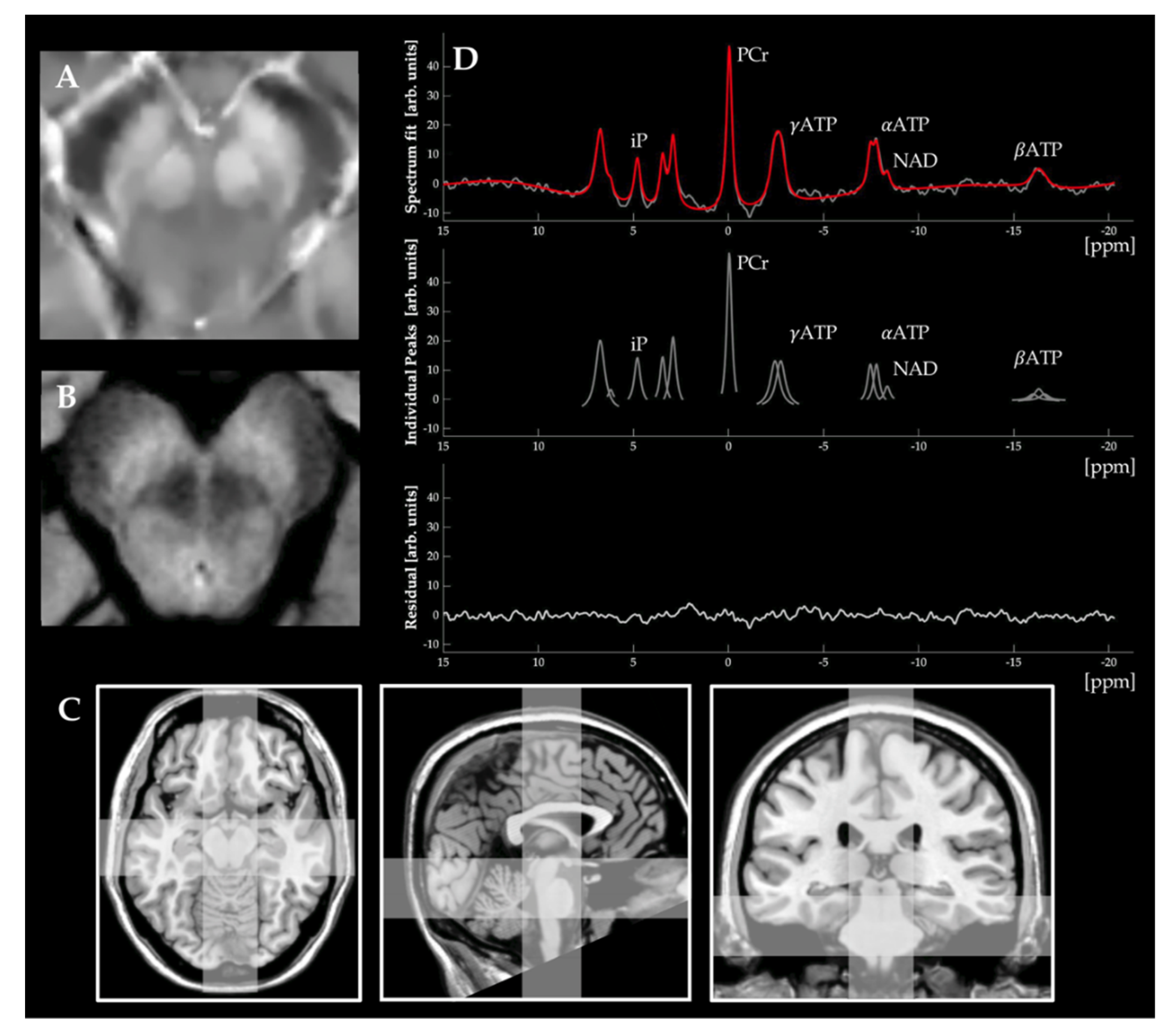
2.4.6. Mapping Mitochondrial Dysfunction
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, J.; Bruggemann, N. Genotype-driven therapeutic developments in Parkinson’s disease. Mol. Med. 2021, 27, 42. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, J.; Davis, R.L.; Kumar, K.R. Targeting Mitochondrial Impairment in Parkinson’s Disease: Challenges and Opportunities. Front. Cell Dev. Biol. 2020, 8, 615461. [Google Scholar] [CrossRef]
- Prasuhn, J.; Bruggemann, N. Gene Therapeutic Approaches for the Treatment of Mitochondrial Dysfunction in Parkinson’s Disease. Genes 2021, 12, 1840. [Google Scholar] [CrossRef]
- Prasuhn, J.; Kunert, L.; Bruggemann, N. Neuroimaging Methods to Map In Vivo Changes of OXPHOS and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2022, 23, 7263. [Google Scholar] [CrossRef]
- Cho, S.J.; Bae, Y.J.; Kim, J.M.; Kim, D.; Baik, S.H.; Sunwoo, L.; Choi, B.S.; Kim, J.H. Diagnostic performance of neuromelanin-sensitive magnetic resonance imaging for patients with Parkinson’s disease and factor analysis for its heterogeneity: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 1268–1280. [Google Scholar] [CrossRef]
- Cho, S.J.; Bae, Y.J.; Kim, J.-M.; Kim, H.J.; Baik, S.H.; Sunwoo, L.; Choi, B.S.; Jung, C.; Kim, J.H. Iron-sensitive magnetic resonance imaging in Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2021, 268, 4721–4736. [Google Scholar] [CrossRef]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Nikparast, F.; Ganji, Z.; Danesh Doust, M.; Faraji, R.; Zare, H. Brain pathological changes during neurodegenerative diseases and their identification methods: How does QSM perform in detecting this process? Insights Imaging 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef]
- Foley, P.B.; Hare, D.J.; Double, K.L. A brief history of brain iron accumulation in Parkinson disease and related disorders. J. Neural Transm. 2022, 129, 505–520. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yuan, L.; Li, W.; Li, J.Y. Ferroptosis in Parkinson’s disease: Glia-neuron crosstalk. Trends Mol. Med. 2022, 28, 258–269. [Google Scholar] [CrossRef]
- Flones, I.H.; Ricken, G.; Klotz, S.; Lang, A.; Strobel, T.; Dolle, C.; Kovacs, G.G.; Tzoulis, C. Mitochondrial respiratory chain deficiency correlates with the severity of neuropathology in sporadic Creutzfeldt-Jakob disease. Acta Neuropathol. Commun. 2020, 8, 50. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhuang, Q.Q.; Zhu, L.B.; Zhu, H.; Li, T.; Li, R.; Chen, S.F.; Huang, C.P.; Zhang, X.; Zhu, J.H. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci. Rep. 2016, 6, 36669. [Google Scholar] [CrossRef]
- Wang, Z.B.; Liu, J.Y.; Xu, X.J.; Mao, X.Y.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. Neurodegeneration with brain iron accumulation: Insights into the mitochondria dysregulation. Biomed. Pharmacother. 2019, 118, 109068. [Google Scholar] [CrossRef]
- Iankova, V.; Karin, I.; Klopstock, T.; Schneider, S.A. Emerging Disease-Modifying Therapies in Neurodegeneration With Brain Iron Accumulation (NBIA) Disorders. Front. Neurol. 2021, 12, 629414. [Google Scholar] [CrossRef]
- Klopstock, T.; Tricta, F.; Neumayr, L.; Karin, I.; Zorzi, G.; Fradette, C.; Kmiec, T.; Buchner, B.; Steele, H.E.; Horvath, R.; et al. Safety and efficacy of deferiprone for pantothenate kinase-associated neurodegeneration: A randomised, double-blind, controlled trial and an open-label extension study. Lancet Neurol. 2019, 18, 631–642. [Google Scholar] [CrossRef]
- Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-Dose Oral N-Acetylcysteine in Parkinson’s Disease: Pharmacokinetics and Effect on Brain Glutathione and Oxidative Stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garçon, G.; Rouaix, N.; et al. Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Disease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Holmay, M.J.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.; Öz, G.; Cloyd, J.C.; Tuite, P.J. N-acetylcysteine boosts brain and blood glutathione in gaucher and Parkinson diseases. Clin. Neuropharmacol. 2013, 36, 103–106. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Shankland, E.G.; Wilbur, T.K.; Padowski, J.M. Phase IIb Study of Intranasal Glutathione in Parkinson’s Disease. J. Park. Dis. 2017, 7, 289–299. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Ward, R.J.; Newbould, R.; Piccini, P.; Sharp, D.; Kabba, C.; Patel, M.C.; Spino, M.; Connelly, J.; Tricta, F.; et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 2017, 7, 1398. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.-W.; Wintering, N.A.; Bazzan, A.J.; Zhong, L.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; et al. N-Acetyl Cysteine Is Associated With Dopaminergic Improvement in Parkinson’s Disease. Clin. Pharmacol. Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef]
