Anticancer Nanotherapeutics in Clinical Trials: The Work behind Clinical Translation of Nanomedicine
Abstract
1. Introduction
2. Inorganic NPs
2.1. AGuIX
2.2. NBTXR3
2.3. Super Magnetic Iron Oxide
2.4. Gold Nanoparticles
2.5. ELU001 (Folic-Acid Functionalized C’Dot-Drug-Conjugate)
3. Polymeric Particles
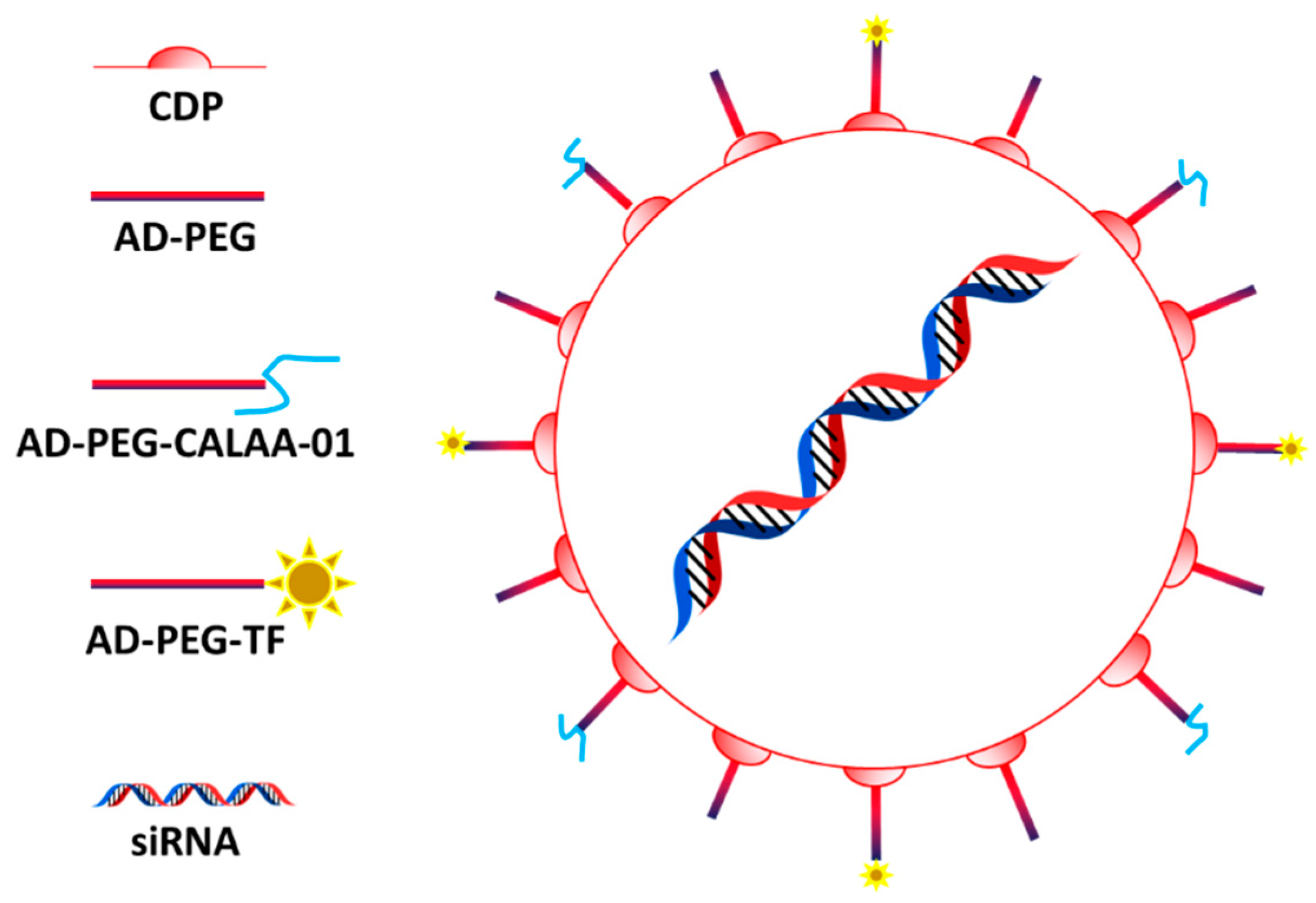
3.1. CALAA-01
3.2. Micelles
3.3. EP0057
3.4. NanoPac
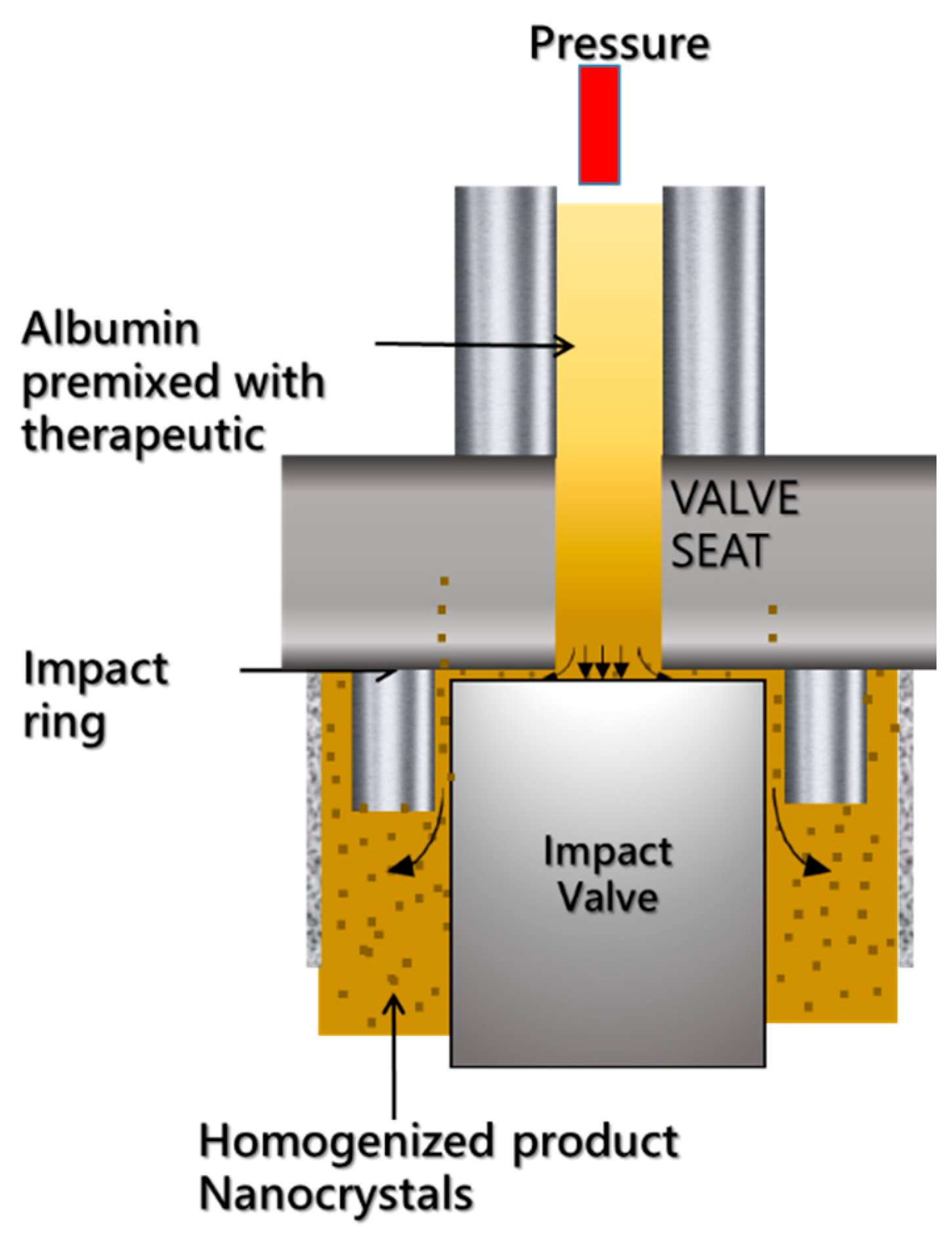
4. Abraxane and Related Technologies
4.1. Abraxane
4.2. Abi 009
5. Lipid/Proteolipid Technologies
5.1. Doxil
5.2. Other Liposomal Formulations
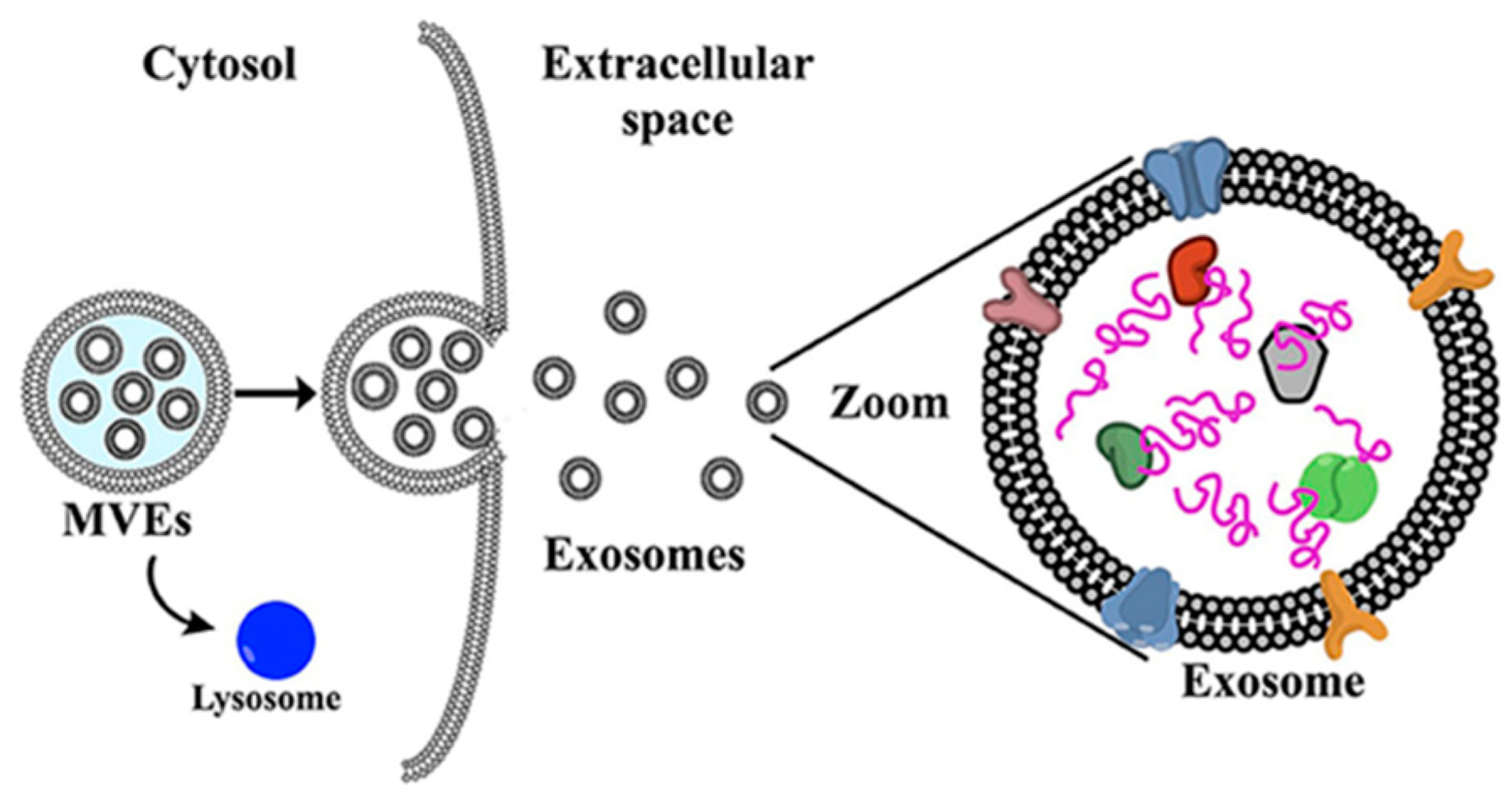
5.3. Exosomes
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lammers, T.; Ferrari, M. The success of nanomedicine. Nano Today 2020, 31, 100853. [Google Scholar] [CrossRef]
- Wu, L.-P.; Wang, D.; Li, Z. Grand challenges in nanomedicine. Mater. Sci. Eng. C 2020, 106, 110302. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; de Rosales, R.T.; La-Beck, N.M. Translational considerations in nanomedicine: The oncology perspective. Adv. Drug Deliv. Rev. 2020, 158, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.C. Doxil®—the first FDA-approved nano-drug: From an idea to a product. In Handbook of Harnessing Biomaterials in Nanomedicine; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2021; pp. 463–528. [Google Scholar]
- Petre, C.E.; Dittmer, D.P. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 277. [Google Scholar]
- Shi, Y.; Van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921. [Google Scholar] [CrossRef]
- Chen, L.; Zang, F.; Wu, H.; Li, J.; Xie, J.; Ma, M.; Gu, N.; Zhang, Y. Using PEGylated magnetic nanoparticles to describe the EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale 2018, 10, 1788–1797. [Google Scholar] [CrossRef]
- Agrawal, P.; Singh, R.P.; Kumari, L.; Sharma, G.; Koch, B.; Rajesh, C.V.; Mehata, A.K.; Singh, S.; Pandey, B.L.; Muthu, M.S. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater. Sci. Eng. C 2017, 74, 167–176. [Google Scholar] [CrossRef]
- Adriani, G.; de Tullio, M.D.; Ferrari, M.; Hussain, F.; Pascazio, G.; Liu, X.; Decuzzi, P. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials 2012, 33, 5504–5513. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- Evangelopoulos, M.; Parodi, A.; Martinez, J.O.; Tasciotti, E. Trends towards biomimicry in theranostics. Nanomaterials 2018, 8, 637. [Google Scholar] [CrossRef]
- Santagiuliana, R.; Milosevic, M.; Milicevic, B.; Sciumè, G.; Simic, V.; Ziemys, A.; Kojic, M.; Schrefler, B.A. Coupling tumor growth and bio distribution models. Biomed. Microdevices 2019, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef]
- Bosetti, R.; Jones, S.L. Cost–effectiveness of nanomedicine: Estimating the real size of nano-costs. Nanomedicine 2019, 14, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Borodina, T.; Kostyushev, D.; Zamyatnin, A.A., Jr.; Parodi, A. Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration. Int. J. Transl. Med. 2021, 1, 306–322. [Google Scholar] [CrossRef]
