Scoping Review on Platelets and Tumor Angiogenesis: Do We Need More Evidence or Better Analysis?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Literature Search
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
- (a)
- Platelets (count, distribution width, volume)
- (b)
- angiogenesis (endothelial cell tube formation, endothelial cell proliferation, endothelial cell migration, microvessel density, angiogenic growth factor concentration)
- (c)
- as for preclinical studies, tumor progression (volume, proliferation, metastasis, epithelial mesenchymal transition (EMT), stemness, invasiveness)
- (d)
- as for clinical studies, clinical outcomes (overall survival, progression-free survival, objective response rate).
- (e)
- antiangiogenic drugs (type)
3. Results and Discussion
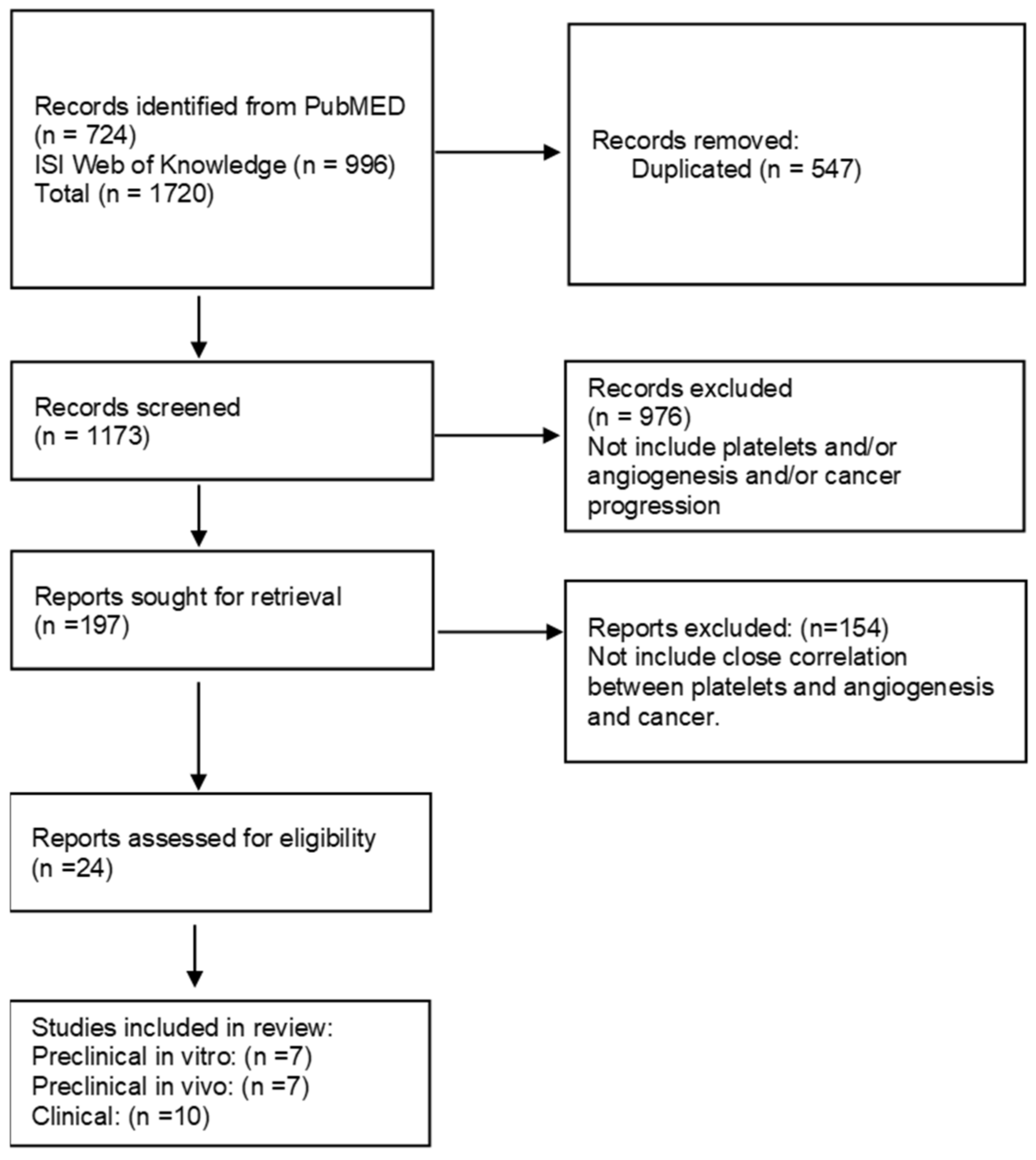
3.1. Literature Search Results
3.2. Preclinical Studies on Cells
3.3. Preclinical Studies on In Vivo Tumor Models
3.4. Clinical Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef]
- Olsson, A.; Cedervall, J. The pro-inflammatory role of platelets in cancer. Platelets 2018, 29, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Hilger, A.; Zarbock, A.; Rossaint, J. Platelets at the Crossroads of Pro-Inflammatory and Resolution Pathways during Inflammation. Cells 2022, 11, 1957. [Google Scholar] [CrossRef]
- Katayama, Y.; Uchino, J.; Chihara, Y.; Tamiya, N.; Kaneko, Y.; Yamada, T.; Takayama, K. Tumor Neovascularization and Developments in Therapeutics. Cancers 2019, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a Source and Target of H2S to Improve Its Trophism and Function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005, 8, 299–309. [Google Scholar] [CrossRef]
- Parenti, A.; Morbidelli, L.; Ledda, F.; Granger, H.J.; Ziche, M. The bradykinin/B1 receptor promotes angiogenesis by up-regulation of endogenous FGF-2 in endothelium via the nitric oxide synthase pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 1487–1489. [Google Scholar] [CrossRef]
- Finetti, F.; Solito, R.; Morbidelli, L.; Giachetti, A.; Ziche, M.; Donnini, S. Prostaglandin E2 regulates angiogenesis via activation of fibroblast growth factor receptor-1. J. Biol. Chem. 2008, 283, 2139–2146. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Filippelli, A.; Ciccone, V.; Donnini, S.; Ziche, M.; Morbidelli, L. Molecular Mechanisms of Resistance to Anti-Angiogenic Drugs. Crit. Rev. Oncog. 2021, 26, 39–66. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Terzuoli, E.; Donnini, S.; Giachetti, A.; Morbidelli, L.; Ziche, M. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1α/VEGF signalling in MCF-7 breast cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 311. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Baluk, P.; Kaidoh, T.; Haskell, A.; Jain, R.K.; McDonald, D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002, 160, 985–1000. [Google Scholar] [CrossRef]
- Roweth, H.G.; Battinelli, E.M. Lessons to learn from tumor-educated platelets. Blood 2021, 137, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Italiano, J.E. 2-Megakaryocyte Development and Platelet Formation. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 25–46. ISBN 978-0-12-813456-6. [Google Scholar]
- Gianazza, E.; Brioschi, M.; Baetta, R.; Mallia, A.; Banfi, C.; Tremoli, E. Platelets in Healthy and Disease States: From Biomarkers Discovery to Drug Targets Identification by Proteomics. Int. J. Mol. Sci. 2020, 21, 4541. [Google Scholar] [CrossRef]
- Quach, M.E.; Chen, W.; Li, R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018, 131, 1512–1521. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Watson, S.P.; Harrison, P.; Halford, G.M. Platelets: The next decade. Platelets 2020, 31, 1–2. [Google Scholar] [CrossRef]
- Savage, B.; McFadden, P.R.; Hanson, S.R.; Harker, L.A. The Relation of Platelet Density to Platelet Age: Survival of Low- and High-Density 111Indium-Labeled Platelets in Baboons. Blood 1986, 68, 386–393. [Google Scholar] [CrossRef]
