MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis)
Abstract
1. Introduction
2. Results
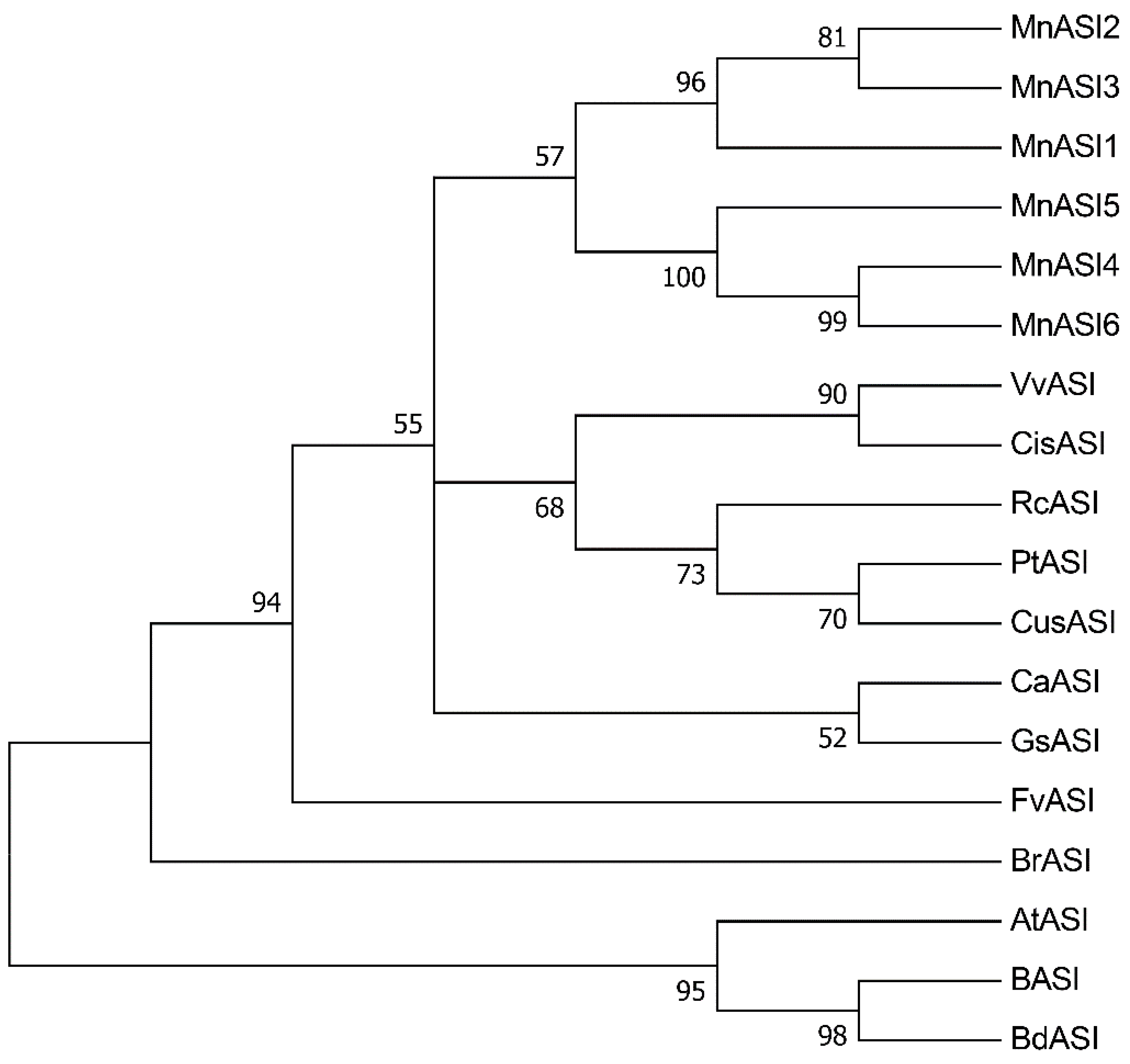
2.1. Bioinformatics Analyses of MnASIs
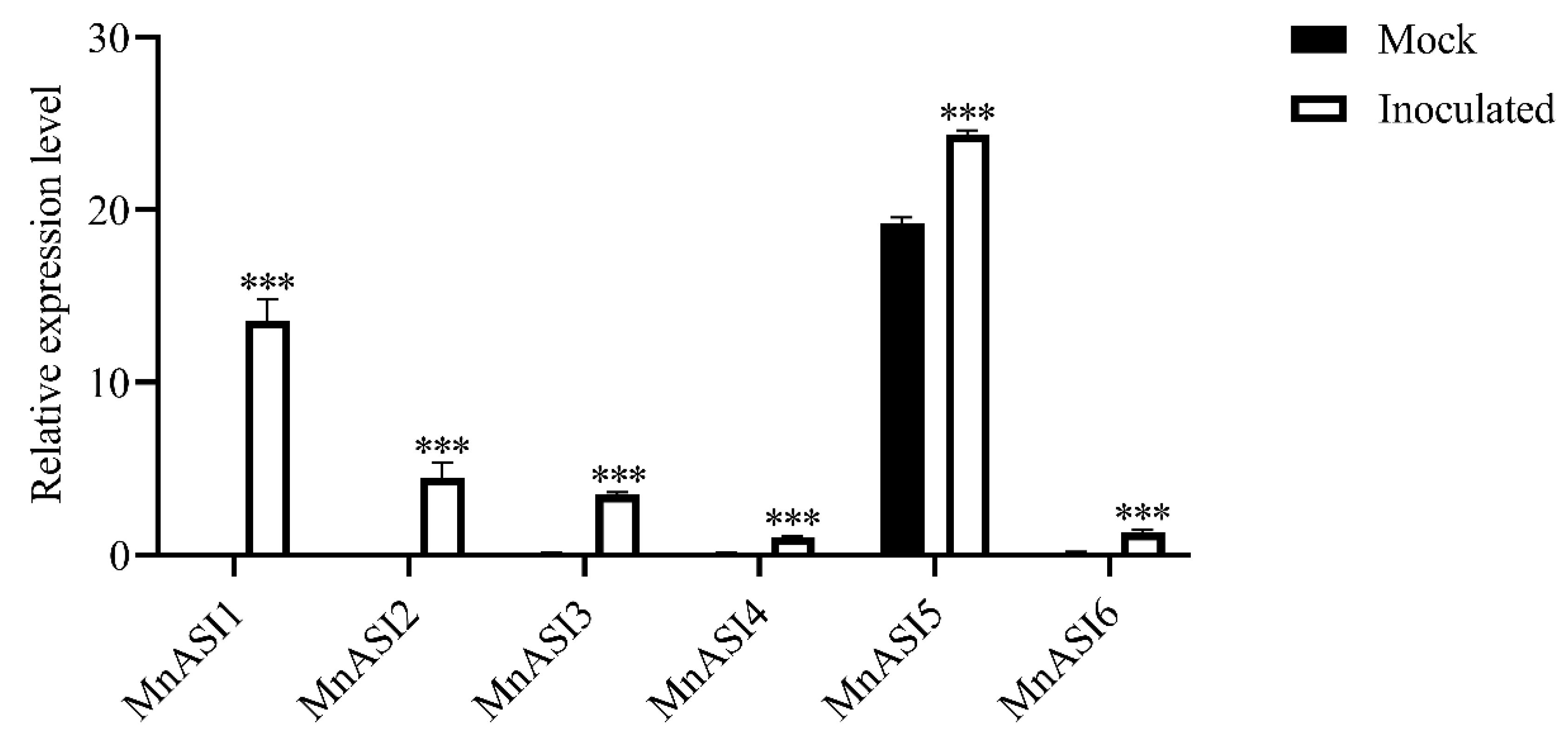
2.2. B. cinerea-Induced MnASIs Expression
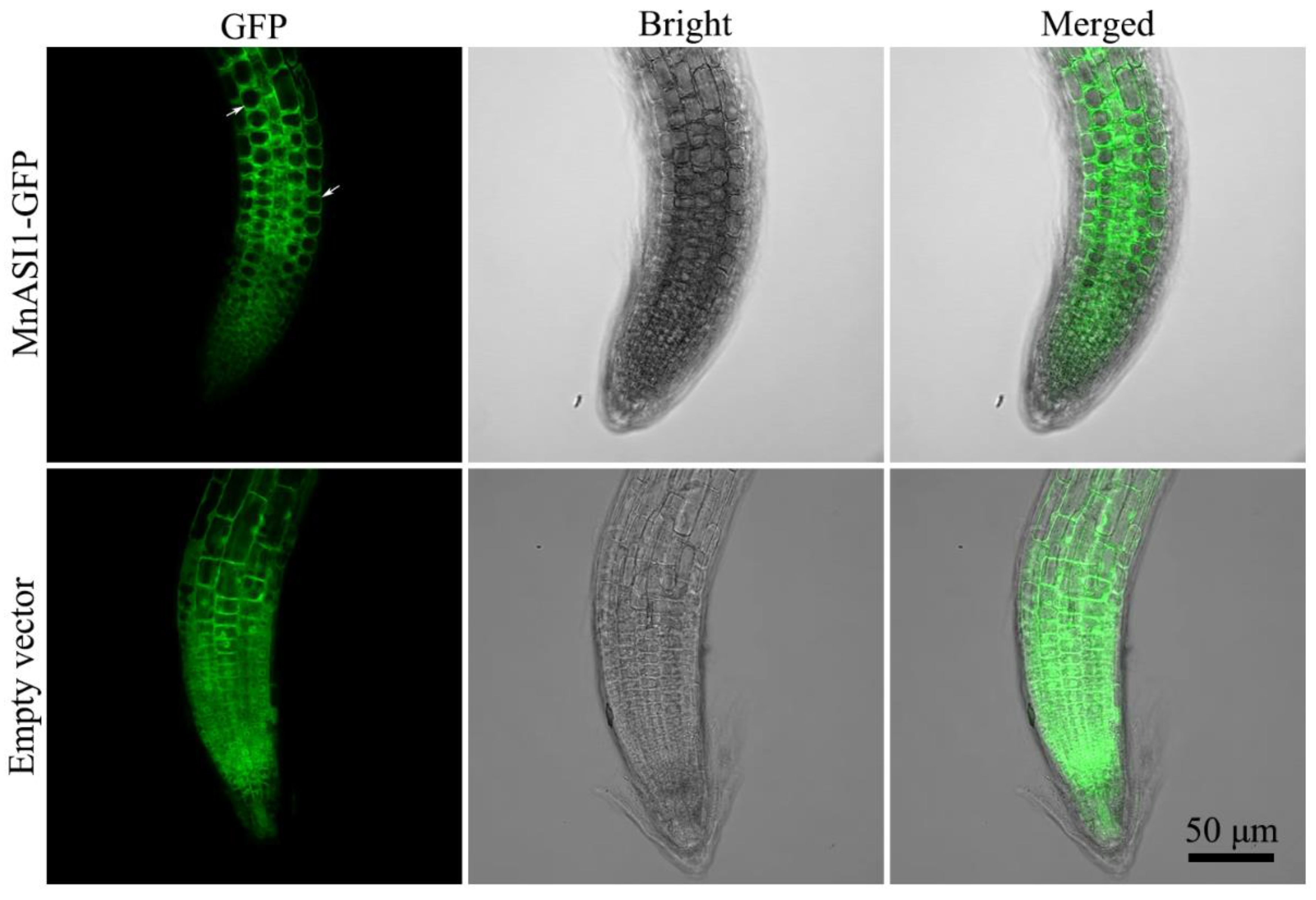
2.3. Subcellular Localization of MnASI1
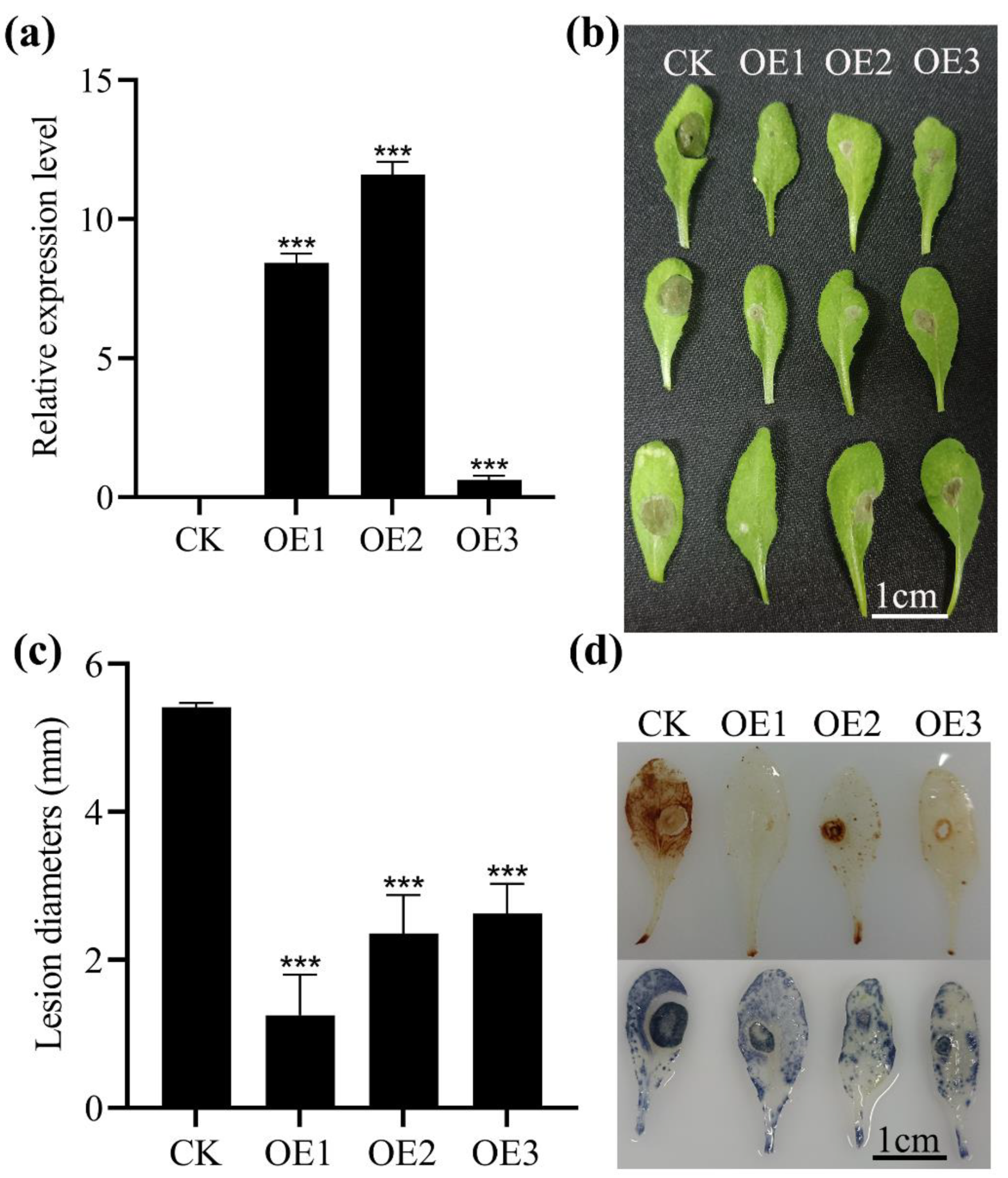
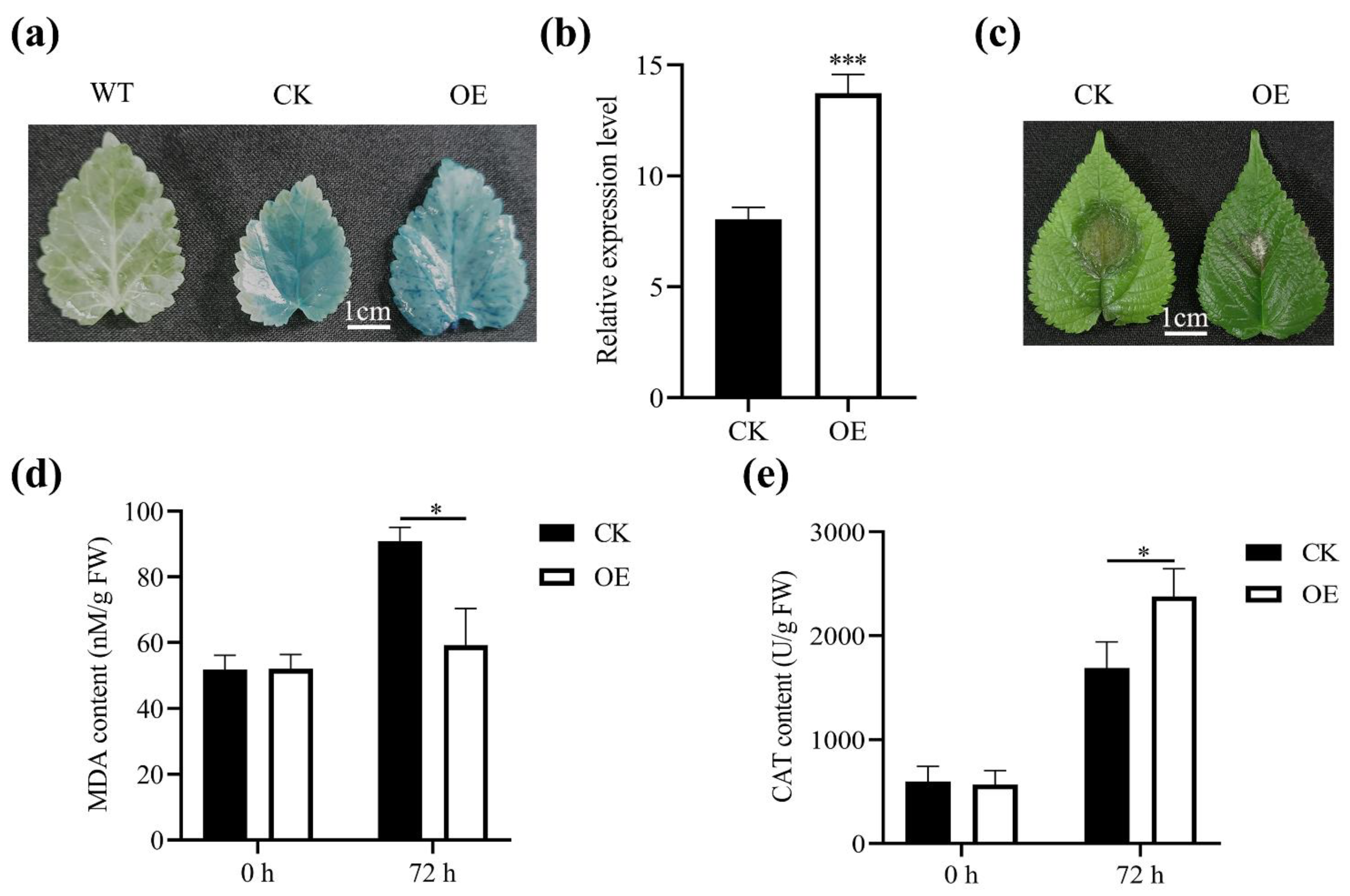
2.4. Positive Regulation of MnASI1 for Resistance to B. cinerea
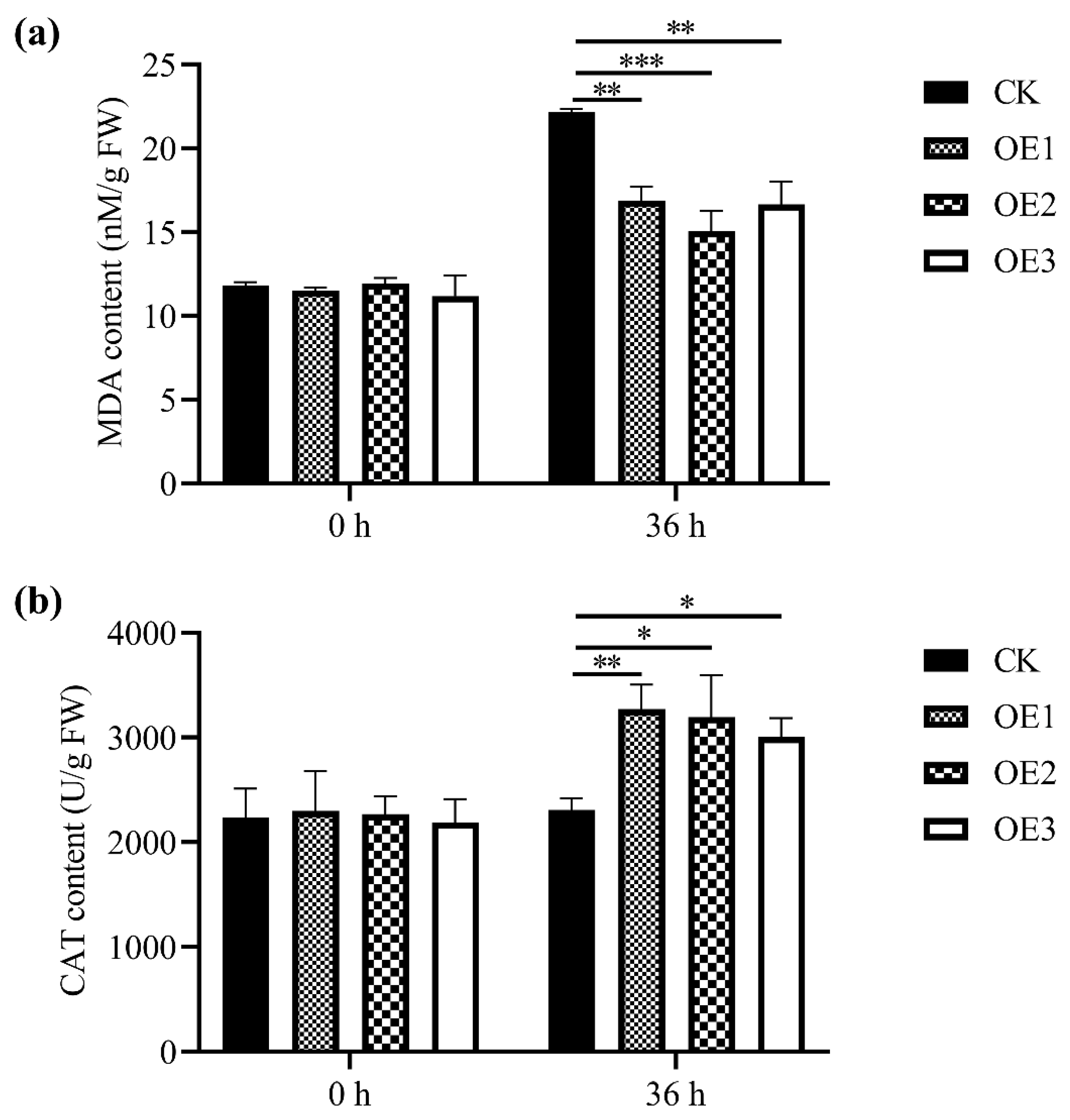
2.5. Detection of Biochemical Indices
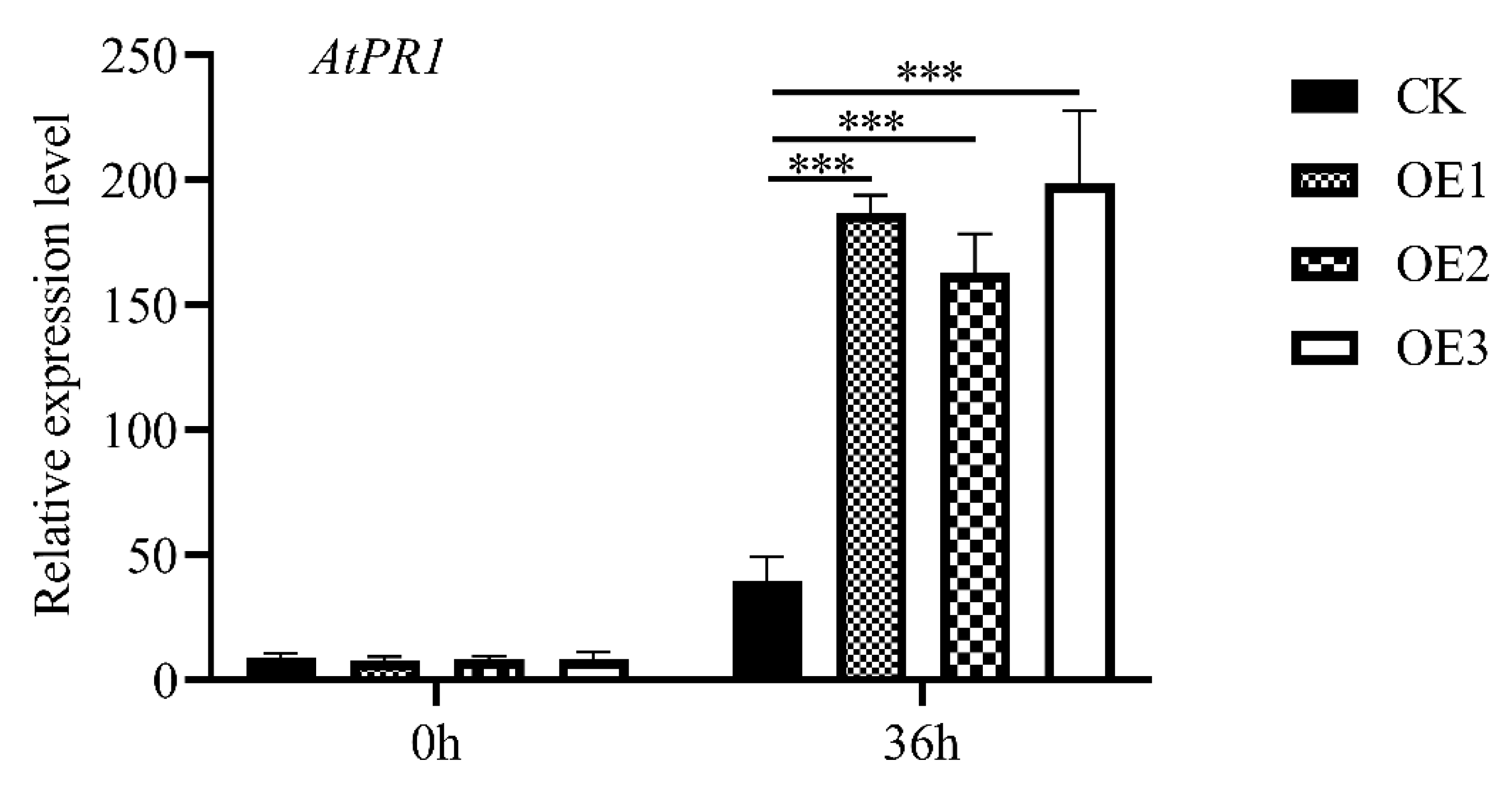
2.6. MnASI1 Transgenic Plants Enhance PR1 Expression
3. Discussions
4. Materials and Methods
4.1. Phylogenetic Tree of ASIs
4.2. Quantitative Real-Time PCR
4.3. Transformation of Arabidopsis
4.4. Transient Expression Analysis of Mulberry Gene Function
4.5. Resistance Analysis of Transgenic Arabidopsis and Mulberry to B. cinerea
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, D.; Thurau, T.; Tian, Y.; Lange, T.; Yeh, K.W.; Jung, C. Sporamin-mediated resistance to beet cyst nematodes (Heterodera schachtii Schm.) is dependent on trypsin