Abstract
Caligus rogercresseyi is the main ectoparasite that affects the salmon industry in Chile. The mechanisms used by the parasite to support its life strategy are of great interest for developing control strategies. Due to the critical role of insect peritrophins in host–parasite interactions and response to pest control drugs, this study aimed to identify and characterize the peritrophin-like genes present in C. rogercresseyi. Moreover, the expression of peritrophin-like genes was evaluated on parasites exposed to delousing drugs such as pyrethroids and azamethiphos. Peritrophin genes were identified by homology analysis among the sea louse transcriptome database and arthropods peritrophin-protein database obtained from GenBank and UniProt. Moreover, the gene loci in the parasite genome were located. Furthermore, peritrophin gene expression levels were evaluated by RNA-Seq analysis in sea louse developmental stages and sea lice exposed to delousing drugs deltamethrin, cypermethrin, and azamethiphos. Seven putative peritrophin-like genes were identified in C. rogercresseyi with high homology with other crustacean peritrophins. Differences in the presence of signal peptides, the number of chitin-binding domains, and the position of conserved cysteines were found. In addition, seven peritrophin-like gene sequences were identified in the C. rogercresseyi genome. Gene expression analysis revealed a stage-dependent expression profile. Notably, differential regulation of peritrophin genes in resistant and susceptible populations to delousing drugs was found. These data are the first report and characterization of peritrophin genes in the sea louse C. rogercresseyi, representing valuable knowledge to understand sea louse biology. Moreover, this study provides evidence for a deeper understanding of the molecular basis of C. rogercresseyi response to delousing drugs.
1. Introduction
The copepod ectoparasite Caligus rogercresseyi, also known as sea louse, represents one of the major problems in the Chilean salmon aquaculture [1,2]. This copepod feeds on fish mucus and blood [3], producing skin lesions, weight loss, and immunosuppression in fish [4,5]. Salmon farming economic costs generated by this ectoparasite have motivated research on the mechanisms behind C. rogercresseyi infection on salmonid fishes. A significant step forward was the publication of the transcriptome of the parasite development stages [6] and the publication of the sea louse genome draft [7]. Functional genomics data have provided information on genes involved in the interaction between this parasite and the host fishes [8,9]. Moreover, sea lice control has been historically based on delousing drugs, highlighting the interest in understanding drug response mechanisms [10,11]. However, the genetic mechanisms involved in these processes in C. rogercresseyi have not yet been fully described.
Peritrophins are proteins with a history of participating in parasitic mechanisms and as part of the response to insecticide drugs [12,13]. In insects, peritrophins have an important role in host–parasite interaction [14,15]. For example, an increased expression of peritrophins was found as part of the host innate immune response after infections by the scabies mite [16]. Additionally, an upregulation of peritrophin genes was detected in the mosquitoes Lutzomyia longipalpis artificially fed with blood containing Leishmania parasite [17]. On the other hand, peritrophin proteins participate in the protective response against toxic compounds damaging [18,19,20]. For instance, the peritrophin AeIMUC1 removes the heme groups from the host species to avoid the deleterious effects of free radical formation in the African malaria mosquito Anopheles gambiae [12,21]. Moreover, upregulation of the peritrophin-1-like gene was observed in the potato pest Leptinotarsa decemlineata exposed to the biological insecticide Spinosad [13]. Regarding sea lice species, a transcriptomic study conducted in Lepeophtheirus salmonis exposed to the chitin synthesis inhibitor Lufenuron showed a downregulation of transcripts containing chitin-binding domains in the affected parasites, including genes with the peritrophin-A domain that is characteristic of the structural domains of peritrophic membrane proteins [22].
The peritrophins are chitin-binding proteins originally isolated from insect intestinal peritrophic membrane or peritrophic matrix (PM) [23,24]. The PM is a non-cellular structure covering the midgut of various arthropods [25,26,27], including copepod parasitic species Lernaea cyprinacea, Ergasilus orientalis, and Neoergasilus japonicus [28]. The PM is similar to a sieve with physiological functions that include intestine epithelium protection against mechanical damage, neutralization of ingested toxins or pathogens, and compartmentalization of the digestive process [29,30,31,32,33,34]. The PM structure is composed of chitin fibrils associated with a protein matrix [18,35]. Among the structural proteins of this matrix, peritrophins are the most abundant, comprising 35 to 55% of the structure [18,36,37,38]. In addition, the peritrophins have functional domains which are tightly associated with the chitin fibrils of the PM [39,40]. The most recurrent domains reported are the chitin-binding domains with N-glycosylations sites and mucin domains with O-glycosylation sites [41]. In crustaceans, the presence of PM and homologous insect peritrophin genes have been described [23,42,43,44,45,46]. For instance, peritrophin-like genes have been identified in crustaceans such as shrimp [23,44,45,47,48,49] and Chinese mitten crab [50,51]. Moreover, Lai and Aboobaker [24] reported a new family of 80 peritrophin-like genes from a transcriptomic database of 55 crustaceans from the Malacostraca class.
The lack of information regarding the C. rogercresseyi peritrophins leaves relevant information gaps for controlling the pathogen. Currently, the main sea louse control methods are based on delousing drugs such as pyrethroids, organophosphates, and chitin inhibitors [52,53]. However, a reduction in the efficacy of these compounds has already developed in C. rogercresseyi populations [54,55,56,57,58]. Thus, several studies have investigated the underlying mechanisms involved in sea lice response to delousing drugs, suggesting potential resistance mechanisms [10,11,59,60,61,62]. Nevertheless, the importance of the PM and the role of peritrophins in the response of C. rogercresseyi to delousing drugs have not been described yet. Given the importance of peritrophin proteins in host–parasite interaction and their role in providing a physical barrier against toxic compounds, this study aimed to identify and characterize the peritrophin-like genes present in C. rogercresseyi developmental stages, and we additionally evaluated the modulation of peritrophin-like genes in C. rogercresseyi exposed to cypermethrin, deltamethrin, and azamethiphos.
2. Results
2.1. Identification and Characterization of Putative Peritrophin-likes Genes
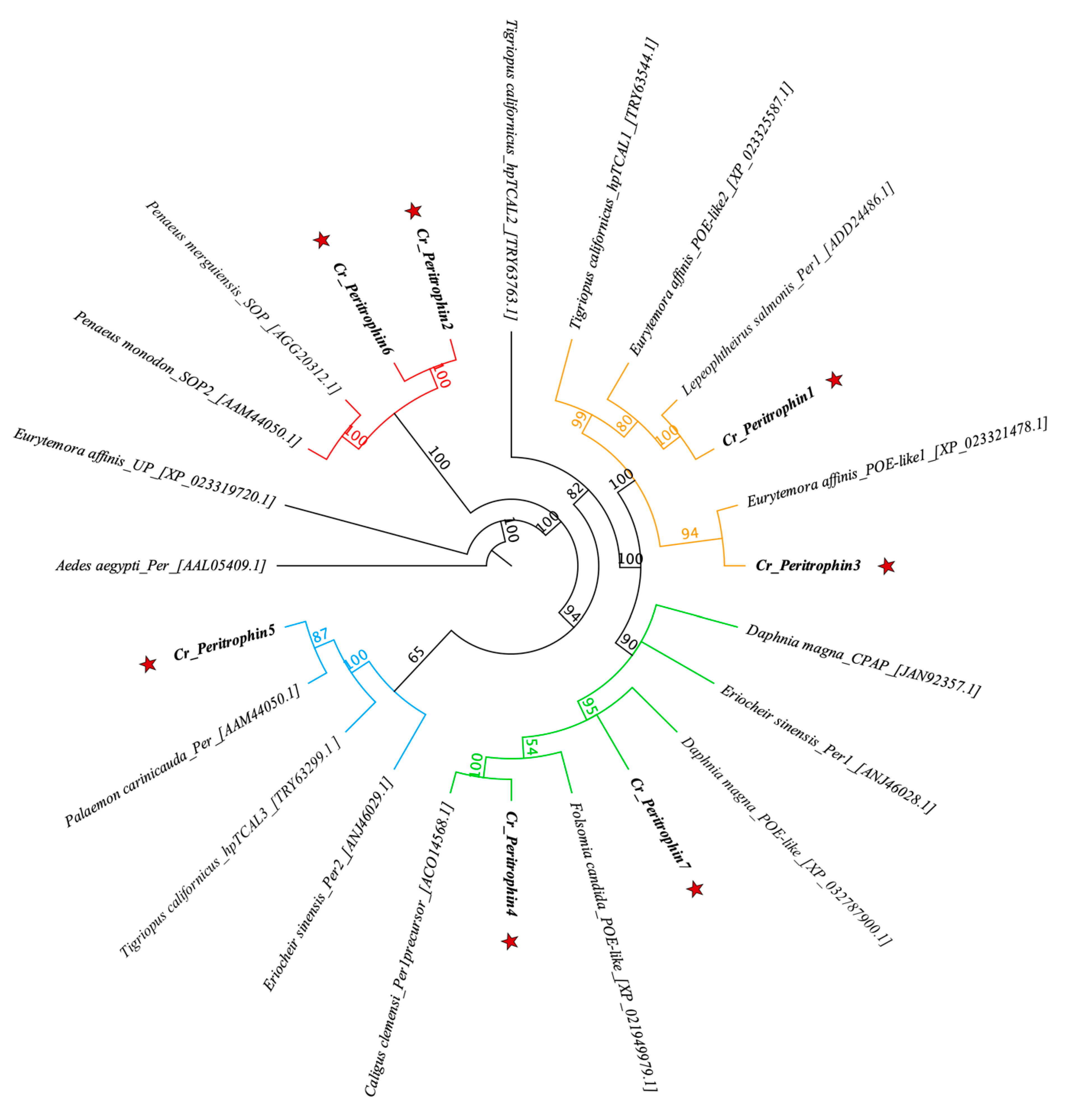
From in silico analysis of the C. rogercresseyi transcriptome, 16 contigs were identified with homology for peritrophin genes (Table S1). Seven of these sequences presented the full open reading frame (ORF). The identified sequences were consecutively named Cr_Peritrophin1 (Cr_Per1) to Cr_Pereritrophin7 (Cr_Per7). The longest ORF was Cr_Per4 with 1008 bp, while the shortest ORF was Cr_Per2 with 315 bp (Table 1). C. rogercresseyi peritrophin sequences show high identity with the other peritrophin-like proteins reported in crustaceans (Figure 1). Most clades are supported with high bootstrapping values, with ranges between 60 and 100%. The Cr_Per6 and Cr_Per2 were grouped in the same clade of Penaeus merguiensis_SOP and Penaeus monodon_SOP. Cr_Per5 was clustered together with Palaemon carinicauda_Per, separated from a copepods clade. The Cr_Per1 and Cr_Per3 were clustered together with L. salmonis_Per1 and Eurytemora affinis_POE-like1, respectively. A second clade contained Cr_Per7 and Cr_Per4 grouped with Caligus clemensi_PT.

