WideEffHunter: An Algorithm to Predict Canonical and Non-Canonical Effectors in Fungi and Oomycetes
Abstract
1. Introduction
2. Results
2.1. Protein Databases
2.2. In Silico Characterization of True Effectors
2.3. Functional Annotation of Fungal/Oomycete Effector Proteins: Domains and Motifs
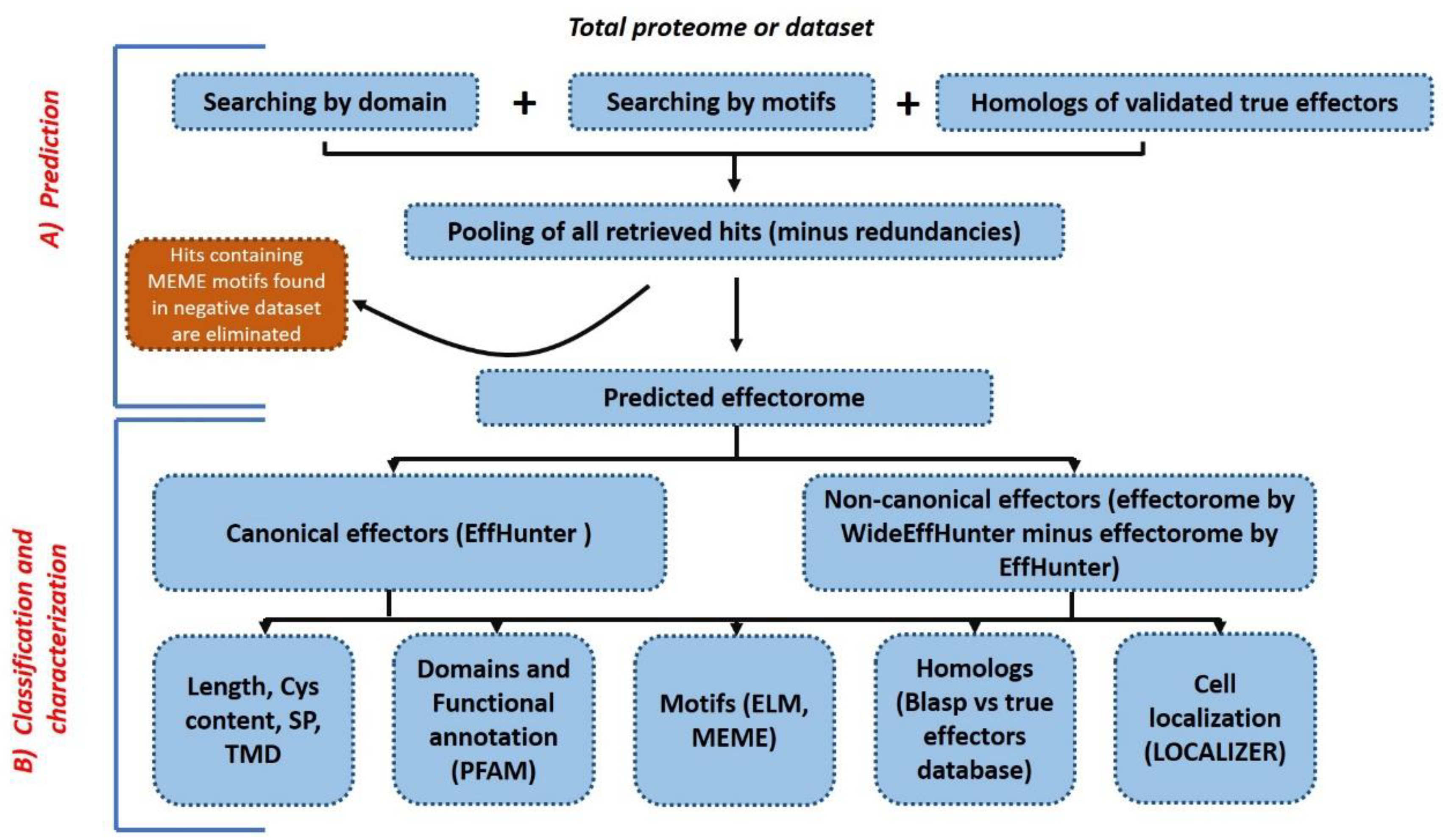
2.4. Construction and Validation of WideEffHunter Algorithm
2.5. WideEffHunter Prediction of Effectoromes in Fungal and Oomycete Proteomes
3. Discussion
4. Materials and Methods
4.1. Data Protein Collection
4.2. In Silico Characterization of Effectors
4.3. Construction of Databases
4.3.1. Database of Domains
4.3.2. Database of Motifs
4.3.3. Database of True Effectors
4.4. Construction of WideEffHunter
4.5. Validation of WideEffHunter
4.6. Prediction of Effector Proteins in Fungal and Oomycete Genomes
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giesbers, A.K.J.; Pelgrom, A.J.E.; Visser, R.G.F.; Niks, R.E.; Van den Ackerveken, G.; Jeuken, M.J.W. Effector-Mediated Discovery of a Novel Resistance Gene against Bremia lactucae in a Nonhost Lettuce Species. New Phytol. 2017, 216, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Coaker, G. Harnessing Effector-Triggered Immunity for Durable Disease Resistance. Phytopathology 2017, 107, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Kanja, C.; Hammond-Kosack, K.E. Proteinaceous Effector Discovery and Characterization in Filamentous Plant Pathogens. Mol. Plant Pathol. 2020, 21, 1353–1376. [Google Scholar] [CrossRef] [PubMed]
- Gorash, A.; Armonienė, R.; Kazan, K. Can Effectoromics and Loss-of-Susceptibility Be Exploited for Improving Fusarium Head Blight Resistance in Wheat? Crop J. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Chang, T.-C.; Salvucci, A.; Crous, P.W.; Stergiopoulos, I. Comparative Genomics of the Sigatoka Disease Complex on Banana Suggests a Link between Parallel Evolutionary Changes in Pseudocercospora fijiensis and Pseudocercospora eumusae and Increased Virulence on the Banana Host. PLOS Genet. 2016, 12, e1005904. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Ma, X.; Torriani, S.F.F.; Zala, M.; Francisco, C.S.; Hartmann, F.E.; Croll, D.; McDonald, B.A. Comparative Transcriptome Analyses in Zymoseptoria tritici Reveal Significant Differences in Gene Expression Among Strains During Plant Infection. Mol. Plant Microbe Interact. 2017, 30, 231–244. [Google Scholar] [CrossRef]
- Ökmen, B.; Mathow, D.; Hof, A.; Lahrmann, U.; Aßmann, D.; Doehlemann, G. Mining the Effector Repertoire of the Biotrophic Fungal Pathogen Ustilago hordei during Host and Non-Host Infection. Mol. Plant Pathol. 2018, 19, 2603–2622. [Google Scholar] [CrossRef]
- Ozketen, A.C.; Andac-Ozketen, A.; Dagvadorj, B.; Demiralay, B.; Akkaya, M.S. In-Depth Secretome Analysis of Puccinia striiformis f. sp. tritici in Infected Wheat Uncovers Effector Functions. Biosci. Rep. 2020, 40, BSR20201188. [Google Scholar] [CrossRef]
- Sperschneider, J.; Gardiner, D.M.; Dodds, P.N.; Tini, F.; Covarelli, L.; Singh, K.B.; Manners, J.M.; Taylor, J.M. EffectorP: Predicting Fungal Effector Proteins from Secretomes Using Machine Learning. New Phytol. 2016, 210, 743–761. [Google Scholar] [CrossRef]
- Sonah, H.; Deshmukh, R.K.; Bélanger, R.R. Computational Prediction of Effector Proteins in Fungi: Opportunities and Challenges. Front. Plant Sci. 2016, 7, 126. [Google Scholar] [CrossRef]