- Seet, R.C.-S.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chow, A.W.L.; Chong, W.-L.; Ng, M.P.E.; Ong, C.-N.; Halliwell, B. Does High-Dose Coenzyme Q10 Improve Oxidative Damage and Clinical Outcomes in Parkinson’s Disease? Antioxid. Redox Signal. 2014, 21, 211–217. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- The Parkinson Study Group QE3 Investigators; Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.; et al. A randomized clinical trial of high-dosage coenzyme Q10 in early parkinson disease no evidence of benefit. JAMA Neurol. 2014, 75, 543–552. [Google Scholar] [CrossRef]
- Du, G.; Wang, E.; Sica, C.; Chen, H.; De Jesus, S.; Lewis, M.M.; Kong, L.; Connor, J.; Mailman, R.B.; Huang, X. Dynamics of Nigral Iron Accumulation in Parkinson’s Disease: From Diagnosis to Late Stage. Mov. Disord. 2022, 37, 1654–1662. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, F.J.; Alonso-Navarro, H.; Garcia-Martin, E.; Agundez, J.A.G. Biological fluid levels of iron and iron-related proteins in Parkinson’s disease: Review and meta-analysis. Eur. J. Neurol. 2021, 28, 1041–1055. [Google Scholar] [CrossRef]
- Glass, K.; Ito, S.; Wilby, P.R.; Sota, T.; Nakamura, A.; Bowers, C.R.; Vinther, J.; Dutta, S.; Summons, R.; Briggs, D.E.; et al. Direct chemical evidence for eumelanin pigment from the Jurassic period. Proc. Natl. Acad. Sci. USA 2012, 109, 10218–10223. [Google Scholar] [CrossRef]
- Wogelius, R.A.; Manning, P.L.; Barden, H.E.; Edwards, N.P.; Webb, S.M.; Sellers, W.I.; Taylor, K.G.; Larson, P.L.; Dodson, P.; You, H.; et al. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science 2011, 333, 1622–1626. [Google Scholar] [CrossRef]
- Zhang, F.; Kearns, S.L.; Orr, P.J.; Benton, M.J.; Zhou, Z.; Johnson, D.; Xu, X.; Wang, X. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 2010, 463, 1075–1078. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- McNamara, M.E.; Rossi, V.; Slater, T.S.; Rogers, C.S.; Ducrest, A.L.; Dubey, S.; Roulin, A. Decoding the Evolution of Melanin in Vertebrates. Trends Ecol. Evol. 2021, 36, 430–443. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.N. The red and the black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef]
- Thody, A.J.; Higgins, E.M.; Wakamatsu, K.; Ito, S.; Burchill, S.A.; Marks, J.M. Pheomelanin as well as eumelanin is present in human epidermis. J. Investig. Dermatol. 1991, 97, 340–344. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Murase, T.; Zucca, F.A.; Zecca, L.; Ito, S. Biosynthetic pathway to neuromelanin and its aging process. Pigment. Cell Melanoma Res. 2012, 25, 792–803. [Google Scholar] [CrossRef]
- Haining, R.L.; Achat-Mendes, C. Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen. Res. 2017, 12, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Basso, E.; Cupaioli, F.A.; Ferrari, E.; Sulzer, D.; Casella, L.; Zecca, L. Neuromelanin of the human substantia nigra: An update. Neurotox. Res. 2014, 25, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Tampellini, D.; Gerlach, M.; Riederer, P.; Fariello, R.G.; Sulzer, D. Substantia nigra neuromelanin: Structure, synthesis, and molecular behaviour. Mol. Pathol. 2001, 54, 414–418. [Google Scholar] [PubMed]
- Wakamatsu, K.; Tabuchi, K.; Ojika, M.; Zucca, F.A.; Zecca, L.; Ito, S. Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J. Neurochem. 2015, 135, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, Oxidative Stress and Protein-Quinone Modifications in Parkinson’s and Other Neurodegenerative Diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Vila, M. Neuromelanin, aging, and neuronal vulnerability in Parkinson’s disease. Mov. Disord. 2019, 34, 1440–1451. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s Disease: Tyrosine Hydroxylase and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176. [Google Scholar] [CrossRef]
- Diederich, N.J.; James Surmeier, D.; Uchihara, T.; Grillner, S.; Goetz, C.G. Parkinson’s disease: Is it a consequence of human brain evolution? Mov. Disord. 2019, 34, 453–459. [Google Scholar] [CrossRef]
- Marsden, C.D. Pigmentation in the nucleus substantiae nigrae of mammals. J. Anat. 1961, 95, 256–261. [Google Scholar]
