In Vitro and In Vivo Biological Activity of Ruthenium 1,10-Phenanthroline-5,6-dione Arene Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Complexes Stability
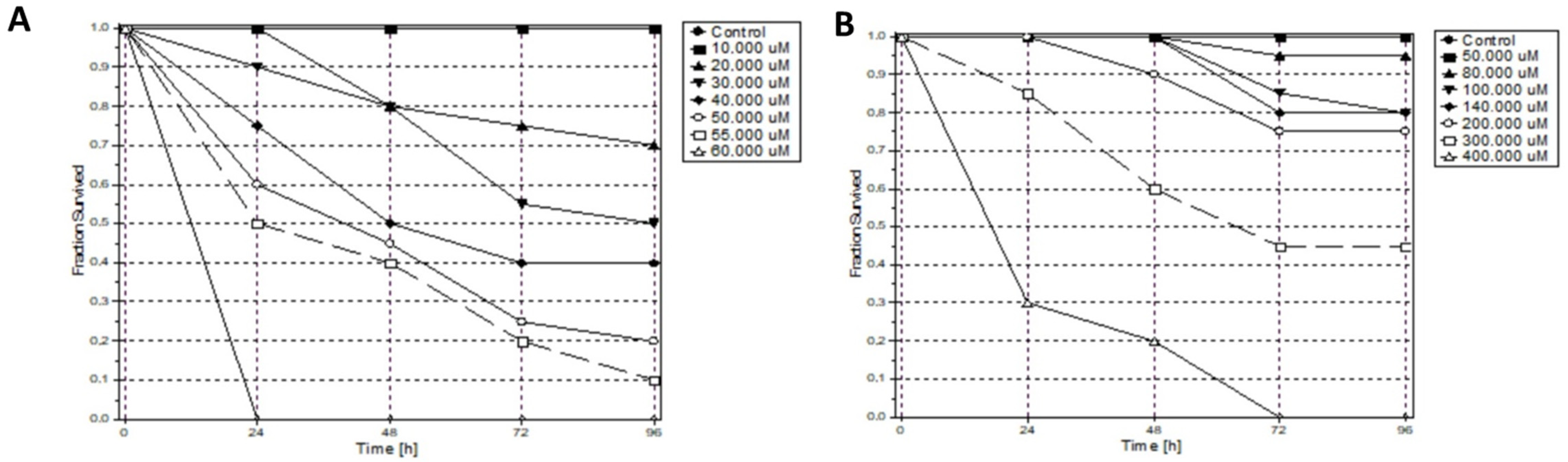
2.3. Antiproliferative Activity
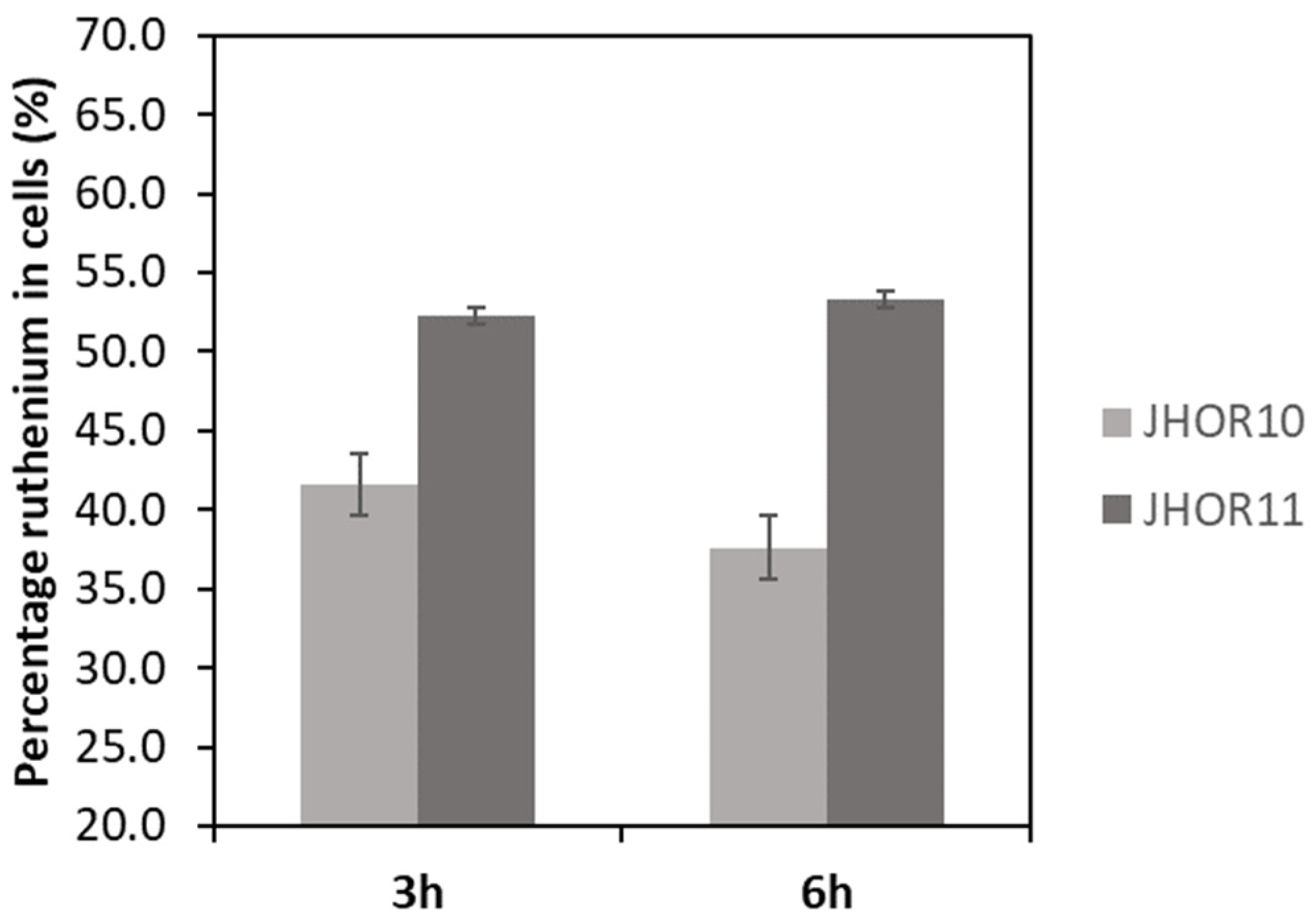
2.4. Complexes Internalization in Cells
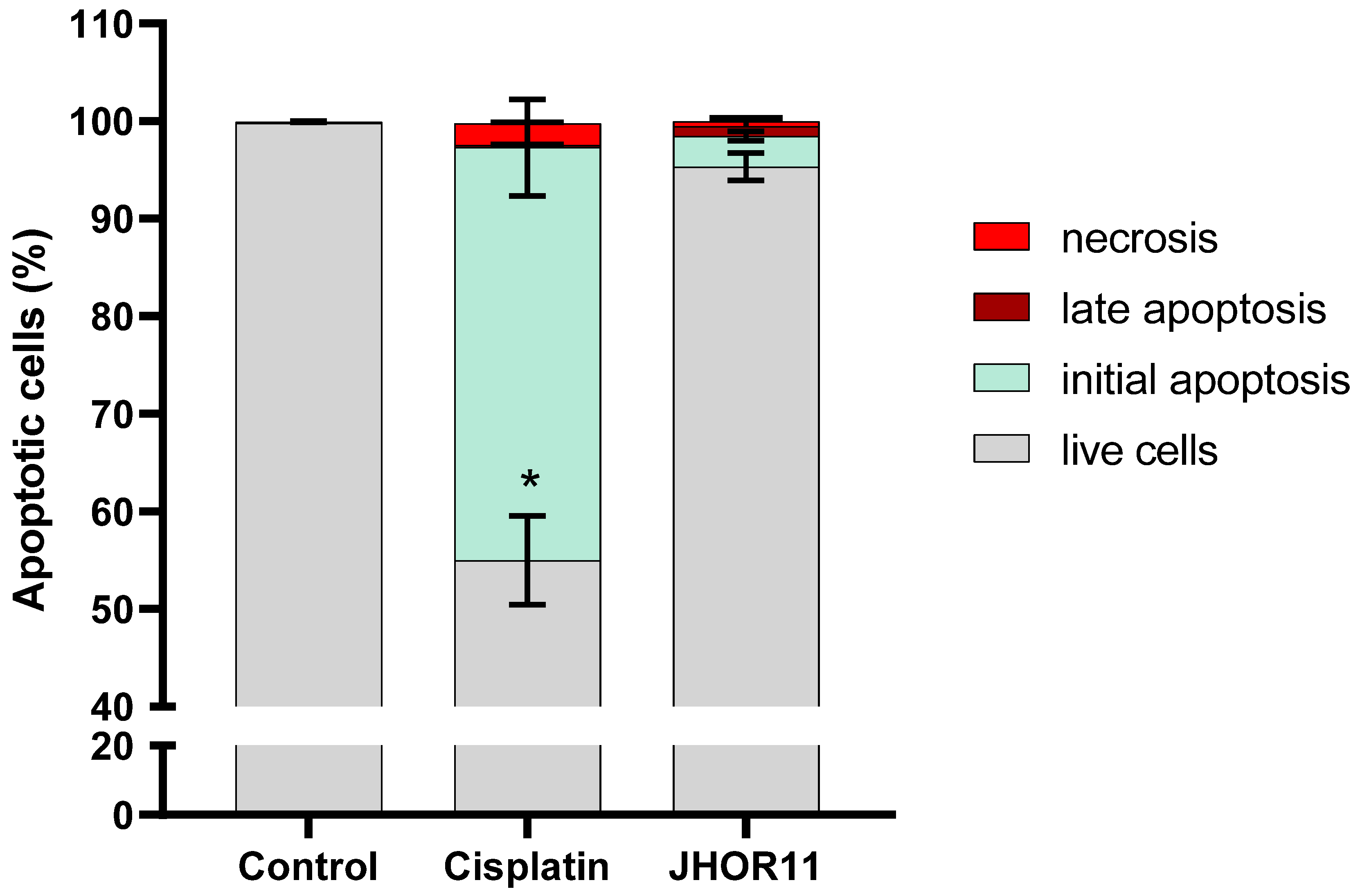
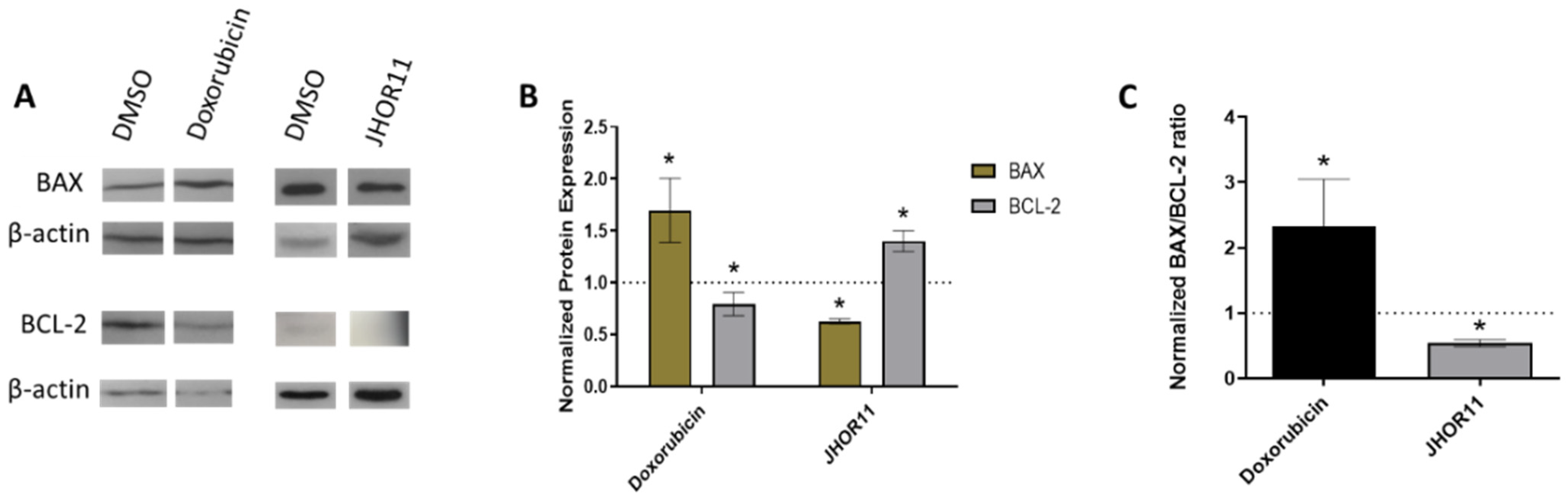
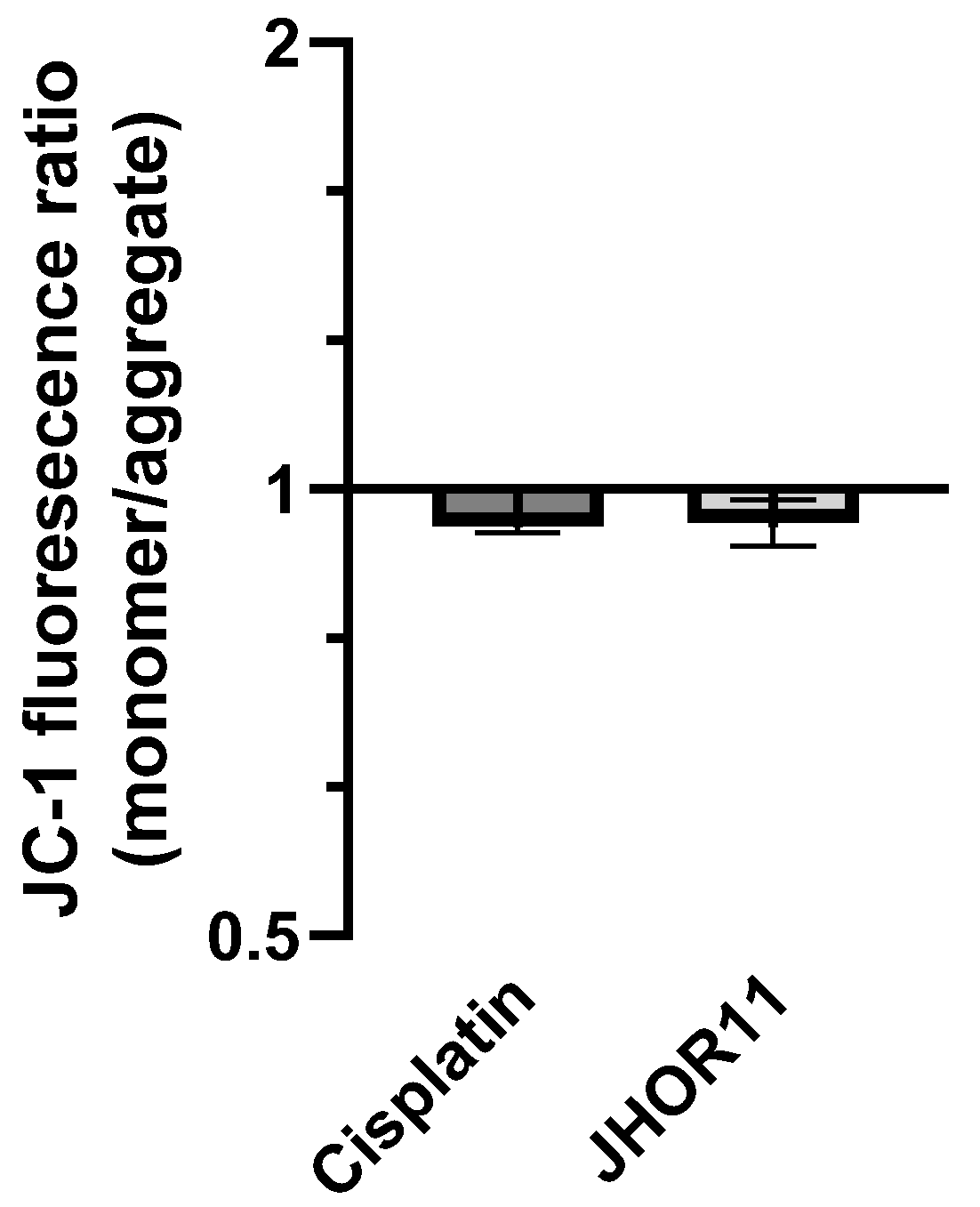
2.5. Mechanisms of Cytotoxicity
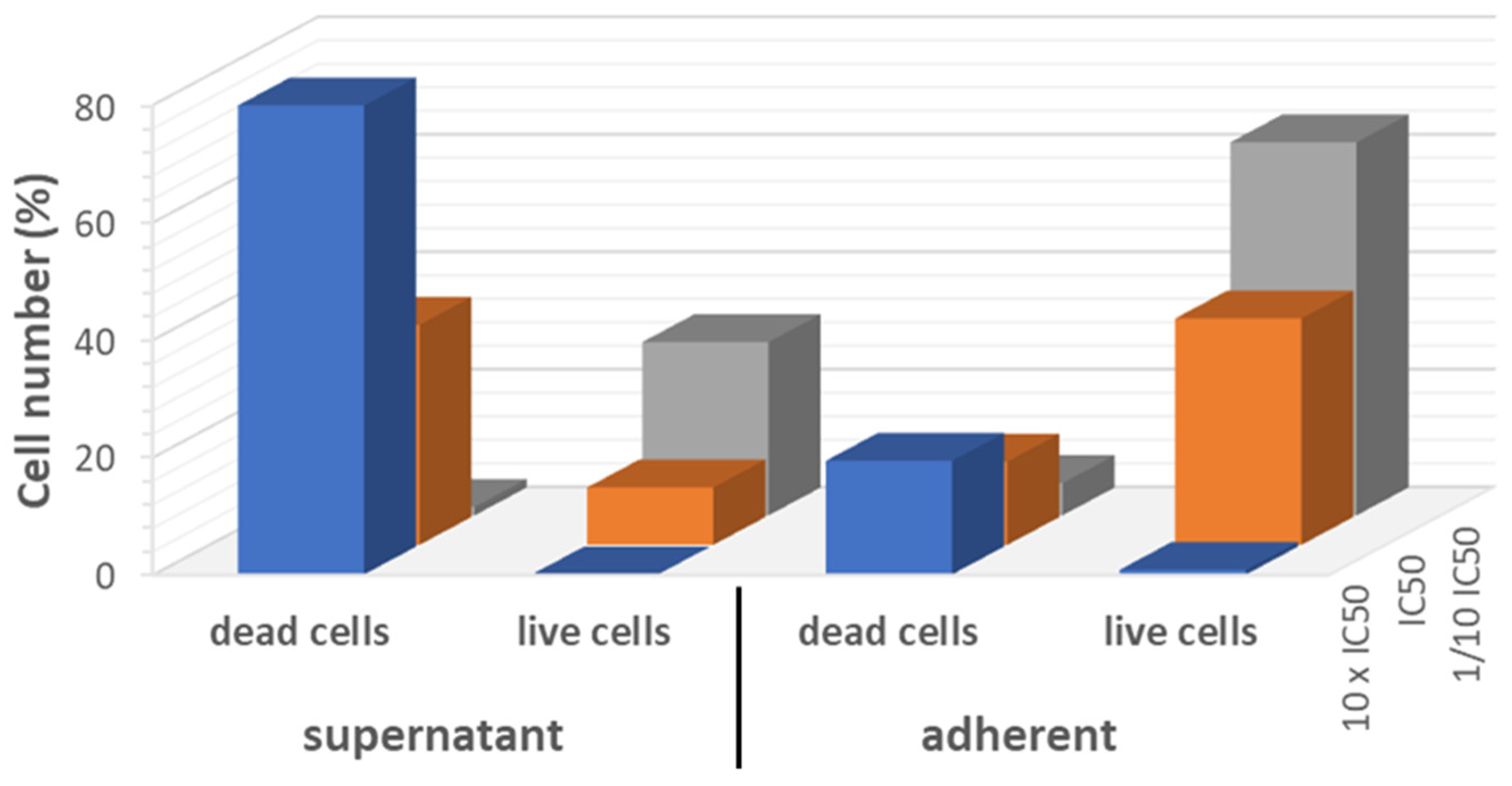
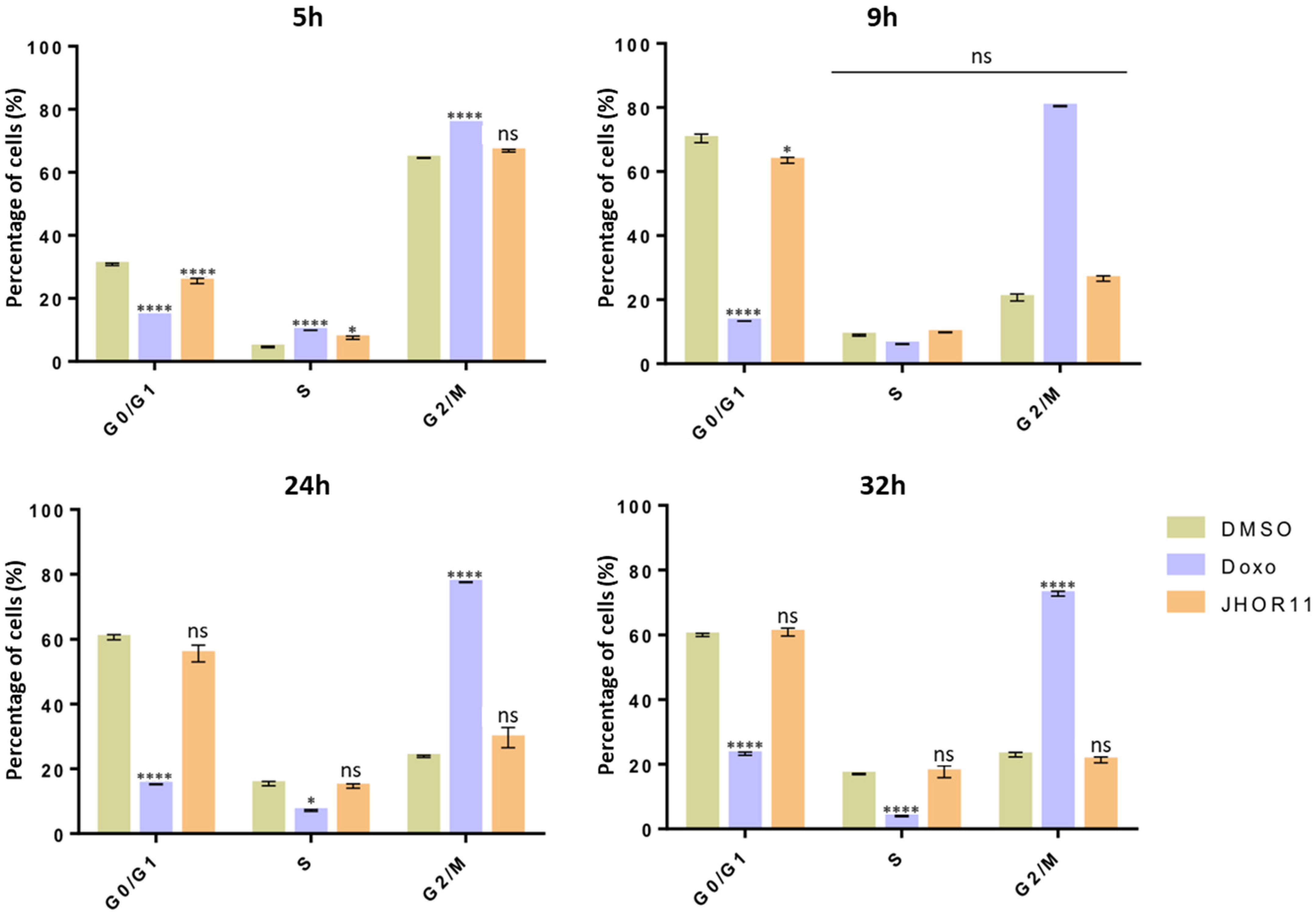
2.6. Cytostatic Effect
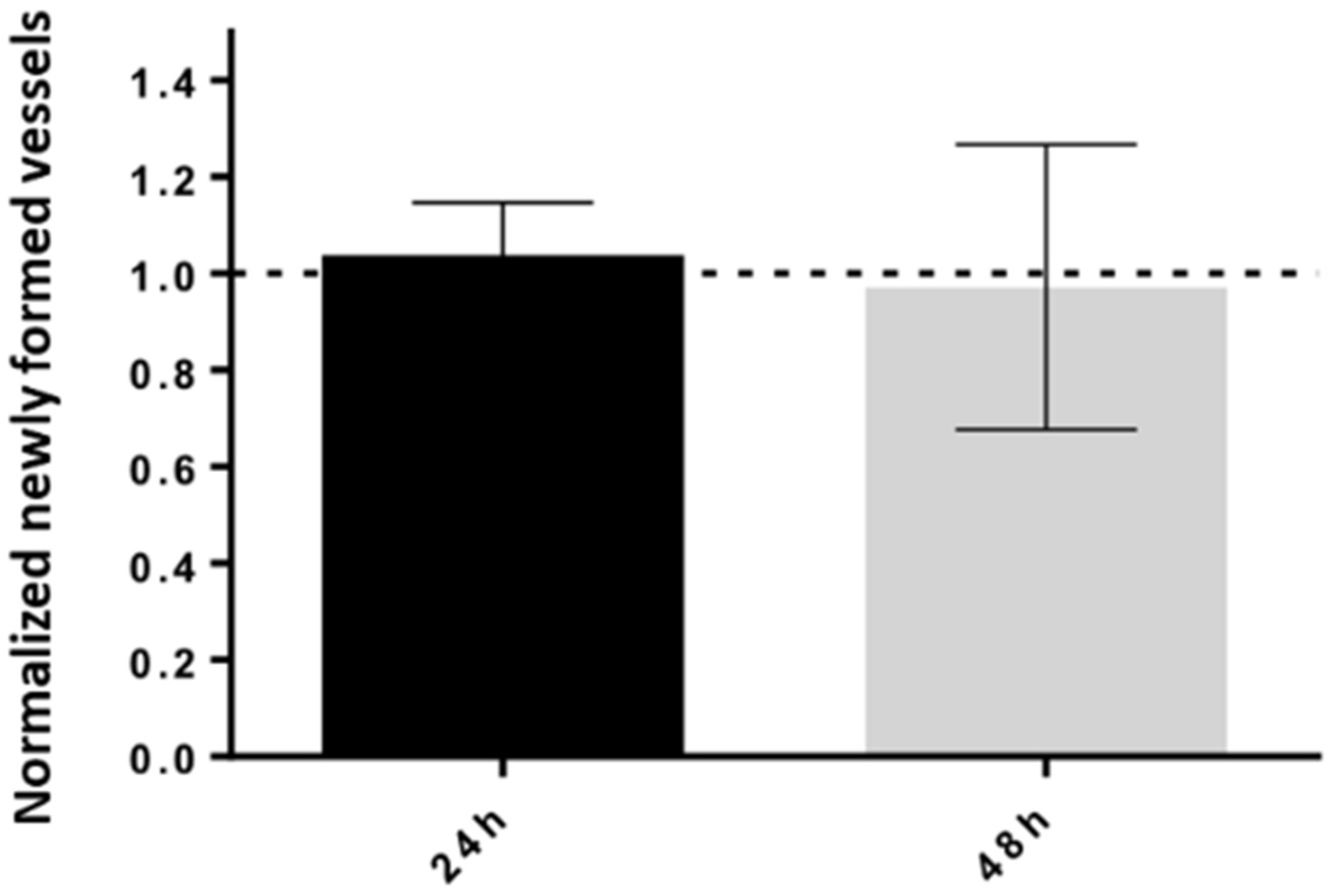
2.7. Tumorigenicity
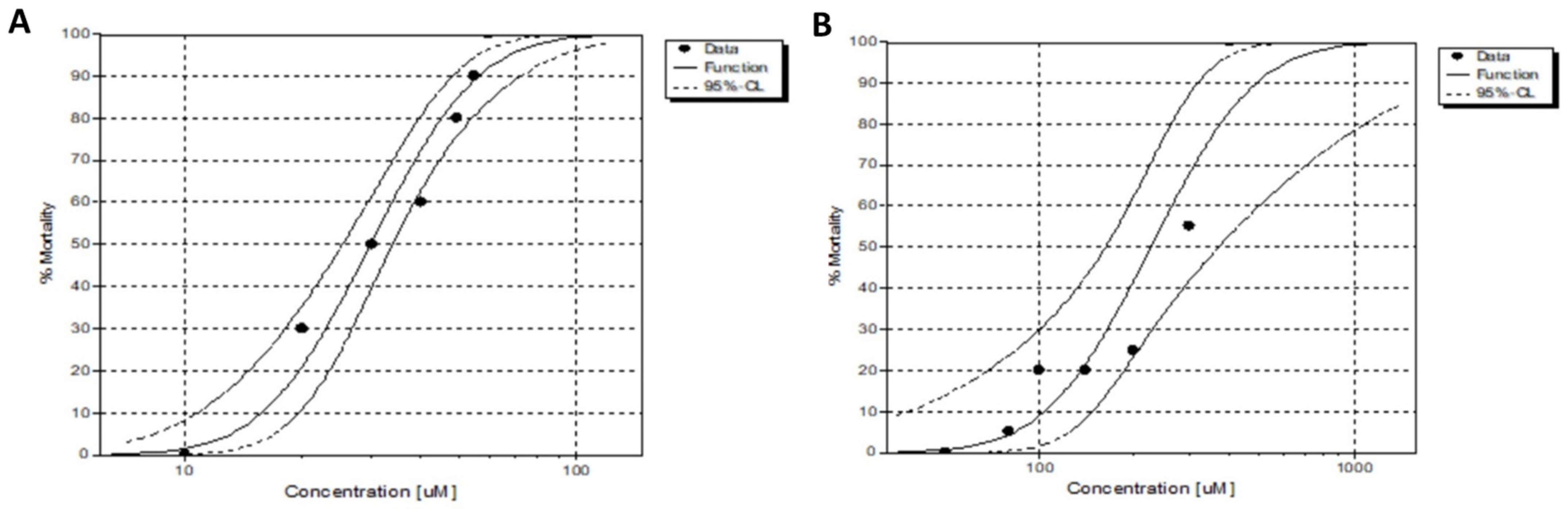
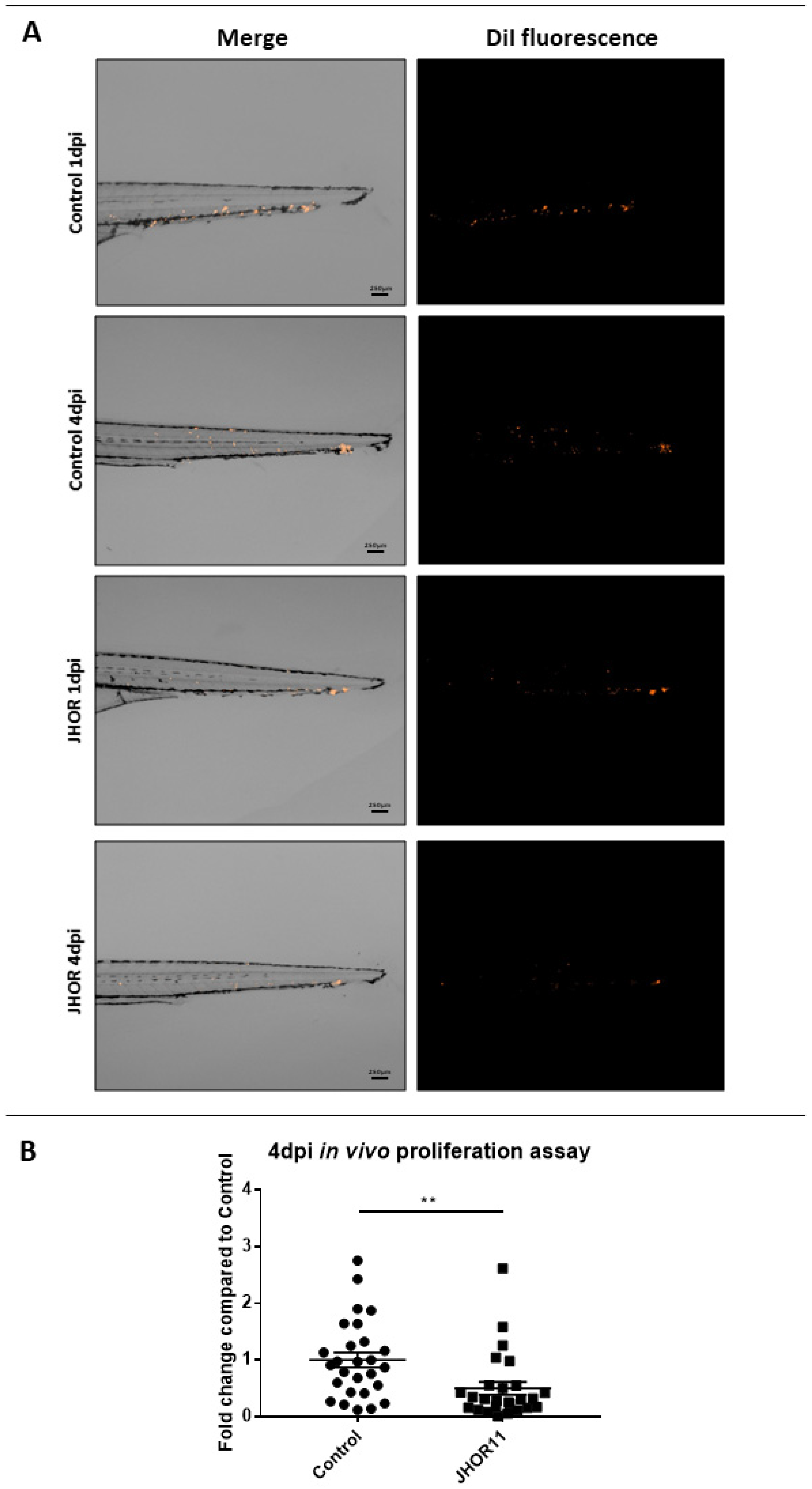
2.8. In Vivo Toxicity Assessment and Xenograft Using Zebrafish Embryos
3. Materials and Methods
3.1. Synthesis
Preparation [Ru{p-C6H4(Me)(iPr)}{(Dione)Cl][CF3SO3] (JHOR10)
3.2. Complexes Stability
3.3. Cell Culture
3.4. Complexes Internalization
3.5. Antiproliferative Activity
3.6. Evaluation of Apoptosis with Annexin V—Alexa Fluor 488/Propidium Iodide Double Staining
3.7. Western Blot to Determine the Apoptotic Index
3.8. Mitochondrial Membrane Potential
3.9. Trypan Blue Exclusion Method
3.10. Autophagy
3.11. Production of Reactive Oxygen Species
3.12. Cell Cycle Analysis
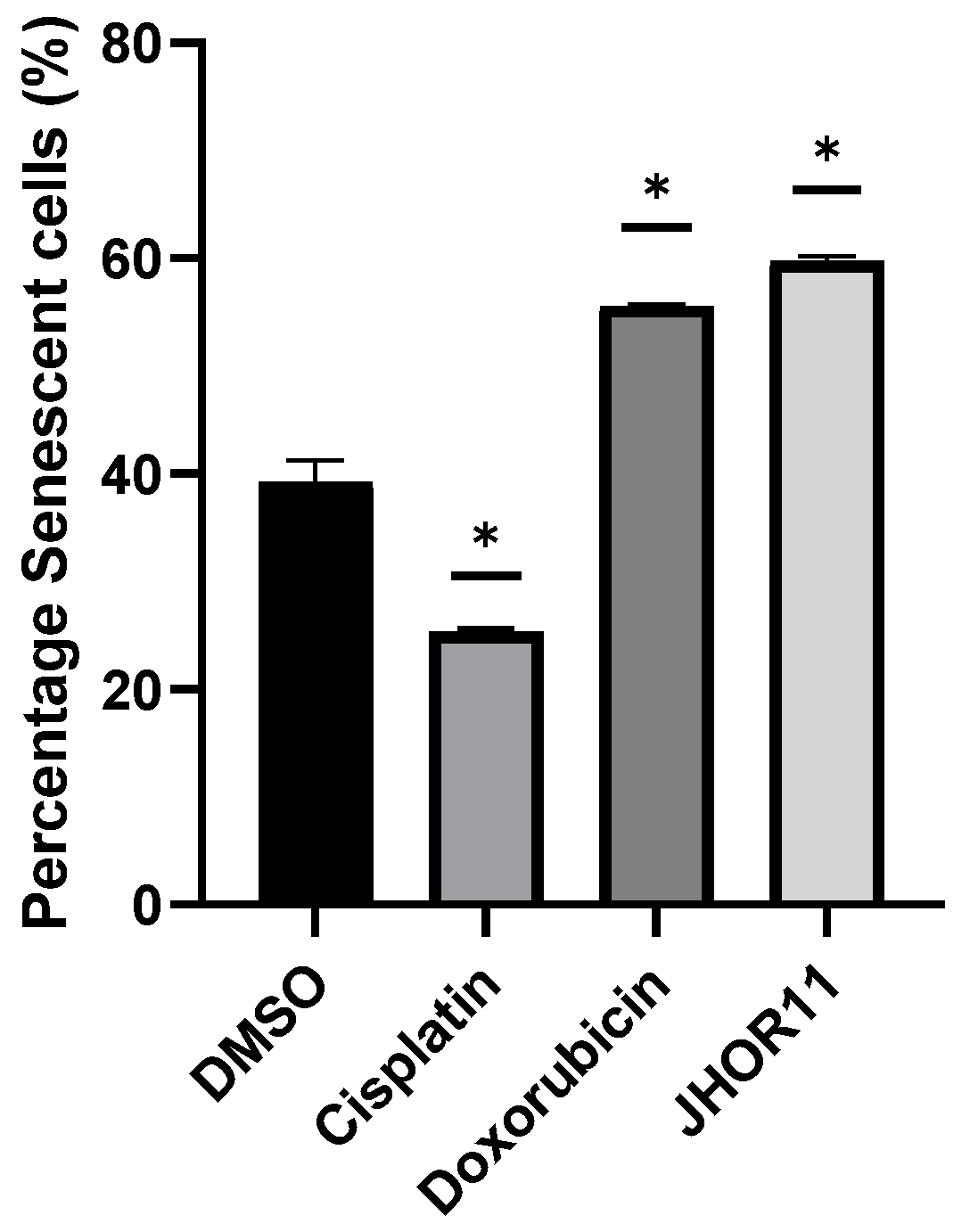
3.13. Senescence Assay
3.14. Wound Scratch Assay
3.15. Ex Ovo CAM Assay
3.16. Zebrafish Handling and Care
3.17. In Vivo Toxicity Assessment Using Zebrafish Embryos
3.18. Cell Culture
3.19. Zebrafish Embryo Xenograft Assays
3.20. Zebrafish Embryo Image Analysis