- Parodi, A.; Buzaeva, P.; Nigovora, D.; Baldin, A.; Kostyushev, D.; Chulanov, V.; Savvateeva, L.V.; Zamyatnin, A.A. Nanomedicine for increasing the oral bioavailability of cancer treatments. J. Nanobiotechnol. 2021, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’sullivan, J.M. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Lux, F.; Mignot, A.; Mowat, P.; Louis, C.; Dufort, S.; Bernhard, C.; Denat, F.; Boschetti, F.; Brunet, C.; Antoine, R. Ultrasmall rigid particles as multimodal probes for medical applications. Angew. Chem. 2011, 123, 12507–12511. [Google Scholar] [CrossRef]
- Lux, F.; Tran, V.L.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F. AGuIX® from bench to bedside—Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2019, 92, 20180365. [Google Scholar] [CrossRef]
- Sancey, L.; Lux, F.; Kotb, S.; Roux, S.; Dufort, S.; Bianchi, A.; Cremillieux, Y.; Fries, P.; Coll, J.-L.; Rodriguez-Lafrasse, C. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br. J. Radiol. 2014, 87, 20140134. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, Y.; Busser, B.; Trichard, F.; Kulesza, A.; Laurent, J.; Zaun, V.; Lux, F.; Benoit, J.-M.; Panczer, G.; Dugourd, P. 3D imaging of nanoparticle distribution in biological tissue by laser-induced breakdown spectroscopy. Sci. Rep. 2016, 6, 29936. [Google Scholar] [CrossRef] [PubMed]
- Kotb, S.; Detappe, A.; Lux, F.; Appaix, F.; Barbier, E.L.; Tran, V.-L.; Plissonneau, M.; Gehan, H.; Lefranc, F.; Rodriguez-Lafrasse, C. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: Proof of concept before phase I trial. Theranostics 2016, 6, 418. [Google Scholar] [CrossRef] [PubMed]
- Le Duc, G.; Roux, S.; Paruta-Tuarez, A.; Dufort, S.; Brauer, E.; Marais, A.; Truillet, C.; Sancey, L.; Perriat, P.; Lux, F. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Lux, F.; Detappe, A.; Dufort, S.; Sancey, L.; Louis, C.; Carme, S.; Tillement, O. Nanoparticules ultrafines en radiothérapie: Le cas des AguIX. Cancer/Radiothérapie 2015, 19, 508–514. [Google Scholar] [CrossRef]
- Mowat, P.; Mignot, A.; Rima, W.; Lux, F.; Tillement, O.; Roulin, C.; Dutreix, M.; Bechet, D.; Huger, S.; Humbert, L. In vitro radiosensitizing effects of ultrasmall gadolinium based particles on tumour cells. J. Nanosci. Nanotechnol. 2011, 11, 7833–7839. [Google Scholar] [CrossRef]
- Luchette, M.; Korideck, H.; Makrigiorgos, M.; Tillement, O.; Berbeco, R. Radiation dose enhancement of gadolinium-based AguIX nanoparticles on HeLa cells. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1751–1755. [Google Scholar] [CrossRef]
- Radiosensitization of Multiple Brain Metastases Using AguIX Gadolinium Based Nanoparticles. Available online: https://ClinicalTrials.gov/show/NCT02820454 (accessed on 1 October 2022).
