GABAergic Regulation of Astroglial Gliotransmission through Cx43 Hemichannels
Abstract
1. Introduction
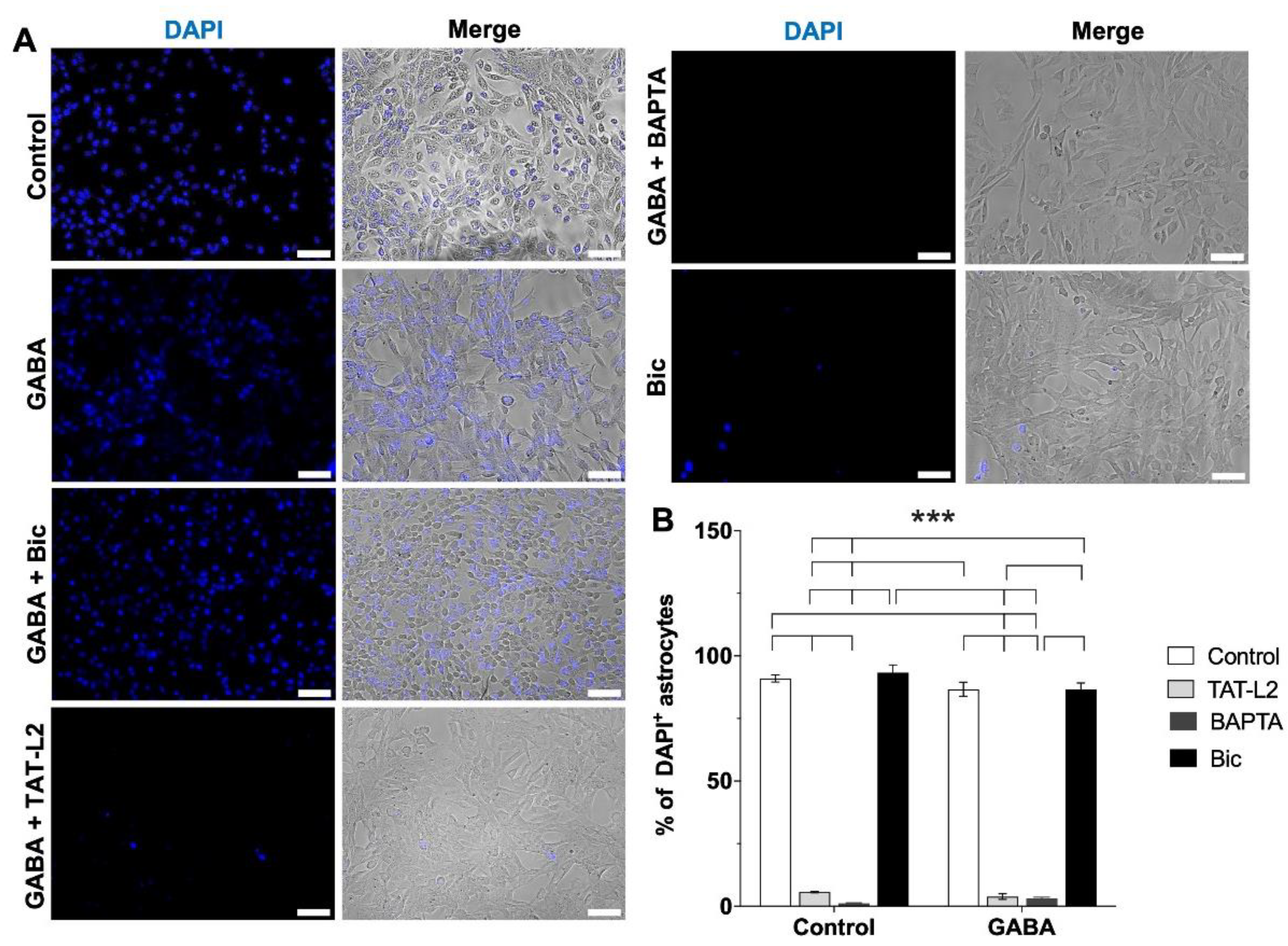
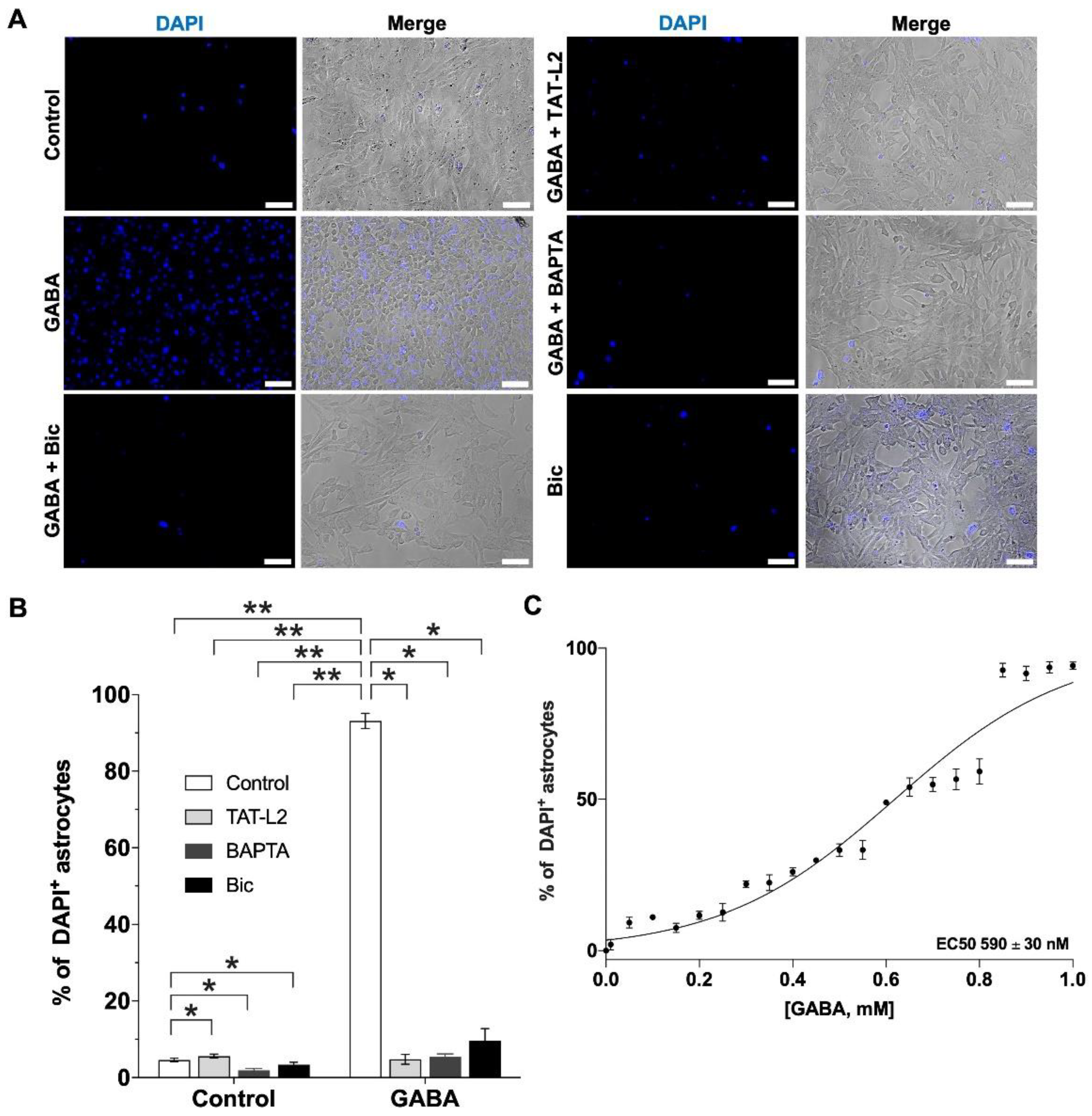
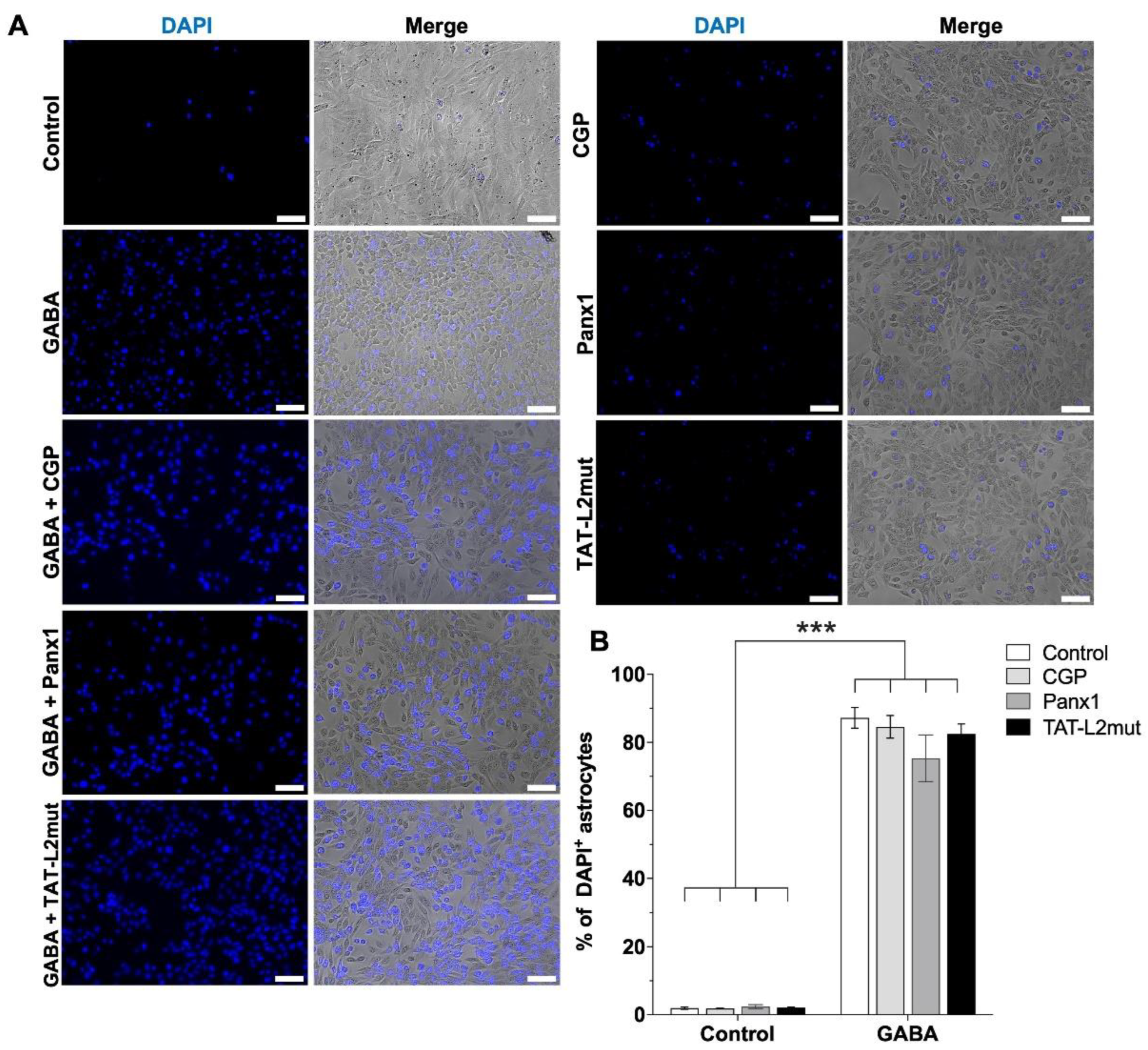
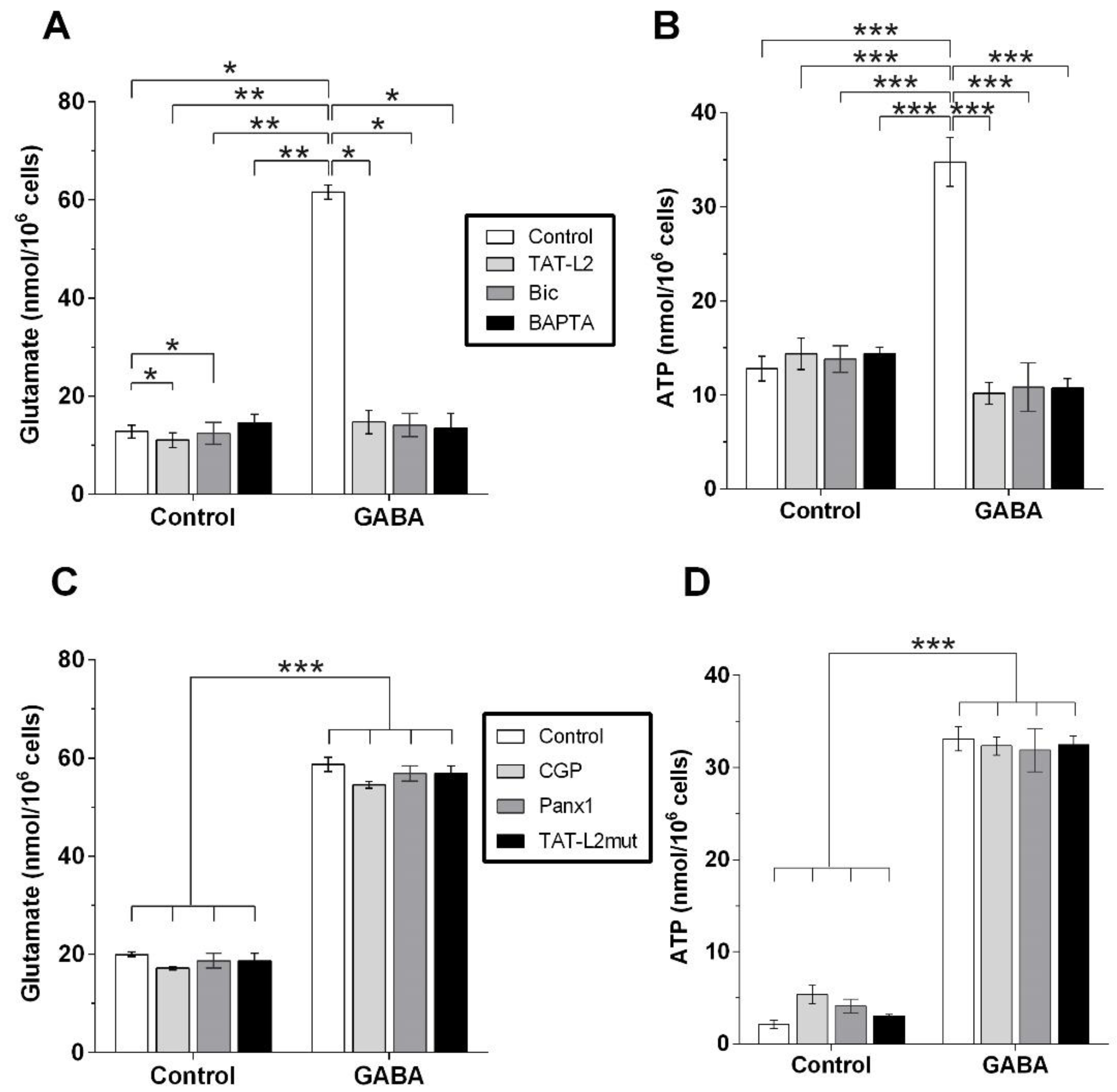
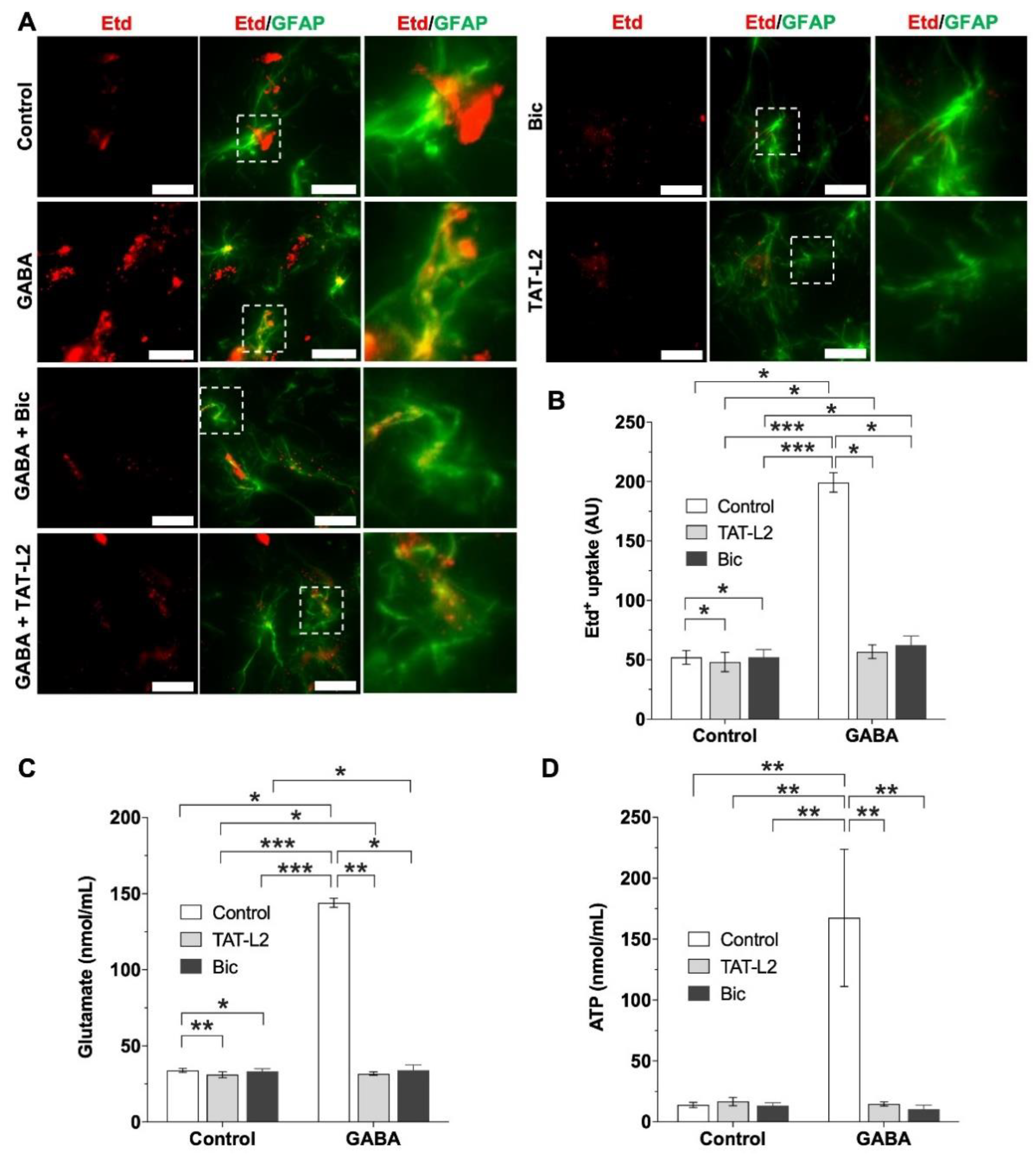
2. Results
3. Discussion
4. Materials and Methods
4.1. DI TNC1 Astroglial Cell Culture
4.2. DAPI Uptake
4.3. Drugs Evaluated
4.4. Measurement of Extracellular Glutamate and ATP
4.5. Animals
4.6. Hippocampal Slice Preparation
4.7. Dye Uptake Measurements in Hippocampal Slices
4.8. Measurements of Extracellular Glutamate and ATP
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roux, L.; Buzsáki, G. Tasks for Inhibitory Interneurons in Intact Brain Circuits. Neuropharmacology 2015, 88, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiong, B.-R.; Zhang, L.-Q.; Huang, X.; Yuan, X.; Tian, Y.-K.; Tian, X.-B. The Role of the GABAergic System in Diseases of the Central Nervous System. Neuroscience 2021, 470, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.W. Cortical and Subcortical Gamma Amino Acid Butyric Acid Deficits in Anxiety and Stress Disorders: Clinical Implications. World J. Psychiatry 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Della Vecchia, A.; Arone, A.; Piccinni, A.; Mucci, F.; Marazziti, D. GABA System in Depression: Impact on Pathophysiology and Psychopharmacology. Curr. Med. Chem. 2022, 29, 5710–5730. [Google Scholar] [CrossRef]
- Scimemi, A. Structure, Function, and Plasticity of GABA Transporters. Front. Cell. Neurosci. 2014, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-González, C.; Pirttimaki, T.; Cope, D.W.; Parri, H.R. Non-Neuronal, Slow GABA Signalling in the Ventrobasal Thalamus Targets δ-Subunit-Containing GABAA Receptors: Thalamic Glial GABAergic Signalling. Eur. J. Neurosci. 2011, 33, 1471–1482. [Google Scholar] [CrossRef]
- Kozlov, A.S.; Angulo, M.C.; Audinat, E.; Charpak, S. Target Cell-Specific Modulation of Neuronal Activity by Astrocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 10058–10063. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, B.-E.; Berglund, K.; Oh, S.-J.; Park, H.; Shin, H.-S.; Augustine, G.J.; Lee, C.J. Channel-Mediated Tonic GABA Release from Glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Schaffner, A.E.; Chang, Y.H.; Maric, D.; Barker, J.L. Persistent Activation of GABA A Receptor/Cl − Channels by Astrocyte-Derived GABA in Cultured Embryonic Rat Hippocampal Neurons. J. Neurophysiol. 2000, 84, 1392–1403. [Google Scholar] [CrossRef]