inhibitory activity in sugar beet (Beta vulgaris L.) hairy roots. Plant Mol. Biol. 2003, 51, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Bunyatang, O.; Chirapongsatonkul, N.; Bangrak, P.; Henry, R.; Churngchow, N. Molecular cloning and characterization of a novel bi-functional alpha-amylase/subtilisin inhibitor from Hevea brasiliensis. Plant Physiol. Biochem. 2016, 101, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, M.; Mishra, P.; Mahato, A.K.; Singh, N.K.; Rao, U.; Sreevathsa, R. Comparative transcriptome analyses provide novel insights into the differential response of Pigeonpea (Cajanus cajan L.) and its wild relative (Cajanus platycarpus (Benth.) Maesen) to herbivory by Helicoverpa armigera (Hubner). Plant Mol. Biol. 2019, 101, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.D.; Ramasamy, S.; Suresh, C.G. Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights. Int. J. Biol. Macromol. 2018, 113, 933–943. [Google Scholar] [CrossRef]
- Laskowski, M., Jr.; Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980, 49, 593–626. [Google Scholar] [CrossRef]
- De Leo, F.; Volpicella, M.; Licciulli, F.; Liuni, S.; Gallerani, R.; Ceci, L.R. PLANT-PIs: A database for plant protease inhibitors and their genes. Nucleic Acids Res. 2002, 30, 347–348. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Tolle, D.P.; Barrett, A.J. Evolutionary families of peptidase inhibitors. Biochem. J. 2004, 378, 705–716. [Google Scholar] [CrossRef]
- Major, I.T.; Constabel, C.P. Functional analysis of the Kunitz trypsin inhibitor family in poplar reveals biochemical diversity and multiplicity in defense against herbivores. Plant Physiol. 2008, 146, 888–903. [Google Scholar] [CrossRef]
- Oliva, M.L.; Silva, M.C.; Sallai, R.C.; Brito, M.V.; Sampaio, M.U. A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie 2010, 92, 1667–1673. [Google Scholar] [CrossRef]
- Zhou, D.; Hansen, D.; Shabalin, I.G.; Gustchina, A.; Vieira, D.F.; de Brito, M.V.; Araujo, A.P.; Oliva, M.L.; Wlodawer, A. Structure of BbKI, a disulfide-free plasma kallikrein inhibitor. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 1055–1062. [Google Scholar] [CrossRef]
- Franco, O.L.; Rigden, D.J.; Melo, F.R.; Grossi-De-Sa, M.F. Plant alpha-amylase inhibitors and their interaction with insect alpha-amylases. Eur. J. Biochem. 2002, 269, 397–412. [Google Scholar] [CrossRef]