Table 1.
C. rogercresseyi peritrophins characterization.
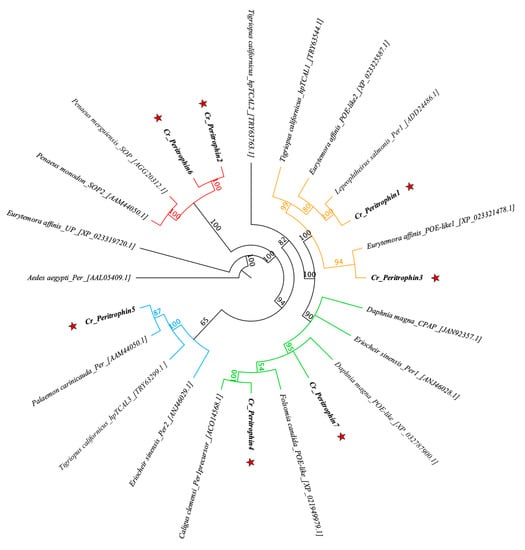
Figure 1.
Phylogenetic analysis of sea louse peritrophin proteins. Jukes–Cantor genetic distance model was used to determine the bootstrapping indicated in the nodes. Sequence access numbers are found next to species names. The Cr_Per sequences are marked with a red star. The different clades are shown in red, orange, green, and blue.
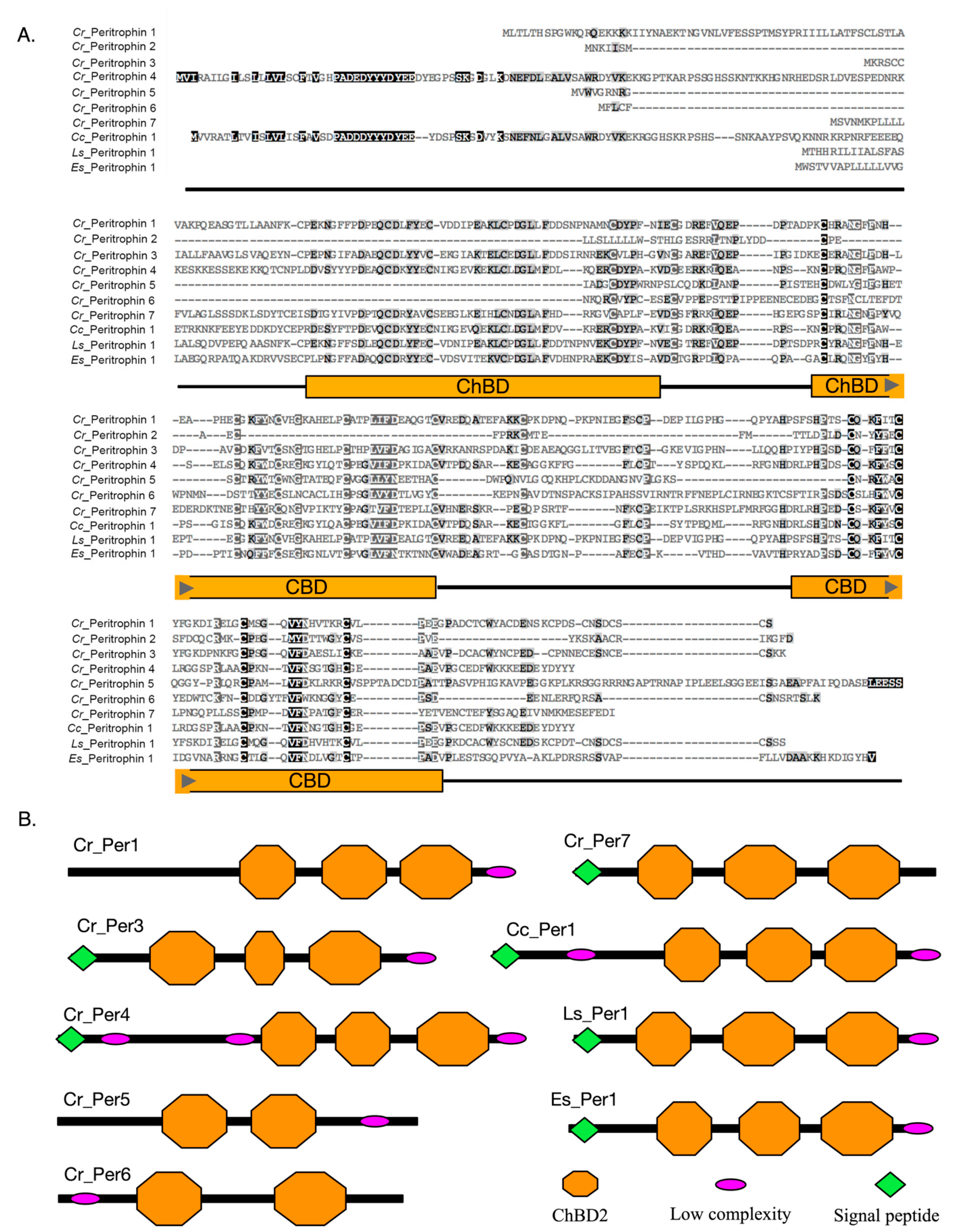
From the characterization of peritrophin protein sequences, differences in the number of ChtBD2 domains and distribution of the conserved Cys were observed (Figure 2A). For instance, three ChtBD2 were found in Cr_Per1, Cr_Per3, Cr_Per4, and Cr_Per7, and two of these domains in Cr_Per5 and Cr_Per6 (Figure 2B). Cr_Per2 did not show any of these domains despite their high homology with peritrophin-like protein sequences. Furthermore, all the Cr_Per sequences exhibited O-glycosylation sites, while N-glycosylation sites were only found in Cr_Per3, Cr_Per5, Cr_Per6, and Cr_Per7. Protein subcellular prediction determined by protein sequences that Cr_Per1 was a cell membrane protein, and the rest of Cr_Per were soluble extracellular proteins (Table 1). Finally, the predicted tertiary structure for Cr_Per proteins, the invertebrate chitin-binding protein (d1dqca), was used as a template with a confidence of more than 96% for every Cr_Per (Figure S1).
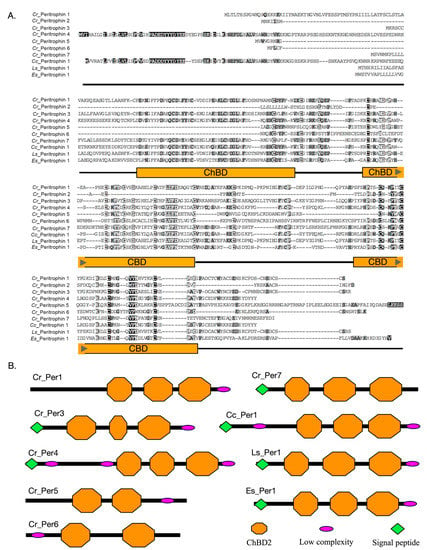
Figure 2.
Amino acid sequences analysis of peritrophins identified in C. rogercresseyi. (A). Multiple alignments of the Cr_Per protein with other crustacea peritrophin proteins deposited in GenBank. The dark gray region indicates the positions where all the sequences share the same amino acid residue, and oranges boxes represent chitin-binding domains. (B). The predicted domain structure of the peritrophin-like proteins of C. rogercresseyi (Cr). Cc: Caligus clemensi [ACO14568.1], Ls: Lepeophtheirus salmonis [ADD24486.1], Es: Eriocheir sinensis [ANJ46028.1].
2.2. Identification of Cr_Per Sequences in the Genome
The sequences of the peritrophin-like genes of C. rogercresseyi were located in the sea louse genome (BioProyect Accession: PRJNA551027). The characterized peritrophins were located in different chromosomes, and most of them presented a single exon (Table 2). Although, Cr_Per5, located in chromosome 11, presented two exons separated by a short intron of 61 pb (Figure S2).

Table 2.
Genome structure of C. rogercresseyi peritrophin-like.