- Bormann, J.; Kettenmann, H. Patch-Clamp Study of Gamma-Aminobutyric Acid Receptor Cl- Channels in Cultured Astrocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 9336–9340. [Google Scholar] [CrossRef]
- MacVicar, B.; Tse, F.; Crichton, S.; Kettenmann, H. GABA-Activated Cl- Channels in Astrocytes of Hippocampal Slices. J. Neurosci. 1989, 9, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Fritschy, J.; Grosche, J.; Pratt, G.; Mohler, H.; Kettenmann, H. Developmental Regulation of Voltage-Gated K+ Channel and GABAA Receptor Expression in Bergmann Glial Cells. J. Neurosci. 1994, 14, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, A.; Broman, J. Light and Electron Microscopic Immunohistochemical Demonstration of GABA-Immunoreactive Astrocytes in the Brain Stem of the Rat. J. Neurocytol. 1988, 17, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, R.; Tonda, A.; Gragera, R.R.; Paz-Doel, R.; García-Cordovilla, R.; Fernández-Fernández, E.; Fernández, A.M.; González-Romero, F.; López-Bravo, A. Synaptic and Non-Synaptic Immunolocalization of GABA and Glutamate Acid Decarboxylase (GAD) in Cerebellar Cortex of Rat. Cell. Mol. Biol. 1993, 39, 115–123. [Google Scholar] [PubMed]
- Mariotti, L.; Losi, G.; Lia, A.; Melone, M.; Chiavegato, A.; Gómez-Gonzalo, M.; Sessolo, M.; Bovetti, S.; Forli, A.; Zonta, M.; et al. Interneuron-Specific Signaling Evokes Distinctive Somatostatin-Mediated Responses in Adult Cortical Astrocytes. Nat. Commun. 2018, 9, 82. [Google Scholar] [CrossRef]
- Mederos, S.; Perea, G. GABAergic-astrocyte Signaling: A Refinement of Inhibitory Brain Networks. Glia 2019, 67, 1842–1851. [Google Scholar] [CrossRef]
- Nagai, J.; Rajbhandari, A.K.; Gangwani, M.R.; Hachisuka, A.; Coppola, G.; Masmanidis, S.C.; Fanselow, M.S.; Khakh, B.S. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 2019, 177, 1280–1292. [Google Scholar] [CrossRef]
- Nilsson, M.; Eriksson, P.S.; Rönnbäck, L.; Hansson, E. GABA Induces Ca2+ Transients in Astrocytes. Neuroscience 1993, 54, 605–614. [Google Scholar] [CrossRef]
