Neurotrophic and Immunomodulatory Lanostane Triterpenoids from Wood-Inhabiting Basidiomycota
Abstract
:1. Introduction
2. Results
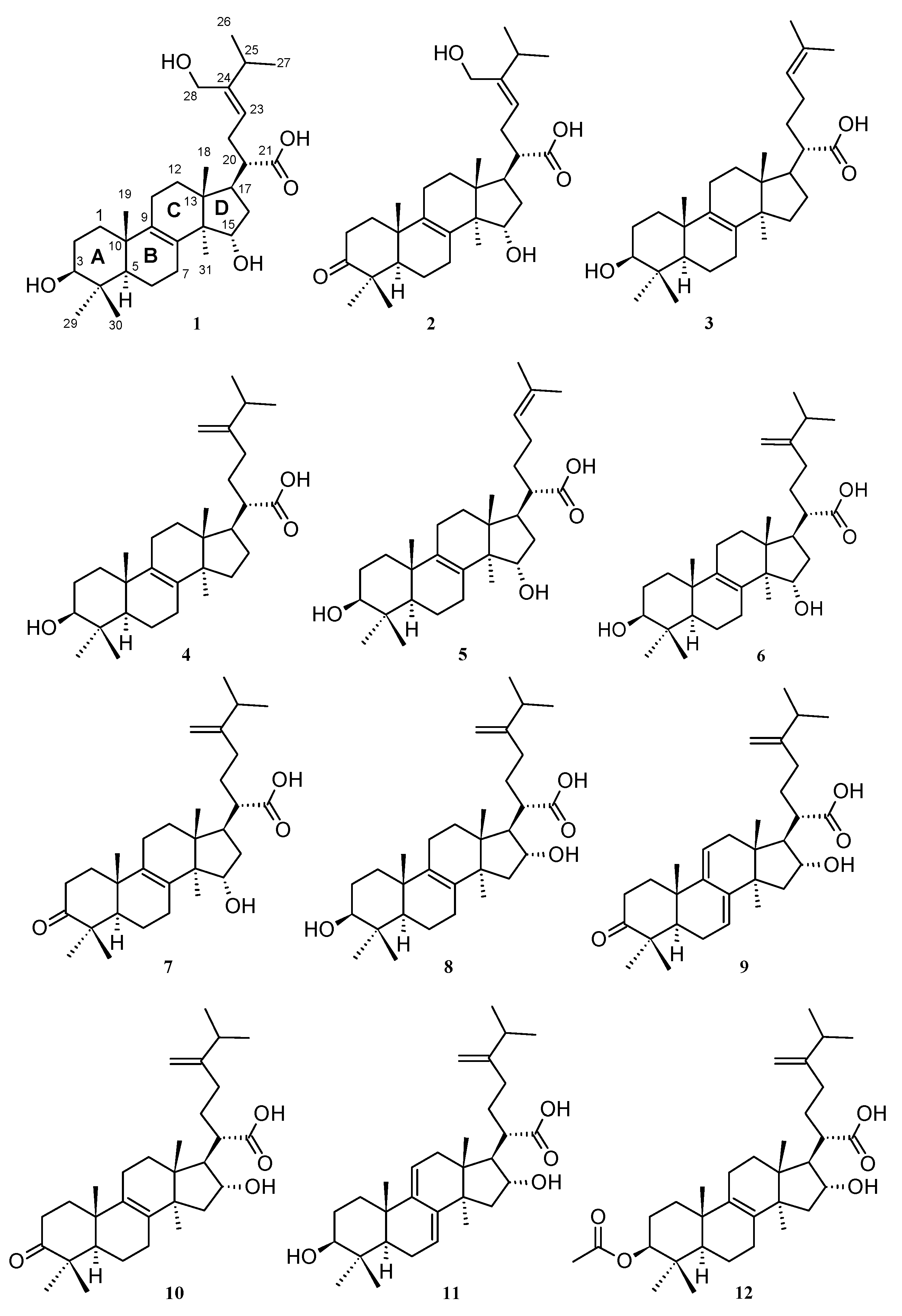
2.1. Isolation of Triterpenoids from L. sulphureus and Antrodia sp. (MUCL 56049)
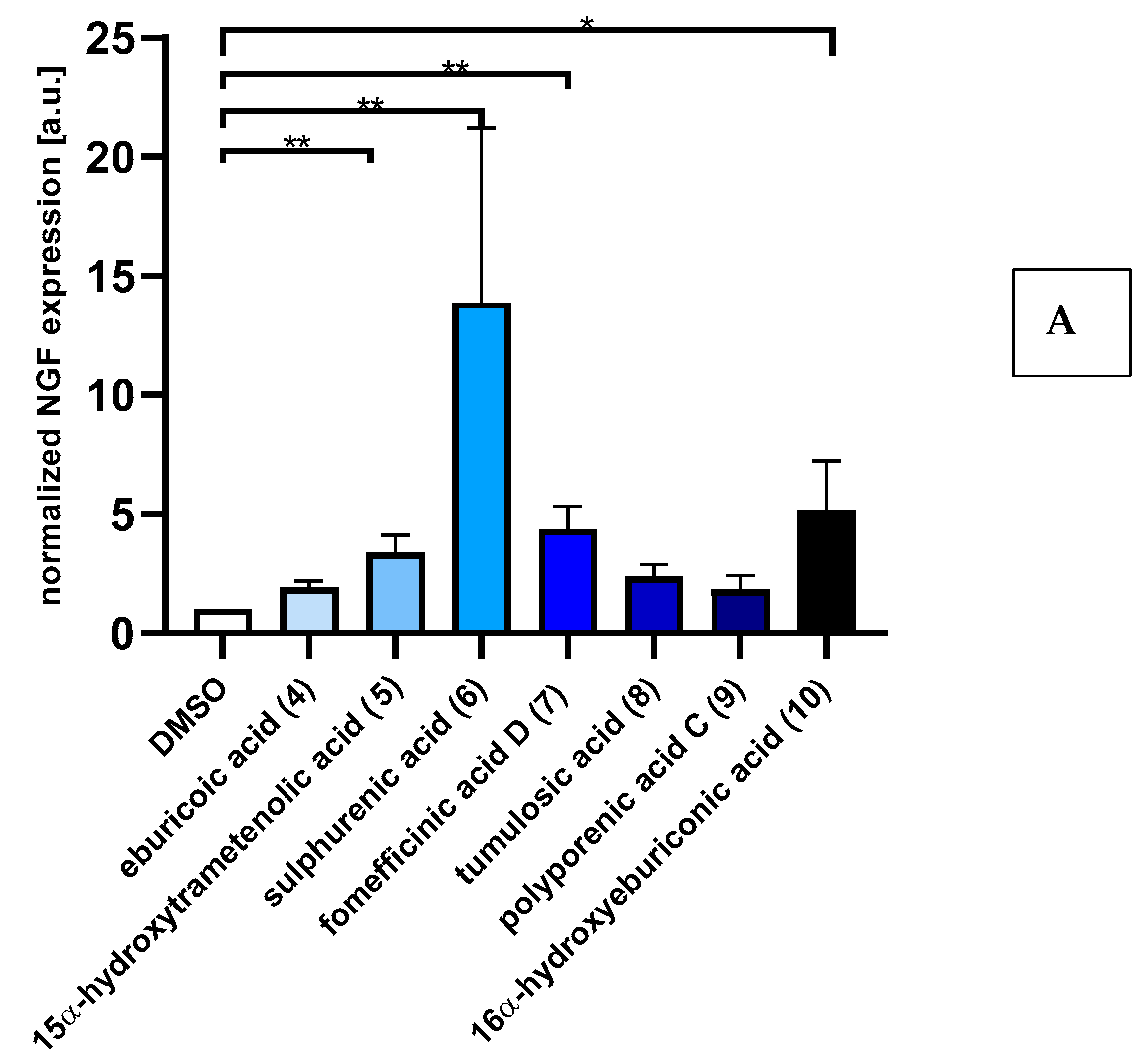
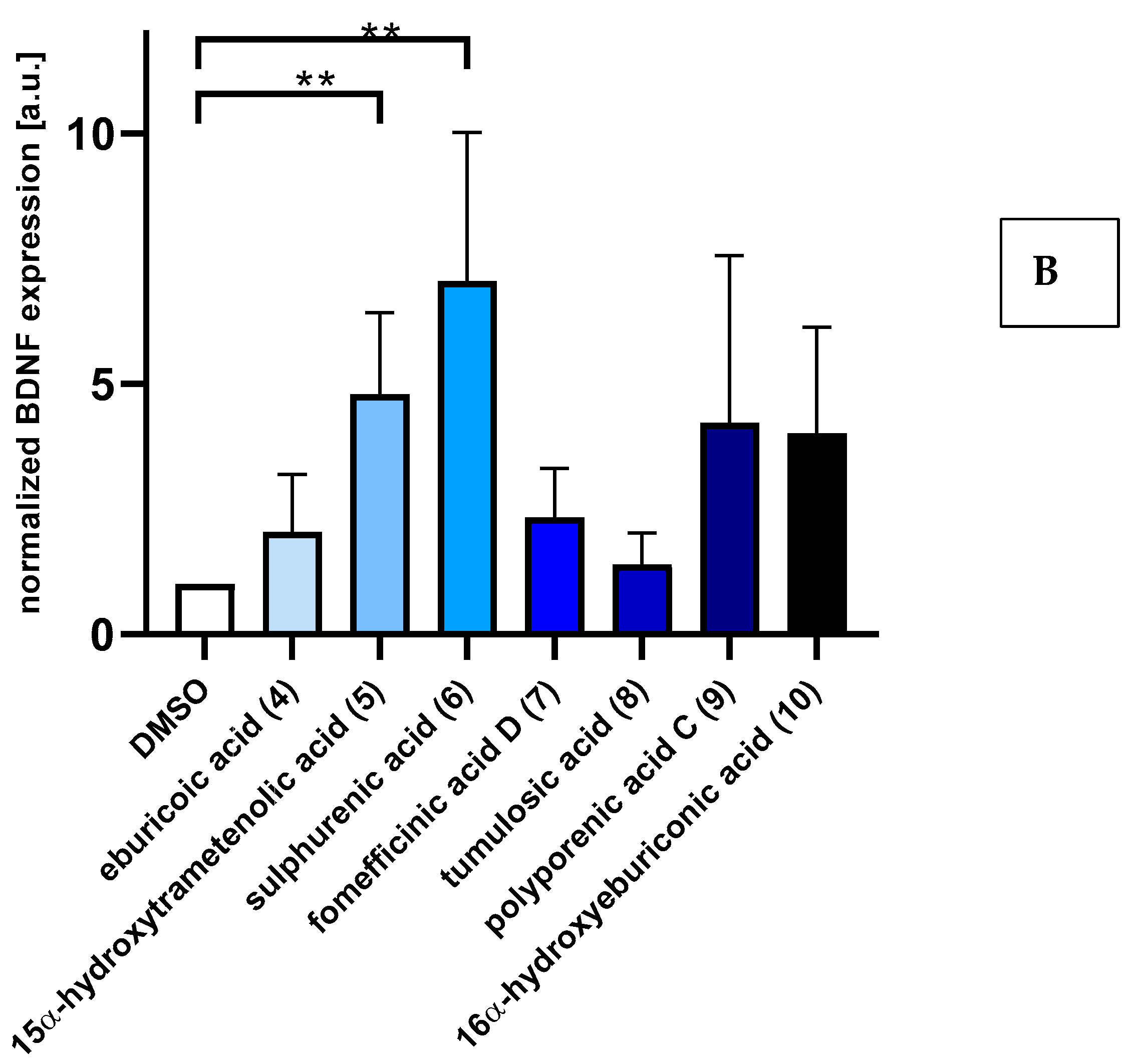
2.2. Triterpenoids Induce Expression of ngf and bdnf in Astrocytoma Cells
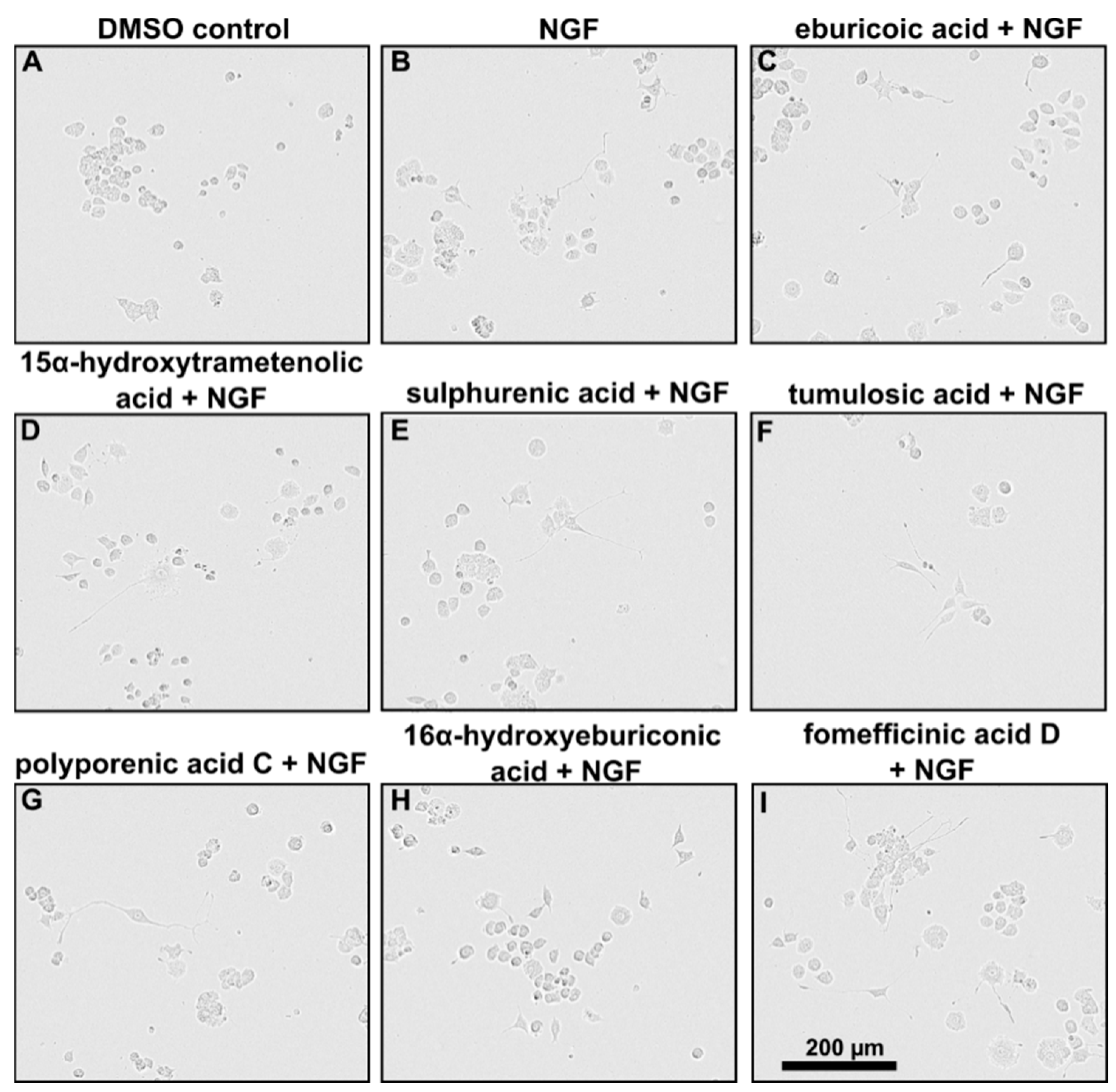
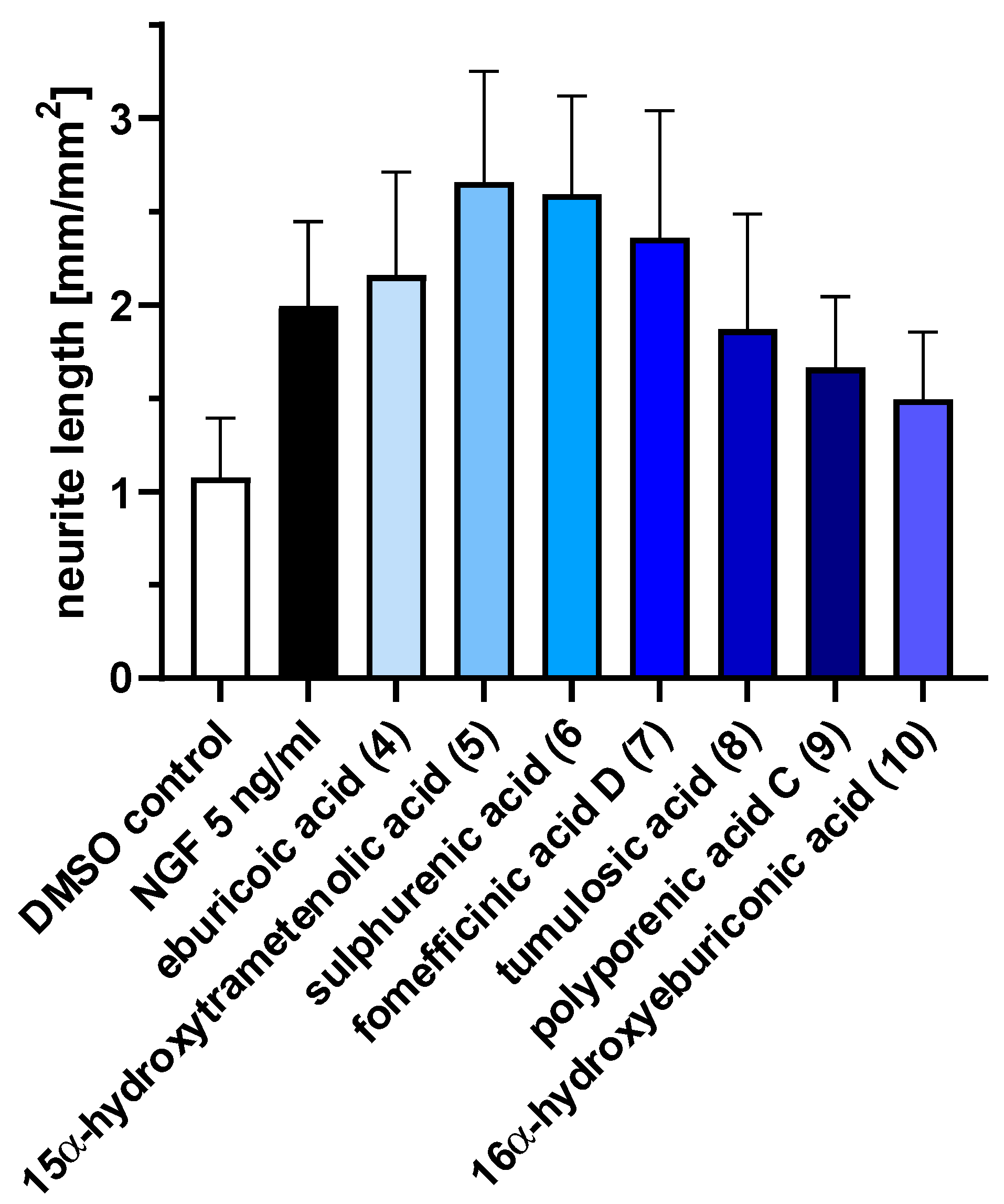
2.3. Triterpenoids Enhance ngf-Induced Neurite Outgrowth in PC-12 Cells
3. Discussion
3.1. Previous Reports of Neurotrophic Activities of Extracts Derived from Other Basidiomycota and Selected Reports on Neurotropic Effects of Plant Metabolites
3.2. Known Biological Activities of Compounds 1–12 and Related Triterpenes
3.3. Previous Use of Laetiporus and Antrodia Species and Their Relatives in Traditional Medicine and for Other Applications
4. Materials and Methods
4.1. General Information
4.2. Fungal Material
4.3. Extraction and Isolation of the Secondary Metabolites
4.4. Cell Culture
4.5. cDNA Synthesis and Real-Time Quantitative RT-PCR
4.6. Neurite Outgrowth Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective natural products for Alzheimer’s disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Mapook, A.; Hyde, K.D.; Hassan, K.; Kemkuignou, B.M.; Čmoková, A.; Surup, F.; Kuhnert, E.; Paomephan, P.; Cheng, T.; de Hoog, S.; et al. Ten decadal advances in fungal biology leading towards human well-being. Fungal Diversity 2022, 116, 547–614. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lacoske, M.H.; Theodorakis, E.A. Neurotrophic natural products: Chemistry and biology. Angew. Chem. Int. Ed. 2014, 53, 956–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, T.