- Solano, F.; Hearing, V.J.; Garcia-Borron, J.C. Neurotoxicity due to o-quinones: Neuromelanin formation and possible mechanisms for o-quinone detoxification. Neurotox. Res. 2000, 1, 153–169. [Google Scholar] [CrossRef]
- Youdim, M.B.; Ben-Shachar, D.; Riederer, P. The enigma of neuromelanin in Parkinson’s disease substantia nigra. J. Neural Transm. 1994, 43, 113–122. [Google Scholar]
- Double, K.L.; Gerlach, M.; Schunemann, V.; Trautwein, A.X.; Zecca, L.; Gallorini, M.; Youdim, M.B.; Riederer, P.; Ben-Shachar, D. Iron-binding characteristics of neuromelanin of the human substantia nigra. Biochem. Pharmacol. 2003, 66, 489–494. [Google Scholar] [CrossRef]
- Graham, D.G. On the origin and significance of neuromelanin. Arch. Pathol. Lab. Med. 1979, 103, 359–362. [Google Scholar]
- Korzhevskii, D.E.; Kirik, O.V.; Guselnikova, V.V.; Tsyba, D.L.; Fedorova, E.A.; Grigorev, I.P. Changes in cytoplasmic and extracellular neuromelanin in human substantia nigra with normal aging. Eur. J. Histochem. 2021, 65. [Google Scholar] [CrossRef]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Munoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef]
- Ueno, F.; Iwata, Y.; Nakajima, S.; Caravaggio, F.; Rubio, J.M.; Horga, G.; Cassidy, C.M.; Torres-Carmona, E.; de Luca, V.; Tsugawa, S.; et al. Neuromelanin accumulation in patients with schizophrenia: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 132, 1205–1213. [Google Scholar] [CrossRef]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 634–650. [Google Scholar] [CrossRef]
- Prasuhn, J.; Prasuhn, M.; Fellbrich, A.; Strautz, R.; Lemmer, F.; Dreischmeier, S.; Kasten, M.; Munte, T.F.; Hanssen, H.; Heldmann, M.; et al. Association of Locus Coeruleus and Substantia Nigra Pathology With Cognitive and Motor Functions in Patients With Parkinson Disease. Neurology 2021, 97, e1007–e1016. [Google Scholar] [CrossRef]
- Hirsch, E.; Graybiel, A.M.; Agid, Y.A. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 1988, 334, 345–348. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Graybiel, A.M.; Agid, Y. Selective vulnerability of pigmented dopaminergic neurons in Parkinson’s disease. Acta Neurol. Scand. 1989, 126, 19–22. [Google Scholar] [CrossRef]
- Kastner, A.; Hirsch, E.C.; Lejeune, O.; Javoy-Agid, F.; Rascol, O.; Agid, Y. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson’s disease related to their neuromelanin content? J. Neurochem. 1992, 59, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.V.; Larsen, K.E.; Kanter, E.; Phillips, K.A.; Wilson, K.; Schmitz, Y.; Krantz, D.E.; Kobayashi, K.; Edwards, R.H.; Sulzer, D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009, 62, 218–229. [Google Scholar] [CrossRef]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Gimenez, J.; Cuadros, T.; Bove, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sepulveda, M.; Penuelas, N.; Torra, A.; et al. Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat. Commun. 2019, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Viceconte, N.; Burguillos, M.A.; Herrera, A.J.; De Pablos, R.M.; Joseph, B.; Venero, J.L. Neuromelanin activates proinflammatory microglia through a caspase-8-dependent mechanism. J. Neuroinflammation 2015, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.M.; Zucca, F.A.; Girgis, R.R.; Baker, S.C.; Weinstein, J.J.; Sharp, M.E.; Bellei, C.; Valmadre, A.; Vanegas, N.; Kegeles, L.S.; et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc. Natl. Acad. Sci. USA 2019, 116, 5108–5117. [Google Scholar] [CrossRef]
- Prasuhn, J.; Strautz, R.; Lemmer, F.; Dreischmeier, S.; Kasten, M.; Hanssen, H.; Heldmann, M.; Bruggemann, N. Neuroimaging Correlates of Substantia Nigra Hyperechogenicity in Parkinson’s Disease. J. Park. Dis. 2022, 12, 1191–1200. [Google Scholar] [CrossRef]
- Sulzer, D.; Cassidy, C.; Horga, G.; Kang, U.J.; Fahn, S.; Casella, L.; Pezzoli, G.; Langley, J.; Hu, X.P.; Zucca, F.A.; et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Park. Dis. 2018, 4, 11. [Google Scholar] [CrossRef]
- Borsche, M.; Pereira, S.L.; Klein, C.; Grunewald, A. Mitochondria and Parkinson’s Disease: Clinical, Molecular, and Translational Aspects. J. Park. Dis. 2021, 11, 45–60. [Google Scholar] [CrossRef]
- Munoz, Y.; Carrasco, C.M.; Campos, J.D.; Aguirre, P.; Nunez, M.T. Parkinson’s Disease: The Mitochondria-Iron Link. Park. Dis. 2016, 2016, 7049108. [Google Scholar] [CrossRef]
- Prasuhn, J.; Gottlich, M.; Gerkan, F.; Kourou, S.; Ebeling, B.; Kasten, M.; Hanssen, H.; Klein, C.; Bruggemann, N. Relationship between brain iron deposition and mitochondrial dysfunction in idiopathic Parkinson’s disease. Mol. Med. 2022, 28, 28. [Google Scholar] [CrossRef]