- Perea, G.; Gómez, R.; Mederos, S.; Covelo, A.; Ballesteros, J.J.; Schlosser, L.; Hernández-Vivanco, A.; Martín-Fernández, M.; Quintana, R.; Rayan, A.; et al. Activity-Dependent Switch of GABAergic Inhibition into Glutamatergic Excitation in Astrocyte-Neuron Networks. eLife 2016, 5, e20362. [Google Scholar] [CrossRef]
- Fraser, D.; Duffy, S.; Angelides, K.; Perez-Velazquez, J.; Kettenmann, H.; MacVicar, B. GABAA/Benzodiazepine Receptors in Acutely Isolated Hippocampal Astrocytes. J. Neurosci. 1995, 15, 2720–2732. [Google Scholar] [CrossRef]
- Kettenmann, H.; Schachner, M. Pharmacological Properties of Gamma-Aminobutyric Acid-, Glutamate-, and Aspartate-Induced Depolarizations in Cultured Astrocytes. J. Neurosci. 1985, 5, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Park, Y.K.; Chater, T.E.; Chipman, P.H.; Gautam, S.G.; Oshima-Takago, T.; Goda, Y. Astrocytes Regulate Heterogeneity of Presynaptic Strengths in Hippocampal Networks. Proc. Natl. Acad. Sci. USA 2016, 113, E2685–E2694. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.D.; Kafitz, K.W.; Rose, C.R. Developmental Profile and Mechanisms of GABA-Induced Calcium Signaling in Hippocampal Astrocytes. Glia 2008, 56, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Losi, G.; Sessolo, M.; Marcon, I.; Carmignoto, G. The Inhibitory Neurotransmitter GABA Evokes Long-lasting Ca2+ Oscillations in Cortical Astrocytes. Glia 2016, 64, 363–373. [Google Scholar] [CrossRef]
- Andersson, M.; Blomstrand, F.; Hanse, E. Astrocytes Play a Critical Role in Transient Heterosynaptic Depression in the Rat Hippocampal CA1 Region: Transient Heterosynaptic Depression. J. Physiol. 2007, 585, 843–852. [Google Scholar] [CrossRef]
- Kang, M.H.; Spigelman, I.; Olsen, R.W. Alteration in the Sensitivity of GABA(A) Receptors to Allosteric Modulatory Drugs in Rat Hippocampus after Chronic Intermittent Ethanol Treatment. Alcohol. Clin. Exp. Res. 1998, 22, 2165–2173. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.-L.; Qu, J.; Wang, W.; Zhang, T.; Yanagawa, Y.; Wu, S.-X.; Li, Y.-Q. Neurokinin-1 Receptor-Expressing Neurons That Contain Serotonin and Gamma-Aminobutyric Acid in the Rat Rostroventromedial Medulla Are Involved in Pain Processing. J. Pain 2013, 14, 778–792. [Google Scholar] [CrossRef]
- Serrano, A. GABAergic Network Activation of Glial Cells Underlies Hippocampal Heterosynaptic Depression. J. Neurosci. 2006, 26, 5370–5382. [Google Scholar] [CrossRef]