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol. Pharm. Bull. 2008, 31, 1727–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Ye, Y.; Xiang, L.; Osada, H.; Qi, J. Lindersin B from Lindernia crustacea induces neuritogenesis by activation of tyrosine kinase A/phosphatidylinositol 3 kinase/extracellular signal-regulated kinase signaling pathway. Phytomedicine 2017, 24, 31–38. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Ip, F.C.; Zhang, D.; Chen, L.-X.; Zhang, W.; Li, Y.-L.; Ip, N.Y.; Ye, W.-C. Triterpenoids from Ganoderma lucidum and their cytotoxic activities. J. Nat. Prod. Res. 2011, 27, 37–41. [Google Scholar]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef] [Green Version]
- De Silva, D.D.; Rapior, S.; Sudarman, E.; Stadler, M.; Xu, J.; Aisyah Alias, S.; Hyde, K.D. Bioactive metabolites from macrofungi: Ethnopharmacology, biological activities and chemistry. Fungal Divers. 2013, 62, 1–40. [Google Scholar] [CrossRef]
- Rascher, M.; Wittstein, K.; Winter, B.; Rupcic, Z.; Wolf-Asseburg, A.; Stadler, M.; Köster, R.W. Erinacine C activates transcription from a consensus ETS DNA binding site in astrocytic cells in addition to NGF induction. Biomolecules 2020, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.; Kemkuignou, B.M.; Stadler, M. Two new triterpenes from basidiomata of the medicinal and edible mushroom, Laetiporus sulphureus. Molecules 2021, 26, 7090. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Ikuta, M.; Arihara, S.; Matsumura, E.; Katayama, S. Two new steroidal derivatives from the fruit body of Chlorophyllum molybdites. Chem. Pharm. Bull. 2001, 49, 1030–1032. [Google Scholar] [CrossRef] [Green Version]