- He, Y.; Thong, P.S.; Lee, T.; Leong, S.K.; Mao, B.Y.; Dong, F.; Watt, F. Dopaminergic cell death precedes iron elevation in MPTP-injected monkeys. Free Radic. Biol. Med. 2003, 35, 540–547. [Google Scholar] [CrossRef]
- Li, S.J.; Ren, Y.D.; Li, J.; Cao, B.; Ma, C.; Qin, S.S.; Li, X.R. The role of iron in Parkinson’s disease monkeys assessed by susceptibility weighted imaging and inductively coupled plasma mass spectrometry. Life Sci. 2020, 240, 117091. [Google Scholar] [CrossRef]
- Shi, L.; Huang, C.; Luo, Q.; Rogers, E.; Xia, Y.; Liu, W.; Ma, W.; Zeng, W.; Gong, L.; Fang, J.; et al. The Association of Iron and the Pathologies of Parkinson’s Diseases in MPTP/MPP(+)-Induced Neuronal Degeneration in Non-human Primates and in Cell Culture. Front. Aging Neurosci. 2019, 11, 215. [Google Scholar] [CrossRef]
- Riederer, P.; Monoranu, C.; Strobel, S.; Iordache, T.; Sian-Hulsmann, J. Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson’s disease. J. Neural Transm. 2021, 128, 1577–1598. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011, 121, 171–181. [Google Scholar] [CrossRef]
- Lawana, V.; Um, S.Y.; Foguth, R.M.; Cannon, J.R. Neuromelanin formation exacerbates HAA-induced mitochondrial toxicity and mitophagy impairments. Neurotoxicology 2020, 81, 147–160. [Google Scholar] [CrossRef]
- Maruyama, W.; Shamoto-Nagai, M.; Akao, Y.; Riederer, P.; Naoi, M. The effect of neuromelanin on the proteasome activity in human dopaminergic SH-SY5Y cells. J. Neural Transm. 2006, 70, 125–132. [Google Scholar] [CrossRef]
- Prasuhn, J.; Martensson, C.U.; Krajka, V.; Klein, C.; Rakovic, A. Genome-Edited, TH-expressing Neuroblastoma Cells as a Disease Model for Dopamine-Related Disorders: A Proof-of-Concept Study on DJ-1-deficient Parkinsonism. Front. Cell. Neurosci. 2017, 11, 426. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef]
- Shamoto-Nagai, M.; Maruyama, W.; Yi, H.; Akao, Y.; Tribl, F.; Gerlach, M.; Osawa, T.; Riederer, P.; Naoi, M. Neuromelanin induces oxidative stress in mitochondria through release of iron: Mechanism behind the inhibition of 26S proteasome. J. Neural Transm. 2006, 113, 633–644. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Iron, Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 7267. [Google Scholar] [CrossRef] [PubMed]
- Bagwe-Parab, S.; Kaur, G. Molecular targets and therapeutic interventions for iron induced neurodegeneration. Brain Res. Bull. 2020, 156, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feraco, P.; Gagliardo, C.; La Tona, G.; Bruno, E.; D’Angelo, C.; Marrale, M.; Del Poggio, A.; Malaguti, M.C.; Geraci, L.; Baschi, R.; et al. Imaging of Substantia Nigra in Parkinson’s Disease: A Narrative Review. Brain Sci. 2021, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Lehericy, S.; Chiu, S.Y.; Strafella, A.P.; Stoessl, A.J.; Vaillancourt, D.E. Emerging Neuroimaging Biomarkers Across Disease Stage in Parkinson Disease: A Review. JAMA Neurol. 2021, 78, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C. Quantitative relaxometry of the brain. Top. Magn. Reson. Imaging 2010, 21, 101–113. [Google Scholar] [CrossRef]
- Heim, B.; Krismer, F.; De Marzi, R.; Seppi, K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J. Neural Transm. 2017, 124, 915–964. [Google Scholar] [CrossRef]
- Saeed, U.; Compagnone, J.; Aviv, R.I.; Strafella, A.P.; Black, S.E.; Lang, A.E.; Masellis, M. Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: Current and emerging concepts. Transl. Neurodegener. 2017, 6, 8. [Google Scholar] [CrossRef]
- Haacke, E.M.; Liu, S.; Buch, S.; Zheng, W.; Wu, D.; Ye, Y. Quantitative susceptibility mapping: Current status and future directions. Magn. Reson. Imaging 2015, 33, 1–25. [Google Scholar] [CrossRef]
- Kim, P.H.; Lee, D.H.; Suh, C.H.; Kim, M.; Shim, W.H.; Kim, S.J. Diagnostic performance of loss of nigral hyperintensity on susceptibility-weighted imaging in parkinsonism: An updated meta-analysis. Eur. Radiol. 2021, 31, 6342–6352. [Google Scholar] [CrossRef]
- Prasuhn, J.; Neumann, A.; Strautz, R.; Dreischmeier, S.; Lemmer, F.; Hanssen, H.; Heldmann, M.; Schramm, P.; Bruggemann, N. Clinical MR imaging in Parkinson’s disease: How useful is the swallow tail sign? Brain Behav. 2021, 11, e02202. [Google Scholar] [CrossRef]