- Freson, K.; Devriendt, K.; Matthijs, G.; Van Hoof, A.; De Vos, R.; Thys, C.; Minner, K.; Hoylaerts, M.F.; Vermylen, J.; Van Geet, C. Platelet characteristics in patients with X-linked macrothrombocytopenia because of a novel GATA1 mutation. Blood 2001, 98, 85–92. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Gasic, G.J.; Gasic, T.B.; Galanti, N.; Johnson, T.; Murphy, S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int. J. Cancer 1973, 11, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Gasic, G.J.; Gasic, T.B.; Stewart, C.C. Antimetastatic effects associated with platelet reduction. Proc. Natl. Acad. Sci. USA 1968, 61, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Camerer, E.; Qazi, A.A.; Duong, D.N.; Cornelissen, I.; Advincula, R.; Coughlin, S.R. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 2004, 104, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Revenko, A.S.; Tibbitts, J.; Ngo, A.T.P.; Mitrugno, A.; Healy, L.D.; Johnson, J.; Tucker, E.I.; Hinds, M.T.; Coussens, L.M.; et al. Hepatic thrombopoietin gene silencing reduces platelet count and breast cancer progression in transgenic MMTV-PyMT mice. Blood Adv. 2019, 3, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets guide the formation of early metastatic niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Sharda, A. 19-Platelet Secretion. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 349–370. ISBN 978-0-12-813456-6. [Google Scholar]
- O’Donnell, V.B.; Murphy, R.C.; Watson, S.P. Platelet Lipidomics. Circ. Res. 2014, 114, 1185–1203. [Google Scholar] [CrossRef]
- Möhle, R.; Green, D.; Moore, M.A.S.; Nachman, R.L.; Rafii, S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA 1997, 94, 663–668. [Google Scholar] [CrossRef]
- Gasecka, A.; Nieuwland, R.; Siljander, P.R.-M. 22-Platelet-Derived Extracellular Vesicles. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 401–416. ISBN 978-0-12-813456-6. [Google Scholar]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.I.; Hackeng, T.M.; Weber, C. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005, 105, 924–930. [Google Scholar] [CrossRef]
- Ansari, M.J.; Bokov, D.; Markov, A.; Jalil, A.T.; Shalaby, M.N.; Suksatan, W.; Chupradit, S.; Al-Ghamdi, H.S.; Shomali, N.; Zamani, A.; et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. Signal. 2022, 20, 49. [Google Scholar] [CrossRef]
- Pinedo, H.; Verheul, H.; D’Amato, R.; Folkman, J. Involvement of platelets in tumour angiogenesis? Lancet 1998, 352, 1775–1777. [Google Scholar] [CrossRef]
- Kerr, B.; McCabe, N.; Feng, W.; Byzova, T. Platelets govern pre-metastatic tumor communication to bone. Oncogene 2013, 32, 4319–4324. [Google Scholar] [CrossRef] [PubMed]
- Klement, G.; Yip, T.; Cassiola, F.; Kikuchi, L.; Cervi, D.; Podust, V.; Italiano, J.; Wheatley, E.; Abou-Slaybi, A.; Bender, E.; et al. Platelets actively sequester angiogenesis regulators. Blood 2009, 113, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Heeke, S.; Mograbi, B.; Alix-Panabières, C.; Hofman, P. Never Travel Alone: The Crosstalk of Circulating Tumor Cells and the Blood Microenvironment. Cells 2019, 8, 714. [Google Scholar] [CrossRef]
- Italiano, J.; Richardson, J.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef]
- Sehgal, S.; Storrie, B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J. Thromb. Haemost. 2007, 5, 2009–2016. [Google Scholar] [CrossRef]
- Perini, R.; Wallace, J. Proteinase-activated receptors (PARs), platelets and angiogenesis. Drug Dev. Res. 2003, 59, 395–399. [Google Scholar] [CrossRef]
- Chatterjee, M.; Huang, Z.; Zhang, W.; Jiang, L.; Hultenby, K.; Zhu, L.; Hu, H.; Nilsson, G.P.; Li, N. Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood 2011, 117, 3907–3911. [Google Scholar] [CrossRef]
- Battinelli, E.; Markens, B.; Italiano, J. Release of angiogenesis regulatory proteins from platelet alpha granules: Modulation of physiologic and pathologic angiogenesis. Blood 2011, 118, 1359–1369. [Google Scholar] [CrossRef]