- Chepkirui, C.; Matasyoh, J.C.; Decock, C.; Stadler, M. Two cytotoxic triterpenes from cultures of a Kenyan Laetiporus sp. (Basidiomycota). Phytochem. Lett. 2017, 20, 106–110. [Google Scholar] [CrossRef]
- Yang, S.W.; Shen, Y.C.; Chen, C.H. Steroids and triterpenoids of Antrodia cinnamomea—A fungus parasitic on Cinnamomum micranthum. Phytochemistry 1996, 41, 1389–1392. [Google Scholar] [CrossRef]
- Fried, J.; Grabowich, P.; Sabo, E.; Cohen, A. The structure of sulphurenic acid: A new triterpenoid from Polyporus sulphureus. Tetrahedron 1964, 20, 2297–2312. [Google Scholar] [CrossRef]
- Wu, X.; Yang, J.; Zhou, L.; Dong, Y. New lanostane-type triterpenes from Fomes officinalis. Chem. Pharm. Bull. 2004, 52, 1375–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cort, L.A.; Gascoigne, R.M.; Holker, J.S.E.; Ralph, B.J.; Robertson, A.; Simes, J.J.H. The chemistry of fungi. Part XXIII. Tumulosic acid. J. Chem Soc. 1954, 3713–3721. [Google Scholar] [CrossRef]
- Rösecke, J.; König, W.A. Constituents of the fungi Daedalea quercina and Daedaleopsis confragosa var. tricolor. Phytochemistry 2000, 54, 757–762. [Google Scholar] [CrossRef]
- Tai, T.; Shingu, T.; Kikuchi, T.; Tezuka, Y.; Akahori, A. Triterpenes from the surface layer of Poria cocos. Phytochemistry 1995, 39, 1165–1169. [Google Scholar] [CrossRef]
- Shingu, T.; Tai, T.; Akahori, A. A lanostane triterpenoid from Poria cocos. Phytochemistry 1992, 31, 2548–2549. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom Hericium erinaceus and the rare species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, C.W.; Lee, G.S.; Hong, S.L.; Wong, Y.T.; Brkljača, R.; Urban, S.; Abd Malek, S.N.; Sabaratnam, V. Hericium erinaceus (Bull.: Fr) Pers. cultivated under tropical conditions: Isolation of hericenones and demonstration of NGF-mediated neurite outgrowth in PC12 cells via MEK/ERK and PI3K-Akt signaling pathways. Food Funct. 2014, 5, 3160–3169. [Google Scholar] [CrossRef]