3.21. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Liu, F.; Dong, X.; Shi, Q.; Chen, J.; Su, W. Improving the anticancer activity of platinum(iv) prodrugs using a dual-targeting strategy with a dichloroacetate axial ligand. RSC Adv. 2019, 9, 22240–22247. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Deegan, C.; Coyle, B.; McCann, M.; Devereux, M.; Egan, D.A. In vitro anti-tumour effect of 1,10-phenanthroline-5,6-dione (phendione), [Cu(phendione)3](ClO4)2·4H2O and [Ag(phendione)2]ClO4 using human epithelial cell lines. Chem. Interact. 2006, 164, 115–125. [Google Scholar] [CrossRef]
- Varol, M.; Benkli, K.; Koparal, A.T.; Bostancıoğlu, R.B. Design and synthesis of novel organometallic complexes using boronated phenylalanine derivatives as potential anticancer agents. Drug Chem. Toxicol. 2019, 42, 436–443. [Google Scholar] [CrossRef]
- Roy, S.; Hagen, K.D.; Maheswari, P.U.; Lutz, M.; Spek, A.L.; Reedijk, J.; Van Wezel, G.P. Phenanthroline Derivatives with Improved Selectivity as DNA-Targeting Anticancer or Antimicrobial Drugs. ChemMedChem 2008, 3, 1427–1434. [Google Scholar] [CrossRef]
- Farhangian, H.; Moghadam, M.E.; Divsalar, A.; Rahiminezhad, A. Anticancer activity of novel amino acid derivative of palladium complex with phendione ligand against of human colon cancer cell line. JBIC J. Biol. Inorg. Chem. 2017, 22, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Kaplanis, M.; Stamatakis, G.; Papakonstantinou, V.D.; Paravatou-Petsotas, M.; Demopoulos, C.A.; Mitsopoulou, C.A. Re(I) tricarbonyl complex of 1,10-phenanthroline-5,6-dione: DNA binding, cytotoxicity, anti-inflammatory and anti-coagulant effects towards platelet activating factor. J. Inorg. Biochem. 