- Moraga-Amaro, R.; Jerez-Baraona, J.M.; Simon, F.; Stehberg, J. Role of Astrocytes in Memory and Psychiatric Disorders. J. Physiol.-Paris 2014, 108, 240–251. [Google Scholar] [CrossRef]
- Parpura, V.; Grubišić, V.; Verkhratsky, A. Ca2+ Sources for the Exocytotic Release of Glutamate from Astrocytes. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 984–991. [Google Scholar] [CrossRef]
- Stout, C.; Charles, A. Modulation of Intercellular Calcium Signaling in Astrocytes by Extracellular Calcium and Magnesium. Glia 2003, 43, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Palacios-Prado, N.; Orellana, J.A.; Sáez, J.C. Currently Used Methods for Identification and Characterization of Hemichannels. Cell Commun. Adhes. 2008, 15, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Charles, A. Extracellular Calcium Sensing by Glial Cells: Low Extracellular Calcium Induces Intracellular Calcium Release and Intercellular Signaling. J. Neurochem. 2002, 69, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Ponsaerts, R.; De Vuyst, E.; Retamal, M.; D’Hondt, C.; Vermeire, D.; Wang, N.; De Smedt, H.; Zimmermann, P.; Himpens, B.; Vereecke, J.; et al. Intramolecular Loop/Tail Interactions Are Essential for Connexin 43-hemichannel Activity. FASEB J. 2010, 24, 4378–4395. [Google Scholar] [CrossRef]
- Stehberg, J.; Moraga-Amaro, R.; Salazar, C.; Becerra, A.; Echeverría, C.; Orellana, J.A.; Bultynck, G.; Ponsaerts, R.; Leybaert, L.; Simon, F.; et al. Release of Gliotransmitters through Astroglial Connexin 43 Hemichannels Is Necessary for Fear Memory Consolidation in the Basolateral Amygdala. FASEB J. 2012, 26, 3649–3657. [Google Scholar] [CrossRef]
- Linsambarth, S.; Carvajal, F.J.; Moraga-Amaro, R.; Mendez, L.; Tamburini, G.; Jimenez, I.; Verdugo, D.A.; Gómez, G.I.; Jury, N.; Martínez, P.; et al. Astroglial Gliotransmitters Released via Cx43 Hemichannels Regulate NMDAR-dependent Transmission and Short-term Fear Memory in the Basolateral Amygdala. FASEB J. 2022, 36, e22134. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodríguez, J.J.; Parpura, V. Calcium Signalling in Astroglia. Mol. Cell. Endocrinol. 2012, 353, 45–56. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Reyes, R.C.; Parpura, V. TRP Channels Coordinate Ion Signalling in Astroglia. In Reviews of Physiology, Biochemistry and Pharmacology 166; Nilius, B., Gudermann, T., Jahn, R., Lill, R., Offermanns, S., Petersen, O.H., Eds.; Reviews of Physiology, Biochemistry and Pharmacology; Springer International Publishing: Cham, Switzerland, 2013; Volume 166, pp. 1–22. ISBN 978-3-319-04905-2. [Google Scholar]