- Jones, D.A.; Bertazzoni, S.; Turo, C.J.; Syme, R.A.; Hane, J.K. Bioinformatic Prediction of Plant–Pathogenicity Effector Proteins of Fungi. Curr. Opin. Microbiol. 2018, 46, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Carreón-Anguiano, K.G.; Islas-Flores, I.; Vega-Arreguín, J.; Sáenz-Carbonell, L.; Canto-Canché, B. EffHunter: A Tool for Prediction of Effector Protein Candidates in Fungal Proteomic Databases. Biomolecules 2020, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, H.; Zhou, Y.; Bao, Y.; Duan, Z.; Wang, C.; Powell, C.A.; Chen, B.; Zhang, M.; Yao, W. Predication of the Effector Proteins Secreted by Fusarium sacchari Using Genomic Analysis and Heterogenous Expression. J. Fungi 2022, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, R.; Dubey, H.; Kiran, K.; Rawal, H.; Rajarammohan, S.; Prasad, P.; Bhardwaj, S.C.; Sonah, H.; Deshmukh, R.; Gupta, N.; et al. Comparative Secretomics Identifies Conserved WAxR Motif-Containing Effectors in Rust Fungi That Suppress Cell Death in Plants. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhao, S.; Shang, X.; Bi, W.; Yu, X.; Liu, D.; Kang, Z.; Wang, X.; Wang, X. Genome-Wide Identification of Effector Candidates with Conserved Motifs from the Wheat Leaf Rust Fungus Puccinia triticina. Front. Microbiol. 2020, 11, 1188. [Google Scholar] [CrossRef] [PubMed]
- Schurack, S.; Depotter, J.R.L.; Gupta, D.; Thines, M.; Doehlemann, G. Comparative Transcriptome Profiling Identifies Maize Line Specificity of Fungal Effectors in the Maize–Ustilago maydis Interaction. Plant J. 2021, 106, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Boevink, P.C.; Wang, X.; McLellan, H.; He, Q.; Naqvi, S.; Armstrong, M.R.; Zhang, W.; Hein, I.; Gilroy, E.M.; Tian, Z.; et al. A Phytophthora infestans RXLR Effector Targets Plant PP1c Isoforms That Promote Late Blight Disease. Nat. Commun. 2016, 7, 10311. [Google Scholar] [CrossRef]
- Pennington, H.G.; Jones, R.; Kwon, S.; Bonciani, G.; Thieron, H.; Chandler, T.; Luong, P.; Morgan, S.N.; Przydacz, M.; Bozkurt, T.; et al. The Fungal Ribonuclease-like Effector Protein CSEP0064/BEC1054 Represses Plant Immunity and Interferes with Degradation of Host Ribosomal RNA. PLoS Pathog. 2019, 15, e1007620. [Google Scholar] [CrossRef]
- Liu, T.; Song, T.; Zhang, X.; Yuan, H.; Su, L.; Li, W.; Xu, J.; Liu, S.; Chen, L.; Chen, T.; et al. Unconventionally Secreted Effectors of Two Filamentous Pathogens Target Plant Salicylate Biosynthesis. Nat. Commun. 2014, 5, 4686. [Google Scholar] [CrossRef]
- Ghareeb, H.; Drechsler, F.; Löfke, C.; Teichmann, T.; Schirawski, J. SUPPRESSOR OF APICAL DOMINANCE1 of Sporisorium reilianum Modulates Inflorescence Branching Architecture in Maize and Arabidopsis. Plant Physiol. 2015, 169, 2789–2804. [Google Scholar] [CrossRef]
- Salcedo, A.; Rutter, W.; Wang, S.; Akhunova, A.; Bolus, S.; Chao, S.; Anderson, N.; De Soto, M.F.; Rouse, M.; Szabo, L.; et al. Variation in the AvrSr35 Gene Determines Sr35 Resistance against Wheat Stem Rust Race Ug99. Science 2017, 358, 1604–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, J.; Qi, Y.; Li, J.; Amin, R.; Yang, W.; Liu, D. Predicating the Effector Proteins Secreted by Puccinia triticina Through Transcriptomic Analysis and Multiple Prediction Approaches. Front. Microbiol. 2020, 11, 538032. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tian, L.; Zhang, D.-D.; Song, J.; Song, S.-S.; Yin, C.-M.; Zhou, L.; Liu, Y.; Wang, B.-L.; Kong, Z.-Q.; et al. Functional Analyses of Small Secreted Cysteine-Rich Proteins Identified Candidate Effectors in Verticillium dahliae. Mol. Plant Pathol. 2020, 21, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Sperschneider, J.; Dodds, P.N. EffectorP 3.0: Prediction of Apoplastic and Cytoplasmic Effectors in Fungi and Oomycetes. Mol. Plant Microbe Interact. 2022, 35, 146–156. [Google Scholar] [CrossRef]
- Wang, C.; Wang, P.; Han, S.; Wang, L.; Zhao, Y.; Juan, L. FunEffector-Pred: Identification of Fungi Effector by Activate Learning and Genetic Algorithm Sampling of Imbalanced Data. IEEE Access 2020, 8, 57674–57683. [Google Scholar] [CrossRef]
- Jones, D.A.B.; Rozano, L.; Debler, J.W.; Mancera, R.L.; Moolhuijzen, P.M.; Hane, J.K. An Automated and Combinative Method for the Predictive Ranking of Candidate Effector Proteins of Fungal Plant Pathogens. Sci. Rep. 2021, 11, 19731. [Google Scholar] [CrossRef]