- Cheng, Z.; He, N.; Huang, P.; Li, Y.; Tang, R.; Sethi, S.K.; Ghassaban, K.; Yerramsetty, K.K.; Palutla, V.K.; Chen, S.; et al. Imaging the Nigrosome 1 in the substantia nigra using susceptibility weighted imaging and quantitative susceptibility mapping: An application to Parkinson’s disease. NeuroImage Clin. 2020, 25, 102103. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, J.; He, N.; Li, Y.; Wen, Y.; Xu, H.; Tang, R.; Jin, Z.; Haacke, E.M.; Yan, F.; et al. Radiomic Features of the Nigrosome-1 Region of the Substantia Nigra: Using Quantitative Susceptibility Mapping to Assist the Diagnosis of Idiopathic Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 167. [Google Scholar] [CrossRef]
- Mahlknecht, P.; Krismer, F.; Poewe, W.; Seppi, K. Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson’s disease. Mov.Disord. Off. J. Mov. Disord. Soc. 2017, 32, 619–623. [Google Scholar] [CrossRef]
- Genoud, S.; Senior, A.M.; Hare, D.J.; Double, K.L. Meta-Analysis of Copper and Iron in Parkinson’s Disease Brain and Biofluids. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 662–671. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Sanz-Morere, C.B.; Gaurav, R.; Biondetti, E.; Valabregue, R.; Santin, M.; Yahia-Cherif, L.; Lehericy, S. Iron Imaging as a Diagnostic Tool for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 366. [Google Scholar] [CrossRef]
- Pelzer, E.A.; Florin, E.; Schnitzler, A. Quantitative Susceptibility Mapping and Resting State Network Analyses in Parkinsonian Phenotypes-A Systematic Review of the Literature. Front. Neural Circuits 2019, 13, 50. [Google Scholar] [CrossRef]
- Dietrich, O.; Levin, J.; Ahmadi, S.A.; Plate, A.; Reiser, M.F.; Botzel, K.; Giese, A.; Ertl-Wagner, B. MR imaging differentiation of Fe(2+) and Fe(3+) based on relaxation and magnetic susceptibility properties. Neuroradiology 2017, 59, 403–409. [Google Scholar] [CrossRef]
- Pietracupa, S.; Bologna, M.; Tommasin, S.; Elifani, F.; Vasselli, F.; Paparella, G.; Petsas, N.; Berardelli, A.; Pantano, P. No evidence of iron deposition in essential tremor: A susceptibility-weighted imaging study. Neurol. Sci. 2021, 42, 4667–4672. [Google Scholar] [CrossRef]
- Du, G.; Lewis, M.M.; Sica, C.; He, L.; Connor, J.R.; Kong, L.; Mailman, R.B.; Huang, X. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov. Disord. 2018, 33, 1423–1431. [Google Scholar] [CrossRef]
- Hopes, L.; Grolez, G.; Moreau, C.; Lopes, R.; Ryckewaert, G.; Carriere, N.; Auger, F.; Laloux, C.; Petrault, M.; Devedjian, J.C.; et al. Magnetic Resonance Imaging Features of the Nigrostriatal System: Biomarkers of Parkinson’s Disease Stages? PLoS ONE 2016, 11, e0147947. [Google Scholar] [CrossRef]
- Li, K.R.; Avecillas-Chasin, J.; Nguyen, T.D.; Gillen, K.M.; Dimov, A.; Chang, E.; Skudin, C.; Kopell, B.H.; Wang, Y.; Shtilbans, A. Quantitative evaluation of brain iron accumulation in different stages of Parkinson’s disease. J. Neuroimaging 2022, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Watanabe, Y.; Tanaka, H.; Mihara, M.; Mochizuki, H.; Takahashi, K.; Yamamoto, K.; Liu, T.; Wang, Y.; Tomiyama, N. Comprehensive MRI quantification of the substantia nigra pars compacta in Parkinson’s disease. Eur. J. Radiol. 2018, 109, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, Y.; Zhang, Y.; Wang, F.; Yu, H.; Zhang, C.; Jiang, Z.; Luo, W. Iron deposition in Parkinson’s disease by quantitative susceptibility mapping. BMC Neurosci. 2019, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, X.; Zhang, M. Region-Specific Iron Measured by MRI as a Biomarker for Parkinson’s Disease. Neurosci. Bull. 2017, 33, 561–567. [Google Scholar] [CrossRef]
- Guan, X.; Xuan, M.; Gu, Q.; Huang, P.; Liu, C.; Wang, N.; Xu, X.; Luo, W.; Zhang, M. Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed. 2017, 30, e3489. [Google Scholar] [CrossRef]
- Wieler, M.; Gee, M.; Camicioli, R.; Martin, W.R.W. Freezing of gait in early Parkinson’s disease: Nigral iron content estimated from magnetic resonance imaging. J. Neurol. Sci. 2016, 361, 87–91. [Google Scholar] [CrossRef]
- Tambasco, N.; Nigro, P.; Chiappiniello, A.; Paolini Paoletti, F.; Scialpi, S.; Simoni, S.; Chiarini, P.; Parnetti, L. An Updated Overview of the Magnetic Resonance Imaging of Brain Iron in Movement Disorders. Behav. Neurol. 2022, 2022, 3972173. [Google Scholar] [CrossRef]
- Li, D.T.H.; Hui, E.S.; Chan, Q.; Yao, N.; Chua, S.E.; McAlonan, G.M.; Pang, S.Y.Y.; Ho, S.L.; Mak, H.K.F. Quantitative susceptibility mapping as an indicator of subcortical and limbic iron abnormality in Parkinson’s disease with dementia. Neuroimage Clin. 2018, 20, 365–373. [Google Scholar] [CrossRef]
- Nikparast, F.; Ganji, Z.; Zare, H. Early differentiation of neurodegenerative diseases using the novel QSM technique: What is the biomarker of each disorder? BMC Neurosci. 2022, 23, 48. [Google Scholar] [CrossRef]