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L. Theranostic AguIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]
- Radiotherapy of Multiple Brain Metastases Using AguIX®. Available online: https://ClinicalTrials.gov/show/NCT02820454 (accessed on 1 October 2022).
- AguIX Nanoparticles with Radiotherapy Plus Concomitant Temozolomide in the Treatment of Newly Diagnosed Glioblastoma. Available online: https://ClinicalTrials.gov/show/NCT04881032 (accessed on 1 October 2022).
- Stereotactic Brain-directed Radiation with or without Aguix Gadolinium-Based Nanoparticles in Brain Metastases. Available online: https://ClinicalTrials.gov/show/NCT04899908 (accessed on 1 October 2022).
- AguIX Gadolinium-based Nanoparticles in Combination with Chemoradiation and Brachytherapy. Available online: https://ClinicalTrials.gov/show/NCT03308604 (accessed on 1 October 2022).
- Nano-SMART: Nanoparticles with MR Guided SBRT in Centrally Located Lung Tumors and Pancreatic Cancer. Available online: https://ClinicalTrials.gov/show/NCT04789486 (accessed on 1 October 2022).
- Marill, J.; Anesary, N.M.; Zhang, P.; Vivet, S.; Borghi, E.; Levy, L.; Pottier, A. Hafnium oxide nanoparticles: Toward an in vitropredictive biological effect? Radiat. Oncol. 2014, 9, 150. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Moreno, V.; Salas, S.; Mirabel, X.; Calvo, E.; Doger, B.; Florescu, C.; Thariat, J.; Fijuth, J.; Rutkowski, T. Hafnium oxide nanoparticles NBTXR3 activated by radiotherapy as a new therapeutic option for elderly/frail HNSCC patients. J. Clin. Oncol. 2019, 37, 6069. [Google Scholar] [CrossRef]
- Bilynsky, C.; Millot, N.; Papa, A.L. Radiation nanosensitizers in cancer therapy—From preclinical discoveries to the outcomes of early clinical trials. Bioeng. Transl. Med. 2022, 7, e10256. [Google Scholar] [CrossRef] [PubMed]
- NBTXR3 Activated by Radiation Therapy for the Treatment of Locally Advanced or Borderline-Resectable Pancreatic Cancer. Available online: https://ClinicalTrials.gov/show/NCT04484909 (accessed on 1 October 2022).
- NBTXR3, Chemotherapy, and Radiation Therapy for the Treatment of Esophageal Cancer. Available online: https://ClinicalTrials.gov/show/NCT04615013 (accessed on 1 October 2022).
- NBTXR3, Radiation Therapy, Ipilimumab, and Nivolumab for the Treatment of Lung and/or Liver Metastases from Solid Malignancy. Available online: https://ClinicalTrials.gov/show/NCT05039632 (accessed on 1 October 2022).
- NBTXR3, Radiation Therapy, and Pembrolizumab for the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Cancer. Available online: https://ClinicalTrials.gov/show/NCT04862455 (accessed on 1 October 2022).
- NBTXR3 with or without Cetuximab in LA-HNSCC. Available online: https://ClinicalTrials.gov/show/NCT04892173 (accessed on 1 October 2022).
- NBTXR3 Crystalline Nanoparticles and Radiation Therapy in Treating Randomized Patients in Two Arms with Soft Tissue Sarcoma of the Extremity and Trunk Wall. Available online: https://ClinicalTrials.gov/show/NCT01433068 (accessed on 1 October 2022).
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.-P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Hoffmann, C.; Calugaru, V.; Borcoman, E.; Moreno, V.; Calvo, E.; Liem, X.; Salas, S.; Doger, B.; Jouffroy, T.; Mirabel, X. Phase I dose-escalation study of NBTXR3 activated by intensity-modulated radiation therapy in elderly patients with locally advanced squamous cell carcinoma of the oral cavity or oropharynx. Eur. J. Cancer 2021, 146, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles—Current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Lassenberger, A.; Scheberl, A.; Stadlbauer, A.; Stiglbauer, A.; Helbich, T.; Reimhult, E. Individually stabilized, superparamagnetic nanoparticles with controlled shell and size leading to exceptional stealth properties and high relaxivities. ACS Appl. Mater. Interfaces 2017, 9, 3343–3353. [Google Scholar] [CrossRef]
- Cano, M.; Núñez-Lozano, R.; Lumbreras, R.; González-Rodríguez, V.; Delgado-García, A.; Jiménez-Hoyuela, J.M.; de la Cueva-Méndez, G. Partial PEGylation of superparamagnetic iron oxide nanoparticles thinly coated with amine-silane as a source of ultrastable tunable nanosystems for biomedical applications. Nanoscale 2017, 9, 812–822. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Song, S.-C. Multiple hyperthermia-mediated release of TRAIL/SPION nanocomplex from thermosensitive polymeric hydrogels for combination cancer therapy. Biomaterials 2017, 132, 16–27. [Google Scholar] [CrossRef]
- Talluri, S.; Malla, R.R. Superparamagnetic iron oxide nanoparticles (SPIONs) for diagnosis and treatment of breast, ovarian and cervical Cancers. Curr. Drug Metab. 2019, 20, 942–945. [Google Scholar] [CrossRef]
- Li, X.; Taratula, O.; Taratula, O.; Schumann, C.; Minko, T. LHRH-targeted drug delivery systems for cancer therapy. Mini Rev. Med. Chem. 2017, 17, 258–267. [Google Scholar] [CrossRef]
- Onbasli, K.; Erkısa, M.; Demirci, G.; Muti, A.; Ulukaya, E.; Sennaroglu, A.; Acar, H.Y. The improved killing of both androgen-dependent and independent prostate cancer cells by etoposide loaded SPIONs coupled with NIR irradiation. Biomater. Sci. 2022, 10, 3951–3962. [Google Scholar] [CrossRef]
- Liu, J.F.; Lan, Z.; Ferrari, C.; Stein, J.M.; Higbee-Dempsey, E.; Yan, L.; Amirshaghaghi, A.; Cheng, Z.; Issadore, D.; Tsourkas, A. Use of oppositely polarized external magnets to improve the accumulation and penetration of magnetic nanocarriers into solid tumors. ACS Nano 2019, 14, 142–152. [Google Scholar] [CrossRef]
- Alvear-Jiménez, A.; Zabala Gutierrez, I.; Shen, Y.; Villaverde, G.; Lozano-Chamizo, L.; Guardia, P.; Tinoco, M.; Garcia-Pinel, B.; Prados, J.; Melguizo, C. Electrospraying as a Technique for the Controlled Synthesis of Biocompatible PLGA@ Ag2S and PLGA@ Ag2S@ SPION Nanocarriers with Drug Release Capability. Pharmaceutics 2022, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbabi Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Imaging Kidney Transplant Rejection Using Ferumoxytol-Enhanced Magnetic Resonance. Available online: https://ClinicalTrials.gov/show/NCT02006108 (accessed on 1 October 2022).