2.3. Peritrophin Expression Analysis in C. rogercresseyi Developmental Stages
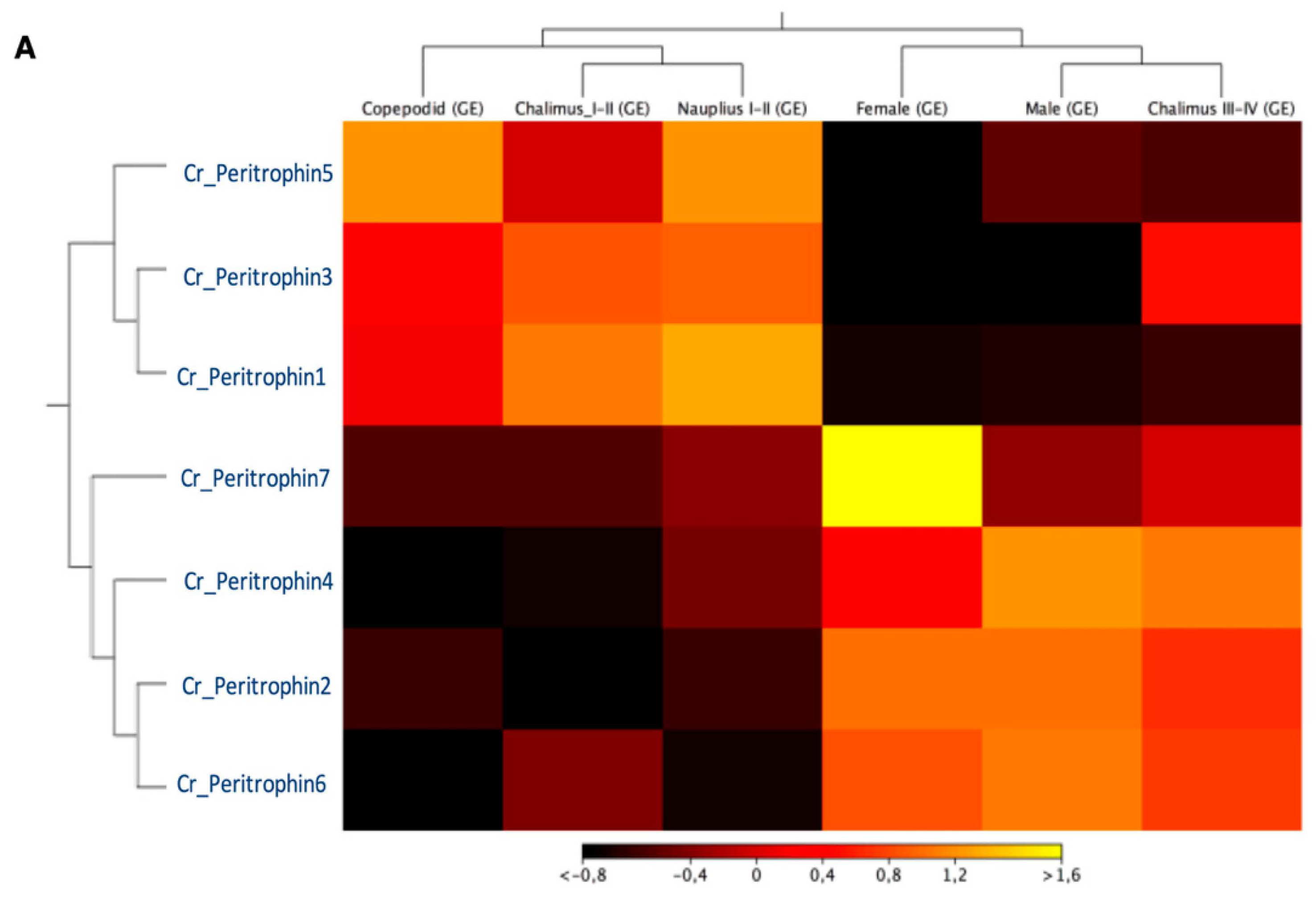
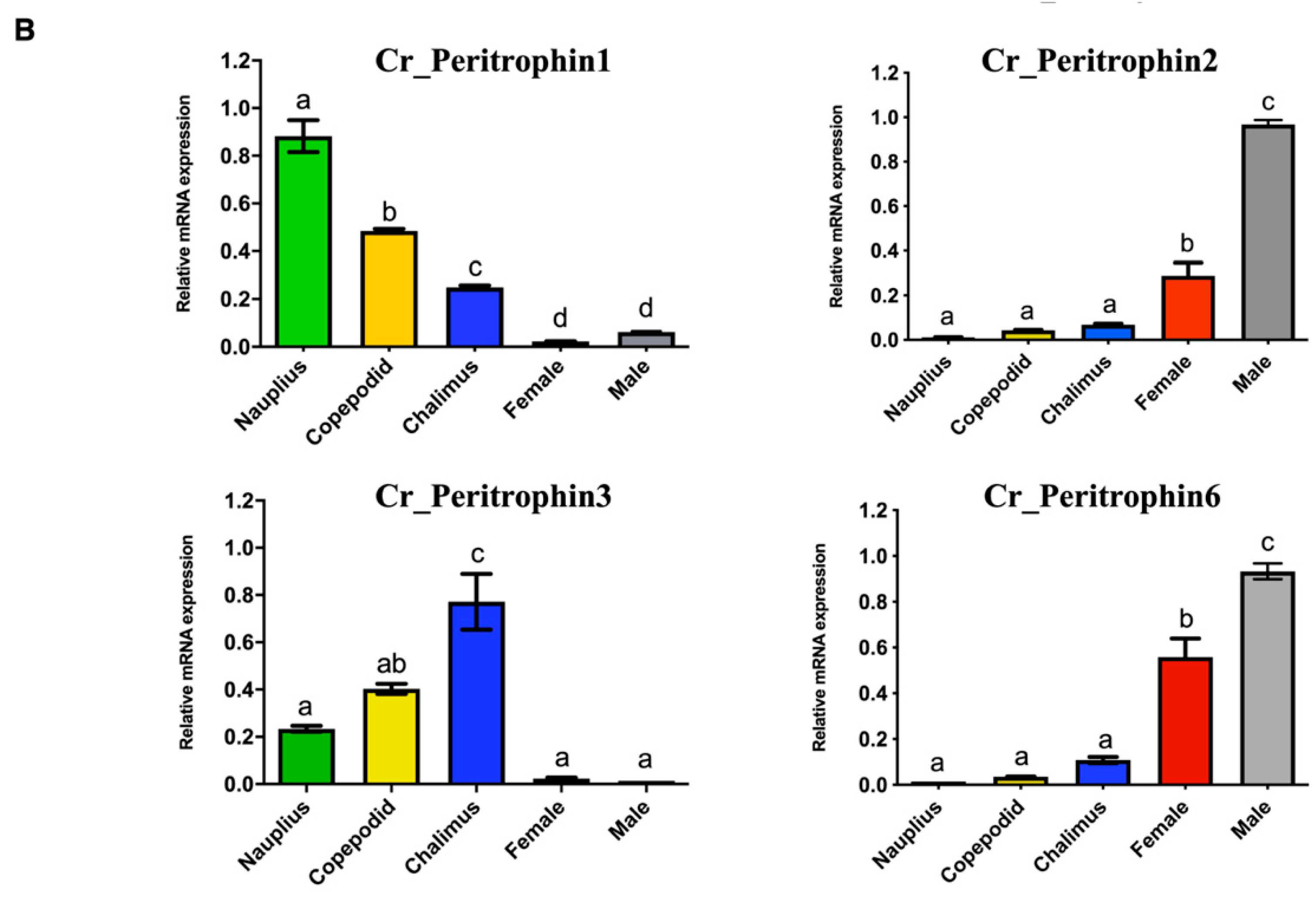
The expression profile of peritrophin-like genes characterized in C. rogercresseyi was evaluated in all developmental stages. The RNA-Seq analysis showed two clusters of peritrophin-like genes. The first one was composed of Cr_Per1, Cr_Per3, and Cr_Per5, which were highly expressed in free live stages, and chalimus I-II. Moreover, a second cluster composed of Cr_Per2, Cr_Per4, Cr_Per6, and Cr_Per7 was mainly expressed in chalimus III-IV and adults. Notably, the Cr_Per7 was strongly upregulated in females (Figure 3A). The RT-qPCR validation was performed for Cr_Per1, Cr_Per2, Cr_Per3, and Cr_Per6 (Figure 3B). Cr_Per1 showed high expression levels in early stages (nauplius and copepodid), which were similar to the in silico analysis (Figure 3B). Furthermore, the expression levels of Cr_Per2 and Cr_Per6 in adults sea louse were upregulated (Figure 3B), while the Cr_Per3 was upregulated significantly in chalimus stages (Figure 3B).
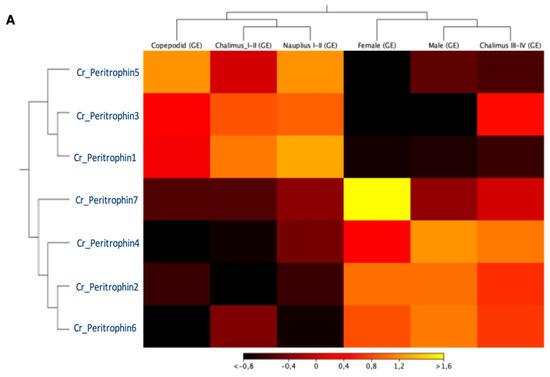
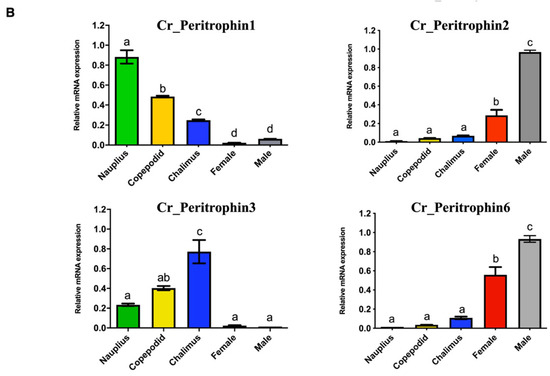
Figure 3.
Transcriptional expression of contigs annotated as peritrophin genes during C. rogercresseyi developmental stages. (A). Heatmap showing the TPM values using transcriptomic data of C. rogercresseyi developmental stages. Hierarchical clustering was based on Euclidean distances with an average linkage. (B). RT-qPCR validation of four Cr_Per in sea louse developmental stages. Bars represent the relative expression abundance of the transcripts (mean ± standard deviation). The lowercase letters above the error bars for each gene showed significant differences at p < 0.05.