- Huang, H.P.; Ou, T.T.; Wang, C.J. Mulberry (sang shen zi) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement. Med. 2013, 3, 7–15. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit-A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and Antibacterial Properties of Anthocyanins and Flavonols from Fruits of Black and Non-Black Mulberries. Molecules 2017, 23, 4. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, W.J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; Moustafa, K.; Tran, L.S. ‘Omics’ and Plant Responses to Botrytis cinerea. Front. Plant Sci. 2016, 7, 1658. [Google Scholar] [CrossRef]
- Xin, Y.; Meng, S.; Ma, B.; He, W.; He, N. Mulberry genes MnANR and MnLAR confer transgenic plants with resistance to Botrytis cinerea. Plant Sci. 2020, 296, 110473. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, D.; Han, S.; Li, S.; Gong, N.; Fan, Y.; Ji, X. Characterization of the Chitinase Gene Family in Mulberry (Morus notabilis) and MnChi18 Involved in Resistance to Botrytis cinerea. Genes 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Ma, B.; Zeng, Q.; He, W.; Qin, M.; He, N. Dynamic changes in transposable element and gene methylation in mulberry (Morus notabilis) in response to Botrytis cinerea. Hortic. Res. 2021, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Kuckenberg, M.; Kastilan, R.; Muth, J.; Gebhardt, C. Novel in vitro inhibitory functions of potato tuber proteinaceous inhibitors. Mol. Genet. Genom. 2015, 290, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Heibges, A.; Salamini, F.; Gebhardt, C. Functional comparison of homologous members of three groups of Kunitz-type enzyme inhibitors from potato tubers (Solanum tuberosum L.). Mol. Genet. Genom. 2003, 269, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Heibges, A.; Glaczinski, H.; Ballvora, A.; Salamini, F.; Gebhardt, C. Structural diversity and organization of three gene families for Kunitz-type enzyme inhibitors from potato tubers (Solanum tuberosum L.). Mol. Genet. Genom. 2003, 269, 526–534. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Qiu, L. Novel alleles among soybean Bowman-Birk proteinase inhibitor gene families. Sci. China C Life Sci. 2008, 51, 687–692. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Lee, M.D.; Starr, C.M.; Miller, M.; Hanover, J.A. The gene encoding rat nuclear pore glycoprotein p62 is intronless. J. Biol. Chem. 1991, 266, 11980–11985. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Q.; Zhao, Y.Q.; Yang, J.; He, H.B.; Jia, G.X. The lre-miR159a-LrGAMYB pathway mediates resistance to grey mould infection in Lilium regale. Mol. Plant Pathol. 2020, 21, 749–760. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Yan, S.; Yu, T.; Yang, J.; Dong, J.; Zhang, S.; Zhao, J.; Yang, T.; Mao, X.; et al. OsWRKY67 positively regulates blast and bacteria blight resistance by direct activation of PR genes in rice. BMC Plant Biol. 2018, 18, 257. [Google Scholar] [CrossRef]
- Lv, Z.; Hao, L.; Ma, B.; He, Z.; Luo, Y.; Xin, Y.; He, N. Ciboria carunculoides Suppresses Mulberry Immune Responses Through Regulation of Salicylic Acid Signaling. Front. Plant Sci. 2021, 12, 658590. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Gechev, T.; Gadjev, I.; Van Breusegem, F.; Inze, D.; Dukiandjiev, S.; Toneva, V.; Minkov, I. Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol. Life Sci. 2002, 59, 708–714. [Google Scholar] [CrossRef]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P.; et al. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Liu, H.; Ouyang, B.; Zhang, J.; Wang, T.; Li, H.; Zhang, Y.; Yu, C.; Ye, Z. Differential modulation of photosynthesis, signaling, and transcriptional regulation between tolerant and sensitive tomato genotypes under cold stress. PLoS ONE 2012, 7, e50785. [Google Scholar] [CrossRef]
- Su, Y.; Xu, L.; Fu, Z.; Yang, Y.; Guo, J.; Wang, S.; Que, Y. ScChi, encoding an acidic class III chitinase of sugarcane, confers positive responses to biotic and abiotic stresses in sugarcane. Int. J. Mol. Sci. 2014, 15, 2738–2760. [Google Scholar] [CrossRef]

| Gene Name | Gene Name | GenBank Acc. | CDS (bp) | Size (aa) | MW (kDa) | Predicted pI |
|---|---|---|---|---|---|---|
| MnASI1 | L484_010983 | EXB74706.1 | 576 | 191 | 20.82 | 4.46 |
| MnASI2 | L484_010984 | EXB74707.1 | 603 | 200 | 21.7 | 5.41 |
| MnASI3 | L484_010986 | EXB74709.1 | 624 | 207 | 22.65 | 8.35 |
| MnASI4 | L484_010988 | EXB74711.1 | 579 | 192 | 21.19 | 7.52 |
| MnASI5 | L484_010987 | EXB74710.1 | 576 | 191 | 21.12 | 4.93 |