2014, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miserachs, H.G.; Cipriani, M.; Grau, J.; Vilaseca, M.; Lorenzo, J.; Medeiros, A.; Comini, M.A.; Gambino, D.; Otero, L.; Moreno, V. Antitumor and antiparasitic activity of novel ruthenium compounds with polycyclic aromatic ligands. J. Inorg. Biochem. 2015, 150, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Smoleński, P.; Martins, L.M.D.R.S.; da Silva, M.F.C.G.; Fernandes, A.R.; Luis, D.; Silva, A.; Santos, S.; Borralho, P.M.; Rodrigues, C.M.P.; et al. Cobalt and Zinc Compounds Bearing 1,10-Phenanthroline-5,6-dione or 1,3,5-Triaza-7-phosphaadamantane Derivatives—Synthesis, Characterization, Cytotoxicity, and Cell Selectivity Studies. Eur. J. Inorg. Chem. 2013, 2013, 3651–3658. [Google Scholar] [CrossRef]
- Pedrosa, P.; Mendes, R.; Cabral, R.; Martins, L.M.D.R.S.; Baptista, P.V.; Fernandes, A.R. Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Raposo, L.R.; Roma-Rodrigues, C.; Jesus, J.; Martins, L.M.D.R.S.; Pombeiro, A.J.; Baptista, P.V.; Fernandes, A.R. Targeting canine mammary tumours via gold nanoparticles functionalized with promising Co(II) and Zn(II) compounds. Vet. Comp. Oncol. 2017, 15, 1537–1542. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Robalo, M.P.; Tomaz, A.I.; Carvalho, A.; Fernandes, A.R.; Marques, F.; Folgueira, M.; Yáñez, J.; Vázquez-García, D.; Torres, M.L.; et al. RuII(p-cymene) Compounds as Effective and Selective Anticancer Candidates with No Toxicity in Vivo. Inorg. Chem. 2018, 57, 13150–13166. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Cabral, R.; Carvalho, B.; Friães, S.; Roma-Rodrigues, C.; Fernández, J.A.A.; Vila, S.F.; Sanchez, L.; Gomes, C.S.B.; Fernandes, A.R.; et al. Triazole-Based Half-Sandwich Ruthenium(II) Compounds: From In Vitro Antiproliferative Potential to In Vivo Toxicity Evaluation. Inorg. Chem. 2021, 60, 8011–8026. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Roma-Rodrigues, C.; Fernandes, A.R.; Marques, F.; Pérez-Fernández, D.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; López-Torres, M.; Fernández, A.; et al. Dinuclear RuII(bipy)2 Derivatives: Structural, Biological, and in Vivo Zebrafish Toxicity Evaluation. Inorg. Chem. 2017, 56, 7127–7144. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Fernandes, A.R.; Roma-Rodrigues, C.; Baptista, P.V.; Marques, F.; Pérez-Fernández, D.; Guerra-Varela, J.; Sánchez, L.; Vázquez-García, D.; Torres, M.L.; et al. Heteroleptic mononuclear compounds of ruthenium(ii): Synthesis, structural analyses, in vitro antitumor activity and in vivo toxicity on zebrafish embryos. Dalton Trans. 2016, 45, 19127–19140. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.; Tian, Z.; Tian, M.; Zhang, S.; Xu, Z.; Gong, P.; Zheng, X.; Zhao, J.; Liu, Z. Significant effects of counteranions on the anticancer activity of iridium(iii) complexes. Chem. Commun. 2018, 54, 4421–4424. [Google Scholar] [CrossRef] [PubMed]