- Thomas, G.E.C.; Leyland, L.A.; Schrag, A.E.; Lees, A.J.; Acosta-Cabronero, J.; Weil, R.S. Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 418–425. [Google Scholar] [CrossRef]
- Sasaki, M.; Shibata, E.; Tohyama, K.; Takahashi, J.; Otsuka, K.; Tsuchiya, K.; Takahashi, S.; Ehara, S.; Terayama, Y.; Sakai, A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport 2006, 17, 1215–1218. [Google Scholar] [CrossRef]
- Jin, L.; Wang, J.; Wang, C.; Lian, D.; Zhou, Y.; Zhang, Y.; Lv, M.; Li, Y.; Huang, Z.; Cheng, X.; et al. Combined Visualization of Nigrosome-1 and Neuromelanin in the Substantia Nigra Using 3T MRI for the Differential Diagnosis of Essential Tremor and de novo Parkinson’s Disease. Front. Neurol. 2019, 10, 100. [Google Scholar] [CrossRef]
- Reimao, S.; Pita Lobo, P.; Neutel, D.; Guedes, L.C.; Coelho, M.; Rosa, M.M.; Azevedo, P.; Ferreira, J.; Abreu, D.; Goncalves, N.; et al. Substantia nigra neuromelanin-MR imaging differentiates essential tremor from Parkinson’s disease. Mov. Disord. 2015, 30, 953–959. [Google Scholar] [CrossRef]
- Chougar, L.; Arsovic, E.; Gaurav, R.; Biondetti, E.; Faucher, A.; Valabregue, R.; Pyatigorskaya, N.; Dupont, G.; Lejeune, F.X.; Cormier, F.; et al. Regional Selectivity of Neuromelanin Changes in the Substantia Nigra in Atypical Parkinsonism. Mov. Disord. 2022, 37, 1245–1255. [Google Scholar] [CrossRef]
- He, N.; Ghassaban, K.; Huang, P.; Jokar, M.; Wang, Y.; Cheng, Z.; Jin, Z.; Li, Y.; Sethi, S.K.; He, Y.; et al. Imaging iron and neuromelanin simultaneously using a single 3D gradient echo magnetization transfer sequence: Combining neuromelanin, iron and the nigrosome-1 sign as complementary imaging biomarkers in early stage Parkinson’s disease. Neuroimage 2021, 230, 117810. [Google Scholar] [CrossRef]
- Oshima, S.; Fushimi, Y.; Okada, T.; Nakajima, S.; Yokota, Y.; Shima, A.; Grinstead, J.; Ahn, S.; Sawamoto, N.; Takahashi, R.; et al. Neuromelanin-Sensitive Magnetic Resonance Imaging Using DANTE Pulse. Mov. Disord. 2021, 36, 874–882. [Google Scholar] [CrossRef]
- De Pietro Franco Zorzenon, C.; Almeida Antonio Bienes, G.H.; Duarte Alves, E.; Tobaru Tibana, L.A.; Carrete Junior, H.; Ballalai Ferraz, H. Magnetic resonance imaging evaluation of nigrosome 1 and neuromelanin can assist Parkinson’s disease diagnosis, but requires an expert neuroradiologist. Park. Relat. Disord. 2021, 83, 8–12. [Google Scholar] [CrossRef]
- Takahashi, H.; Kashiwagi, N.; Arisawa, A.; Matsuo, C.; Kato, H.; Adachi, H.; Kajiyama, Y.; Mochizuki, H.; Tomiyama, N. Imaging of the nigrostriatal system for evaluating the preclinical phase of Parkinson’s disease development: The utility of neuromelanin, diffusion MRI, and DAT-SPECT. Br. J. Radiol. 2022, 95, 20210837. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhu, C.; Li, G.; Kang, J.; Chen, F.; Yang, L. The diagnostic value of SNpc using NM-MRI in Parkinson’s disease: Meta-analysis. Neurol. Sci. 2019, 40, 2479–2489. [Google Scholar] [CrossRef]
- Ohtsuka, C.; Sasaki, M.; Konno, K.; Kato, K.; Takahashi, J.; Yamashita, F.; Terayama, Y. Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Park. Relat. Disord. 2014, 20, 755–760. [Google Scholar] [CrossRef]
- Matsuura, K.; Ii, Y.; Maeda, M.; Tabei, K.I.; Satoh, M.; Umino, M.; Miyashita, K.; Ishikawa, H.; Shindo, A.; Tomimoto, H. Neuromelanin-sensitive magnetic resonance imaging in disease differentiation for parkinsonism or neurodegenerative disease affecting the basal ganglia. Park. Relat. Disord. 2021, 87, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Maeda, M.; Yata, K.; Ichiba, Y.; Yamaguchi, T.; Kanamaru, K.; Tomimoto, H. Neuromelanin magnetic resonance imaging in Parkinson’s disease and multiple system atrophy. Eur. Neurol. 2013, 70, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Huang, Z.; Wan, W.; Zhang, Y.; Wang, C.; Cheng, X.; Ye, F.; Liu, K.; Fei, G.; et al. Neuromelanin-sensitive magnetic resonance imaging features of the substantia nigra and locus coeruleus in de novo Parkinson’s disease and its phenotypes. Eur. J. Neurol. 2018, 25, 949-e73. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, R.; Yahia-Cherif, L.; Pyatigorskaya, N.; Mangone, G.; Biondetti, E.; Valabregue, R.; Ewenczyk, C.; Hutchison, R.M.; Cedarbaum, J.M.; Corvol, J.C.; et al. Longitudinal Changes in Neuromelanin MRI Signal in Parkinson’s Disease: A Progression Marker. Mov. Disord. 2021, 36, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, E.; Santin, M.D.; Valabregue, R.; Mangone, G.; Gaurav, R.; Pyatigorskaya, N.; Hutchison, M.; Yahia-Cherif, L.; Villain, N.; Habert, M.O.; et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson’s disease. Brain 2021, 144, 3114–3125. [Google Scholar] [CrossRef]