- Nur, M.; Wood, K.; Michelmore, R. EffectorO: Motif-Independent Prediction of Effectors in Oomycete Genomes Using Machine Learning and Lineage Specificity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Godfrey, D.; Böhlenius, H.; Pedersen, C.; Zhang, Z.; Emmersen, J.; Thordal-Christensen, H. Powdery Mildew Fungal Effector Candidates Share N-Terminal Y/F/WxC-Motif. BMC Genom. 2010, 11, 317. [Google Scholar] [CrossRef]
- Wood, K.J.; Nur, M.; Gil, J.; Fletcher, K.; Lakeman, K.; Gann, D.; Gothberg, A.; Khuu, T.; Kopetzky, J.; Naqvi, S.; et al. Effector Prediction and Characterization in the Oomycete Pathogen Bremia lactucae Reveal Host-Recognized WY Domain Proteins That Lack the Canonical RXLR Motif. PLOS Pathog. 2020, 16, e1009012. [Google Scholar] [CrossRef]
- Jones, D.A.B.; Moolhuijzen, P.M.; Hane, J.K. Remote Homology Clustering Identifies Lowly Conserved Families of Effector Proteins in Plant-Pathogenic Fungi. Microb. Genom. 2021, 7, 000637. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N.; Gardiner, D.M.; Singh, K.B.; Taylor, J.M. Improved Prediction of Fungal Effector Proteins from Secretomes with EffectorP 2.0. Mol. Plant Pathol. 2018, 19, 2094–2110. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Liu, S.; Xu, F.; Jiang, S.; Yan, J.; He, Q.; Liu, W.; Lin, C.; Zheng, F.; Wang, X.; et al. Powdery Mildews Are Characterized by Contracted Carbohydrate Metabolism and Diverse Effectors to Adapt to Obligate Biotrophic Lifestyle. Front. Microbiol. 2018, 9, 3160. [Google Scholar] [CrossRef] [PubMed]
- Morais do Amaral, A.; Antoniw, J.; Rudd, J.J.; Hammond-Kosack, K.E. Defining the Predicted Protein Secretome of the Fungal Wheat Leaf Pathogen Mycosphaerella graminicola. PLoS ONE 2012, 7, e49904. [Google Scholar] [CrossRef]
- Neu, E.; Debener, T. Prediction of the Diplocarpon rosae Secretome Reveals Candidate Genes for Effectors and Virulence Factors. Fungal Biol. 2019, 123, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s Conserved Domain Database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Attwood, T.K. The PRINTS Database: A Resource for Identification of Protein Families. Brief. Bioinform. 2002, 3, 252–263. [Google Scholar] [CrossRef]
- Schultz, J.; Copley, R.R.; Doerks, T.; Ponting, C.P.; Bork, P. SMART: A Web-Based Tool for the Study of Genetically Mobile Domains. Nucleic Acids Res. 2000, 28, 231–234. [Google Scholar] [CrossRef]
- Haft, D.H.; Selengut, J.D.; White, O. The TIGRFAMs Database of Protein Families. Nucleic Acids Res. 2003, 31, 371–373. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, H.; Boeren, S.; Dings, H.; Kulikova, O.; Bisseling, T.; Limpens, E. A Nuclear-Targeted Effector of Rhizophagus irregularis Interferes with Histone 2B Mono-Ubiquitination to Promote Arbuscular Mycorrhization. New Phytol. 2021, 230, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Rocafort, M.; Bowen, J.K.; Hassing, B.; Cox, M.P.; McGreal, B.; de la Rosa, S.; Plummer, K.M.; Bradshaw, R.E.; Mesarich, C.H. The Venturia inaequalis Effector Repertoire Is Expressed in Waves, and Is Dominated by Expanded Families with Predicted Structural Similarity to Avirulence Proteins from Other Fungi. bioRxiv 2022. [Google Scholar] [CrossRef]
- de Queiroz, C.B.; Santana, M.F. Prediction of the Secretomes of Endophytic and Nonendophytic Fungi Reveals Similarities in Host Plant Infection and Colonization Strategies. Mycologia 2020, 112, 491–503. [Google Scholar] [CrossRef] [PubMed]
- van Dam, P.; Fokkens, L.; Schmidt, S.M.; Linmans, J.H.J.; Kistler, H.C.; Ma, L.-J.; Rep, M. Effector Profiles Distinguish Formae Speciales of Fusarium oxysporum. Environ. Microbiol. 2016, 18, 4087–4102. [Google Scholar] [CrossRef]
- Lu, S.; Edwards, M.C. Genome-Wide Analysis of Small Secreted Cysteine-Rich Proteins Identifies Candidate Effector Proteins Potentially Involved in Fusarium graminearum−Wheat Interactions. Phytopathology 2016, 106, 166–176. [Google Scholar] [CrossRef]