- Queyriaux, N.; Giannoudis, E.; Lefebvre, J.; Artero, V.; Chavarot-Kerlidou, M. Synthesis of Ruthenium Tris-Diimine Photosensitizers Substituted by Four Methylphosphonate Anchoring Groups for Dye-Sensitized Photoelectrochemical Cell Applications. Eur. J. Inorg. Chem. 2019, 2019, 2154–2161. [Google Scholar] [CrossRef]
- Dougan, S.J.; Sadler, P.J. The Design of Organometallic Ruthenium Arene Anticancer Agents. CHIMIA 2007, 61, 704–715. [Google Scholar] [CrossRef]
- Loughrey, B.T.; Healy, P.C.; Parsons, P.G.; Williams, M.L. Selective Cytotoxic Ru(II) Arene Cp* Complex Salts [R-PhRuCp*]+X− for X = BF4−, PF6−, and BPh4−. Inorg. Chem. 2008, 47, 8589–8591. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.; Prins, F.; Bill, E.; Kooijman, H.; Gamez, P.; Roubeau, O.; Spek, A.L.; Haasnoot, J.G.; Reedijk. Counterion effect on the spin-transition properties of the cation [Fe(btzx)3]2+ (btzx=m-Xylylenebis(tetrazole)). Chemistry 2008, 14, 8486–8499. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis Int. J. Program. Cell Death 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Perelman, A.; Wachtel, C.; Cohen, M.E.; Haupt, S.; Shapiro, H.M.; Tzur, A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2001, 21, A.3B.1–A.3B.2. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Malta, G.; Peixoto, D.; Ferreira, L.M.; Baptista, P.V.; Fernandes, A.R.; Branco, P.S. Synthesis of new hetero-arylidene-9(10H)-anthrone derivatives and their biological evaluation. Bioorganic Chem. 2020, 99, 103849. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-H.; Li, W.; Wang, X.-Z.; Zhang, C.; Zeng, C.-C.; Tang, B.; Wan, D.; Liu, Y.-J. Apoptosis, autophagy, cell cycle arrest, cell invasion and BSA-binding studies in vitro of ruthenium(ii) polypyridyl complexes. RSC Adv. 2016, 6, 63143–63155. [Google Scholar] [CrossRef]