- Folkman, J.; Cervi, D.; Klement, G.; Italiano, J.; Yip, T. The platelet angiogenesis proteome for early detection of cancer. FASEB J. 2006, 20, A414–A415. [Google Scholar] [CrossRef]
- Almog, N.; Henke, V.; Flores, L.; Hlatky, L.; Kung, A.L.; Wright, R.D.; Berger, R.; Hutchinson, L.; Naumov, G.N.; Bender, E.; et al. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Naumov, G.N.; Bender, E.; Zurakowski, D.; Kang, S.-Y.; Sampson, D.; Flynn, E.; Watnick, R.S.; Straume, O.; Akslen, L.A.; Folkman, J.; et al. A model of human tumor dormancy: An angiogenic switch from the nonangiogenic phenotype. J. Natl. Cancer Inst. 2006, 98, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Cervi, D.; Yip, T.; Bhattacharya, N.; Podust, V.; Peterson, J.; Abou-Slaybi, A.; Naumov, G.; Bender, E.; Almog, N.; Italiano, J.; et al. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood 2008, 111, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, O.; Varon, D.; Brill, A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int. J. Cancer 2009, 124, 1773–1777. [Google Scholar] [CrossRef]
- Amirkhosravi, A.; Meyer, T.; Sackel, D.; Desai, I.; Biddinger, R.; Amaya, M.; Francis, J. Platelet microparticles upregulate TF and VEGF in endothelial and melanoma cells in a CD40 ligand-dependent manner: Possible role in angiogenesis and metastasis. Blood 2002, 100, 63B. [Google Scholar]
- Grover, S.P.; Bergmeier, W.; Mackman, N. Platelet Signaling Pathways and New Inhibitors. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e28–e35. [Google Scholar] [CrossRef]
- Bohula, E.A.; Aylward, P.E.; Bonaca, M.P.; Corbalan, R.L.; Kiss, R.G.; Murphy, S.A.; Scirica, B.M.; White, H.; Braunwald, E.; Morrow, D.A. Efficacy and Safety of Vorapaxar With and Without a Thienopyridine for Secondary Prevention in Patients With Previous Myocardial Infarction and No History of Stroke or Transient Ischemic Attack. Circulation 2015, 132, 1871–1879. [Google Scholar] [CrossRef]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef]
- Donnini, S.; Morbidelli, L.; Taraboletti, G.; Ziche, M. ERK1-2 and p38 MAPK regulate MMP/TIMP balance and function in response to thrombospondin-1 fragments in the microvascular endothelium. Life Sci. 2004, 74, 2975–2985. [Google Scholar] [CrossRef]
- Feng, W.; Madajka, M.; Kerr, B.; Mahabeleshwar, G.; Whiteheart, S.; Byzova, T. A novel role for platelet secretion in angiogenesis: Mediating bone marrow-derived cell mobilization and homing. Blood 2011, 117, 3893–3902. [Google Scholar] [CrossRef] [PubMed]
- Ho-Tin-Noe, B.; Demers, M.; Wagner, D. How platelets safeguard vascular integrity. J. Thromb. Haemost. 2011, 9, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ho-Tin-Noe, B.; Goerge, T.; Wagner, D. Platelets: Guardians of Tumor Vasculature. Cancer Res. 2009, 69, 5623–5626. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Syvannarath, V.; Lamrani, L.; Ollivier, V.; Loyau, S.; Goerge, T.; Nieswandt, B.; Jandrot-Perrus, M.; Ho-Tin-Noé, B. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood 2015, 126, 1017–1026. [Google Scholar] [CrossRef]
- Kisucka, J.; Butterfield, C.; Duda, D.; Eichenberger, S.; Saffaripour, S.; Ware, J.; Ruggeri, Z.; Jain, R.; Folkman, J.; Wagner, D. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc. Natl. Acad. Sci. USA 2006, 103, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.; Goldfinger, L. Platelets and extracellular vesicles and their cross talk with cancer. Blood 2021, 137, 3192–3200. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; U.S. Preventive Services Task Force. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836–845. [Google Scholar] [CrossRef]
- Giacomazzi, A.; Degan, M.; Calabria, S.; Meneguzzi, A.; Minuz, P. Antiplatelet Agents Inhibit the Generation of Platelet-Derived Microparticles. Front. Pharmacol. 2016, 7, 314. [Google Scholar] [CrossRef]