- Krijger, J.-J.; Thon, M.R.; Deising, H.B.; Wirsel, S.G. Compositions of Fungal Secretomes Indicate a Greater Impact of Phylogenetic History than Lifestyle Adaptation. BMC Genom. 2014, 15, 722. [Google Scholar] [CrossRef]
- Tabima, J.F.; Grünwald, N.J. EffectR: An Expandable R Package to Predict Candidate RxLR and CRN Effectors in Oomycetes Using Motif Searches. Mol. Plant Microbe Interact. 2019, 32, 1067–1076. [Google Scholar] [CrossRef]
- He, Q.; McLellan, H.; Boevink, P.C.; Birch, P.R.J. All Roads Lead to Susceptibility: The Many Modes of Action of Fungal and Oomycete Intracellular Effectors. Plant Commun. 2020, 1, 100050. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Yang, J.; Yang, X.; Zhang, M.; Zhao, Z.; Fan, Y.; Wang, C.; Wang, J. Bioinformatics and Transcriptome Analysis of CFEM Proteins in Fusarium graminearum. J. Fungi 2021, 7, 871. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.-D.; Song, J.; Li, J.-J.; Wang, J.; Li, R.; Klosterman, S.J.; Kong, Z.-Q.; Lin, F.-Z.; Dai, X.-F.; et al. Verticillium dahliae CFEM Proteins Manipulate Host Immunity and Differentially Contribute to Virulence. BMC Biol. 2022, 20, 55. [Google Scholar] [CrossRef]
- Haas, B.J.; Kamoun, S.; Zody, M.C.; Jiang, R.H.Y.; Handsaker, R.E.; Cano, L.M.; Grabherr, M.; Kodira, C.D.; Raffaele, S.; Torto-Alalibo, T.; et al. Genome Sequence and Analysis of the Irish Potato Famine Pathogen Phytophthora infestans. Nature 2009, 461, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Dölfors, F.; Holmquist, L.; Dixelius, C.; Tzelepis, G. A LysM Effector Protein from the Basidiomycete Rhizoctonia solani Contributes to Virulence through Suppression of Chitin-Triggered Immunity. Mol. Genet. Genom. 2019, 294, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Yarden, O.; Hadar, Y. Seeking the Roles for Fungal Small-Secreted Proteins in Affecting Saprophytic Lifestyles. Front. Microbiol. 2020, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, M.; Maqbool, A.; Jiménez-Dalmaroni, M.J.; Pennington, H.G.; Kamoun, S.; Banfield, M.J. Effectors of Filamentous Plant Pathogens: Commonalities amid Diversity. Microbiol. Mol. Biol. Rev. 2017, 81, e00066-16. [Google Scholar] [CrossRef]
- Kale, S.D. Oomycete and Fungal Effector Entry, a Microbial Trojan Horse. New Phytol. 2012, 193, 874–881. [Google Scholar] [CrossRef]
- Ai, G.; Yang, K.; Ye, W.; Tian, Y.; Du, Y.; Zhu, H.; Li, T.; Xia, Q.; Shen, D.; Peng, H.; et al. Prediction and Characterization of RXLR Effectors in Pythium Species. Mol. Plant Microbe Interact. 2019, 33, 1046–1058. [Google Scholar] [CrossRef]
- Deb, D.; Anderson, R.G.; How-Yew-Kin, T.; Tyler, B.M.; McDowell, J.M. Conserved RxLR Effectors from Oomycetes Hyaloperonospora arabidopsidis and Phytophthora sojae Suppress PAMP- and Effector-Triggered Immunity in Diverse Plants. Mol. Plant Microbe Interact. 2018, 31, 374–385. [Google Scholar] [CrossRef]
- Stam, R.; Motion, G.B.; Martinez-Heredia, V.; Boevink, P.C.; Huitema, E. A Conserved Oomycete CRN Effector Targets Tomato TCP14-2 to Enhance Virulence. Mol. Plant Microbe Interact. 2021, 34, 309–318. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes11Edited by F. Cohen. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Sperschneider, J.; Catanzariti, A.-M.; DeBoer, K.; Petre, B.; Gardiner, D.M.; Singh, K.B.; Dodds, P.N.; Taylor, J.M. LOCALIZER: Subcellular Localization Prediction of Both Plant and Effector Proteins in the Plant Cell. Sci. Rep. 2017, 7, 44598. [Google Scholar] [CrossRef] [PubMed]