- Sharp, A.H.; Nucifora, F.C.; Blondel, O.; Sheppard, C.A.; Zhang, C.; Snyder, S.H.; Russell, J.T.; Ryugo, D.K.; Ross, C.A. Differential Cellular Expression of Isoforms of Inositol 1,4,5-Triphosphate Receptors in Neurons and Glia in Brain. J. Comp. Neurol. 1999, 406, 207–220. [Google Scholar] [CrossRef]
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. G i/o Protein-coupled Receptors Inhibit Neurons but Activate Astrocytes and Stimulate Gliotransmission. Glia 2019, 67, 1076–1093. [Google Scholar] [CrossRef]
- Boddum, K.; Jensen, T.P.; Magloire, V.; Kristiansen, U.; Rusakov, D.A.; Pavlov, I.; Walker, M.C. Astrocytic GABA Transporter Activity Modulates Excitatory Neurotransmission. Nat. Commun. 2016, 7, 13572. [Google Scholar] [CrossRef]
- Doengi, M.; Hirnet, D.; Coulon, P.; Pape, H.-C.; Deitmer, J.W.; Lohr, C. GABA Uptake-Dependent Ca2+ Signaling in Developing Olfactory Bulb Astrocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 17570–17575. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Bosson, A.; Riebe, I.; Reynell, C.; Vallée, J.; Laplante, I.; Panatier, A.; Robitaille, R.; Lacaille, J.-C. Astrocytes Detect and Upregulate Transmission at Inhibitory Synapses of Somatostatin Interneurons onto Pyramidal Cells. Nat. Commun. 2018, 9, 4254. [Google Scholar] [CrossRef] [PubMed]
- Longuemare, M.C.; Swanson, R.A. Net Glutamate Release from Astrocytes Is Not Induced by Extracellular Potassium Concentrations Attainable in Brain. J. Neurochem. 2002, 69, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Oshima, T.; Attwell, D. Glutamate Release in Severe Brain Ischaemia Is Mainly by Reversed Uptake. Nature 2000, 403, 316–321. [Google Scholar] [CrossRef]
- Abdullaev, I.F.; Rudkouskaya, A.; Schools, G.P.; Kimelberg, H.K.; Mongin, A.A. Pharmacological Comparison of Swelling-Activated Excitatory Amino Acid Release and Cl− Currents in Cultured Rat Astrocytes: VRACs and Excitatory Amino Acid Release in Astrocytes. J. Physiol. 2006, 572, 677–689. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; Anderson, E.; Kettenmann, H. Swelling-Induced Changes in Electrophysiological Properties of Cultured Astrocytes and Oligodendrocytes. II. Whole-Cell Currents. Brain Res. 1990, 529, 262–268. [Google Scholar] [CrossRef]