- Li, Y.; Ishibashi, M.; Chen, X.; Ohizumi, Y. Littorachalcone, a new enhancer of NGF-mediated neurite outgrowth, from Verbena littoralis. Chem. Pharm. Bull. 2003, 51, 872–874. [Google Scholar] [CrossRef]
- Eik, L.-F.; Naidu, M.; David, P.; Wong, K.-H.; Tan, Y.-S.; Sabaratnam, V. Lignosus rhinocerus (Cooke) Ryvarden: A medicinal mushroom that stimulates neurite outgrowth in PC-12 cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 320308. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Muroi, M.; Cao, S.; Bian, L.; Osada, H.; Xiang, L.; Qi, J. 3β,23,28-Trihydroxy-12-oleanene 3β-Caffeate from Desmodium sambuense-Induced neurogenesis in PC12 cells mediated by ER stress and BDNF-TrkB signaling pathways. Mol. Pharm. 2019, 16, 1423–1432. [Google Scholar] [CrossRef]
- Phan, C.-W.; Wong, W.-L.; David, P.; Naidu, M.; Sabaratnam, V. Pleurotus giganteus (Berk.) Karunarathna & K.D. Hyde: Nutritional value and in vitro neurite outgrowth activity in rat pheochromocytoma cells. BMC Complement. Altern. Med. 2012, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.-H.; Yue, R.-C.; Fang, X.; Zhang, J.-P.; Wang, G.-W.; Shan, L.; Zhang, W.-D.; Shen, Y.-H. Terpenoids with neurite outgrowth-promoting activity from the branches and leaves of Illicium merrillianum. J. Asian Nat. Prod. Res. 2016, 18, 495–503. [Google Scholar] [CrossRef]