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically approved nanoparticle imaging agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef]
- Delayed Sentinel Lymph Node Biopsy in Ductal Cancer In Situ. Available online: https://ClinicalTrials.gov/show/NCT04722692 (accessed on 6 October 2022).
- Pre-Operative Staging of Pancreatic Cancer Using Superparamagnetic Iron Oxide Magnetic Resonance Imaging (SPIO MRI). Available online: https://ClinicalTrials.gov/show/NCT00920023 (accessed on 6 October 2022).
- Magnetic Nanoparticle Thermoablation-Retention and Maintenance in the Prostate: A Phase 0 Study in Men. Available online: https://ClinicalTrials.gov/show/NCT02033447 (accessed on 6 October 2022).
- Radiotherapy with Iron Oxide Nanoparticles (SPION) on MR-Linac for Primary & Metastatic Hepatic Cancers. Available online: https://ClinicalTrials.gov/show/NCT04682847 (accessed on 6 October 2022).
- A Phase I Clinical Trial of Neoadjuvant Chemotherapy with/without SPIONs/SMF for Patients with Osteosarcoma. Available online: https://ClinicalTrials.gov/show/NCT04316091 (accessed on 6 October 2022).
- Diagnostic and Prognostic Accuracy of Gold Nanoparticles in Salivary Gland Tumours. Available online: https://ClinicalTrials.gov/show/NCT04907422 (accessed on 6 October 2022).
- NU-0129 in Treating Patients with Recurrent Glioblastoma or Gliosarcoma Undergoing Surgery. Available online: https://ClinicalTrials.gov/show/NCT03020017 (accessed on 6 October 2022).
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R. A first-in-human phase 0 clinical study of RNA interference–based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mijakovic, I. Advances in gold nanoparticle technology as a tool for diagnostics and treatment of cancer. Expert Rev. Mol. Diagn. 2021, 21, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, L.S.; Boonstra, M.C.; Beck, A.-J.; Charehbili, A.; Hoogstins, C.E.; Prevoo, H.A.; Singhal, S.; Low, P.S.; van de Velde, C.J.; Vahrmeijer, A.L. Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget 2016, 7, 17442. [Google Scholar] [CrossRef]
- A Study to Evaluate ELU001 in Patients with Solid Tumors that Overexpress Folate Receptor Alpha (FRα). Available online: https://ClinicalTrials.gov/show/NCT05001282 (accessed on 6 October 2022).
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody–drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Davis, M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef]
- Kurreck, J. Proof of RNA interference in humans after systemic delivery of siRNAs. Angew. Chem. Int. Ed. 2010, 49, 6258–6259. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef] [PubMed]
- Safety Study of CALAA-01 to Treat Solid Tumor Cancers. Available online: https://ClinicalTrials.gov/show/NCT00689065 (accessed on 6 October 2022).
- Parvani, J.G.; Jackson, M.W. Silencing the roadblocks to effective triple-negative breast cancer treatments by siRNA nanoparticles. Endocr. Relat. Cancer 2017, 24, R81–R97. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Safety of Genexol PM and Carboplatin as First-Line Therapy in Ovarian Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05300828 (accessed on 6 October 2022).
- Borgå, O.; Henriksson, R.; Bjermo, H.; Lilienberg, E.; Heldring, N.; Loman, N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: A dose-escalation study. Adv. Ther. 2019, 36, 1150–1163. [Google Scholar] [CrossRef]
- Vergote, I.; Bergfeldt, K.; Franquet, A.; Lisyanskaya, A.; Bjermo, H.; Heldring, N.; Buyse, M.; Brize, A. A randomized phase III trial in patients with recurrent platinum sensitive ovarian cancer comparing efficacy and safety of paclitaxel micellar and Cremophor EL-paclitaxel. Gynecol. Oncol. 2020, 156, 293–300. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report Apealea; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Apealea®-Elevar Therapeutics. Available online: https://elevartherapeutics.com/apealea-paclitaxel-micellar-elevar/ (accessed on 1 October 2022).
- Shi, M.; Gu, A.; Tu, H.; Huang, C.; Wang, H.; Yu, Z.; Wang, X.; Cao, L.; Shu, Y.; Yang, R. Comparing nanoparticle polymeric micellar paclitaxel and solvent-based paclitaxel as first-line treatment of advanced non-small-cell lung cancer: An open-label, randomized, multicenter, phase III trial. Ann. Oncol. 2021, 32, 85–96. [Google Scholar] [CrossRef]
- Lu, J.; Gu, A.; Wang, W.; Huang, A.; Han, B.; Zhong, H. Polymeric micellar paclitaxel (pm-Pac) prolonged overall survival for NSCLC patients without pleural metastasis. Int. J. Pharm. 2022, 623, 121961. [Google Scholar] [CrossRef] [PubMed]
- Study of Paclitaxel Micelles for Injection in Chinese Patients with Advanced Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT04778839 (accessed on 6 October 2022).
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.-C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Study to Evaluate the Efficacy and Safety of Docetaxel Polymeric Micelle (PM) in Recurrent or Metastatic HNSCC. Available online: https://ClinicalTrials.gov/show/NCT02639858 (accessed on 6 October 2022).
- Study to Evaluate the Safety of Nanoxel M Inj. Available online: https://ClinicalTrials.gov/show/NCT04066335 (accessed on 6 October 2022).
- Compare the Efficacy and the Safety of Doxorubicin and Cyclophosphamide Followed by Taxotere versus Doxorubicin and Cyclophosphamide Nanoxel M as Neoadjuvant Chemotherapy in Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT05207514 (accessed on 6 October 2022).
- Combination of Nanoxel and Herzuma in Salivary Duct Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT03614364 (accessed on 1 October 2022).
- Volovat, S.R.; Ciuleanu, T.-E.; Koralewski, P.; Olson, J.E.G.; Croitoru, A.; Koynov, K.; Stabile, S.; Cerea, G.; Osada, A.; Bobe, I. A multicenter, single-arm, basket design, phase II study of NC-6004 plus gemcitabine in patients with advanced unresectable lung, biliary tract, or bladder cancer. Oncotarget 2020, 11, 3105. [Google Scholar] [CrossRef] [PubMed]
- Osada, A.; Mangel, L.; Fijuth, J.; Żurawski, B.; Ursulovic, T.; Nikolin, B.; Djan, I.; Olson, J.G. Phase IIa/IIb clinical trial of NC-6004 (Nanoparticle Cisplatin) plus Pembrolizumab in patients with head and neck cancer (HNSCC) who have failed platinum or a platinum-containing regimen. Eur. J. Cancer 2020, 138, S35. [Google Scholar] [CrossRef]
- Subbiah, V.; Grilley-Olson, J.E.; Combest, A.J.; Sharma, N.; Tran, R.H.; Bobe, I.; Osada, A.; Takahashi, K.; Balkissoon, J.; Camp, A. Phase Ib/II Trial of NC-6004 (Nanoparticle Cisplatin) Plus Gemcitabine in Patients with Advanced Solid TumorsNC-6004/Gemcitabine in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 43–51. [Google Scholar] [CrossRef]
- A Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Antitumor Efficacy of Cisplatin Micelle Injection (HA132) in Patients with Advanced Malignant Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT05478785 (accessed on 1 October 2022).
- Hamaguchi, T.; Tsuji, A.; Yamaguchi, K.; Takeda, K.; Uetake, H.; Esaki, T.; Amagai, K.; Sakai, D.; Baba, H.; Kimura, M. A phase II study of NK012, a polymeric micelle formulation of SN-38, in unresectable, metastatic or recurrent colorectal cancer patients. Cancer Chemother. Pharmacol. 2018, 82, 1021–1029. [Google Scholar] [CrossRef]
- Ri, M.; Suzuki, K.; Iida, S.; Hatake, K.; Chou, T.; Taniwaki, M.; Watanabe, N.; Tsukamoto, T. A phase I/II study for dose-finding, and to investigate the safety, pharmacokinetics and preliminary efficacy of NK012, an SN-38-incorporating macromolecular polymeric micelle, in patients with multiple myeloma. Intern. Med. 2018, 57, 939–946. [Google Scholar] [CrossRef]
- Riedel, R.F.; Chua, V.S.; Kim, T.; Dang, J.; Zheng, K.; Moradkhani, A.; Osada, A.; Chawla, S.P. Results of NC-6300 (nanoparticle epirubicin) in an expansion cohort of patients with angiosarcoma. Oncologist 2022, 27, 809-e765. [Google Scholar] [CrossRef]
- Chawla, S.P.; Goel, S.; Chow, W.; Braiteh, F.; Singh, A.S.; Olson, J.E.G.; Osada, A.; Bobe, I.; Riedel, R.F. A Phase 1b Dose Escalation Trial of NC-6300 (Nanoparticle Epirubicin) in Patients with Advanced Solid Tumors or Advanced, Metastatic, or Unresectable Soft-tissue SarcomaNanoparticle Epirubicin in Solid Tumors and Sarcoma. Clin. Cancer Res. 2020, 26, 4225–4232. [Google Scholar] [CrossRef]
- Trial of EP0057, a Nanoparticle Camptothecin with Olaparib in People with Relapsed/Refractory Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT02769962 (accessed on 1 October 2022).