- Mongin, A.A.; Kimelberg, H.K. ATP Regulates Anion Channel-Mediated Organic Osmolyte Release from Cultured Rat Astrocytes via Multiple Ca2+-Sensitive Mechanisms. Am. J. Physiol.-Cell Physiol. 2005, 288, C204–C213. [Google Scholar] [CrossRef]
- Ramos-Mandujano, G.; Vázquez-Juárez, E.; Hernández-Benítez, R.; Pasantes-Morales, H. Thrombin Potently Enhances Swelling-Sensitive Glutamate Efflux from Cultured Astrocytes. Glia 2007, 55, 917–925. [Google Scholar] [CrossRef]
- Cavelier, P.; Attwell, D. Tonic Release of Glutamate by a DIDS-Sensitive Mechanism in Rat Hippocampal Slices: Tonic Glutamate Release. J. Physiol. 2005, 564, 397–410. [Google Scholar] [CrossRef]
- Park, H.; Han, K.-S.; Oh, S.-J.; Jo, S.; Woo, J.; Yoon, B.-E.; Lee, C.J. High Glutamate Permeability and Distal Localization of Best1 Channel in CA1 Hippocampal Astrocyte. Mol. Brain 2013, 6, 54. [Google Scholar] [CrossRef]
- Woo, D.H.; Han, K.-S.; Shim, J.W.; Yoon, B.-E.; Kim, E.; Bae, J.Y.; Oh, S.-J.; Hwang, E.M.; Marmorstein, A.D.; Bae, Y.C.; et al. TREK-1 and Best1 Channels Mediate Fast and Slow Glutamate Release in Astrocytes upon GPCR Activation. Cell 2012, 151, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Anderson, C.M.; Keung, E.C.; Chen, Y.; Chen, Y.; Swanson, R.A. P2X 7 Receptor-Mediated Release of Excitatory Amino Acids from Astrocytes. J. Neurosci. 2003, 23, 1320–1328. [Google Scholar] [CrossRef]
- Bezzi, P.; Gundersen, V.; Galbete, J.L.; Seifert, G.; Steinhäuser, C.; Pilati, E.; Volterra, A. Astrocytes Contain a Vesicular Compartment That Is Competent for Regulated Exocytosis of Glutamate. Nat. Neurosci. 2004, 7, 613–620. [Google Scholar] [CrossRef]
- Bohmbach, K.; Schwarz, M.K.; Schoch, S.; Henneberger, C. The Structural and Functional Evidence for Vesicular Release from Astrocytes in Situ. Brain Res. Bull. 2018, 136, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Bowser, D.N.; Khakh, B.S. Two Forms of Single-Vesicle Astrocyte Exocytosis Imaged with Total Internal Reflection Fluorescence Microscopy. Proc. Natl. Acad. Sci. USA 2007, 104, 4212–4217. [Google Scholar] [CrossRef] [PubMed]