- Volz, J.; Mammadova-Bach, E.; Gil-Pulido, J.; Nandigama, R.; Remer, K.; Sorokin, L.; Zernecke, A.; Abrams, S.; Ergun, S.; Henke, E.; et al. Inhibition of platelet GPVI induces intratumor hemorrhage and increases efficacy of chemotherapy in mice. Blood 2019, 133, 2696–2706. [Google Scholar] [CrossRef]
- Demers, M.; Ho-Tin-Noé, B.; Schatzberg, D.; Yang, J.J.; Wagner, D.D. Increased Efficacy of Breast Cancer Chemotherapy in Thrombocytopenic Mice. Cancer Res. 2011, 71, 1540–1549. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Liu, Z.; Chen, G.; Li, X.; Ren, H. Damaging Tumor Vessels with an Ultrasound-Triggered NO Release Nanosystem to Enhance Drug Accumulation and T Cells Infiltration. Int. J. Nanomed. 2021, 16, 2597–2613. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Caine, G.; Lip, G.; Blann, A. Platelet-derived VEGF, Flt-1, angiopoietin-1 and P-selectin in breast and prostate cancer: Further evidence for a role of platelets in tumour angiogenesis. Ann. Med. 2004, 36, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Huong, P.T.; Nguyen, L.T.; Nguyen, X.-B.; Lee, S.K.; Bach, D.-H. The Role of Platelets in the Tumor-Microenvironment and the Drug Resistance of Cancer Cells. Cancers 2019, 11, 240. [Google Scholar] [CrossRef]
- Augustine, T. The aegis: Platelets as biomarkers of tumor progression. Biomark. Med. 2020, 14, 573–586. [Google Scholar] [CrossRef]
- Wu, H.; Fan, F.; Liu, Z.; Zhang, F.; Liu, Y.; Wei, Z.; Shen, C.; Cao, Y.; Wang, A.; Lu, Y. The angiogenic responses induced by release of angiogenic proteins from tumor cell-activated platelets are regulated by distinct molecular pathways. IUBMB Life 2015, 67, 626–633. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, M.; Li, J.; Yang, R.; Du, J.; Luo, Y. High Platelet Levels Attenuate the Efficacy of Platinum-Based Treatment in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2018, 48, 2456–2469. [Google Scholar] [CrossRef]
- Yao, L.; Dong, H.; Luo, Y.; Du, J.; Hu, W. Net Platelet Angiogenic Activity (NPAA) Correlates with Progression and Prognosis of Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e96206. [Google Scholar] [CrossRef]
- Li, B.; Dong, X.; Zhu, J.; Zhu, T.; Tao, X.; Peng, D.; Li, Q. Crosstalk between H1975 tumor cells and platelets to induce the proliferation, migration and tube formation of vascular endothelial cells. Oncol. Lett. 2021, 22, 676. [Google Scholar] [CrossRef]
- Jiang, L.; Luan, Y.; Miao, X.; Sun, C.; Li, K.; Huang, Z.; Xu, D.; Zhang, M.; Kong, F.; Li, N. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br. J. Cancer 2017, 117, 695–703. [Google Scholar] [CrossRef]
- Erices, R.; Cubillos, S.; Aravena, R.; Santoro, F.; Marquez, M.; Orellana, R.; Ramirez, C.; Gonzalez, P.; Fuenzalida, P.; Bravo, M.; et al. Diabetic concentrations of metformin inhibit platelet-mediated ovarian cancer cell progression. Oncotarget 2017, 8, 20865–20880. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, C.; Navone, S.; Marfia, G.; Hadi, L.; Mancuso, M.; Pecci, A.; Crisa, F.; Berno, V.; Rampini, P.; Campanella, R.; et al. Platelets from glioblastoma patients promote angiogenesis of tumor endothelial cells and exhibit increased VEGF content and release. Platelets 2017, 28, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ren, M.; Chen, N.; Luo, M.; Deng, X.; Xia, J.; Yu, G.; Liu, J.; He, B.; Zhang, X.; et al. Presence of intratumoral platelets is associated with tumor vessel structure and metastasis. BMC Cancer 2014, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, A.; Baek, K.; Lynch, R.; Short, S.; Grillo, J.; Folkman, J.; Italiano, J.; Ryeom, S. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood 2010, 115, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, X. Platelets are associated with xenograft tumor growth and the clinical malignancy of ovarian cancer through an angiogenesis-dependent mechanism. Mol. Med. Rep. 2015, 11, 2449–2458. [Google Scholar] [CrossRef]
- Martini, C.; Thompson, E.; Hyslop, S.; Cockshell, M.; Dale, B.; Ebert, L.; Woods, A.; Josefsson, E.; Bonder, C. Platelets disrupt vasculogenic mimicry by cancer cells. Sci. Rep. 2020, 10, 5869. [Google Scholar] [CrossRef]