2.4. Peritrophin Expression in Drug-Exposed C. rogercresseyi
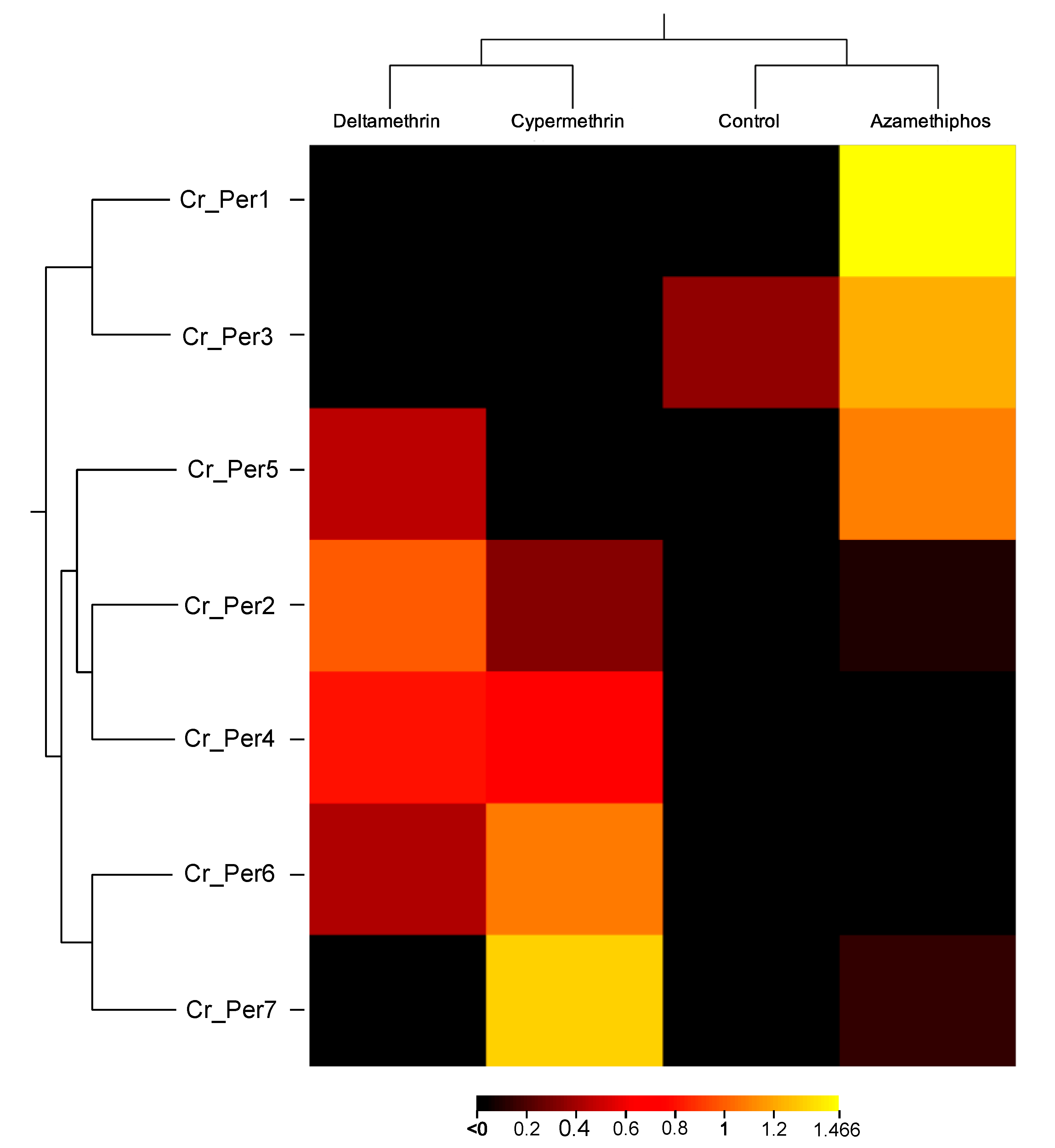
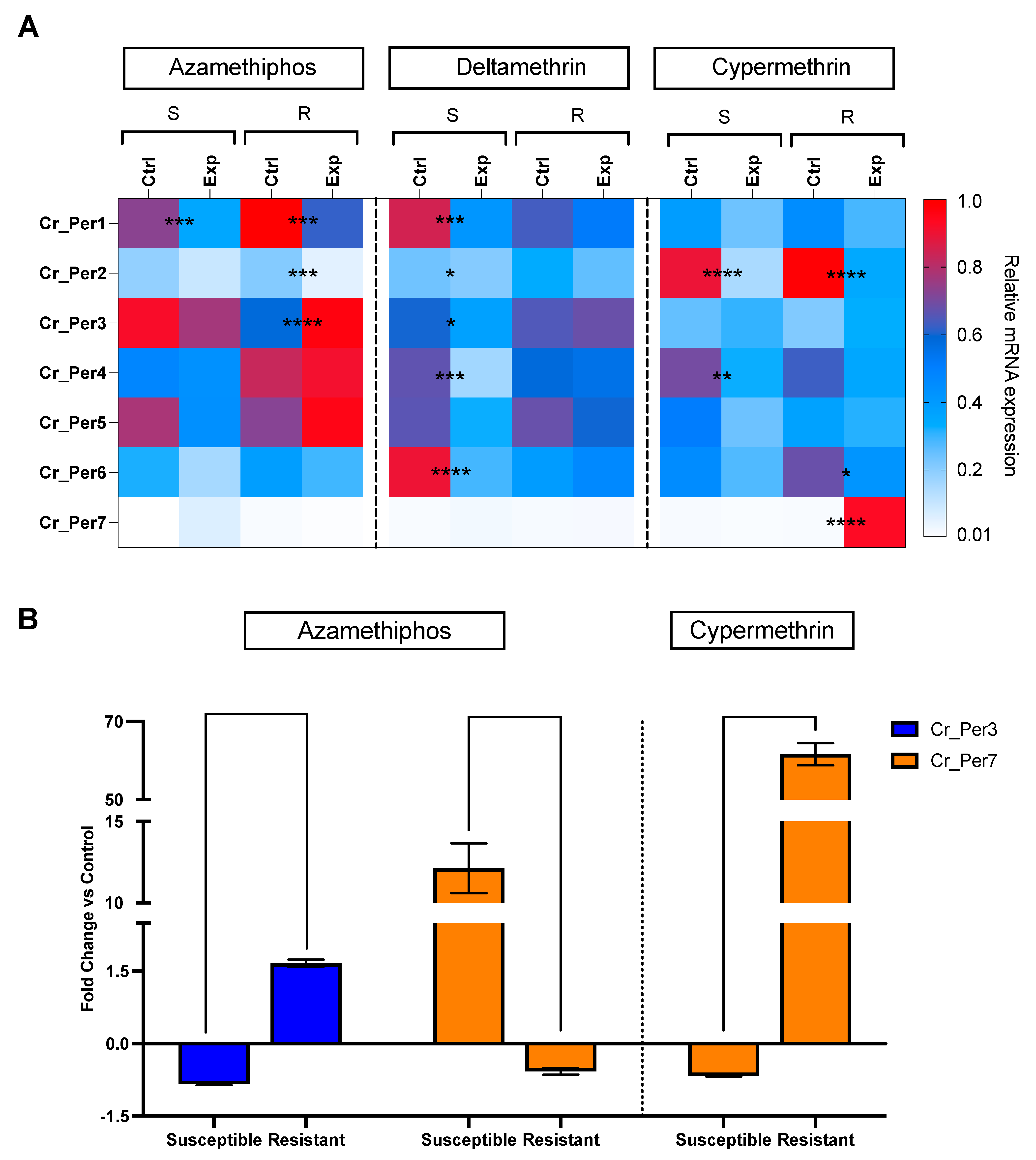
Sea lice challenged with delousing chemicals showed a drug-dependent peritrophin expression profile (Figure 4). Sea lice groups exposed to pyrethroids integrated one sole cluster, upregulating Cr_Per2, Cr_Per4, and Cr_Per7. In contrast, the azamethiphos group strongly upregulated Cr_Per1, Cr_Per3, and Cr_Per5 (Figure 4). Furthermore, expression was validated in susceptible and resistant C. rogercressyi populations to these drugs [11]. Interestingly, all drug-exposed groups evidence modulation of peritrophin genes expression (Figure 5A). In the azamethiphos group, the resistant population showed the greatest differences with Cr_Per1, Cr_Per2, and Cr_Per3, while the deltamethrin susceptible group modulated five of the seven Cr_Per genes. Interestingly, Cr_Per7 gene expression was only upregulated in resistant sea lice populations challenged with cypermethrin (Figure 5A). Moreover, cypermethrin Cr_Per7 was the gene with the highest fold change between populations of susceptible and resistant sea lice (Figure 5B).
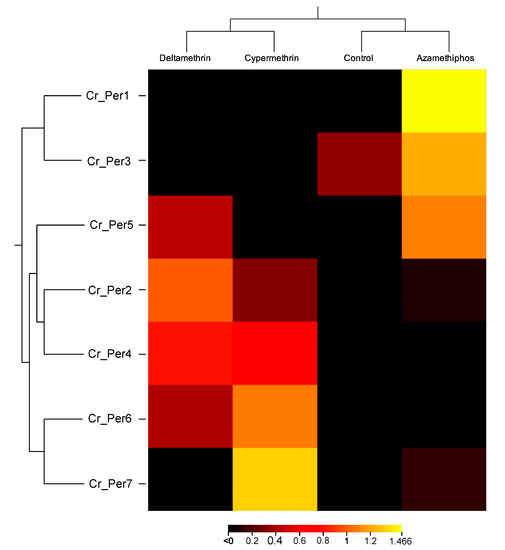
Figure 4.
Heatmap of gene transcription values from drugs-exposed C. rogercresseyi to 100 ppb of azamethiphos, 3 ppb deltamethrin, and 15 ppb cypermethrin. Transcript abundance is represented as TPM values. Color scales show relative transcript expression. Hierarchical clustering was conducted based on Euclidean distance for TPM data with an average linkage.
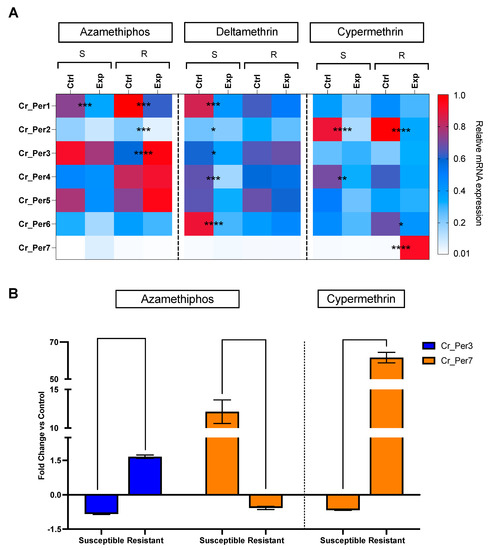
Figure 5.