- Pierleoni, A.; Martelli, P.L.; Casadio, R. PredGPI: A GPI-Anchor Predictor. BMC Bioinform. 2008, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro Protein Families and Domains Database: 20 Years On. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.N.; Hamilton, J.P.; Zerillo, M.M.; Tisserat, N.; Lévesque, C.A.; Buell, C.R. Comparative Genomics Reveals Insight into Virulence Strategies of Plant Pathogenic Oomycetes. PLoS ONE 2013, 8, e75072. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, L.; Jia, Q.; Pan, R.; Oelmüller, R.; Zhang, W.; Wu, C. Arms Race: Diverse Effector Proteins with Conserved Motifs. Plant Signal. Behav. 2019, 14, 1557008. [Google Scholar] [CrossRef]
| Type of Dataset | Sequence Origin | Protein Sequences | Reference |
|---|---|---|---|
| Fungal | EffHunter | 134 | [12] |
| EffectorP v2.0 | 20 | [31] | |
| FunEffector-Pred | 25 | [25] | |
| Predector | 36 | [26] | |
| - * | 13 | This study | |
| Oomycete | EffHunter | 9 | [12] |
| EffectorO | 74 | [27] | |
| - * | 3 | This study |
| Canonical | Non-Canonical | Total | Percentage (%) * | |
|---|---|---|---|---|
| Length <400 amino acids | 142 | 139 | 281 | 89.5 |
| Length >400 amino acids | - | 33 ** | 33 | 10.5 |
| zero cysteine | - | 47 | 47 | 15 |
| 1–3 cysteines | - | 96 | 96 | 30.8 |
| 4–8 cysteines | 111 | 19 | 130 | 41 |
| 9–10 cysteines | 15 | 0 | 15 | 4.7 |
| 11–16 cysteines | 14 | 7 | 21 | 6.8 |
| 17–19 cysteines | 0 | 1 | 1 | 0.3 |
| 20–25 cysteines | 2 | 2 | 4 | 1.3 |
| No signal peptide | - | 47 | 47 | 15 |
| Signal peptide | 142 | 125 | 267 | 85 |
| No TMD | 142 | 143 | 285 | 90.7 |
| TMD | - | 29 & | 29 | 9.3 |
| No GPI | 133 | 170 | 303 | 96. |
| GPI-anchor | 9 | 2 | 11 | 3.5 |
| Extracellular | 113 | 112 | 225 | 71.6 |
| Intracellular | 29 | 60 # | 89 | 28.4 |
| Fungal | % in Fungal Database * | Oomycete | % in Oomycete Database ** | Total | % in Fungal + Oomycete Database | |
|---|---|---|---|---|---|---|
| Canonical | 130 | 57 | 12 | 13.9 | 142 | 45.2 |
| Non-canonical | 98 | 43 | 74 | 86.1 | 172 | 54.8 |
| Length <400 amino acids | 211 | 92.5 | 70 | 81.4 | 281 | 89.4 |
| Length >400 amino acids | 17 | 7.5 | 16 | 18.6 | 36 | 10.6 |
| zero cysteine | 16 | 7 | 31 | 36.1 | 47 | 15 |
| 1–3 cysteines | 59 | 25.9 | 37 | 43.1 | 96 | 30.6 |
| 4–8 cysteines | 116 | 50.9 | 14 | 16.4 | 130 | 41.4 |
| 9–10 cysteines | 14 | 6.1 | 1 | 1.1 | 15 | 4.7 |
| 11–16 cysteines | 19 | 8.4 | 2 | 2.2 | 21 | 6.7 |
| 17–19 cysteines | 0 | - | 1 | 1.1 | 1 | 0.3 |
| 20–25 cysteines | 4 | 1.7 | 0 | - | 4 | 1.3 |
| No signal peptide | 17 | 7.5 | 30 | 34.9 | 47 | 14.9 |
| Signal peptide | 211 | 92.5 | 56 | 65.1 | 267 | 85.1 |
| No TMD | 205 | 89.9 | 80 | 93 | 285 | 90.7 |
| TMD | 23 | 10.1 | 6 | 7 | 29 | 9.3 |
| No GPI | 218 | 95.6 | 85 | 98.8 | 303 | 96.5 |
| GPI-anchor | 10 | 4.4 | 1 | 1.2 | 11 | 3.5 |
| Extracellular | 174 | 76.3 | 51 | 59.3 | 225 | 71.6 |
| Intracellular | 54 | 23.7 | 35 | 40.7 | 89 | 28.4 |
| Domain | Fungi | Oomycete | Total | Function |
|---|---|---|---|---|
| Glycosyl hydrolase | 13 | 2 | 15 | Glycoside hydrolase |
| LysM | 13 | - | 13 | Peptidoglycan binding |
| RXLR signature | - | 11 | 11 | Effector translocation into host cells |
| Pectin lyase fold | 7 | 1 | 8 | Pectolytic enzyme, pectin lyase, which acts as a virulence factor. |
| RlpA | 7 | - | 7 | Transglycolase, endoglucanase. Lytic transglycosylase with a strong preference for naked glycan strands |
| CFEM domain | 6 | - | 6 | Fungal specific cysteine-rich domain, found in some proteins involved in fungal pathogenesis |
| NPP1 | 4 | 1 | 5 | Necrosis-inducing protein |
| Cerato-platanin | 4 | - | 4 | Functional similarities with expansins; may facilitate the mechanical penetration of fungi |
| Peptidase_A1 | 4 | - | 4 | Protease |
| Metalloprotease | 4 | - | 4 | Protease |
| Crinkler | 1 | 3 | 4 | CRN proteins participate in processes controlling plant cell death and immunity |
| PROKAR lipoprotein | 1 | 3 | 4 | Relatedto prokaryotic membrane lipoproteins. Domain present in enzymes, inhibitors, transporters, structural proteins, and virulence factors |