- EP0057 in Combination with Olaparib in Advanced Ovarian Cancer. Available online: https://ClinicalTrials.gov/show/NCT04669002 (accessed on 6 October 2022).
- A Study of CRLX101(NLG207) in Combination with Weekly Paclitaxel in Patients with Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer. Available online: https://ClinicalTrials.gov/show/NCT02389985 (accessed on 1 October 2022).
- Duska, L.; Krasner, C.; O’Malley, D.; Hays, J.; Modesitt, S.; Mathews, C.; Moore, K.; Thaker, P.; Miller, A.; Purdy, C. A phase Ib/II and pharmacokinetic study of EP0057 (formerly CRLX101) in combination with weekly paclitaxel in patients with recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer. Gynecol. Oncol. 2021, 160, 688–695. [Google Scholar] [CrossRef]
- Voss, M.H.; Hussain, A.; Vogelzang, N.; Lee, J.; Keam, B.; Rha, S.; Vaishampayan, U.; Harris, W.; Richey, S.; Randall, J. A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann. Oncol. 2017, 28, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.J.; Chao, J.; Neidhart, J.D.; Ramanathan, R.K.; Bassett, D.; Neidhart, J.A.; Choi, C.H.J.; Chow, W.; Chung, V.; Forman, S.J. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Investig. New Drugs 2013, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Chen, L.; Yen, T.; Wang, Y.; Zhou, B.; Davis, M.; Yen, Y. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 721–730. [Google Scholar] [CrossRef]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Nguyen, M.; Foote, H.P.; Caster, J.M.; Roche, K.C.; Peters, C.G.; Wu, P.; Jayaraman, L.; Garmey, E.G.; Tepper, J.E. CRLX101, a Nanoparticle–Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1αCRLX101 Improves Cancer Chemoradiotherapy. Cancer Res. 2017, 77, 112–122. [Google Scholar] [CrossRef]
- Tian, X.; Nguyen, M.; Foote, H.; Garmey, E.; Eliasof, S.; Wang, A. CRLX101, an Investigational Nanoparticle Drug Conjugate of Camptothecin, as a Potentially Effective Radiosensitizer in Chemoradiation Treatment of Colorectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, S49. [Google Scholar] [CrossRef]
- Chao, J.; Lin, J.; Frankel, P.; Clark, A.J.; Wiley, D.T.; Garmey, E.; Fakih, M.; Lim, D.; Chung, V.; Luevanos, E. Pilot trial of CRLX101 in patients with advanced, chemotherapy-refractory gastroesophageal cancer. J. Gastrointest. Oncol. 2017, 8, 962. [Google Scholar] [CrossRef]
- EP0057 in Combination with Olaparib in Relapsed Advanced Gastric Cancer and Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT05411679 (accessed on 1 October 2022).
- Schmidt, K.T.; Huitema, A.D.; Dorlo, T.P.; Peer, C.J.; Cordes, L.M.; Sciuto, L.; Wroblewski, S.; Pommier, Y.; Madan, R.A.; Thomas, A. Population pharmacokinetic analysis of nanoparticle-bound and free camptothecin after administration of NLG207 in adults with advanced solid tumors. Cancer Chemother. Pharmacol. 2020, 86, 475–486. [Google Scholar] [CrossRef]
- Keefe, S.; Hoffman-Censits, J.; Cohen, R.; Mamtani, R.; Heitjan, D.; Eliasof, S.; Nixon, A.; Turnbull, B.; Garmey, E.; Gunnarsson, O. Efficacy of the nanoparticle–drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: Results of an investigator-initiated phase I–IIa clinical trial. Ann. Oncol. 2016, 27, 1579–1585. [Google Scholar] [CrossRef]
- Combining CRLX101, a Nanoparticle Camptothecin, with Enzalutamide in People with Progressive Metastatic Castration Resistant Prostate Cancer Following Prior Enzalutamide Treatment. Available online: https://ClinicalTrials.gov/show/NCT03531827 (accessed on 1 October 2022).
- A Phase 2 Study of CRLX101(NLG207) in Patients with Advanced Non-Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT01380769 (accessed on 1 October 2022).
- Neoadjuvant Chemoradiotherapy with CRLX-101 and Capecitabine for Rectal Cancer. Available online: https://ClinicalTrials.gov/show/NCT02010567 (accessed on 1 October 2022).
- Topotecan Hydrochloride or Cyclodextrin-Based Polymer-Camptothecin CRLX101 in Treating Patients with Recurrent Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT01803269 (accessed on 1 October 2022).
- Verco, J.; Johnston, W.; Baltezor, M.; Kuehl, P.J.; Gigliotti, A.; Belinsky, S.A.; Lopez, A.; Wolff, R.; Hylle, L.; diZerega, G. Pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac®) in a rodent model. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 99–109. [Google Scholar] [CrossRef]
- Trial of NanoPac Focal Therapy for Prostate Cancer. Available online: https://ClinicalTrials.gov/show/NCT03077659 (accessed on 1 October 2022).
- Intracystic Injection of NanoPac® in Subjects with Mucinous Cystic Pancreatic Neoplasms. Available online: https://ClinicalTrials.gov/show/NCT03188991 (accessed on 1 October 2022).
- Phase II Study of Intraperitoneal NanoPac® in Patients with Ovarian Cancer. Available online: ://ClinicalTrials.gov/show/NCT03029585 (accessed on 6 October 2022).
- Desai, N. Nanoparticle albumin-bound paclitaxel (Abraxane®). In Albumin in Medicine; Springer: Berlin/Heidelberg, Germany, 2016; pp. 101–119. [Google Scholar]
- Parodi, A.; Miao, J.; Soond, S.M.; Rudzińska, M.; Zamyatnin, A.A., Jr. Albumin nanovectors in cancer therapy and imaging. Biomolecules 2019, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.M.; Williams, T.M.; Nana-Sinkam, S.P.; Shilo, K.; Chatterjee, M.; Mo, X.; Rahmani, M.; Phillips, G.S.; Villalona-Calero, M.A.; Otterson, G.A. Stromal caveolin-1 is associated with response and survival in a phase II trial of nab-paclitaxel with carboplatin for advanced NSCLC patients. Clin. Lung Cancer 2015, 16, 466–474.e4. [Google Scholar] [CrossRef][Green Version]
- Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients with Stage IIIB, Stage IV, or Recurrent Non-Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT00729612 (accessed on 1 October 2022).
- Hosein, P.J.; de Lima Lopes, G., Jr.; Pastorini, V.H.; Gomez, C.; Macintyre, J.; Zayas, G.; Reis, I.; Montero, A.J.; Merchan, J.R.; Lima, C.M.R. A phase II trial of nab-Paclitaxel as second-line therapy in patients with advanced pancreatic cancer. Am. J. Clin. Oncol. 2013, 36, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Abraxane Therapy in Patients with Pancreatic Cancer Who Failed First-Line Gemcitabine Therapy. Available online: https://ClinicalTrials.gov/show/NCT00691054 (accessed on 6 October 2022).
- S1505: Combination Chemotherapy or Gemcitabine Hydrochloride and Paclitaxel Albumin-Stabilized Nanoparticle Formulation before Surgery in Treating Patients with Pancreatic Cancer that Can be Removed by Surgery. Available online: https://ClinicalTrials.gov/show/NCT02562716 (accessed on 1 October 2022).