- Hirata, F.C.C.; Sato, J.R.; Vieira, G.; Lucato, L.T.; Leite, C.C.; Bor-Seng-Shu, E.; Pastorello, B.F.; Otaduy, M.C.G.; Chaim, K.T.; Campanholo, K.R.; et al. Substantia nigra fractional anisotropy is not a diagnostic biomarker of Parkinson’s disease: A diagnostic performance study and meta-analysis. Eur. Radiol. 2017, 27, 2640–2648. [Google Scholar] [CrossRef]
- Langley, J.; Huddleston, D.E.; Sedlacik, J.; Boelmans, K.; Hu, X.P. Parkinson’s disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov. Disord. 2017, 32, 441–449. [Google Scholar] [CrossRef]
- Isaias, I.U.; Trujillo, P.; Summers, P.; Marotta, G.; Mainardi, L.; Pezzoli, G.; Zecca, L.; Costa, A. Neuromelanin Imaging and Dopaminergic Loss in Parkinson’s Disease. Front. Aging Neurosci. 2016, 8, 196. [Google Scholar] [CrossRef]
- Kuya, K.; Shinohara, Y.; Miyoshi, F.; Fujii, S.; Tanabe, Y.; Ogawa, T. Correlation between neuromelanin-sensitive MR imaging and (123)I-FP-CIT SPECT in patients with parkinsonism. Neuroradiology 2016, 58, 351–356. [Google Scholar] [CrossRef]
- Kuya, K.; Ogawa, T.; Shinohara, Y.; Ishibashi, M.; Fujii, S.; Mukuda, N.; Tanabe, Y. Evaluation of Parkinson’s disease by neuromelanin-sensitive magnetic resonance imaging and (123)I-FP-CIT SPECT. Acta Radiol. 2018, 59, 593–598. [Google Scholar] [CrossRef]
- Langley, J.; Huddleston, D.E.; Crosson, B.; Song, D.D.; Factor, S.A.; Hu, X. Multimodal assessment of nigrosomal degeneration in Parkinson’s disease. Park. Relat. Disord. 2020, 80, 102–107. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Lao-Kaim, N.P.; Roussakis, A.A.; Searle, G.E.; Xing, Y.; Gunn, R.N.; Schwarz, S.T.; Barker, R.A.; Auer, D.P.; Piccini, P. Relationship between neuromelanin and dopamine terminals within the Parkinson’s nigrostriatal system. Brain 2019, 142, 2023–2036. [Google Scholar] [CrossRef]
- Reimao, S.; Ferreira, S.; Nunes, R.G.; Pita Lobo, P.; Neutel, D.; Abreu, D.; Goncalves, N.; Campos, J.; Ferreira, J.J. Magnetic resonance correlation of iron content with neuromelanin in the substantia nigra of early-stage Parkinson’s disease. Eur. J. Neurol. 2016, 23, 368–374. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Shimada, H.; Kodaka, F.; Suzuki, M.; Shinotoh, H.; Hirano, S.; Kershaw, J.; Inoue, Y.; Nakamura, M.; Sasai, T.; et al. Principal Component Analysis of Multimodal Neuromelanin MRI and Dopamine Transporter PET Data Provides a Specific Metric for the Nigral Dopaminergic Neuronal Density. PLoS ONE 2016, 11, e0151191. [Google Scholar] [CrossRef]
- Biondetti, E.; Gaurav, R.; Yahia-Cherif, L.; Mangone, G.; Pyatigorskaya, N.; Valabregue, R.; Ewenczyk, C.; Hutchison, M.; Francois, C.; Arnulf, I.; et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain 2020, 143, 2757–2770. [Google Scholar] [CrossRef]
- Gramsch, C.; Reuter, I.; Kraff, O.; Quick, H.H.; Tanislav, C.; Roessler, F.; Deuschl, C.; Forsting, M.; Schlamann, M. Nigrosome 1 visibility at susceptibility weighted 7T MRI-A dependable diagnostic marker for Parkinson’s disease or merely an inconsistent, age-dependent imaging finding? PLoS ONE 2017, 12, e0185489. [Google Scholar] [CrossRef]
- Prasuhn, J.; Heldmann, M.; Münte, T.F.; Brüggemann, N. A machine learning-based classification approach on Parkinson’s disease diffusion tensor imaging datasets. Neurol. Res. Pract. 2020, 2, 46. [Google Scholar] [CrossRef]
- Safai, A.; Prasad, S.; Chougule, T.; Saini, J.; Pal, P.K.; Ingalhalikar, M. Microstructural abnormalities of substantia nigra in Parkinson’s disease: A neuromelanin sensitive MRI atlas based study. Hum. Brain Mapp. 2020, 41, 1323–1333. [Google Scholar] [CrossRef]
- Buonocore, M.H.; Maddock, R.J. Magnetic resonance spectroscopy of the brain: A review of physical principles and technical methods. Rev. Neurosci. 2015, 26, 609–632. [Google Scholar] [CrossRef]
- Weiduschat, N.; Mao, X.; Beal, M.F.; Nirenberg, M.J.; Shungu, D.C.; Henchcliffe, C. Usefulness of proton and phosphorus MR spectroscopic imaging for early diagnosis of Parkinson’s disease. J. Neuroimaging 2015, 25, 105–110. [Google Scholar] [CrossRef]