RT-qPCR of peritrophin genes in C. rogercresseyi populations susceptible (S) and resistant (R) to drugs. (A). Heatmaps show the relative expression (fold change values) of Cr_Per in control (Ctrl) and exposed (Exp) lice to azamethiphos, deltamethrin, and cypermethrin. The relative expression levels were normalized with β tubulin as an endogenous control. Asterisks indicate statistically significant values between control and exposed lice of the same population and treatment, where p-value < 0.05 (*), < 0.005 (**), < 0.0003 (***), and < 0.0001 (****). Color scales show relative gene expression. (B). Peritrophin genes with higher fold change in C. rogercresseyi exposed to drugs. Column bars represent the means for the fold change differences based on the relative expression of the exposed group against the control group for each population. Data are presented as the mean expression ± standard error (SEM). Asterisks indicate statistical differences between the resistance and susceptible control group for each population.
3. Discussion
Caligus rogercresseyi is the most important ectoparasite in Chilean salmon farming; thereby, interest in developing new control methods has encouraged the study of their physiology and molecular mechanisms of interaction with the host. Peritrophins, as the main component of the peritrophic membrane, have been evaluated as a pharmacological target for insect control methods [63,64]. Furthermore, peritrophin proteins have been related in some insects to the protective response against damage caused by toxic compounds, such as insecticides [14,65]. Here, we identified and characterized C. rogercresseyi peritrophin-like genes with a potential role in C. rogercresseyi response to delousing drugs.
Studies performed in arthropods have described different numbers of peritrophin gene isoforms expressed in one species. For instance, Lai and Aboobaker [24] reported 80 peritrophin-like transcripts from five orders of Malacostraca. In insect species, such as Spodoptera frugiperda and Abracris flavolineata, 38 and 23 perithrophins-like, respectively, have been reported [66]. In this study, 16 peritrophin-like sequences were identified from C. rogercresseyi transcriptome database [6]. The phylogenetic analysis showed divergences among C. rogercresseyi peritrophin-like proteins, suggesting different functions. For instance, Cr_Per2 and Cr_Per6 converged with peritrophin-like proteins expressed during shrimp oogenesis called Shrimp Ovarian Peritrophin (SOP) [23,45,47,67]. SOPs are the main protein in Penaeus semisulcatus oocytes and an important component for the development of spawned eggs [45]. Furthermore, SOPs play an immune role in protecting spawned eggs against pathogens [23]. The high expression levels of Cr_Per2 and Cr_Per6 in C. rogercresseyi females suggest an SOP-like function of these two sea louse peritrophins. Regarding Cr_Per3, this gene was clustered with the protein obstructor-E-like (obst-E) of the copepod E. affinis. Obstructing genes represent the invertebrate multigene family [68] mainly studied in insects [69,70,71]. These proteins with chitin-binding domains participate in the development of the cuticle [72]. For instance, in Drosophila melanogaster Obst-E participates in the contraction and expansion of the cuticle in the pre-adult stages, regulating the chitin disposition in the cuticle in the third larvae stage and participates in determining the shape of the pupa [73,74]. In C. rogercresseyi, a total of 479 transcripts were previously reported as proteins linked to cuticle formation, with high levels of transcription during the larval stage and highly regulated in the adults stage, and also with differential expression levels after delousing drug exposure [10]. Here, Cr_Per3 gene was highly expressed in early sea louse stages, suggesting a function in the molting process.
Differences among C. rogercresseyi peritrophin isoforms were observed. For instance, the theoretical molecular weight found for the seven peritrophin-like protein sequences was between 12,141 and 38,324 KDa. These results are in concordance with previous results in crustacean species where several peritrophins sizes have been reported [23,44,51]. The Chinese mitten crab Eriocheir sinensis has the Es-peritrophin1 whit a MW of 28.93 KDa. However, for this crab was also reported the Es-peritrophin2 with 51.17 KDa [50]. Additionally, the pI found in the peritrophin-like proteins of C. rogercresseyi were in a range between 4.5 and 6, which is common among other peritrophins [39]. The extreme environment in these parts of the gastrointestinal tract requires special characteristics, and these values of pIs have been reported in other PM-related proteins [75,76].
The chitin-binding domains are the functional peritrophin domains reported in the PM of most invertebrates [72,77]. Herein, six peritrophin-like proteins identified in C. rogercresseyi presented the chitin-binding domain (ChBD2). This domain allows the assembly of the proteins and chitin fibers forming the PM [18,50,78]. Differences in the number of Cys were reported in a family of crustacean peritrophin-like proteins [24]. The consensus sequence of C. rogercresseyi peritrophin ChBD2 identified in this work was CX1-23CX5CX9-10CX12-15CX7-12C. However, Cr_Per6 ChBD2 presented one Cys more than the other sea louse peritrophins. Furthermore, characterized C. rogercresseyi peritrophins presented two or three ChBD2s. Similar results have been reported in crustacean species, Fenneropenaeus Chinensis, P. monodon, Penaeus semisulcatus, and E. sinensis [44,50,51]. In addition, more than three ChBD2 domains have been described for insect peritrophins [77]. Interestingly, no presence of functional ChBD2 domains was found in the Cr_Per2 sequence. These results are consistent with those reported in A. gambiae, where PM proteins without ChBD2 were identified, suggesting a function associated with protein–protein interactions and formation of three-dimensional PM structure [12]. Additionally, the presence of signal peptide in the ChBD2 protein is a characteristic of chitin-binding functions in extracellular matrices [72]. The analysis of signal peptide and cellular localization of C. rogercresseti peritrophins suggest that they are expressed and secreted in the extracellular matrix, except for the Cr_Per1, which was determined as a membrane protein. These data are in accordance with the PM reported structure, which is a non-cellular structure composed of secreted proteins embedded in a proteoglycan matrix with chitin [79]. Interestingly, chitin synthesis inhibitors have been used for caligidosis control [80]. However, research has shown a decrease in the effectiveness of these treatments [53]. Notably, the structural characteristics of C. rogercresseyi peritrophins suggest their participation as part of drug response [81].
To identify the genome position of Cr_Per genes, the sequence was mapped against C. rogercresseyi genome (BioProject Accession: PRJNA551027). Each characterized sea louse peritrophins was located in a different chromosome. Furthermore, six of them presented a single exon. These results are in agreement with a study conducted on the common cutworm, Spodoptera litura, where the peritrophin-37 gene with a single exon was found in the cutworm genome [82]. Additionally, peritrophin genes such as PMP9 of Tribolium castaneum [72], and the Peritrophin-44 gene of the ectoparasite Lucilia cuprina [37,83] had only a single exon. However, an L. cuprina peritrophin called peritrophin-95 has been described with a small intron of 77 bp [84]. In our work, a small intron of 61 bp was identified for Cr_Per5 gene, located in sea louse chromosome 11.
As we described above, peritrophins are essential molecules to the physiological functions of PM and play an important role in the control of toxicity damage [85,86]. Indeed, an in vitro study reported that Aedes aegypti peritrophin AelMUCI can bind to the hemotoxic groups [14], suggesting a detoxification mechanism used by the mosquito to reduce the damage caused by free radicals during hemoglobin digestion obtained from blood-feeding [12]. Therefore, studies aimed at the control of pests have targeted the PM in different insect species [87,88,89]. For example, increased mortality was observed when the termite Reticulitermes flavipes treated with double-stranded RNA (dsRNA) to reduce the expression levels of PM genes such as peritrophin and chitin, was challenged with the themicide imidacloprid [65,90]. Moreover, the transcriptome analysis of the potato pest L. decemlineata showed a strong upregulation of peritrophin-1-like gene when the beetle was exposed to the insecticide Spinosad [13]. Here, our data show a differential expression pattern of peritrophin-like genes in C. rogercresseyi exposed to delousing drugs azamethiphos and pyrethroids. Moreover, we present the first peritrophin genes expression analysis in populations of C. rogercresseyi differing in sensitivity to deltamethrin, cypermethrin, and azamethiphos. Interestingly, some genes were differentially expressed specifically after drug exposure. The expression of Cr_Per7 gene significantly increased the expression in exposed individuals from the cypermethrin-resistant population. Similarly, an increased expression of the Cr_Per3 gene was observed in exposed individuals belonging to the azamethiphos-resistant population. Thus, these proteins might have a function in the detoxification process, preventing the accumulation of these compounds in resistant individuals. Previously, Chávez-Mardones, Valenzuela-Muños, and Gallardo-Escárate [10] reported a high correlation between azamethiphos exposure and cuticle precursor gene expression in C. rogercresseyi, suggesting an influence of this organophosphate on the regulation of cuticle-related proteins in sea lice. Notably, Cr_Per3 is structurally close to cuticle-related proteins. Another interesting finding was the upregulation of the Cr_Per1, Cr_Per2, Cr_Per3, and Cr_Per4 genes in control groups, which suggests these genes as markers for drug sensitivity testing.
Our results support the hypothesis of the potential functionality of these proteins in decreased mortality after drug exposure. As we identified peritrophin genes that are constituents of the PM, we provide evidence for a deeper understanding of the molecular basis of C. rogercresseyi response to delousing drugs. Nevertheless, complementary studies will be needed to determine the role played by these peritrophin genes in the delousing drugs response. Finally, the recognition of molecular markers to predict drug resistance in sea louse can be an important asset for the salmon industry [61]. This work identified significant modulation of the Cr_Per7 gene in exposed individuals from the resistant cypermethrin population in comparison with the susceptible population. Similarly, the Cr_Per3 gene showed significant differences between susceptible and resistant populations to azamethiphos. Thus, the characterization of sea louse peritrophin genes are reported here, evidenced their utility in C. rogercresseyi control. Therefore, we suggest that these genes should be considered candidate genes for sea louse control in further studies.