- Brockmann, M.; Bender, B.; Plaxina, E.; Nolte, I.; Erber, R.; Lamszus, K.; Groden, C.; Schilling, L. Differential effects of tumor-platelet interaction in vitro and in vivo in glioblastoma. J. Neurooncol. 2011, 105, 45–56. [Google Scholar] [CrossRef]
- Chater, C.; Bauters, A.; Beugnet, C.; M’Ba, L.; Rogosnitzky, M.; Zerbib, P. Intraplatelet Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor: New Biomarkers in Carcinoembryonic Antigen-Negative Colorectal Cancer? Gastrointest. Tumors 2018, 5, 32–37. [Google Scholar] [CrossRef]
- Han, H.; Cao, F.-L.; Wang, B.-Z.; Mu, X.-R.; Li, G.-Y.; Wang, X.-W. Expression of angiogenesis regulatory proteins and epithelial-mesenchymal transition factors in platelets of the breast cancer patients. Sci. World J. 2014, 2014, 878209. [Google Scholar] [CrossRef]
- Kim, H.; Song, K.; Park, Y.; Kang, Y.; Lee, Y.; Lee, K.; Kim, H.; Ryu, K.; Bae, J.; Kim, S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: Possible role of a metastasis predictor. Eur. J. Cancer 2003, 39, 184–191. [Google Scholar] [CrossRef]
- Mayer, E.; Isakoff, S.; Klement, G.; Downing, S.; Chen, W.; Hannagan, K.; Gelman, R.; Winer, E.; Burstein, H. Combination antiangiogenic therapy in advanced breast cancer: A phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res. Treat. 2012, 136, 169–178. [Google Scholar] [CrossRef] [PubMed]
- McDowell, G.; Temple, I.; Li, C.; Kirwan, C.; Bundred, N.; McCollum, C.; Burton, I.; Kumar, S.; Byrne, G. Alteration in platelet function in patients with early breast cancer. Anticancer Res. 2005, 25, 3963–3966. [Google Scholar]
- Sabrkhany, S.; Kuijpers, M.; van Kuijk, S.; Sanders, L.; Pineda, S.; Damink, S.; Dingemans, A.; Griffioen, A.; Egbrink, M. A combination of platelet features allows detection of early-stage cancer. Eur. J. Cancer 2017, 80, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Park, J.; Park, K.; Kim, S.; Oh, S.; Kim, B.; Kim, Y.; Kim, J. Prognostic Significance of Serum Vascular Endothelial Growth Factor Per Platelet Count in Unresectable Advanced Gastric Cancer Patients. Jpn. J. Clin. Oncol. 2010, 40, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Tokyol, C.; Ersoz, G.; Dilek, F.; Gencer, E.; Kosar, M.; Dilek, O. Thrombospondin 1 expression and angiogenesis in breast carcinoma and their relation with platelet activity. Upsala J. Med. Sci. 2009, 114, 108–115. [Google Scholar] [CrossRef]
- Nafady, A.; Saleh, M.; Nafady-Hego, H.; Wahman, M.; Nasif, K.; Sedik, W. Role of circulating endothelial cells and platelet microparticles as markers of angiogenesis in chronic myeloid leukemia. Egypt. J. Haematol. 2018, 43, 171–178. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Basch, R.; Karpatkin, S. Thrombin induces the release of angiopoietin-1 from platelets. Thromb. Haemost. 2001, 85, 204–206. [Google Scholar] [CrossRef]
- Sylman, J.L.; Mitrugno, A.; Tormoen, G.W.; Wagner, T.H.; Mallick, P.; McCarty, O.J.T. Platelet count as a predictor of metastasis and venous thromboembolism in patients with cancer. Converg. Sci. Phys. Oncol. 2017, 3, 023001. [Google Scholar] [CrossRef]
- Michael, J.; Wurtzel, J.; Mao, G.; Rao, A.; Hoffman, N.; Rajan, S.; Madesh, M.; Jana, F.; Nieman, M.; Rowley, J.; et al. Platelet microparticles infiltrating solid tumors transfer miRNAs and modulate tumor angiogenesis and growth. Cancer Res. 2016, 76, 13. [Google Scholar] [CrossRef]
- Okada, K.; Yokoyama, K.; Okihara, K.; Ukimura, O.; Kojima, M.; Miki, T.; Takamatsu, T. Immunohistochemical localization of platelet-derived endothelial cell growth factor expression and its relation to angiogenesis in prostate. Urology 2001, 57, 376–381. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Platelet-induced inhibition of tumor cell growth. Thromb. Res. 2008, 123, 324–330. [Google Scholar] [CrossRef]