- Tan, C.; Lai, S.; Wu, S.; Hu, S.; Zhou, L.; Chen, Y.; Wang, M.; Zhu, Y.; Lian, W.; Peng, W.; et al. Nuclear Permeable Ruthenium(II) β-Carboline Complexes Induce Autophagy to Antagonize Mitochondrial-Mediated Apoptosis. J. Med. Chem. 2010, 53, 7613–7624. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, A.J.-S. Cellular senescence: A promising strategy for cancer therapy. BMB Rep. 2019, 52, 35–41. [Google Scholar] [CrossRef]
- Berndsen, R.H.; Weiss, A.; Abdul, U.K.; Wong, T.J.; Meraldi, P.; Griffioen, A.W.; Dyson, P.; Nowak-Sliwinska, P. Combination of ruthenium(II)-arene complex [Ru(η6-p-cymene)Cl2(pta)] (RAPTA-C) and the epidermal growth factor receptor inhibitor erlotinib results in efficient angiostatic and antitumor activity. Sci. Rep. 2017, 7, srep43005. [Google Scholar] [CrossRef]
- Riahi, R.; Yang, Y.; Zhang, D.D.; Wong, P.K. Advances in Wound-Healing Assays for Probing Collective Cell Migration. J. Lab. Autom. 2012, 17, 59–65. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Reigosa-Chamorro, F.; Raposo, L.R.; Munín-Cruz, P.; Pereira, M.T.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R.; Vila, J.M. In Vitro and In Vivo Effect of Palladacycles: Targeting A2780 Ovarian Carcinoma Cells and Modulation of Angiogenesis. Inorg. Chem. 2021, 60, 3939–3951. [Google Scholar] [CrossRef]