- Jeftinija, S.D.; Jeftinija, K.V.; Stefanovic, G. Cultured Astrocytes Express Proteins Involved in Vesicular Glutamate Release. Brain Res. 1997, 750, 41–47. [Google Scholar] [CrossRef]
- Montana, V.; Malarkey, E.B.; Verderio, C.; Matteoli, M.; Parpura, V. Vesicular Transmitter Release from Astrocytes. Glia 2006, 54, 700–715. [Google Scholar] [CrossRef]
- Parpura, V.; Zorec, R. Gliotransmission: Exocytotic Release from Astrocytes. Brain Res. Rev. 2010, 63, 83–92. [Google Scholar] [CrossRef]
- Xu, J.; Peng, H.; Kang, N.; Zhao, Z.; Lin, J.H.-C.; Stanton, P.K.; Kang, J. Glutamate-Induced Exocytosis of Glutamate from Astrocytes. J. Biol. Chem. 2007, 282, 24185–24197. [Google Scholar] [CrossRef]
- Bennett, M.V.L.; Garré, J.M.; Orellana, J.A.; Bukauskas, F.F.; Nedergaard, M.; Giaume, C.; Sáez, J.C. Connexin and Pannexin Hemichannels in Inflammatory Responses of Glia and Neurons. Brain Res. 2012, 1487, 3–15. [Google Scholar] [CrossRef]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.G.; Charles, A.C. Intercellular Calcium Signaling in Astrocytes via ATP Release through Connexin Hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lippi, G.; Carlson, D.M.; Berg, D.K. Activation of A7-Containing Nicotinic Receptors on Astrocytes Triggers AMPA Receptor Recruitment to Glutamatergic Synapses. J. Neurochem. 2013, 127, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Beppu, K.; Kubo, N.; Matsui, K. Glial Amplification of Synaptic Signals. J. Physiol. 2021, 599, 2085–2102. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martin, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-Specific Astrocyte Gating of Amygdala-Related Behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Caudal, L.C.; Gobbo, D.; Scheller, A.; Kirchhoff, F. The Paradox of Astroglial Ca2 + Signals at the Interface of Excitation and Inhibition. Front. Cell. Neurosci. 2020, 14, 609947. [Google Scholar] [CrossRef]
- Choudary, P.V.; Molnar, M.; Evans, S.J.; Tomita, H.; Li, J.Z.; Vawter, M.P.; Myers, R.M.; Bunney, W.E.; Akil, H.; Watson, S.J.; et al. Altered Cortical Glutamatergic and GABAergic Signal Transmission with Glial Involvement in Depression. Proc. Natl. Acad. Sci. USA 2005, 102, 15653–15658. [Google Scholar] [CrossRef]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic Deficit Hypothesis of Major Depressive Disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef]