- LI, Y.; OHIZUMI, Y. Search for constituents with neurotrophic factor-potentiating activity from the medicinal plants of Paraguay and Thailand. YAKUGAKU ZASSHI 2004, 124, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Lee, H.J.; Lee, H.E.; Park, S.J.; Gwon, Y.; Kim, H.; Zhang, J.; Shin, C.Y.; Kim, D.H.; Ryu, J.H. Oleanolic acid ameliorates cognitive dysfunction caused by cholinergic blockade via TrkB-dependent BDNF signaling. Neuropharmacology 2017, 113, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Ho, Y.-P.; Chou, J.-C. Phenologic variation of major triterpenoids in regular and white Antrodia cinnamomea. Bot. Stud. 2016, 57, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girometta, C. Antimicrobial properties of Fomitopsis officinalis in the light of its bioactive metabolites: A review. Mycology 2019, 10, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.H.; Hsiao, L.W.; Kuo, Y.H.; Shih, C.C. Antidiabetic and antihyperlipidemic effects of sulphurenic acid, a triterpenoid compound from Antrodia camphorata, in streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2019, 20, 4897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.-C.; Chen, C.-F.; Wang, Y.-H.; Chang, T.-T.; Chou, C.-J. Evaluation of the immuno-modulating activity of some active principles isolated from the fruiting bodies of Antrodia camphorata. Chin. Pharm. J. 2003, 55, 313–318. [Google Scholar] [CrossRef]
- Yeh, C.T.; Rao, Y.K.; Yao, C.J.; Yeh, C.F.; Li, C.H.; Chuang, S.E.; Luong, J.H.T.; Lai, G.M.; Tzeng, Y.M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009, 285, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yang, J.; Dong, Y. Chemical Constituents of Fomes officinalis (I) | Chinese Traditional and Herbal Drugs | WPRIM. 1994. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-573660?lang=en (accessed on 2 March 2022).