- Raposo, L.R.; Silva, A.; Silva, D.; Roma-Rodrigues, C.; Espadinha, M.; Baptista, P.V.; Santos, M.M.; Fernandes, A.R. Exploiting the antiproliferative potential of spiropyrazoline oxindoles in a human ovarian cancer cell line. Bioorganic Med. Chem. 2021, 30, 115880. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.S.; Jousseaume, Y.; Piedade, M.F.M.; Roma-Rodrigues, C.; Fernandes, A.R.; Marques, F.; de Brito, M.J.V.; Garcia, M.H. Important cytotoxic and cytostatic effects of new copper(i)–phosphane compounds with N,N, N,O and N,S bidentate ligands. Dalton Trans. 2018, 47, 7819–7829. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; Kanaras, A.G.; Heuer-Jungemann, A.; Roma-Rodrigues, C.; Fernandes, A.R. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomed. 2016, 11, 2633–2639. [Google Scholar] [CrossRef]
- Martin, M.; Maibach, H. OECD guidelines for testing of chemicals. Dermatotoxicology 2013, 509–511. [Google Scholar] [CrossRef]
- R.H.D. Probit Analysis. By D. J. Finney, M.A., Sc.D., [2nd ed. Pp. xiv 318. Cambridge University Press, 1952. 35s.]. J. Inst. Actuar. 1952, 78, 388–390. [CrossRef]
- Stirling, D.R.; Suleyman, O.; Gil, E.; Elks, P.M.; Torraca, V.; Noursadeghi, M.; Tomlinson, G.S. Analysis tools to quantify dissemination of pathology in zebrafish larvae. Sci. Rep. 2020, 10, 3149. [Google Scholar] [CrossRef]
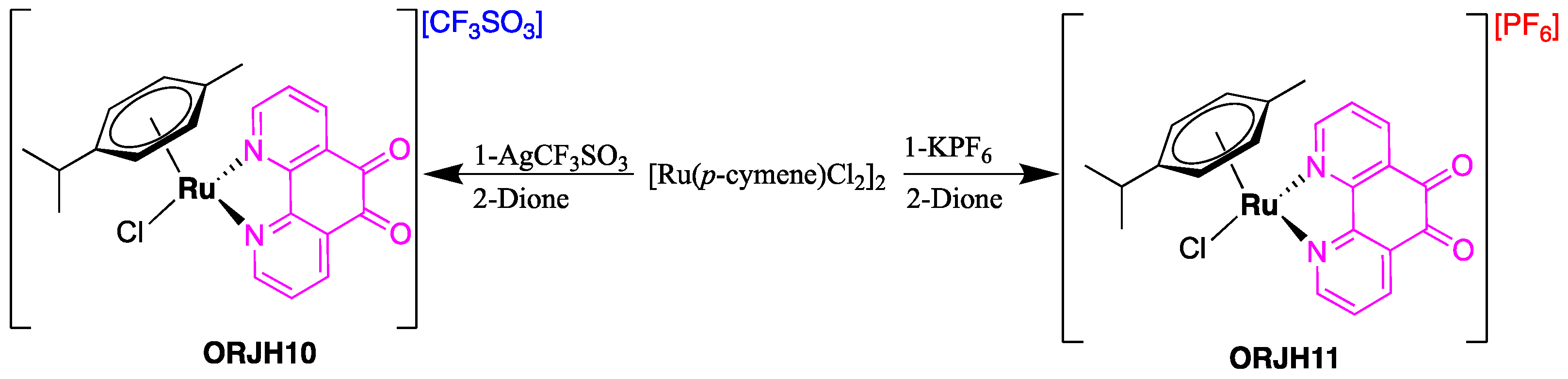
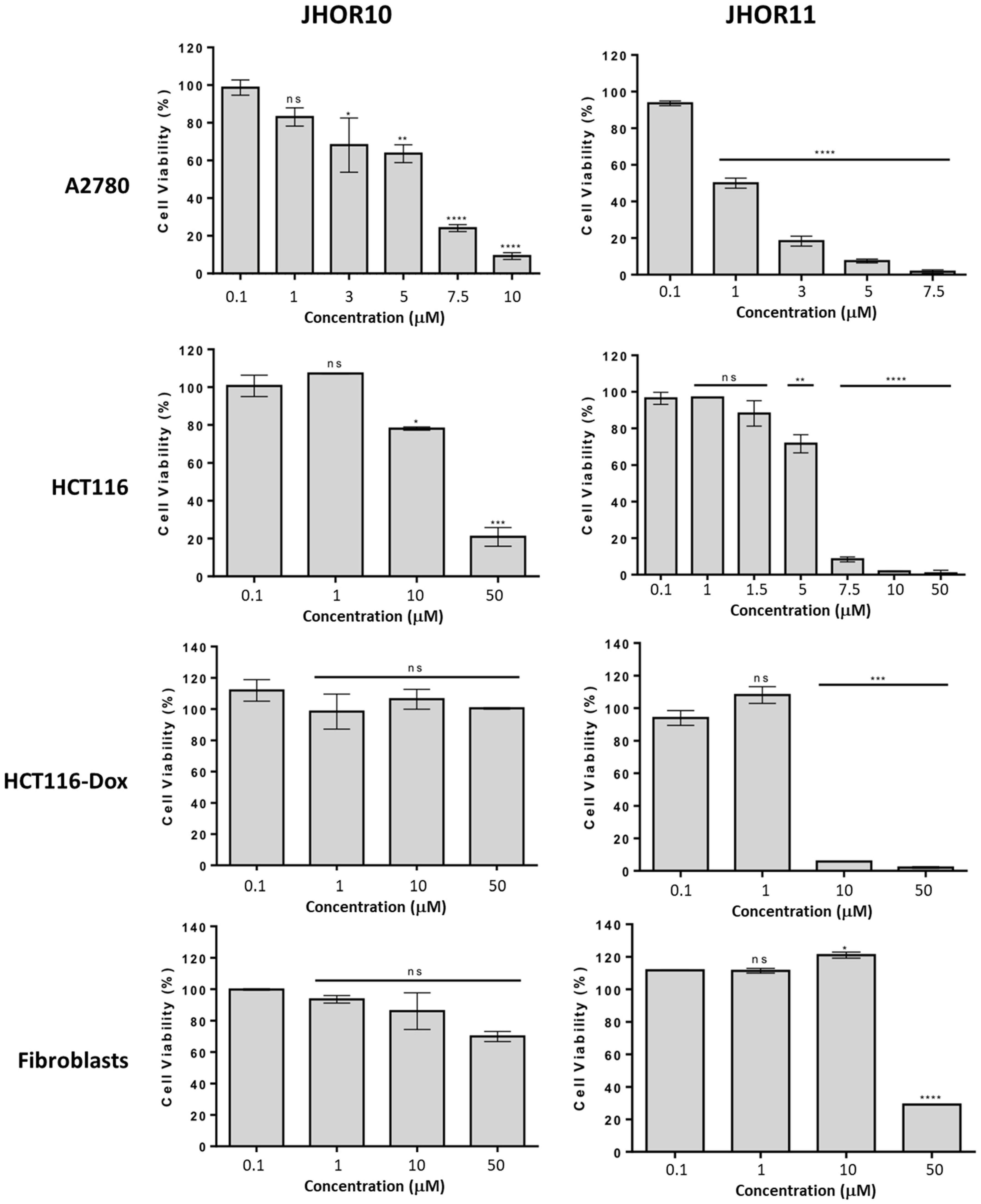



| IC50 (μM) | ||||
|---|---|---|---|---|
| Complexes | A2780 | HCT116 | HCT116-Dox | Fibroblasts |
| JHOR10 | 5.3 ± 0.3 | 25.7 ± 1.3 | >50 | >50 |
| JHOR11 | 1.2 ± 0.1 | 5.5 ± 0.3 | 6.8 ± 0.3 | 44.5 ± 2.2 |
| Cisplatin | 1.9 ± 0.2 | 15.6 ± 5.3 | n.d. | n.d. |
| Compound | LC50 (µM) | C.L (95%) | NOEC (µM) | LOEC (µM) |
|---|---|---|---|---|