- Ibele, G.; Kay, N.; Johnson, G.; Jacob, H. Human platelets exert cytotoxic effects on tumor cells. Blood 1985, 65, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Wurtzel, J.; Lazar, S.; Sikder, S.; Cai, K.; Astsaturov, I.; Weyrich, A.; Rowley, J.; Goldfinger, L. Platelet microRNAs inhibit primary tumor growth via broad modulation of tumor cell mRNA expression in ectopic pancreatic cancer in mice. PLoS ONE 2021, 16, e0261633. [Google Scholar] [CrossRef] [PubMed]
- Handtke, S.; Thiele, T. Large and small platelets-(When) do they differ? J. Thromb. Haemost. 2020, 18, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Chubak, J.; Whitlock, E.P.; Williams, S.B.; Kamineni, A.; Burda, B.U.; Buist, D.S.M.; Anderson, M.L. Aspirin for the Prevention of Cancer Incidence and Mortality: Systematic Evidence Reviews for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 814–825. [Google Scholar] [CrossRef]
- Rodríguez-Miguel, A.; García-Rodríguez, L.A.; Gil, M.; Montoya, H.; Rodríguez-Martín, S.; de Abajo, F.J. Clopidogrel and Low-Dose Aspirin, Alone or Together, Reduce Risk of Colorectal Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 2024–2033.e2. [Google Scholar] [CrossRef]
- Rohwer, N.; Kühl, A.A.; Ostermann, A.I.; Hartung, N.M.; Schebb, N.H.; Zopf, D.; McDonald, F.M.; Weylandt, K.-H. Effects of chronic low-dose aspirin treatment on tumor prevention in three mouse models of intestinal tumorigenesis. Cancer Med. 2020, 9, 2535–2550. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; Wang, Z.; Wu, L.; Zhang, W. Aspirin may inhibit angiogenesis and induce autophagy by inhibiting mTOR signaling pathway in murine hepatocarcinoma and sarcoma models. Oncol. Lett. 2016, 12, 2804–2810. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Wang, Z.; Wang, Z.; Zhang, Y.; Jia, Q.; Wu, L.; Zhang, W. Impact of acetylsalicylic acid on tumor angiogenesis and lymphangiogenesis through inhibition of VEGF signaling in a murine sarcoma model. Oncol. Rep. 2013, 29, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, L.; Vijayan, K. Are Platelets the Primary Target of Aspirin’s Remarkable Anticancer Activity? Cancer Res. 2019, 79, 3820–3823. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yousef, G.; Ni, H. Cancer and platelet crosstalk: Opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018, 131, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef]
- McNeil, J.J.; Gibbs, P.; Orchard, S.G.; Lockery, J.E.; Bernstein, W.B.; Cao, Y.; Ford, L.; Haydon, A.; Kirpach, B.; Macrae, F.; et al. Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. J. Natl. Cancer Inst. 2021, 113, 258–265. [Google Scholar] [CrossRef]
- Miyashita, T.; Tajima, H.; Makino, I.; Nakagawara, H.; Kitagawa, H.; Fushida, S.; Harmon, J.W.; Ohta, T. Metastasis-promoting role of extravasated platelet activation in tumor. J. Surg. Res. 2015, 193, 289–294. [Google Scholar] [CrossRef]
- Saito, H.; Fushida, S.; Miyashita, T.; Oyama, K.; Yamaguchi, T.; Tsukada, T.; Kinoshita, J.; Tajima, H.; Ninomiya, I.; Ohta, T. Potential of extravasated platelet aggregation as a surrogate marker for overall survival in patients with advanced gastric cancer treated with preoperative docetaxel, cisplatin and S-1: A retrospective observational study. BMC Cancer 2017, 17, 294. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, Z.; Feng, W.; Zheng, M.; Xu, Z.; Gao, H.; Li, W.; Zhang, Y.; Zong, Y.; Lu, A.; et al. Platelet infiltration predicts survival in postsurgical colorectal cancer patients. Int. J. Cancer 2022, 150, 509–520. [Google Scholar] [CrossRef]
- Jobe, S. Platelet Heterogeneity. In Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: An Update; Gresele, P., Kleiman, N.S., Lopez, J.A., Page, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 55–67. ISBN 978-3-319-47462-5. [Google Scholar]
- Izquierdo, I.; García, Á. Platelet proteomics applied to the search for novel antiplatelet therapeutic targets. Expert Rev. Proteom. 2016, 13, 993–1006. [Google Scholar] [CrossRef]
- Looße, C.; Swieringa, F.; Heemskerk, J.W.M.; Sickmann, A.; Lorenz, C. Platelet proteomics: From discovery to diagnosis. Expert Rev. Proteom. 2018, 15, 467–476. [Google Scholar] [CrossRef]