- Sohal, D.P.; Duong, M.; Ahmad, S.A.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2021, 7, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Duong, M.; Sohal, D.P.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L., III; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M. Surgical outcome results from SWOG S1505: A randomized clinical trial of mFOLFIRINOX vs. gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann. Surg. 2020, 272, 481. [Google Scholar] [CrossRef]
- Hama, M.; Ishima, Y.; Chuang, V.T.G.; Ando, H.; Shimizu, T.; Ishida, T. Evidence for Delivery of Abraxane via a Denatured-Albumin Transport System. ACS Appl. Mater. Interfaces 2021, 13, 19736–19744. [Google Scholar] [CrossRef]
- Saif, M.W. US Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP J. Pancreas 2013, 14, 686–688. [Google Scholar]
- Adams, S.; Diéras, V.; Barrios, C.; Winer, E.; Schneeweiss, A.; Iwata, H.; Loi, S.; Patel, S.; Henschel, V.; Chui, S. Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann. Oncol. 2020, 31, 582–589. [Google Scholar] [CrossRef]
- Yardley, D.A.; Brufsky, A.; Coleman, R.E.; Conte, P.F.; Cortes, J.; Glück, S.; Nabholtz, J.-M.A.; O’Shaughnessy, J.; Beck, R.M.; Ko, A. Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): Study protocol for a randomized controlled trial. Trials 2015, 16, 575. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Hersh, E.M.; Del Vecchio, M.; Brown, M.; Kefford, R.; Loquai, C.; Testori, A.; Bhatia, S.; Gutzmer, R.; Conry, R.; Haydon, A. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann. Oncol. 2015, 26, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- A Study of Atezolizumab in Combination with Carboplatin + Paclitaxel or Carboplatin + Nab-Paclitaxel Compared with Carboplatin + Nab-Paclitaxel in Participants with Stage IV Squamous Non-Small Cell Lung Cancer (NSCLC) IMpower131. Available online: https://ClinicalTrials.gov/show/NCT02367794 (accessed on 1 October 2022).
- S0800, Nab-Paclitaxel, Doxorubicin, Cyclophosphamide, and Pegfilgrastim with or without Bevacizumab in Treating Women with Inflammatory or Locally Advanced Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT00856492 (accessed on 1 October 2022).
- Phase II NCT (Neoadjuvant Chemotherapy) w/Weekly Abraxane in Combination with Carboplatin & Bevacizumab in Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT00675259 (accessed on 1 October 2022).
- Mrózek, E.; Layman, R.; Ramaswamy, B.; Lustberg, M.; Vecchione, A.; Knopp, M.V.; Shapiro, C.L. Phase II trial of neoadjuvant weekly nanoparticle albumin-bound paclitaxel, carboplatin, and biweekly bevacizumab therapy in women with clinical stage II or III HER2-negative breast cancer. Clin. Breast Cancer 2014, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Phase II Study with Abraxane, Bevacizumab and Carboplatin in Triple Negative Metastatic Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT00479674 (accessed on 1 October 2022).
- Carboplatin, Paclitaxel, and Bevacizumab in Treating Patients with Locally Recurrent or Metastatic Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT00654836 (accessed on 1 October 2022).
- Paclitaxel Albumin-Stabilized Nanoparticle Formulation, Gemcitabine, and Bevacizumab in Treating Patients with Metastatic Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT00662129 (accessed on 1 October 2022).
- Nab-Paclitaxel and Bevacizumab or Ipilimumab as First-Line Therapy in Treating Patients with Stage IV Melanoma that Cannot be Removed by Surgery. Available online: https://ClinicalTrials.gov/show/NCT02158520 (accessed on 1 October 2022).
- Bevacizumab and Temozolomide or Bevacizumab and Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Carboplatin in Treating Patients with Stage IV Malignant Melanoma that Cannot be Removed by Surgery. Available online: https://ClinicalTrials.gov/show/NCT00626405 (accessed on 1 October 2022).
- Topical Imiquimod and Abraxane in Treating Patients with Advanced Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT00821964 (accessed on 1 October 2022).
- Salazar, L.G.; Lu, H.; Reichow, J.L.; Childs, J.S.; Coveler, A.L.; Higgins, D.M.; Waisman, J.; Allison, K.H.; Dang, Y.; Disis, M.L. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: A phase 2 clinical trial. JAMA Oncol. 2017, 3, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Schedules of Nab-Paclitaxel in Metastatic Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT01746225 (accessed on 1 October 2022).
- Utembe, W.; Clewell, H.; Sanabria, N.; Doganis, P.; Gulumian, M. Current approaches and techniques in physiologically based pharmacokinetic (PBPK) modelling of nanomaterials. Nanomaterials 2020, 10, 1267. [Google Scholar] [CrossRef]
- Nab-Paclitaxel/Rituximab-Coated Nanoparticle AR160 in Treating Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. Available online: https://ClinicalTrials.gov/show/NCT03003546 (accessed on 1 October 2022).
- Nevala, W.K.; Butterfield, J.T.; Sutor, S.L.; Knauer, D.J.; Markovic, S.N. Antibody-targeted paclitaxel loaded nanoparticles for the treatment of CD20+ B-cell lymphoma. Sci. Rep. 2017, 7, 45682. [Google Scholar] [CrossRef] [PubMed]
- Nanoparticle Albumin-Bound Rapamycin in Treating Patients with Advanced Cancer with mTOR Mutations. Available online: https://ClinicalTrials.gov/show/NCT02646319 (accessed on 1 October 2022).
- Phase 1/2 Study of ABI-009 in Nonmuscle Invasive Bladder Cancer. Available online: https://ClinicalTrials.gov/show/NCT02009332 (accessed on 6 October 2022).
- Nanoparticle Albumin-Bound Rapamycin and Pazopanib Hydrochloride in Treating Patients with Advanced Nonadipocytic Soft Tissue Sarcomas. Available online: https://ClinicalTrials.gov/show/NCT03660930 (accessed on 1 October 2022).
- Nanoparticle Albumin-Bound Rapamycin, Temozolomide, and Irinotecan Hydrochloride in Treating Pediatric Patients with Recurrent or Refractory Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT02975882 (accessed on 1 October 2022).
- ABI-009 (Nab-Rapamycin) in Recurrent High Grade Glioma and Newly Diagnosed Glioblastoma. Available online: https://ClinicalTrials.gov/show/NCT03463265 (accessed on 1 October 2022).
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta (BBA)-Biomembr. 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Vyas, M.; Simbo, D.A.; Mursalin, M.; Mishra, V.; Bashary, R.; Khatik, G.L. Drug Delivery Approaches for Doxorubicin in the Management of Cancers. Curr. Cancer Ther. Rev. 2020, 16, 320–331. [Google Scholar] [CrossRef]
- Smith, J.A.; Mathew, L.; Burney, M.; Nyshadham, P.; Coleman, R.L. Equivalency challenge: Evaluation of Lipodox® as the generic equivalent for Doxil® in a human ovarian cancer orthotropic mouse model. Gynecol. Oncol. 2016, 141, 357–363. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.; Tomczak, P.; Ackland, S. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating nanotechnology into cancer care. ACS Nano 2019, 13, 7370–7376. [Google Scholar] [CrossRef]
- A Study of Thalidomide plus Dexamethasone (Thal-Dex) versus DOXIL Plus Thalidomide Plus Dexamethasone (DOXIL-Thal-Dex) in Patients with Newly Diagnosed Multiple Myeloma. Available online: https://ClinicalTrials.gov/show/NCT00097981 (accessed on 1 October 2022).
- Pegylated Liposomal Doxorubicin (Caelyx(R)) as Monotherapy in Elderly Patients with Locally Advanced and/or Metastatic Breast Cancer (Study P05059). Available online: https://ClinicalTrials.gov/show/NCT00604968 (accessed on 1 October 2022).
- Safety Study of CAELYX in Patients with Metastatic Breast Cancer Previously Treated with Anthracyclines (Study P04057)(TERMINATED). Available online: https://ClinicalTrials.gov/show/NCT00779285 (accessed on 1 October 2022).
- A Study to Compare CAELYX with Topotecan HCL in Patients with Recurrent Epithelial Ovarian Carcinoma Following Failure of First-Line, Platinum-Based Chemotherapy. Available online: https://ClinicalTrials.gov/show/NCT01840943 (accessed on 1 October 2022).