- Sanacora, G.; Mason, G.F.; Rothman, D.L.; Hyder, F.; Ciarcia, J.J.; Ostroff, R.B.; Berman, R.M.; Krystal, J.H. Increased Cortical GABA Concentrations in Depressed Patients Receiving ECT. Am. J. Psychiatry 2003, 160, 577–579. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Wilson, B.A.; Hussain, S.; Meshram, A.; Rajkowska, G.; Stockmeier, C.A. Reduced Connexin 43 Immunolabeling in the Orbitofrontal Cortex in Alcohol Dependence and Depression. J. Psychiatr. Res. 2014, 55, 101–109. [Google Scholar] [CrossRef]
- Orellana, J.A.; Moraga-Amaro, R.; Dà az-Galarce, R.; Rojas, S.; Maturana, C.J.; Stehberg, J.; Sáez, J.C. Restraint Stress Increases Hemichannel Activity in Hippocampal Glial Cells and Neurons. Front. Cell. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Dinamarca, I.; Reyes-Lizana, R.; Lemunao-Inostroza, Y.; Cárdenas, K.; Castro-Lazo, R.; Peña, F.; Lucero, C.M.; Prieto-Villalobos, J.; Retamal, M.A.; Orellana, J.A.; et al. GABAergic Regulation of Astroglial Gliotransmission through Cx43 Hemichannels. Int. J. Mol. Sci. 2022, 23, 13625. https://doi.org/10.3390/ijms232113625
Jiménez-Dinamarca I, Reyes-Lizana R, Lemunao-Inostroza Y, Cárdenas K, Castro-Lazo R, Peña F, Lucero CM, Prieto-Villalobos J, Retamal MA, Orellana JA, et al. GABAergic Regulation of Astroglial Gliotransmission through Cx43 Hemichannels. International Journal of Molecular Sciences. 2022; 23(21):13625. https://doi.org/10.3390/ijms232113625
Chicago/Turabian StyleJiménez-Dinamarca, Ivanka, Rachel Reyes-Lizana, Yordan Lemunao-Inostroza, Kevin Cárdenas, Raimundo Castro-Lazo, Francisca Peña, Claudia M. Lucero, Juan Prieto-Villalobos, Mauricio Antonio Retamal, Juan Andrés Orellana, and et al. 2022. "GABAergic Regulation of Astroglial Gliotransmission through Cx43 Hemichannels" International Journal of Molecular Sciences 23, no. 21: 13625. https://doi.org/10.3390/ijms232113625
APA StyleJiménez-Dinamarca, I., Reyes-Lizana, R., Lemunao-Inostroza, Y., Cárdenas, K., Castro-Lazo, R., Peña, F., Lucero, C. M., Prieto-Villalobos, J., Retamal, M. A., Orellana, J. A., & Stehberg, J. (2022). GABAergic Regulation of Astroglial Gliotransmission through Cx43 Hemichannels. International Journal of Molecular Sciences, 23(21), 13625. https://doi.org/10.3390/ijms232113625