- Muszyńska, B.; Fijałkowska, A.; Sułkowska-Ziaja, K.; Włodarczyk, A.; Kaczmarczyk, P.; Nogaj, E.; Piętka, J. Fomitopsis officinalis: A Species of arboreal mushroom with promising biological and medicinal properties. Chem. Biodivers. 2020, 17, e2000213. [Google Scholar] [CrossRef]
- Gapter, L.; Wang, Z.; Glinski, J.; Ng, K. Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem. Biophys. Res. Commun. 2005, 332, 1153–1161. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lee, N.-H.; Bhattarai, G.; Kim, G.-E.; Lee, I.-K.; Yun, B.-S.; Hwang, P.-H.; Yi, H.-K. Anti-inflammatory effect of pachymic acid promotes odontoblastic differentiation via HO-1 in dental pulp cells. Oral Dis. 2013, 19, 193–199. [Google Scholar] [CrossRef]
- Saba, E.; Son, Y.; Jeon, B.R.; Kim, S.-E.; Lee, I.-K.; Yun, B.-S.; Rhee, M.H. Acetyl Eburicoic Acid from Laetiporus sulphureus var. miniatus suppresses inflammation in murine macrophage RAW 264.7 cells. Mycobiology 2015, 43, 131–136. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Ip, F.C.; Zhang, D.M.; Chen, L.X.; Zhang, W.; Li, Y.L.; Ip, N.Y.; Ye, W.C. Triterpenoids with neurotrophic activity from Ganoderma lucidum. Nat. Prod. Res. 2011, 25, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.-Z.; Ye, K. Deficiency in BDNF/TrkB neurotrophic activity stimulates δ-secretase by upregulating C/EBPβ in Alzheimer’s disease. Cell Rep. 2019, 28, 655–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-L.; Han, F.; Luan, S.-S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.-X. Triterpenoids from Ganoderma lucidum and their potential anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 5147–5158. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Wittstein, K.; Kirk, P.M.; Stadler, M. An assessment of the taxonomy and chemotaxonomy of Ganoderma. Fungal Divers. 2015, 71, 1–15. [Google Scholar] [CrossRef]