- A Study Comparing the Combination of Trabectedin (YONDELIS) and DOXIL/CAELYX with DOXIL/CAELYX for the Treatment of Advanced-Relapsed Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. Available online: https://ClinicalTrials.gov/show/NCT01846611 (accessed on 1 October 2022).
- Monk, B.J.; Herzog, T.J.; Wang, G.; Triantos, S.; Maul, S.; Knoblauch, R.; McGowan, T.; Shalaby, W.S.; Coleman, R.L. A phase 3 randomized, open-label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol. Oncol. 2020, 156, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.; Ghatage, P.; Parekh, T.; Henitz, E.; Knoblauch, R.; Matos-Pita, A.; Nieto, A.; Park, Y.; Cheng, P.; Li, W. Effect of BRCA1 and XPG mutations on treatment response to trabectedin and pegylated liposomal doxorubicin in patients with advanced ovarian cancer: Exploratory analysis of the phase 3 OVA-301 study. Ann. Oncol. 2015, 26, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Herzog, T.J.; Patel, S.R.; von Mehren, M.; Schuetze, S.M.; Van Tine, B.A.; Coleman, R.L.; Knoblauch, R.; Triantos, S.; Hu, P. Cardiac safety of trabectedin monotherapy or in combination with pegylated liposomal doxorubicin in patients with sarcomas and ovarian cancer. Cancer Med. 2021, 10, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hong, K.; Park, J.W.; Kirpotin, D.B. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006, 66, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Papi, M.; Caputo, D.; Palmieri, V.; Coppola, R.; Palchetti, S.; Bugli, F.; Martini, C.; Digiacomo, L.; Pozzi, D.; Caracciolo, G. Clinically approved PEGylated nanoparticles are covered by a protein corona that boosts the uptake by cancer cells. Nanoscale 2017, 9, 10327–10334. [Google Scholar] [CrossRef]
- Study of Irinotecan Liposome Injection (ONIVYDE®) in Patients with Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT03088813 (accessed on 1 October 2022).
- Study of Convection-Enhanced, Image-Assisted Delivery of Liposomal-Irinotecan in Recurrent High Grade Glioma. Available online: https://ClinicalTrials.gov/show/NCT02022644 (accessed on 1 October 2022).
- Nal-iri/lv5-fu versus Paclitaxel as Second Line Therapy in Patients with Metastatic Oesophageal Squamous Cell Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT03719924 (accessed on 1 October 2022).
- Study of Onivyde with Talazoparib or Temozolomide in Children with Recurrent Solid Tumors and Ewing Sarcoma. Available online: https://ClinicalTrials.gov/show/NCT04901702 (accessed on 1 October 2022).
- Batist, G.; Barton, J.; Chaikin, P.; Swenson, C.; Welles, L. Myocet (liposome-encapsulated doxorubicin citrate): A new approach in breast cancer therapy. Expert Opin. Pharmacother. 2002, 3, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Niemann, B.; Massing, U.; Drevs, J.; Unger, C.; Bhamra, R.; Swenson, C.E. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: An open-label, single-dose study. Cancer Chemother. Pharmacol. 2004, 54, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Myocet® in Children with Relapsed or Refractory Non-Brainstem Malignant Glioma. Available online: https://ClinicalTrials.gov/show/NCT02861222 (accessed on 1 October 2022).
- A Multi-Centre Study of MBVD in Elderly and/or Cardiopathic Patients Affected by Hodgkin’s Lymphoma (HL). Available online: https://ClinicalTrials.gov/show/NCT01523847 (accessed on 1 October 2022).
- Open Label, Single Arm, Phase II Study Using R-COMP in Elderly Patients with Aggressive NHL. Available online: https://ClinicalTrials.gov/show/NCT00244127 (accessed on 1 October 2022).
- Liposome-Encapsulated Doxorubicin Citrate and Carboplatin in Treating Patients with Advanced or Metastatic Recurrent Endometrial Cancer. Available online: https://ClinicalTrials.gov/show/NCT01100359 (accessed on 1 October 2022).
- Liposome-Encapsulated Doxorubicin Citrate with or without Gemcitabine Hydrochloride in Treating Patients with Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cavity Cancer. Available online: https://ClinicalTrials.gov/show/NCT01100372 (accessed on 6 October 2022).
- Addition of Carboplatin to Neoadjuvant Therapy for Triple-Negative and HER2-Positive Early Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT01426880 (accessed on 1 October 2022).
- Allen, T.M.; Martin, F.J. Advantages of liposomal delivery systems for anthracyclines. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 5–15. [Google Scholar]
- Forssen, E.A. The design and development of DaunoXome® for solid tumor targeting in vivo. Adv. Drug Deliv. Rev. 1997, 24, 133–150. [Google Scholar] [CrossRef]
- Fassas, A.; Anagnostopoulos, A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk. Lymphoma 2005, 46, 795–802. [Google Scholar] [CrossRef]
- Liposomal Daunorubicin in Treating Patients with HIV-Related Kaposi’s Sarcoma. Available online: https://ClinicalTrials.gov/show/NCT00427414 (accessed on 6 October 2022).
- S0106 Cytarabine and Daunorubicin w/ or w/o Gemtuzumab Followed by HD Cytarabine and Either Gemtuzumab or Nothing in De Novo AML. Available online: https://ClinicalTrials.gov/show/NCT00085709 (accessed on 6 October 2022).
- Alfayez, M.; Kantarjian, H.; Kadia, T.; Ravandi-Kashani, F.; Daver, N. CPX-351 (vyxeos) in AML. Leuk. Lymphoma 2020, 61, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Daunorubicin/cytarabine liposome: A review in acute myeloid leukaemia. Drugs 2018, 78, 1903–1910. [Google Scholar] [CrossRef]
- Phase III Study of CPX-351 versus 7 + 3 in Patients 60–75 Years Old with Untreated High Risk (Secondary) Acute Myeloid Leukemia. Available online: https://ClinicalTrials.gov/show/NCT01696084 (accessed on 1 October 2022).
- A Trial to Evaluate the Potential Impact of Renal Impairment on the Pharmacokinetics and Safety of CPX-351. Available online: https://ClinicalTrials.gov/show/NCT03555955 (accessed on 6 October 2022).
- Aleku, M.; Schulz, P.; Keil, O.; Santel, A.; Schaeper, U.; Dieckhoff, B.; Janke, O.; Endruschat, J.; Durieux, B.; Röder, N. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008, 68, 9788–9798. [Google Scholar] [CrossRef]
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef] [PubMed]
- Study with Atu027 in Patients with Advanced Solid Cancer. Available online: https://ClinicalTrials.gov/show/NCT00938574 (accessed on 1 October 2022).
- Schultheis, B.; Strumberg, D.; Kuhlmann, J.; Wolf, M.; Link, K.; Seufferlein, T.; Kaufmann, J.; Feist, M.; Gebhardt, F.; Khan, M. Safety, efficacy and pharcacokinetics of targeted therapy with the liposomal RNA interference therapeutic Atu027 combined with gemcitabine in patients with pancreatic adenocarcinoma. a randomized phase Ib/IIa study. Cancers 2020, 12, 3130. [Google Scholar] [CrossRef]
- Atu027 Plus Gemcitabine in Advanced or Metastatic Pancreatic Cancer (Atu027-I-02). Available online: https://ClinicalTrials.gov/show/NCT01808638 (accessed on 1 October 2022).
- Boman, N.L.; Cullis, P.R.; Mayer, L.D.; Bally, M.B.; Webb, M.S. Liposomal vincristine: The central role of drug retention in defining therapeutically optimized anticancer formulations. In Long Circulating Liposomes: Old Drugs, New Therapeutics; Springer: Berlin/Heidelberg, Germany, 1998; pp. 29–49. [Google Scholar]
- Silverman, J.A.; Deitcher, S.R. Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Davis, T.; Farag, S.S. Treating relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: Liposome-encapsulated vincristine. Int. J. Nanomed. 2013, 8, 3479. [Google Scholar]
- Vincristine Sulfate Liposome Injection (Marqibo®) in Combination with UK ALL R3 Induction Chemotherapy for Children, Adolescents, and Young Adults with Relapsed ALL. Available online: https://ClinicalTrials.gov/show/NCT02879643 (accessed on 1 October 2022).