4. Materials and Methods
4.1. Identification of Peritrophin-like Genes in C. rogercresseyi
4.1.1. Identification and Characterization of C. rogercresseyi Peritrophins
From the transcriptome database described for C. rogercresseyi [6], peritrophin cDNA sequences were identified. Sea louse transcripts annotation was performed by tBLASTx analysis against peritrophin transcripts in CLC Genomics Workbench software (Version 22, CLC Qiagen Bioinformatics, CA, USA), using the EST database available for arthropods in GenBank. Transcripts with an E-value ≤ 1 × 10−5 were selected. The cDNA open reading frame (ORF) was determined and translated using Geneious software (Version 11.0.9, Biomatters Ltd., Auckland, NI, New Zealand). Moreover, a multiple alignment analysis using a BLOSUM62 matrix was performed with peritrophin sequences described for arthropods in GenBank. Furthermore, a phylogenetic tree was generated with the Neighbor-Joining method, using Jukes–Cantor as a genetic distance model, and applying a bootstrap of 1000. In addition, protein functional domains were identified with the online tools SMART [91] and PROSITE [92]. SignalP-5.0 was used to determine the presence of signal peptides [93] and DeepLoc-1.0 for predicting the protein subcellular localization [94]. The molecular weight (MW) and theoretical isoelectric point (pI) of the putative protein sequences were determined with Compute pI/Mw software [95]. Additionally, O- and N-linked glycosylation sites were predicted with NetOGlyc 4.0 Server [96] and NetNGlyc 1.0 Server [97], respectively. The Phyre2 web portal was used for modeling, prediction, and analysis of the tertiary protein structure [98].
4.1.2. Peritrophins Genome Organization
The C. rogercresseyi Genome draft (Genome NCBI Accession: PRJNA551027) was used to predict the chromosomal location and genome structure of the Cr_Per genes. Re-sequencing analysis was performed using the Map Reads to Reference (1.6) plugin included in CLC Genomics Workbench (Version 22, CLC Bio, Denmark). The parameters used were mismatch cost = 2, cost of insertions and deletions = linear gap cost, insertion cost = 1, deletion cost = 1, length fraction = 0.9, and similarity fraction = 0.8.
4.2. Expression Analysis of Peritrophin Genes
4.2.1. Ontogeny Expression
Raw reads obtained for C. rogercresseyi developmental stages [6] were mapped against peritrophin contigs with a significant annotation (E-value > 10−5). The RNA-seq settings were a minimum length fraction = 0.8 and a minimum similarity fraction (long reads) = 0.8. The expression value was set as a Transcripts Per Million of Reads (TPM). The distance metric was calculated with the Euclidean distance method [99], where the mean expression level in 5–6 rounds of k-means clustering was subtracted. Finally, multi-factorial statistics analysis based on a negative binomial GLM was used to compare gene expression.
4.2.2. Sea Lice Challenged with Delousing Drugs
Peritrophin contigs were used as reference to map the raw reads obtained from C. rogercresseyi populations exposed to drugs: 8 ppb of azamethiphos (Byelice®, Bayer Cono Sur, Santiago, Chile), 3 ppb of deltamethrin (AMX®, Pharmaq South America, Santiago, Chile), and 5 ppb of cypermethrin (Betamax®, Novartis Chile S.A., Santiago, Chile) [11]. RNA-seq analysis parameters and statistical tests were performed as described in Section 4.2.1.
4.2.3. RT-qPCR Validation
Peritrophin genes expression in ontogeny and drug exposure were validated by RT-qPCR. For ontogeny, C. rogercresseyi samples were obtained from the experimental laboratory of the Marine Biological Station, University of Concepción, Dichato, Chile. Sea lice larvae were kept in trays provided with a flow of seawater at 12 °C and soft aeration. Atlantic salmons were infected to obtain juvenile and adult stages at a load of 35 copepodites per fish. Samples of each stage of the parasite were collected: nauplius, copepodite, chalimus, adult females, and males. Furthermore, peritrophin genes expression was validated using C. rogercresseyi strains previously characterized as susceptible and resistant to each delousing drug: deltamethrin, cypermethrin, and azamethiphos [11]. For each delousing drug, an exposed plus a control group was chosen for the susceptible and the resistant population. Each group consisted of 30 individuals (fifteen females and fifteen males). The exposure period for azamethiphos was 30 min (100 ppb), 40 min for deltamethrin (3 ppb), and 30 min for cypermethrin (15 ppb). Once the trial time was over, the parasites were fixed in the RNALater solution (Ambion®, Thermo Fisher ScientificTM, Waltham, MA, USA) and stored at −80 °C.
Total RNA was isolated using Trizol reagent (Ambion, Life Technologies™, Carlsbad, CA, USA) according to the manufacturer’s instructions and quantified on Nanodrop One spectrophotometer (Thermo Scientific, Waltham, MA, USA). The RT-qPCR standardization was carried out according to the MIQE guidelines [100]. cDNA was synthesized starting at 200 ng/µL of initial total RNA and using the RevertAid H Minus First Strand cDNA Synthesis kit (Thermo Fisher Scientific, MA, USA), following the manufacturer’s directions. The RT-qPCR reaction was performed on the StepOnePlus™ thermocycler (Applied Biosystems, Foster City, CA, USA). For the expression quantification of peritrophin-like genes, the comparative ΔΔCt method was used [101]. The data were normalized using the β-tubulin II gene as housekeeping [8]. The PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) was used in a final reaction volume of 10 uL. Amplification was carried out under the following conditions: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 30 s at 60 °C, followed by a melting curve (95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s). Specific primers were designed in Geneious (Version 11.0.9, Biomatters Ltd., Auckland, NI, New Zealand) (Table 3). Statistical analyses were performed in the GraphPad Prism 9.0 software (San Diego, CA, USA). The data present the mean of the standard error (SEM). Significant differences were determined by one-way ANOVA and Tukey post-hoc analysis. Statistically significant values were set at p-values < 0.05.

Table 3.
Oligonucleotide primers used for RT-qPCR analysis.
5. Conclusions
This is the first study to identify and characterize peritrophin-like genes in the sea louse C. rogercresseyi, and the expression profile of these genes in response to delousing drugs. C. rogercresseyi peritrophin-like genes were differentially expressed in drug-susceptible and drug-resistant sea lice populations exposed to delousing drugs azamethiphos, deltamethrin, and cypermethrin. The characterized sea louse peritrophins encode extracellular proteins with putative functions as a structural component of the peritrophic membrane or cuticula. Gene expression is differentially regulated during the parasite developmental stages, suggesting different functions through ontogeny. Further studies will be conducted to evaluate the use of peritrophin genes as a tool for sea louse control.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232113341/s1.
Author Contributions
Conceptualization, A.C., G.N.-A., V.V.-M. and C.G.-E.; methodology, A.C., G.N.-A., V.V.-M. and C.G.-E.; formal analysis, A.C., V.V.-M., G.N.-A. and C.S.-V.; writing—original draft preparation, A.C.; writing—review and editing, A.C., G.N.-A., V.V.-M., C.S.-V. and C.G.-E. All authors have read and agreed to the published version of the manuscript.
Funding
ANID-Chile funded this study through the grants FONDAP (1510027) and Doctoral grant Doctorado Nacional (2018-21180084).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fast, M.D. Fish immune responses to parasitic copepod (namely sea lice) infection. Dev. Comp. Immunol. 2014, 43, 300–312. [Google Scholar] [CrossRef]
- Torrissen, O.; Jones, S.; Asche, F.; Guttormsen, A.; Skilbrei, O.T.; Nilsen, F.; Horsberg, T.E.; Jackson, D. Salmon lice–impact on wild salmonids and salmon aquaculture. J. Fish Dis. 2013, 36, 171–194. [Google Scholar] [CrossRef]
- Valenzuela-Muñoz, V.; Gallardo-Escárate, C. Iron metabolism modulation in Atlantic salmon infested with the sea lice Lepeophtheirus salmonis and Caligus rogercresseyi: A matter of nutritional immunity? Fish Shellfish Immunol. 2017, 60, 97–102. [Google Scholar] [CrossRef]
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The impact of co-infections on fish: A review. Vet. Res. 2017, 47, 98. [Google Scholar] [CrossRef]
- Gallardo-Escárate, C.; Valenzuela-Muñoz, V.; Nuñez-Acuña, G. RNA-Seq analysis using de novo transcriptome assembly as a reference for the salmon louse Caligus rogercresseyi. PLoS ONE 2014, 9, e92239. [Google Scholar] [CrossRef]
- Gallardo-Escárate, C.; Valenzuela-Muñoz, V.; Nuñez-Acuña, G.; Valenzuela-Miranda, D.; Gonçalves, A.T.; Escobar-Sepulveda, H.; Liachko, I.; Nelson, B.; Roberts, S.; Warren, W. Chromosome-scale genome assembly of the sea louse Caligus rogercresseyi by SMRT sequencing and Hi-C analysis. Sci. Data 2021, 8, 60. [Google Scholar] [CrossRef]
- Vera-Bizama, F.; Valenzuela-Muñoz, V.; Gonçalves, A.T.; Marambio, J.P.; Hawes, C.; Wadsworth, S.; Gallardo-Escárate, C. Transcription expression of immune-related genes from Caligus rogercresseyi evidences host-dependent patterns on Atlantic and coho salmon. Fish Shellfish Immunol. 2015, 47, 725–731. [Google Scholar] [CrossRef]