| JHOR10 | 29.83 | 25.31–33.97 | 10.00 | 20.00 |
| JHOR11 | 225.38 | 161.86–370.40 | 80.00 | 10.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenis-Rojas, O.A.; Roma-Rodrigues, C.; Carvalho, B.; Cabezas-Sainz, P.; Fernández Vila, S.; Sánchez, L.; Baptista, P.V.; Fernandes, A.R.; Royo, B. In Vitro and In Vivo Biological Activity of Ruthenium 1,10-Phenanthroline-5,6-dione Arene Complexes. Int. J. Mol. Sci. 2022, 23, 13594. https://doi.org/10.3390/ijms232113594
Lenis-Rojas OA, Roma-Rodrigues C, Carvalho B, Cabezas-Sainz P, Fernández Vila S, Sánchez L, Baptista PV, Fernandes AR, Royo B. In Vitro and In Vivo Biological Activity of Ruthenium 1,10-Phenanthroline-5,6-dione Arene Complexes. International Journal of Molecular Sciences. 2022; 23(21):13594. https://doi.org/10.3390/ijms232113594
Chicago/Turabian StyleLenis-Rojas, Oscar A., Catarina Roma-Rodrigues, Beatriz Carvalho, Pablo Cabezas-Sainz, Sabela Fernández Vila, Laura Sánchez, Pedro V. Baptista, Alexandra R. Fernandes, and Beatriz Royo. 2022. "In Vitro and In Vivo Biological Activity of Ruthenium 1,10-Phenanthroline-5,6-dione Arene Complexes" International Journal of Molecular Sciences 23, no. 21: 13594. https://doi.org/10.3390/ijms232113594
APA StyleLenis-Rojas, O. A., Roma-Rodrigues, C., Carvalho, B., Cabezas-Sainz, P., Fernández Vila, S., Sánchez, L., Baptista, P. V., Fernandes, A. R., & Royo, B. (2022). In Vitro and In Vivo Biological Activity of Ruthenium 1,10-Phenanthroline-5,6-dione Arene Complexes. International Journal of Molecular Sciences, 23(21), 13594. https://doi.org/10.3390/ijms232113594