- Neoadjuvant Carboplatin and Vincristine and Standard Local Ophthalmic Therapy in Treating Patients with Intraocular Retinoblastoma. Available online: https://ClinicalTrials.gov/show/NCT00079417 (accessed on 1 October 2022).
- Safety and Efficacy of Marqibo in Metastatic Malignant Uveal Melanoma. Available online: https://ClinicalTrials.gov/show/NCT00495079 (accessed on 1 October 2022).
- Ollila, T.A.; Butera, J.; Egan, P.C.; Reagan, J.L.; Thomas, A.G.; Yakirevich, I.; MacKinnon, K.; Margolis, J.; Rosati, V.; McMahon, J. Vincristine Sulfate Liposome Injection (VSLI) with Bendamustine and Rituximab As First-Line Therapy for Indolent B-Cell Lymphomas: A Phase 1 Study. Blood 2020, 136, 15–16. [Google Scholar] [CrossRef]
- Mifamurtide (L-MTP-PE) for High-Risk Osteosarcoma. Available online: https://ClinicalTrials.gov/show/NCT00631631 (accessed on 1 October 2022).
- Nieto González, N.; Obinu, A.; Rassu, G.; Giunchedi, P.; Gavini, E. Polymeric and Lipid Nanoparticles: Which Applications in Pediatrics? Pharmaceutics 2021, 13, 670. [Google Scholar] [CrossRef]
- Nardin, A.; Lefebvre, M.; Labroquere, K.; Faure, O.; Abastado, J. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr. Cancer Drug Targets 2006, 6, 123–133. [Google Scholar] [CrossRef] [PubMed]
- de la Torre Gomez, C.; Goreham, R.V.; Bech Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”—A review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front. Genet. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- iExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation. Available online: https://ClinicalTrials.gov/show/NCT03608631 (accessed on 1 October 2022).
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Study Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Colon Cancer Tissue. Available online: https://ClinicalTrials.gov/show/NCT01294072 (accessed on 1 October 2022).
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics 2022, 14, 822. [Google Scholar] [CrossRef]
- Edible Plant Exosome Ability to Prevent Oral Mucositis Associated with Chemoradiation Treatment of Head and Neck Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01668849 (accessed on 1 October 2022).
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Trial of a Vaccination with Tumor Antigen-Loaded Dendritic Cell-Derived Exosomes. Available online: https://ClinicalTrials.gov/show/NCT01159288 (accessed on 1 October 2022).
- A Study to Evaluate Diagnostic Performance and Safety of Pegsitacianine, an Intraoperative Fluorescence Imaging Agent for the Detection of Lung Malignancies in Patients Undergoing Routine Surgery. Available online: https://ClinicalTrials.gov/show/NCT05048082 (accessed on 1 October 2022).
- Clinical Study on the Harvesting Lymph Nodes with Carbon Nanoparticles for Advanced Gastric Cancer. Available online: https://ClinicalTrials.gov/show/NCT02123407 (accessed on 1 October 2022).
- Carbon Nanoparticles and Indocyanine Green for Sentinel Lymph Node Biopsy in Early Stage Cervical Cancer. Available online: https://ClinicalTrials.gov/show/NCT05167149 (accessed on 1 October 2022).
- M.D. Anderson Cancer Center. Four Quadrants Transverse Abdominus Plane (4Q-TAP) Block with Plain and Liposomal Bupivacaine vs. Thoracic Epidermal Analgesia (TEA) in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) on an Enhanced Recovery Pathway. 2017. Available online: https://ClinicalTrials.gov/show/NCT03359811 (accessed on 1 October 2022).
- Novel RNA-Nanoparticle Vaccine for the Treatment of Early Melanoma Recurrence Following Adjuvant Anti-PD-1 Antibody Therapy. Available online: https://ClinicalTrials.gov/show/NCT05264974 (accessed on 1 October 2022).
- EMD Serono; Merck KGaA, Darmstadt, Germany. Cancer Vaccine Study for Unresectable Stage III Non-Small Cell Lung Cancer (START). 2007. Available online: https://ClinicalTrials.gov/show/NCT00409188 (accessed on 1 October 2022).
- Parodi, A.; Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Borodina, T.; Akasov, R.; Frolova, A.; Chulanov, V.; Zamyatnin, A.A., Jr. Biomimetic approaches for targeting tumor inflammation. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
| Particle | Material | Size (nm) | Targeting Mechanism | Killing Mechanism |
|---|---|---|---|---|
| AguIX | Polysyloxane | 5 | EPR | radiosensitizer |
| NBTXR3 | Hafnium oxide | 50 | intratumor | radiosensitizer |
| SPION | Iron oxide | 20–150 | EPR, intratumor, polarized magnetic fields | Thermoablation, conjugated drug |
| NU-0129 | Gold | N.A. | EPR, transcytosis | RNAi |
| ELU001 | Silica | 6 | Folic acid | topoisomerase-1 inhibitor |
| CALAA-01 | Cyclodextrin | 50–70 | Transferrin | RNAi |
| Genexol-PM | Polymeric micelles | 20–50 | EPR | PTX |
| Apalea/ Paclical | Polymeric micelles | 20–30 | EPR | PTX |
| Pm-PAC | Polymeric micelles | ~20 | EPR | PTX |
| Nanoxel-PM | Polymeric micelles | 10–50 | EPR | DTX |
| NC-6004 | Polymeric micelles | ~30 | EPR | GEM |
| NK012 | Polymeric micelles | ~20 | EPR | SN-30 |
| NC-6300 | Polymeric micelles | 40–80 | EPR, acidic pH | epirubicin |
| EP0057 | Polymeric micelles | 30–40 | EPR | Camptothecin, radiosensitizer |
| Abraxane | Albumin | 130 | Receptor mediated trafficking | PTX |
| Abi-009 | Albumin | 130 | Receptor mediated trafficking local administration | Rapamycin |
| NanoPac | PTX | 600–800 | Local administration | PTX |
| Doxil | Lipids | 70–100 | EPR | DOX |
| Myocet | Lipids | 150 | EPR | DOX |
| Marqibo | Lipids | 100 | EPR | Vincristine |
| MEPACT | Lipids | >100 | Targeting MPS | Mifamurtide |
| Onyvide | Lipids | 110 | EPR | Irinotecan |
| Daunoxome | Lipids | 45 | EPR | Daunorubicin |
| Vyxeos | Lipids | Daunorubicin/Cytarabine | ||
| ATU-027 | Lipids | 100 | EPR | RNAi |
| Exosomes | Proteo/lipids | 30–120 | Natural tropism | RNAi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parodi, A.; Kolesova, E.P.; Voronina, M.V.; Frolova, A.S.; Kostyushev, D.; Trushina, D.B.; Akasov, R.; Pallaeva, T.; Zamyatnin, A.A., Jr. Anticancer Nanotherapeutics in Clinical Trials: The Work behind Clinical Translation of Nanomedicine. Int. J. Mol. Sci. 2022, 23, 13368. https://doi.org/10.3390/ijms232113368
Parodi A, Kolesova EP, Voronina MV, Frolova AS, Kostyushev D, Trushina DB, Akasov R, Pallaeva T, Zamyatnin AA Jr. Anticancer Nanotherapeutics in Clinical Trials: The Work behind Clinical Translation of Nanomedicine. International Journal of Molecular Sciences. 2022; 23(21):13368. https://doi.org/10.3390/ijms232113368
Chicago/Turabian StyleParodi, Alessandro, Ekaterina P. Kolesova, Maya V. Voronina, Anastasia S. Frolova, Dmitry Kostyushev, Daria B. Trushina, Roman Akasov, Tatiana Pallaeva, and Andrey A. Zamyatnin, Jr. 2022. "Anticancer Nanotherapeutics in Clinical Trials: The Work behind Clinical Translation of Nanomedicine" International Journal of Molecular Sciences 23, no. 21: 13368. https://doi.org/10.3390/ijms232113368
APA StyleParodi, A., Kolesova, E. P., Voronina, M. V., Frolova, A. S., Kostyushev, D., Trushina, D. B., Akasov, R., Pallaeva, T., & Zamyatnin, A. A., Jr. (2022). Anticancer Nanotherapeutics in Clinical Trials: The Work behind Clinical Translation of Nanomedicine. International Journal of Molecular Sciences, 23(21), 13368. https://doi.org/10.3390/ijms232113368