- Chen, M.; Hou, L.; Hu, L.; Tan, C.; Wang, X.; Bao, P.; Ran, Q.; Chen, L.; Li, Z. Platelet detection as a new liquid biopsy tool for human cancers. Front. Oncol. 2022, 12, 983724. [Google Scholar] [CrossRef] [PubMed]
- Walraven, M.; Homs, M.; van der Veldt, A.; Dekker, H.; Koldenhof, J.; Honeywell, R.; Barendrecht, A.; Sebastian, S.; Parr, N.; Koekman, A.; et al. Platelet function is disturbed by the angiogenesis inhibitors sunitinib and sorafenib, but unaffected by bevacizumab. Angiogenesis 2018, 21, 325–334. [Google Scholar] [CrossRef]
- Meyer, T.; Robles-Carrillo, L.; Robson, T.; Langer, F.; Desai, H.; Davila, M.; Amaya, M.; Francis, J.; Amirkhosravi, A. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J. Thromb. Haemost. 2009, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef]
- Kempton, C.L.; Hoffman, M.; Roberts, H.R.; Monroe, D.M. Platelet Heterogeneity. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Best, M.G.; Wesseling, P.; Wurdinger, T. Tumor-Educated Platelets as a Noninvasive Biomarker Source for Cancer Detection and Progression Monitoring. Cancer Res. 2018, 78, 3407–3412. [Google Scholar] [CrossRef]
 Platelets; growth/inhibitory factor.
Platelets; growth/inhibitory factor.
 Platelets; growth/inhibitory factor.
Platelets; growth/inhibitory factor.
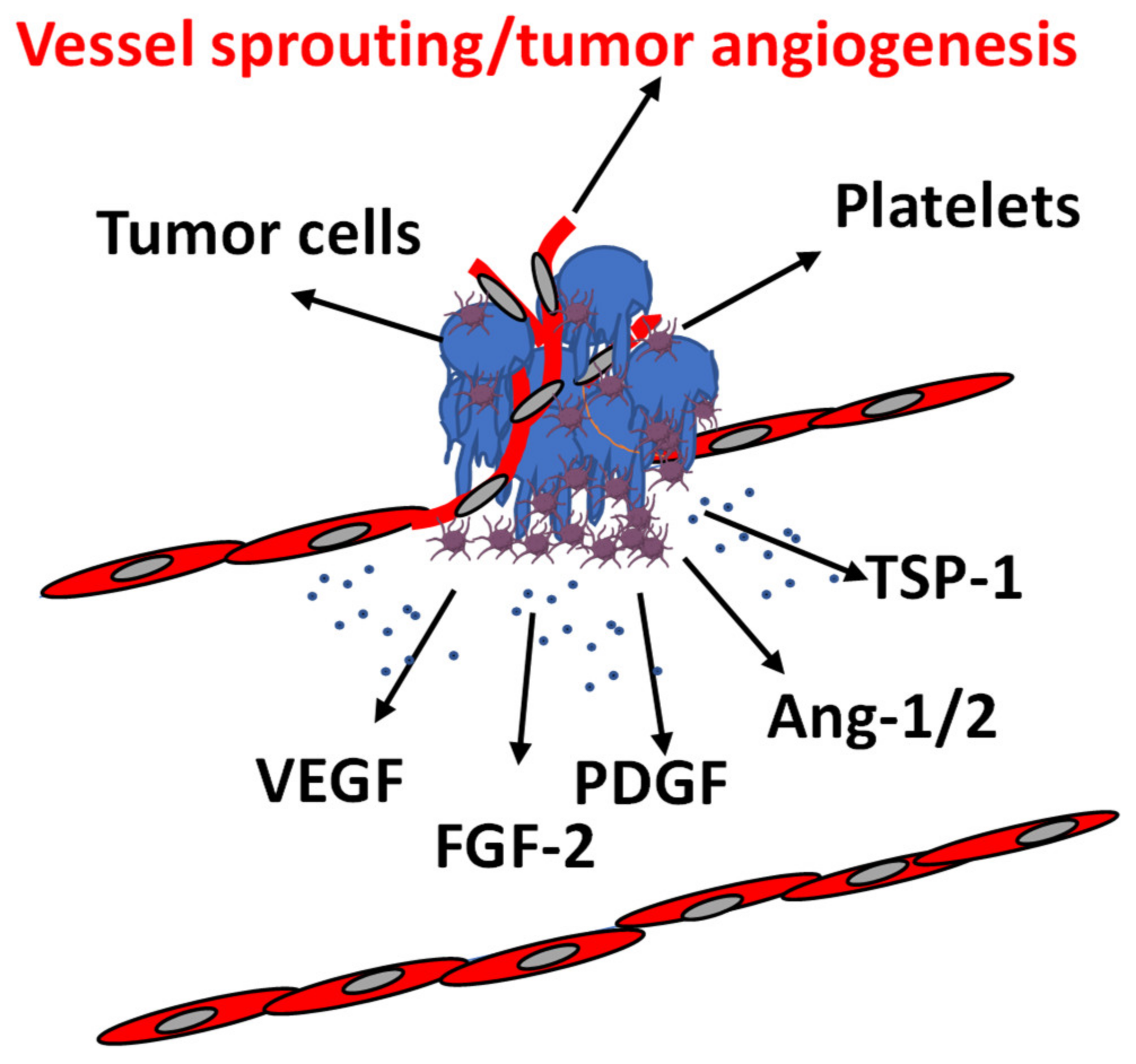
| References | Angiogenic Model | Tumor Cells | Stimulus/i | Effects on Angiogenesis | Effects on Tumor Cells |
|---|---|---|---|---|---|
| Wu et al., 2015; Wang et al., 2018; [69,70] | Endothelial cell tube formation | Lung cancer cells (A549, H1299, H1975) | Platelet releasate | Increase of 25–50% | Proliferation increased 50–70% Cisplatin sensitivity reduced by 50–70% |
| Yao et al., 2014; [71] | Platelet-derived cytokines | Increase of 50–70% | |||
| Li et al., 2021; [72] | Platelet releasate | Increase of 25% | |||
| Jiang et al., 2017; [73] | Breast cancer cells (MCF-7, MDA-MB-231) | PAR1/4-induced platelet releasate | Increase of 25% | Proliferation increased by 50–70% | |
| Erices et al., 2017; [74] | Ovarian cancer cells (SKOV3, UCI101) | Platelet with/without metformin | Increase of 25–50% | Migration increased by 25%; Invasiveness increased by 25–30% | |
| Di Vito et al., 2017; [75] | GBM cells | Platelet releasate | Increase of 50–75% |
| Tumor Model/Cells | Animal Model | Platelets | Tumor Volume | Tumor Weight | Vessel Density | Angiogenic Factors | |
|---|---|---|---|---|---|---|---|
| Li et al., 2014; [76] | B16-F10 murine melanoma | C57BL/6J black mice or BALB/c white mice | Depletion | No differences | No differences | Decreased MVD | Decreased Ang-1 and increased Ang-2 |
| Zaslavsky et al., 2010; [77] | Lewis lung carcinoma and B16F10 murine melanoma | C57BL/6J black mice | Tsp-1−/− | Increase | NR | Decreased MVD | NR |
| Stone et al., 2012; [78] | Epithelial ovarian cancer | White mice | Depletion | NR | Decrease | Decreased MVD | NR |
| Feng et al., 2011; [54] | B16-F10 murine melanoma | C57BL/6J black mice | Infusion | NR | NR | Increased MVD | NR |
| Depletion | NR | NR | Decreased MVD | NR | |||
| Yuan et al., 2015; [79] | SKOV3 Human ovarian cancer cells | White mice | Infusion | Increase | NR | Increased MVD | Increased VEGF |
| Depletion | Decrease | NR | |||||
| Martini et al., 2020; [80] | B16-F10 murine melanoma | Bcl-x Ptl20/Ptl20 black mice on C57BL/6 background | Decrease of 25% | No differences | No differences | Increased MVD | NR |
| Brockmann et al., 2011 [81] | G55T2 glioblastoma cells | NMRI-nu/nu white mice | Depletion | No differences | NR | No differences | NR |
| Ref | Tumor Type | Number of Patients | Stage | PLTs | PLTs Markers | PMP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | MPV | VEGF (pg/106 plts) | IL-6 (pg/mL) | PF4 (ng/106 plts) | TSP-1 (ng/106 plts) | TGFBβ (ng/106 plts) | PDGF (ng/106 plts) | |||||
| Chater et al., 2018; [82] | Colorectal cancer | Pt. 30 | 259 × 109/L | 15.55 pg/actin unit | 304 pg/actin unit | |||||||
| Ctr. 30 | 246 × 109/L | 2.59 pg/actin unit | 125 pg/actin unit | |||||||||