| Chitin binding Peritrophin-A domain | 3 | - | 3 | A six-conserved-cysteine domain found in chitin binding proteins, chitinases |
| Elicitin signature | - | 3 | 3 | Signature present in some oomycete extracellular avirulence or virulence factors |
| Nudix Box | 1 | 1 | 2 | Present in pyrophosphohydrolases, isopentenyl diphosphate isomerases, adenine/guanine mismatch-specific adenine glycosylases (A/G-specific adenine glycosylases), and non-enzymatic activities involved in protein/protein interaction and transcriptional regulation |
| Fungal cellulose binding domain | 2 | - | 2 | Cellulose binding |
| Aspartic peptidase, active sit | 2 | - | 2 | Protease |
| Thiamine binding | 2 | - | 2 | Role in protein-protein interactions |
| alpha/beta hydrolase | 2 | - | 2 | Domain in hydrolytic enzymes of widely differing phylogenetic origin and catalytic function |
| Egh16 | 2 | - | 2 | Virulence factor |
| Nis1 | 2 | - | 2 | Play critical roles in plant-microbe interactions (be required for pathogen virulence), but specific functions are still unknown |
| Fungal hydrophobin signature | 2 | - | 2 | Spontaneously assemble into amphipathic layers at hydrophilic-hydrophobic interfaces |
| ToxA | 2 | - | 2 | Proteinaceous host-selective toxin. Cause cell death in susceptible wheat cultivars |
| Subtilisin | 2 | - | 2 | Peptidase S8 |
| Chymotrypsin | - | 2 | 2 | Peptidase S1A, serine protease |
| Kazal | - | 2 | 2 | Serine protease inhibitor |
| Concanavalin A-like lectin | 1 | 1 | 2 | Carbohydrate binding |
| Cutinase signature | 1 | 1 | 2 | Cutin alpha/beta hydrolase |
| Domain of unknown function | 1 | 1 | 2 | No characterized function |
| Zinc finger CCHC-type | 1 | 1 | 2 | High-affinity binding to single-stranded nucleic acids, especially single-stranded RNAs. |
| RAB5, RABX5 | 1 | - | 1 | Key factor in early endocytosis |
| Hce2 | 1 | - | 1 | Putative necrosis-inducing factor |
| M35_deuterolysin_like | 1 | - | 1 | Lysine-specific metallo-endopeptidase |
| Alternaria alternata allergen 1 | 1 | - | 1 | In fungal exclusive protein family, with unknown function. Commonly secreted by fungi in Alternaria genus |
| ToxB | 1 | - | 1 | Proteinaceous host-selective toxin that causes chlorophyll degradation and foliar chlorosis |
| Isochorismatase | 1 | - | 1 | Conversion of isochorismate into 2,3-dihydroxybenzoate and pyruvate; disrupts the plant salicylate metabolism pathway |
| Fungal_RNase | 1 | - | 1 | Guanine-specific ribonuclease |
| VPS9 | 1 | - | 1 | Vacuolar protein sorting-associated protein |
| Beta-lactamase-inhibitor protein II | 1 | - | 1 | Inhibitors of class A β-lactamases |
| Allergen V5/Tpx-1 family signature | 1 | - | 1 | Domain present in mammalian testis-specific protein (Tpx-1); venom allergen 5 from vespid wasps and venom allergen 3 from fire ants. The function in pathogen proteins is unclear |
| Rhomboid domain | 1 | - | 1 | Conserved domain in some proteases, that cleaves type-1 transmembrane domains using a catalytic dyad composed of serine and histidine. Peptidase S54 |
| Mitochondrial carrier domain | 1 | - | 1 | Mitochondrial basic amino acids transporter |
| Integrin | 1 | - | 1 | Ubiquitously cell surface receptors involved in regulating the cell interaction |
| AroQ | 1 | - | 1 | Chorismate mutase. Suppression of plant immunity by manipulating the salicylic acid pathway |
| Pyridoxal phosphate-dependent transferase, major domain | 1 | - | 1 | Cys/Met metabolism |
| PAN domain | 1 | - | 1 | Mediation of protein-protein and protein-carbohydrate interactions |
| MD-2 | 1 | - | 1 | Lipid-recognition domain |
| Ribonuclease/ribotoxin; | 1 | - | 1 | Extracellular guanyl-specific ribonuclease |
| Ribonuclease Inhibitor | 1 | - | 1 | Enzyme that inhibits RNase activity |
| Fungal calcium binding | 1 | - | 1 | Involved in events where calcium is a second messenger |