- Dai, Y.-C.; Zhou, L.-W.; Hattori, T.; Cao, Y.; Stalpers, J.A.; Ryvarden, L.; Buchanan, P.; Oberwinkler, F.; Hallenberg, N.; Liu, P.-G.; et al. Ganoderma lingzhi (Polyporales, Basidiomycota): The scientific binomial for the widely cultivated medicinal fungus Lingzhi. Mycol. Prog. 2017, 16, 1051–1055. [Google Scholar] [CrossRef]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A–C, Nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264–2269. [Google Scholar] [CrossRef]
- Dai, Y.C.; Cui, B.K.; Yuan, H.S.; Li, B.D. Pathogenic wood-decaying fungi in China. For. Pathol. 2007, 37, 105–120. [Google Scholar] [CrossRef]
- Song, J.; Cui, B.K. Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evol. Biol. 2017, 17, 102. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Sun, Y.F.; Ji, X.; Dai, Y.C.; Cui, B.K. Phylogeny and taxonomy of Laetiporus (Basidiomycota, polyporales) with descriptions of two new species from western China. MycoKeys 2018, 37, 57–71. [Google Scholar] [CrossRef]
- Grienke, U.; Zöll, M.; Peintner, U.; Rollinger, J.M. European medicinal polypores—A modern view on traditional uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Fries, E. Observationes Mycologicae; Lund, Sweden, 1818; Volume 2, pp. 1–372. [Google Scholar]
- Joshi, R.A. Antrodia camphorata with potential anti- cancerous activities: A review. J. Med. Plants 2017, 5, 284–291. [Google Scholar]
- Spirin, V.; Runnel, K.; Vlasák, J.; Miettinen, O.; Põldmaa, K. Species diversity in the Antrodia crassa group (Polyporales, Basidiomycota). Fungal Biol. 2015, 119, 1291–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid.-Based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef]
- Wu, Y.; Lo, J.; Shih, Y.; Liang, H.J. Method for Treating Stroke or Reducing Nerve Injury. U.S. Patent US20180353520, 2018. [Google Scholar]