- Gallardo-Escárate, C.; Arriagada, G.; Carrera, C.; Gonçalves, A.T.; Nuñez-Acuña, G.; Valenzuela-Miranda, D.; Valenzuela-Muñoz, V. The race between host and sea lice in the Chilean salmon farming: A genomic approach. Rev. Aquac. 2019, 11, 325–339. [Google Scholar] [CrossRef]
- Chávez-Mardones, J.; Valenzuela-Muños, V.; Gallardo-Escárate, C. In silico transcriptome analysis of cuticle-related genes associated with delousing drug responses in the sea louse Caligus rogercresseyi. Aquaculture 2016, 450, 123–135. [Google Scholar] [CrossRef]
- Núñez-Acuña, G.; Sáez-Vera, C.; Valenzuela-Muñoz, V.; Valenzuela-Miranda, D.; Arriagada, G.; Gallardo-Escárate, C. Tackling the molecular drug sensitivity in the sea louse Caligus rogercresseyi based on mRNA and lncRNA interactions. Genes 2020, 11, 857. [Google Scholar] [CrossRef]
- Dinglasan, R.R.; Devenport, M.; Florens, L.; Johnson, J.R.; McHugh, C.A.; Donnelly-Doman, M.; Carucci, D.J.; Yates, J.R.; Jacobs-Lorena, M. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem. Mol. Biol. 2009, 39, 125–134. [Google Scholar] [CrossRef]
- Bastarache, P.; Wajnberg, G.; Dumas, P.; Chacko, S.; Lacroix, J.; Crapoulet, N.; Moffat, C.E.; Morin, P. Transcriptomics-based approach identifies spinosad-associated targets in the colorado potato beetle, Leptinotarsa decemlineata. Insects 2020, 11, 820. [Google Scholar] [CrossRef]
- Devenport, M.; Alvarenga, P.H.; Shao, L.; Fujioka, H.; Bianconi, M.L.; Oliveira, P.L.; Jacobs-Lorena, M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry 2006, 45, 9540–9549. [Google Scholar] [CrossRef]
- Oliveira, A.H.; Fernandes, K.M.; Goncalves, W.G.; Zanuncio, J.C.; Serrao, J.E. A peritrophin mediates the peritrophic matrix permeability in the workers of the bees Melipona quadrifasciata and Apis mellifera. Arthropod Struct. Dev. 2019, 53, 100885. [Google Scholar] [CrossRef]
- Mika, A.; Goh, P.; Holt, D.C.; Kemp, D.J.; Fischer, K. Scabies mite peritrophins are potential targets of human host innate immunity. PLoS Negl. Trop. Dis. 2011, 5, e1331. [Google Scholar] [CrossRef]
- Jochim, R.C.; Teixeira, C.R.; Laughinghouse, A.; Mu, J.; Oliveira, F.; Gomes, R.B.; Elnaiem, D.-E.; Valenzuela, J.G. The midgut transcriptome of Lutzomyia longipalpis: Comparative analysis of cDNA libraries from sugar-fed, blood-fed, post-digested and Leishmania infantum chagasi-infected sand flies. BMC Genom. 2008, 9, 15. [Google Scholar] [CrossRef]
- Wang, P.; Granados, R.R. Molecular structure of the peritrophic membrane (PM): Identification of potential PM target sites for insect control. Arch. Insect Biochem. Physiol. 2001, 47, 110–118. [Google Scholar] [CrossRef]
- Sobotnik, J.; Kudlikova-Krizkova, I.; Vancova, M.; Munzbergova, Z.; Hubert, J. Chitin in the peritrophic membrane of Acarus siro (Acari: Acaridae) as a target for novel acaricides. J. Econ. Entomol. 2008, 101, 1028–1033. [Google Scholar] [CrossRef]
- Konno, K.; Mitsuhashi, W. The peritrophic membrane as a target of proteins that play important roles in plant defense and microbial attack. J. Insect Physiol. 2019, 117, 103912. [Google Scholar] [CrossRef]
- Magalhaes, T. What is the association of heme aggregates with the peritrophic matrix of adult female mosquitoes? Parasites Vectors 2014, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Poley, J.D.; Braden, L.M.; Messmer, A.M.; Igboeli, O.O.; Whyte, S.K.; Macdonald, A.; Rodriguez, J.; Gameiro, M.; Rufener, L.; Bouvier, J. High level efficacy of lufenuron against sea lice (Lepeophtheirus salmonis) linked to rapid impact on moulting processes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Loongyai, W.; Avarre, J.C.; Cerutti, M.; Lubzens, E.; Chotigeat, W. Isolation and functional characterization of a new shrimp ovarian peritrophin with antimicrobial activity from Fenneropenaeus merguiensis. Mar. Biotechnol. 2007, 9, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Aboobaker, A.A. Comparative genomic analysis of innate immunity reveals novel and conserved components in crustacean food crop species. BMC Genom. 2017, 18, 389. [Google Scholar] [CrossRef]
- Hao, Z.; Aksoy, S. Proventriculus-specific cDNAs characterized from the tsetse, Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 2002, 32, 1663–1671. [Google Scholar] [CrossRef]
- Terra, W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 2001, 47, 47–61. [Google Scholar] [CrossRef]
- Eiseman, C.H.; Binnington, K.C. The peritrophic membrane: Its formation, structure, chemical-composition and permeability in relation to vaccination against ectoparasitic arthropods. Int. J. Parasitol. 1994, 24, 15–26. [Google Scholar] [CrossRef]
- Yoshikoshi, K.; Ko, Y. Structure and function of the peritrophic membranes of copepods. Nippon Suisan Gakkaishi 1988, 54, 1077–1082. [Google Scholar] [CrossRef]
- Mittapalli, O.; Sardesai, N.; Shukle, R.H. cDNA cloning and transcriptional expression of a peritrophin-like gene in the Hessian fly, Mayetiola destructor [Say]. Arch. Insect Biochem. Physiol. 2007, 64, 19–29. [Google Scholar] [CrossRef]
- Shi, X.Z.; Chamankhah, M.; Visal-Shah, S.; Hemmingsen, S.M.; Erlandson, M.; Braun, L.; Alting-Mees, M.; Khachatourians, G.G.; O’Grady, M.; Hegedus, D.D. Modeling the structure of the Type I peritrophic matrix: Characterization of a Mamestra configurata intestinal mucin and a novel peritrophin containing 19 chitin binding domains. Insect Biochem. Mol. Biol. 2004, 34, 1101–1115. [Google Scholar] [CrossRef]
- Agrawal, S.; Kelkenberg, M.; Begum, K.; Steinfeld, L.; Williams, C.E.; Kramer, K.J.; Beeman, R.W.; Park, Y.; Muthukrishnan, S.; Merzendorfer, H. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem. Mol. Biol. 2014, 49, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, W.; Li, S.Y.; Li, R.J.; Xu, D.Q.; Lu, X.J. Identification of a new peritrophic membrane protein from larval Holotrichia parallela (Coleoptera: Motschulsky). Molecules 2014, 19, 17799–17809. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.M.; Falleiros, A.M.F.; Moscardi, F.; Gregorio, E.A. The role of peritrophic membrane in the resistance of Anticarsia gemmatalis larvae (Lepidoptera: Noctuidae) during the infection by its nucleopolyhedrovirus (AgMNPV). Arthropod Struct. Dev. 2011, 40, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Jariyapan, N.; Saeung, A.; Intakhan, N.; Chanmol, W.; Sor-suwan, S.; Phattanawiboon, B.; Taai, K.; Choochote, W. Peritrophic matrix formation and Brugia malayi microfilaria invasion of the midgut of a susceptible vector, Ochlerotatus togoi (Diptera: Culicidae). Parasitol. Res. 2013, 112, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Lehane, M. Peritrophic matrix structure and function. Annu. Rev. Entomol. 1997, 42, 525–550. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Elvin, C.M.; Vuocolo, T.; Pearson, R.D.; East, I.J.; Riding, G.A.; Eisemann, C.H.; Tellam, R.L. Characterization of a major peritrophic membrane protein, peritrophin-44, from the larvae of Lucilia cuprina: cDNA and deduced amino acid sequences. J. Biol. Chem. 1996, 271, 8925–8935. [Google Scholar] [CrossRef]
- Hu, X.L.; Chen, L.; Xiang, X.W.; Yang, R.; Yu, S.F.; Wu, X.F. Proteomic analysis of peritrophic membrane (PM) from the midgut of fifth-instar larvae, Bombyx mori. Mol. Biol. Rep. 2012, 39, 3427–3434. [Google Scholar] [CrossRef]
- Tellam, R.L.; Wijffels, G.; Willadsen, P. Peritrophic matrix proteins. Insect Biochem. Mol. Biol. 1999, 29, 87–101. [Google Scholar] [CrossRef]
- Toprak, U.; Erlandson, M.; Hegedus, D.D. Peritrophic matrix proteins. Trends Entomol 2010, 6, 23–51. [Google Scholar]
- Hegedus, D.D.; Toprak, U.; Erlandson, M. Peritrophic matrix formation. J. Insect Physiol. 2019, 117, 103898. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Hsu, T.-C.; Huang, P.-Y.; Kang, S.-T.; Lo, C.-F.; Huang, W.-P.; Chen, L.-L. Penaeus monodon chitin-binding protein (PmCBP) is involved in white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2009, 27, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Lu, L.-C.; Wu, W.-J.; Lo, C.-F.; Huang, W.-P. White spot syndrome virus envelope protein VP53A interacts with Penaeus monodon chitin-binding protein (PmCBP). Dis. Aquat. Org. 2007, 74, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Wang, J.X.; Liu, N.; Zhao, X.F.; Li, F.H.; Xiang, J.H. Identification and molecular characterization of a peritrophin-like protein from fleshy prawn (Fenneropenaeus chinensis). Mol. Immunol. 2006, 43, 1633–1644. [Google Scholar] [CrossRef]
- Khayat, M.; Babin, P.J.; Funkenstein, B.; Sammar, M.; Nagasawa, H.; Tietz, A.; Lubzens, E. Molecular characterization and high expression during oocyte development of a shrimp ovarian cortical rod protein homologous to insect intestinal peritrophins. Biol. Reprod. 2001, 64, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Wang, J.-X. Diversity and multiple functions of lectins in shrimp immunity. Dev. Comp. Immunol. 2013, 39, 27–38. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kawazoe, I.; Tsutsui, N.; Jasmani, S.; Wilder, M.N.; Aida, K. Isolation and cDNA cloning of ovarian cortical rod protein in kuruma prawn Marsupenaeus japonicus (Crustacea: Decapoda: Penaeidae). Zool. Sci. 2004, 21, 1109–1119. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, J.Q.; Xiang, J.H. Immune function against bacteria of chitin deacetylase 1 (EcCDA1) from Exopalaemon carinicauda. Fish Shellfish Immunol. 2018, 75, 115–123. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, F.H.; Wang, B.; Xiang, J.H. Structure and partial protein profiles of the peritrophic membrane (PM) from the gut of the shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2012, 33, 1285–1291. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Cai, C.F.; Shui, D.Z.; Ren, S.J.; Chen, W.; Cao, X.M.; Wu, P.; Li, T.; Ye, Y.T. Identification and characterization of two novel peritrophic membrane (PM) genes in the Chinese mitten crab Eriocheir sinensis that exhibit activity against high-pH stress and Aeromonas hydrophila challenge. Aquac. Res. 2018, 49, 3746–3758. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, F.T.; Wang, W.; Ren, Q. Identification and molecular characterization of a peritrophin-like gene, involved in the antibacterial response in Chinese mitten crab, Eriocheir sinensis. Dev. Comp. Immunol. 2015, 50, 129–138. [Google Scholar] [CrossRef]