| MnASI6 | L484_010989 | EXB74712.1 | 579 | 192 | 21.24 | 8.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Gong, N.; Liu, C.; Li, S.; Guo, Z.; Wang, G.; Shang, Q.; Wang, D.; Ji, X.; Xin, Y. MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis). Int. J. Mol. Sci. 2022, 23, 13372. https://doi.org/10.3390/ijms232113372
Wang D, Gong N, Liu C, Li S, Guo Z, Wang G, Shang Q, Wang D, Ji X, Xin Y. MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis). International Journal of Molecular Sciences. 2022; 23(21):13372. https://doi.org/10.3390/ijms232113372
Chicago/Turabian StyleWang, Donghao, Na Gong, Chaorui Liu, Suxia Li, Zhaocheng Guo, Gefan Wang, Qiqi Shang, Dongming Wang, Xianling Ji, and Youchao Xin. 2022. "MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis)" International Journal of Molecular Sciences 23, no. 21: 13372. https://doi.org/10.3390/ijms232113372
APA StyleWang, D., Gong, N., Liu, C., Li, S., Guo, Z., Wang, G., Shang, Q., Wang, D., Ji, X., & Xin, Y. (2022). MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis). International Journal of Molecular Sciences, 23(21), 13372. https://doi.org/10.3390/ijms232113372