| Chitin biosynthesis protein CHS5 | 1 | - | 1 | Found at the N-terminus of fungal chitin biosynthesis protein CHS5. It functions as a dimerization domain |
| Fungalysin (M36)/Thermolysin signature | 1 | - | 1 | Metallopeptidase |
| Lipase (class 3) | 1 | - | 1 | Triacylglycerol lipase |
| Tetratricopeptide repeat domain | - | 1 | 1 | Module for protein interaction and mediators for multiprotein complex |
| Cystatin/monellin | - | 1 | 1 | Cysteine protease inhibitors |
| RuvA domain | - | 1 | 1 | Domain related to prokaryotic proteins; DNA helicase that binds DNA at Holliday junction and promotes ATP-dependent branch migration on the hetero-duplex |
| Database | Protein Sequences | Domain | No Domain |
|---|---|---|---|
| Fungi | 228 | 99 (65 C, 34 NC) | 129 (65 C, 64 NC) |
| Oomycetes | 86 | 34 (12 C, 22 NC) | 52 (52 NC) |
| Total | 317 | 133 (77 C, 56 NC) | 181 (65 C, 116 NC) |
| MEME ID | Num. of Hits in the Positive db * | Width | E-Value | Best Possible Match |
|---|---|---|---|---|
| Fungal positive database | ||||
| MEME-1 | 7 | 50 | 4.60 × 10−143 | GHNTDGFDIGSSNHITIDGAHVYNQDDCMAINSGTNITFTNGYCSGGHGL |
| MEME-2 | 6 | 49 | 8.30 × 10−98 | DGTRVIFEGRTTFGYQEWEGPLISISGKNIKVKGAPGNKIDGDGARWWD |
| MEME-3 | 7 | 50 | 4.60 × 10−98 | NVTYEDITLSEISKYGIVVQQDYKNGKPTGTPTTGVPITNITFNKVTGNV |
| MEME-4 | 7 | 40 | 2.80 × 10−61 | SIGSVGGRSDNTVKDVHIANSKVTKSMNGVRIKTVAGATG |
| MEME-5 | 4 | 50 | 2.40 × 10−58 | YDNVPVTLKKQGIIAKNAYSLYLNSPDAATGQIIFGGVDNAKYSGSLIAL |
| MEME-6 | 4 | 50 | 8.50 × 10−55 | QPYDKCQLLFGVNDANILGDNFLRSAYIVYDLDDNEISLAQVKYTSASNI |
| MEME-7 | 4 | 50 | 8.30 × 10−51 | PFSIEYGDGSSSQGTWYKDTVGFGGISIKKQQFADVTSTSIDQGILGIGY |
| MEME-8 | 173 | 8 | 1.60 × 10−43 | MKFFTILL |
| MEME-9 | 4 | 41 | 1.60 × 10−40 | KRQAVPVTLINEQVSYAADITVGSNKQKLNVIIDTGSSDLW |
| MEME-10 | 4 | 50 | 3.10 × 10−37 | YLAPMYKGKLAFDYPPDDGEIDFLFEQIFNKYGQQWFSELHQQHPRWHRG |
| MEME-11 | 2 | 50 | 8.33 × 10−28 | ICQQYNANFRFNSGFCSGKDRRWDCYDLNFPTTQSERRVQRRRVCRGEHQ |
| MEME-12 | 2 | 50 | 5.13 × 10−27 | QFYDQDNGDYEYFNLSEICDRYQEQDGTVVIEHILVNDRQGRACAMMMIK |
| MEME-13 | 4 | 37 | 8.40 × 10−27 | CKDTSKGQTYVRGAWHGGKYGIMYAWYMPKDQPATGN |
| MEME-14 | 6 | 29 | 4.00 × 10−27 | AAQAIQKKTSCSTITLRNLKVPAGKTLDL |
| MEME-15 | 6 | 39 | 3.70 × 10−36 | GNSEITNLNILNWPVHCFSINHAEGLTIFNINIDNSAGD |
| Oomycete positive database | ||||
| MEME-1 | 3 | 50 | 8.20 × 10−29 | SFQGCADDSGFSLLYSTALPDDDQYVKMCASDNCKSLIESVASLNPPNCD |
| MEME-2 | 59 | 11 | 1.00 × 10−29 | RHLRSHYQDEE |
| MEME-3 | 28 | 22 | 9.90 × 10−19 | LYEHWHMRGCTPEHVYTILKLN |
| MEME-4 | 2 | 31 | 9.50 × 10−10 | CPEMCLDVYDPVGDGEGNEYSNQCYMEMAKC |
| MEME-5 | 36 | 14 | 1.70 × 10−16 | MRLCYFLFVAAAAI |
| MEME-6 | 2 | 39 | 1.30 × 10−7 | CCDMVCPDNEAPVCGSDGERYPNPCELGITACEHPEQNI |
| MEME-7 | 7 | 49 | 4.00 × 10−7 | SPQFQQWMDYISHYNKENPTMQTSLYAALTTHYGDEEMANMLVEAMHSP |
| MEME-8 | 3 | 21 | 4.30 × 10−6 | MVKLYCAVVGVAGSAFPVDID |
| MEME-9 | 2 | 43 | 5.00 × 10−5 | GGGIIPVGQKTYSVGIRSTAGGDTFCGGALISPTHVLTTTMCT |
| MEME-10 | 2 | 40 | 7.90 × 10−5 | FAPVKLPKADGSDIKPGMWSKAMGWGWTSFPNGARANEMQ |
| MEME-11 | 2 | 36 | 4.00 × 10−3 | CNCVYVIGPSEVCAGGEEGKDKCVGDTGGPLIKENG |
| MEME-12 | 3 | 50 | 6.30 × 10−5 | PCSGLCLNVVDLTCGFSGKCSSSSCTSNTASCAATSGTTEAPAATCAAPT |
| MEME-13 | 7 | 9 | 8.50 × 10−3 | PVFNIWLEY |
| MEME-14 | 3 | 39 | 1.20 × 10−1 | SPLQRTDEVQHQPDVDDKTNRFLTSEDKDLPLLVTSDGY |
| MEME-15 | 2 | 30 | 1.30 × 10−1 | WVAVGTHYVNGTKDGEQLKVIQAQNHTDFN |
| WideEffHunter | ||||||||||
| Data | Proteins type | Total proteins | Results | Prediction | Sen/Rec | Spe | PPV/Prec | ACC | FPR | F1 score |
| Set 1 | Fungi | 228 | 228 | 1859 | 1 | 0.658 | 0.168 | 0.68 | 0.341 | 0.287 |
| Set 2 | Oomycete | 86 | 86 | |||||||
| Set 3 | Negatives | 4528 | 1545 | |||||||
| Set 3 | Negatives | 4528 | 192 | 506 | 1 | 0.957 | 0.62 | 0.96 | 0.042 | 0.765 |
| EffectorP 3.0 | ||||||||||
| Data | Proteins type | Total proteins | Results | Prediction | Sen/Rec | Spe | PPV/Prec | ACC | FPR | F1 score |