| Di Vito et al., 2016; [75] | Glioblastoma | Pt. 22 | Low VEGF: 238 nL High VEGF: 248 nL | 41.05 (low) 82.66 (high) | ||||||||
| Han et al., 2014; [83] | Breast cancer | Pt. 37 | I/II: Pt 24 III/IV: Pt 13 | Stage I/II 2.37 Stage III/IV 3.38 | Stage I/II 21.6 Stage III/IV 24.4 | 29.2 | Stage I/II 16.5 Stage III/IV 24.6 | Stage I/II 40.4 pg/106 plts Stage III/IV 49.9 pg/106 plts | ||||
| Ctr. 65 | 0.9 | 10.2 | 27.0 | Median 4.3 | Median 19.1 pg/106 plts | |||||||
| Kim et al., 2003; [84] | Gastric cancer | Pt. 109 | I: Pt 28 II/III: Pt 40 IV: Pt 41 | Stage I 234 × 109/mL Stage II/III 227 × 109/mL Stage IV 274 × 109/mL | Plasmatic level Stage I 23.5 pg/mL Stage II/III 24.4 pg/mL Stage IV 30.2 pg/mL | Stage I 3.40 Stage II/III 3.99 Stage IV 6.00 | Stage I 0.76 × 109/mL Stage II/III 0.87 × 109/mL Stage IV 7.16 × 109/mL | |||||
| Ctr. 29 | Plasmatic level 24.6 pg/mL | 2.95 | 0.42 × 109/mL | |||||||||
| Mayer et al., 2012; [85] | Breast cancer | Pt. 23 | ≈1400 ng/mL | ≈600 pg/mL | ||||||||
| Ctr. NR | ≈100 ng/mL | ≈200 pg/mL | ||||||||||
| McDowell et al., 2005; [86] | Breast cancer | Pt. 11 | Thrombin stimulation: 1.423 TF stimulation: 1.045 Time exposure: 0.104 | Thrombin stimulation: 0.218 TF stimulation: 0.045 Time exposure: 0.030 | ||||||||
| Ctr. 11 | Thrombin stimulation: 0.476 TF stimulation: 0.510 Time exposure: 0.017 | Thrombin stimulation: 0.126 TF stimulation: 0.030.1 Time exposure: 0.070 | ||||||||||
| Sabrkhany et al., 2017; [87] | Lung cancer | Pt. 86 | I/II: Pt 39 III/IV: Pt 47 | Stage I/II ≈ 225 × 109/L Stage III/IV ≈ 310 × 109/L | Stage I/II ≈ 7.0 fL Stage III/IV ≈ 6.0 fL | Stage I/II ≈ 0.8 Stage III/IV ≈ 0.75 | Stage I/II ≈ 38 Stage III/IV ≈ 26 | Stage I/II ≈ 63 Stage III/IV ≈ 55 | Stage I/II ≈ 37 Stage III/IV ≈ 46 | |||
| Ctr. NR | ≈210 × 109/L | ≈6.5 fL | ≈0.4 | ≈36 | ≈62 | ≈36 | ||||||
| Pancreatic cancer | Pt.42 | I/II: Pt 29 III/IV: Pt 13 | Stage I/II ≈ 275 × 109/L Stage III/IV ≈ 260 × 109/L | Stage I/II ≈ 7.1 fL Stage III/IV ≈ 7.5 fL | Stage I/II ≈ 0.7 Stage III/IV ≈ 0.6 | |||||||
| Ctr. NR | ≈225 × 109/L | ≈6.5 fL | ≈0.3 | |||||||||
| Seo et al., 2010; [88] | Gastric cancer | Pt.148 | I/II: Pt 21 III: Pt 29 IV: Pt 98 | 240 × 106/mL | Stage I/II: 1.16 Stage III: 2.02 Stage IV: 2 | |||||||
| Tokyol et al., 2009; [89] | Breast cancer | Pt. 20 | 281.359 ± 17.12 (1000/mm3) | |||||||||
| Ctr. 14 | 236.79 ± 12.84 (10,000/mm3) | |||||||||||
| Yao et al., 2014; [71] | Lung cancer (NSCLC) | Pt.68 | I/II: Pt 38 III/IV: Pt 30 | Stage I/II: 20.2 Stage III/IV: 60.86 | Stage I/II 15.1 Stage III/IV 34.5 | |||||||
| Ctr.68 | NR | |||||||||||
| Nafady et al., 2018; [90] | Chronic myeloid leukemia | Pt.60 (30 A + 30 B) | Group A: 391.50 × 109/L Group B: 250.11 × 109/L | Group A 8.67 ± 2.88 Group B 42.5 ± 2.82 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippelli, A.; Del Gaudio, C.; Simonis, V.; Ciccone, V.; Spini, A.; Donnini, S. Scoping Review on Platelets and Tumor Angiogenesis: Do We Need More Evidence or Better Analysis? Int. J. Mol. Sci. 2022, 23, 13401. https://doi.org/10.3390/ijms232113401
Filippelli A, Del Gaudio C, Simonis V, Ciccone V, Spini A, Donnini S. Scoping Review on Platelets and Tumor Angiogenesis: Do We Need More Evidence or Better Analysis? International Journal of Molecular Sciences. 2022; 23(21):13401. https://doi.org/10.3390/ijms232113401
Chicago/Turabian StyleFilippelli, Arianna, Cinzia Del Gaudio, Vittoria Simonis, Valerio Ciccone, Andrea Spini, and Sandra Donnini. 2022. "Scoping Review on Platelets and Tumor Angiogenesis: Do We Need More Evidence or Better Analysis?" International Journal of Molecular Sciences 23, no. 21: 13401. https://doi.org/10.3390/ijms232113401
APA StyleFilippelli, A., Del Gaudio, C., Simonis, V., Ciccone, V., Spini, A., & Donnini, S. (2022). Scoping Review on Platelets and Tumor Angiogenesis: Do We Need More Evidence or Better Analysis? International Journal of Molecular Sciences, 23(21), 13401. https://doi.org/10.3390/ijms232113401