- Choi, I.Y.; Andronesi, O.C.; Barker, P.; Bogner, W.; Edden, R.A.E.; Kaiser, L.G.; Lee, P.; Marjanska, M.; Terpstra, M.; de Graaf, R.A. Spectral editing in (1) H magnetic resonance spectroscopy: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4411. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Conley, K.E.; Shankland, E.G.; Kavanagh, T.J.; Rosenfeld, M.E.; Duda, J.E.; White, C.C.; Wilbur, T.K.; De La Torre, P.U.; Padowski, J.M. Central nervous system uptake of intranasal glutathione in Parkinson’s disease. NPJ Park. Dis. 2016, 2, 16002. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.; Gu, Y.; Liu, Y.; Kim, K.; Huang, S.; Li, Y.; Lam, F.; Liang, Z.P.; Yu, X. High-Resolution Dynamic (31)P-MR Spectroscopic Imaging for Mapping Mitochondrial Function. IEEE Trans. Biomed. Eng. 2020, 67, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cohen, B.M.; Chen, X.; Lukas, S.E.; Shinn, A.K.; Yuksel, A.C.; Li, T.; Du, F.; Ongur, D. Redox Dysregulation in Schizophrenia Revealed by in vivo NAD+/NADH Measurement. Schizophr. Bull. 2017, 43, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhu, X.H.; Qiao, H.; Zhang, X.; Chen, W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn. Reson. Med. 2007, 57, 103–114. [Google Scholar] [CrossRef]
- Baron, J.C.; Jones, T. Oxygen metabolism, oxygen extraction and positron emission tomography: Historical perspective and impact on basic and clinical neuroscience. Neuroimage 2012, 61, 492–504. [Google Scholar] [CrossRef]
- Christen, T.; Bolar, D.S.; Zaharchuk, G. Imaging brain oxygenation with MRI using blood oxygenation approaches: Methods, validation, and clinical applications. AJNR Am. J. Neuroradiol. 2013, 34, 1113–1123. [Google Scholar] [CrossRef]
- Tsukada, H.; Ohba, H.; Kanazawa, M.; Kakiuchi, T.; Harada, N. Evaluation of 18F-BCPP-EF for mitochondrial complex 1 imaging in the brain of conscious monkeys using PET. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 755–763. [Google Scholar] [CrossRef]
- Berkowitz, B.A. Oxidative stress measured in vivo without an exogenous contrast agent using QUEST MRI. J. Magn. Reson. 2018, 291, 94–100. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Podolsky, R.H.; Childers, K.L.; Gow, A.; Schneider, B.L.; Lloyd, S.C.; Bosse, K.E.; Conti, A.C.; Roberts, R.; Berri, A.M.; et al. Age-related murine hippocampal CA1 laminae oxidative stress measured in vivo by QUEnch-assiSTed (QUEST) MRI: Impact of isoflurane anesthesia. Geroscience 2020, 42, 563–574. [Google Scholar] [CrossRef]
- Kuhl, A.; Dixon, A.; Hali, M.; Apawu, A.K.; Muca, A.; Sinan, M.; Warila, J.; Braun, R.D.; Berkowitz, B.A.; Holt, A.G. Novel QUEST MRI In Vivo Measurement of Noise-induced Oxidative Stress in the Cochlea. Sci. Rep. 2019, 9, 16265. [Google Scholar] [CrossRef]
- Bale, G.; Elwell, C.E.; Tachtsidis, I. From Jobsis to the present day: A review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J. Biomed. Opt. 2016, 21, 091307. [Google Scholar] [CrossRef]
- Prasuhn, J.; Bruggemann, N.; Hessler, N.; Berg, D.; Gasser, T.; Brockmann, K.; Olbrich, D.; Ziegler, A.; Konig, I.R.; Klein, C.; et al. An omics-based strategy using coenzyme Q10 in patients with Parkinson’s disease: Concept evaluation in a double-blind randomized placebo-controlled parallel group trial. Neurol. Res. Pract. 2019, 1, 31. [Google Scholar] [CrossRef]
- Prasuhn, J.; Kasten, M.; Vos, M.; Konig, I.R.; Schmid, S.M.; Wilms, B.; Klein, C.; Bruggemann, N. The Use of Vitamin K2 in Patients With Parkinson’s Disease and Mitochondrial Dysfunction (PD-K2): A Theranostic Pilot Study in a Placebo-Controlled Parallel Group Design. Front. Neurol. 2020, 11, 592104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro-Galleguillos, B.M.; Kunert, L.; Brüggemann, N.; Prasuhn, J. Iron- and Neuromelanin-Weighted Neuroimaging to Study Mitochondrial Dysfunction in Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 13678. https://doi.org/10.3390/ijms232213678
Pizarro-Galleguillos BM, Kunert L, Brüggemann N, Prasuhn J. Iron- and Neuromelanin-Weighted Neuroimaging to Study Mitochondrial Dysfunction in Patients with Parkinson’s Disease. International Journal of Molecular Sciences. 2022; 23(22):13678. https://doi.org/10.3390/ijms232213678
Chicago/Turabian StylePizarro-Galleguillos, Benjamin Matis, Liesa Kunert, Norbert Brüggemann, and Jannik Prasuhn. 2022. "Iron- and Neuromelanin-Weighted Neuroimaging to Study Mitochondrial Dysfunction in Patients with Parkinson’s Disease" International Journal of Molecular Sciences 23, no. 22: 13678. https://doi.org/10.3390/ijms232213678
APA StylePizarro-Galleguillos, B. M., Kunert, L., Brüggemann, N., & Prasuhn, J. (2022). Iron- and Neuromelanin-Weighted Neuroimaging to Study Mitochondrial Dysfunction in Patients with Parkinson’s Disease. International Journal of Molecular Sciences, 23(22), 13678. https://doi.org/10.3390/ijms232213678