| Set 1 | Fungi | 228 | 184 | 476 | 0.845 | 0.952 | 0.557 | 0.945 | 0.047 | 0.669 |
| Set 2 | Oomycete | 86 | 79 | |||||||
| Set 3 | Negatives | 4528 | 213 | |||||||
| EffectorP 2.0 | ||||||||||
| Data | Proteins type | Total proteins | Results | Prediction | Sen/Rec | Spe | PPV/Prec | ACC | FPR | F1 score |
| Set 1 | Fungi | 228 | 153 | 243 | 0.564 | 0.985 | 0.736 | 0.958 | 0.014 | 0.638 |
| Set 2 | Oomycete | 86 | 26 | |||||||
| Set 3 | Negatives | 4528 | 64 | |||||||
| EffectorP 1.0 | ||||||||||
| Data | Proteins type | Total proteins | Results | Prediction | Sen/Rec | Spe | PPV/Prec | ACC | FPR | F1 score |
| Set 1 | Fungi | 228 | 142 | 255 | 0.579 | 0.983 | 0.713 | 0.957 | 0.016 | 0.639 |
| Set 2 | Oomycete | 86 | 40 | |||||||
| Set 3 | Negatives | 4528 | 73 | |||||||
| EffectorO | ||||||||||
| Data | Proteins type | Total proteins | Results | Prediction | Sen/Rec | Spe | PPV/Prec | ACC | FPR | F1 score |
| Set 1 | Fungi | 228 | 97 | 961 | 0.573 | 0.827 | 0.187 | 0.811 | 0.172 | 0.281 |
| Set 2 | Oomycete | 86 | 83 | |||||||
| Set 3 | Negatives | 4528 | 781 | |||||||
| Species | Proteome | Effector Prediction in Reference | Reference | Criteria for Effector Prediction | WideEffHunter 1 | WideEffHunter 2 | EffectorP 1.0 | EffectorP 2.0 | EffectorP 3.0 | EffectorO |
|---|---|---|---|---|---|---|---|---|---|---|
| Puccinia triticina | 15,685 | 904 | [15] | Motifs | 4334 | 2805 | 4162 | 2570 | 7488 | 11,782 |
| Venturia inaequalis | 13,233 | 1369 | [42] | Homology to known effectors | 3847 | 2158 | 2744 | 1832 | 5524 | 8968 |
| Phytophthora infestans | 17,797 | 5814 | [27] | Motif- search and lineage-specific phylogenetic distribution | 7143 | 3811 | 4749 | 3091 | 8879 | 11,952 |
| Bremia lactucae | 10,102 | 1777 | [27] | Motif- search and lineage-specific phylogenetic distribution | 3317 | 1812 | 2435 | 1625 | 4884 | 6355 |
| Trichoderma harzianum | 14,095 | 307 | [12] | Size ≤400 amino acids, SP, No TMD, ≥4 Cys | 4935 | 2693 | 2893 | 1772 | 4900 | 8318 |
| Pestalotiopsis fici | 15,413 | 381 | [43] | Small secreted cysteine-rich proteins, with no conserved domain, with nuclear localization signal (NLS), and repeated sequences (Repeat-containing proteins, or (RCPs) | 5201 | 2524 | 1907 | 1236 | 4488 | 9319 |
| Xylona heveae | 8205 | 84 | [43] | Small secreted cysteine-rich proteins, with no conserved domain, with nuclear localization signal (NLS), and repeated sequences (Repeat-containing proteins, or (RCPs) | 2828 | 1517 | 1322 | 756 | 2819 | 5680 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreón-Anguiano, K.G.; Todd, J.N.A.; Chi-Manzanero, B.H.; Couoh-Dzul, O.J.; Islas-Flores, I.; Canto-Canché, B. WideEffHunter: An Algorithm to Predict Canonical and Non-Canonical Effectors in Fungi and Oomycetes. Int. J. Mol. Sci. 2022, 23, 13567. https://doi.org/10.3390/ijms232113567
Carreón-Anguiano KG, Todd JNA, Chi-Manzanero BH, Couoh-Dzul OJ, Islas-Flores I, Canto-Canché B. WideEffHunter: An Algorithm to Predict Canonical and Non-Canonical Effectors in Fungi and Oomycetes. International Journal of Molecular Sciences. 2022; 23(21):13567. https://doi.org/10.3390/ijms232113567
Chicago/Turabian StyleCarreón-Anguiano, Karla Gisel, Jewel Nicole Anna Todd, Bartolomé Humberto Chi-Manzanero, Osvaldo Jhosimar Couoh-Dzul, Ignacio Islas-Flores, and Blondy Canto-Canché. 2022. "WideEffHunter: An Algorithm to Predict Canonical and Non-Canonical Effectors in Fungi and Oomycetes" International Journal of Molecular Sciences 23, no. 21: 13567. https://doi.org/10.3390/ijms232113567
APA StyleCarreón-Anguiano, K. G., Todd, J. N. A., Chi-Manzanero, B. H., Couoh-Dzul, O. J., Islas-Flores, I., & Canto-Canché, B. (2022). WideEffHunter: An Algorithm to Predict Canonical and Non-Canonical Effectors in Fungi and Oomycetes. International Journal of Molecular Sciences, 23(21), 13567. https://doi.org/10.3390/ijms232113567