- Wu, Y.B.; J, L.; Y, S.; HJ, L.; Lin, P.; Tsay, H. Compositions and Methods for Treating Dementia. World Patent WO2017185073, 2020. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, K.; Matio Kemkuignou, B.; Kirchenwitz, M.; Wittstein, K.; Rascher-Albaghdadi, M.; Chepkirui, C.; Matasyoh, J.C.; Decock, C.; Köster, R.W.; Stradal, T.E.B.; et al. Neurotrophic and Immunomodulatory Lanostane Triterpenoids from Wood-Inhabiting Basidiomycota. Int. J. Mol. Sci. 2022, 23, 13593. https://doi.org/10.3390/ijms232113593
Hassan K, Matio Kemkuignou B, Kirchenwitz M, Wittstein K, Rascher-Albaghdadi M, Chepkirui C, Matasyoh JC, Decock C, Köster RW, Stradal TEB, et al. Neurotrophic and Immunomodulatory Lanostane Triterpenoids from Wood-Inhabiting Basidiomycota. International Journal of Molecular Sciences. 2022; 23(21):13593. https://doi.org/10.3390/ijms232113593
Chicago/Turabian StyleHassan, Khadija, Blondelle Matio Kemkuignou, Marco Kirchenwitz, Kathrin Wittstein, Monique Rascher-Albaghdadi, Clara Chepkirui, Josphat C. Matasyoh, Cony Decock, Reinhard W. Köster, Theresia E. B. Stradal, and et al. 2022. "Neurotrophic and Immunomodulatory Lanostane Triterpenoids from Wood-Inhabiting Basidiomycota" International Journal of Molecular Sciences 23, no. 21: 13593. https://doi.org/10.3390/ijms232113593
APA StyleHassan, K., Matio Kemkuignou, B., Kirchenwitz, M., Wittstein, K., Rascher-Albaghdadi, M., Chepkirui, C., Matasyoh, J. C., Decock, C., Köster, R. W., Stradal, T. E. B., & Stadler, M. (2022). Neurotrophic and Immunomodulatory Lanostane Triterpenoids from Wood-Inhabiting Basidiomycota. International Journal of Molecular Sciences, 23(21), 13593. https://doi.org/10.3390/ijms232113593