- Coates, A.; Phillips, B.L.; Bui, S.; Oppedal, F.; Robinson, N.A.; Dempster, T. Evolution of salmon lice in response to management strategies: A review. Rev. Aquac. 2021, 13, 1397–1422. [Google Scholar] [CrossRef]
- Aaen, S.M.; Helgesen, K.O.; Bakke, M.J.; Kaur, K.; Horsberg, T.E. Drug resistance in sea lice: A threat to salmonid aquaculture. Trends Parasitol. 2015, 31, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sevatdal, S.; Horsberg, T.E. Determination of reduced sensitivity in sea lice (Lepeophtheirus salmonis Krøyer) against the pyrethroid deltamethrin using bioassays and probit modelling. Aquaculture 2003, 218, 21–31. [Google Scholar] [CrossRef]
- Sáez-Vera, C.; Núñez-Acuña, G.; Gallardo-Escárate, C. Sensitivity assessment to azamethiphos by time-to-response bioassay and biomarkers in the sea louse Caligus rogercresseyi. Aquaculture 2022, 546, 737340. [Google Scholar] [CrossRef]
- Helgesen, K.; Bravo, S.; Sevatdal, S.; Mendoza, J.; Horsberg, T. Deltamethrin resistance in the sea louse Caligus rogercresseyi (B oxhall and B ravo) in Chile: Bioassay results and usage data for antiparasitic agents with references to Norwegian conditions. J. Fish Dis. 2014, 37, 877–890. [Google Scholar] [CrossRef]
- Arriagada, G.; Figueroa, J.; Marín, S.L.; Arriagada, A.M.; Lara, M.; Gallardo-Escárate, C. First report of the reduction in treatment efficacy of the organophosphate azamethiphos against the sea lice Caligus rogercresseyi (Boxshall & Bravo, 2000). Aquac. Res. 2020, 51, 436–439. [Google Scholar]
- Bravo, S.; Silva, M.T.; Agusti, C.; Sambra, K.; Horsberg, T.E. The effect of chemotherapeutic drugs used to control sea lice on the hatching viability of egg strings from Caligus rogercresseyi. Aquaculture 2015, 443, 77–83. [Google Scholar] [CrossRef]
- Kaur, K.; Helgesen, K.O.; Bakke, M.J.; Horsberg, T.E. Mechanism behind resistance against the organophosphate azamethiphos in salmon lice (Lepeophtheirus salmonis). PLoS ONE 2015, 10, e0124220. [Google Scholar] [CrossRef]
- Valenzuela-Muñoz, V.; Chavez-Mardones, J.; Gallardo-Escárate, C. RNA-seq analysis evidences multiple gene responses in Caligus rogercresseyi exposed to the anti-salmon lice drug azamethiphos. Aquaculture 2015, 446, 156–166. [Google Scholar] [CrossRef]
- Nuñez-Acuña, G.; Valenzuela-Muñoz, V.; Gallardo-Escárate, C. High-throughput SNP discovery and transcriptome expression profiles from the salmon louse Caligus rogercresseyi (Copepoda: Caligidae). Comp. Biochem. Physiol. Part D: Genom. Proteom. 2014, 10, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Mardones, J.; Gallardo-Escárate, C. Next-generation transcriptome profiling of the salmon louse Caligus rogercresseyi exposed to deltamethrin (alphamax™): Discovery of relevant genes and sex-related differences. Mar. Biotechnol. 2015, 17, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Tellam, R.L.; Eisemann, C.H.; Vuocolo, T.; Casu, R.; Jarmey, J.; Bowles, V.; Pearson, R. Role of oligosaccharides in the immune response of sheep vaccinated with Lucilia cuprina larval glycoprotein, peritrophin-95. Int. J. Parasitol. 2001, 31, 798–809. [Google Scholar] [CrossRef]
- Tetreau, G.; Wang, P. Chitinous Structures as Potential Targets for Insect Pest Control. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Springer: Singapore, 2019; Volume 1142, pp. 273–292. [Google Scholar] [CrossRef]
- Sandoval-Mojica, A.F.; Scharf, M.E. Silencing gut genes associated with the peritrophic matrix of Reticulitermes flavipes (Blattodea: Rhinotermitidae) increases susceptibility to termiticides. Insect Mol. Biol. 2016, 25, 734–744. [Google Scholar] [CrossRef]
- Dias, R.O.; Cardoso, C.; Pimentel, A.C.; Damasceno, T.F.; Ferreira, C.; Terra, W.R. The roles of mucus-forming mucins, peritrophins and peritrophins with mucin domains in the insect midgut. Insect Mol. Biol. 2018, 27, 46–60. [Google Scholar] [CrossRef]
- Kim, Y.K.; Tsutsui, N.; Kawazoe, I.; Okumura, T.; Kaneko, T.; Aida, K. Localization and developmental expression of mRNA for cortical rod protein in kuruma prawn Marsupenaeus japonicus. Zool. Sci. 2005, 22, 675–680. [Google Scholar] [CrossRef]
- Behr, M.; Hoch, M. Identification of the novel evolutionary conserved obstructor multigene family in invertebrates. Febs Lett. 2005, 579, 6827–6833. [Google Scholar] [CrossRef]
- Pesch, Y.-Y.; Riedel, D.; Behr, M.; Liu, L.; Liu, L.; Leung, E.; Cooney, A.J.; Chen, C.; Rosengart, T.K.; Ma, Y.; et al. Obstructor A organizes matrix assembly at the apical cell surface to promote enzymatic cuticle maturation in Drosophila. J. Biol. Chem. 2015, 290, 10071–10082. [Google Scholar] [CrossRef]
- Willis, J.H. Structural cuticular proteins from arthropods: Annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 2010, 40, 189–204. [Google Scholar] [CrossRef]
- Petkau, G.; Wingen, C.; Jussen, L.C.; Radtke, T.; Behr, M. Obstructor-A is required for epithelial extracellular matrix dynamics, exoskeleton function, and tubulogenesis. J. Biol. Chem. 2012, 287, 21396–21405. [Google Scholar] [CrossRef]
- Jasrapuria, S.; Arakane, Y.; Osman, G.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Genes encoding proteins with peritrophin A-type chitin-binding domains in Tribolium castaneum are grouped into three distinct families based on phylogeny, expression and function. Insect Biochem. Mol. Biol. 2010, 40, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, R.; Ogawa, N.; Fujiwara, H.; Kojima, T. Mechanical control of whole body shape by a single cuticular protein Obstructor-E in Drosophila melanogaster. PLoS Genet. 2017, 13, 1006548. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, R. Cuticle itself as a central and dynamic player in shaping cuticle. Curr. Opin. Insect Sci. 2017, 19, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xiu, J.F.; Cheng, J.Z.; Man, L.; Peng, Z.; Shang, X.L.; Tao, W.; WU, J.W. Proteomic analysis of the peritrophic matrix from the midgut of third instar larvae, Musca domestica. Biomed. Environ. Sci. 2016, 29, 56–65. [Google Scholar]
- Wijffels, G.; Eisemann, C.; Riding, G.; Pearson, R.; Jones, A.; Willadsen, P.; Tellam, R. A novel family of chitin-binding proteins from insect type 2 peritrophic matrix cDNA sequences, chitin binding activity, and cellular localization. J. Biol. Chem. 2001, 276, 15527–15536. [Google Scholar] [CrossRef]
- Dias, R.O.; Cardoso, C.; Leal, C.S.; Ribeiro, A.F.; Ferreira, C.; Terra, W.R. Domain structure and expression along the midgut and carcass of peritrophins and cuticle proteins analogous to peritrophins in insects with and without peritrophic membrane. J. Insect Physiol. 2019, 114, 1–9. [Google Scholar] [CrossRef]
- Venancio, T.M.; Cristofoletti, P.T.; Ferreira, C.; Verjovski-Almeida, S.; Terra, W.R. The Aedes aegypti larval transcriptome: A comparative perspective with emphasis on trypsins and the domain structure of peritrophins. Insect Mol. Biol. 2009, 18, 33–44. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Kelkenberg, M.; Muthukrishnan, S. Peritrophic matrices. In Extracellular Composite Matrices in Arthropods; Springer: Cham, Switzerland, 2016; pp. 255–324. [Google Scholar]
- Harðardóttir, H.M.; Male, R.; Nilsen, F.; Dalvin, S. Effects of chitin synthesis inhibitor treatment on Lepeophtheirus salmonis (Copepoda, Caligidae) larvae. PLoS ONE 2019, 14, e0222520. [Google Scholar] [CrossRef]
- Hussain, A.; AlJabr, A.M.; Al-Ayedh, H. Development-Disrupting Chitin Synthesis Inhibitor, Novaluron, Reprogramming the Chitin Degradation Mechanism of Red Palm Weevils. Molecules 2019, 24, 4304. [Google Scholar] [CrossRef]
- Chen, W.J.; Huang, L.X.; Hu, D.; Liu, L.Y.; Gu, J.; Huang, L.H.; Feng, Q.L. Cloning, expression and chitin-binding activity of two peritrophin-like protein genes in the common cutworm, Spodoptera litura. Insect Sci. 2014, 21, 449–458. [Google Scholar] [CrossRef]
- Casu, R.E.; Eisemann, C.H.; Vuocolo, T.; Tellam, R.L. The major excretory/secretory protease from Lucilia cuprina larvae is also a gut digestive protease. Int. J. Parasitol. 1996, 26, 623–628. [Google Scholar] [CrossRef]
- Vuocolo, T.; Eisemann, C.H.; Pearson, R.D.; Willadsen, P.; Tellam, R.L. Identification and molecular characterisation of a peritrophin gene, peritrophin-48, from the myiasis fly Chrysomya bezziana. Insect Biochem. Mol. Biol. 2001, 31, 919–932. [Google Scholar] [CrossRef]
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V. Roles of peritrophic membranes in protecting herbivorous insects from ingested plant allelochemicals. Arch. Insect Biochem. Physiol.: Publ. Collab. Entomol. Soc. Am. 2001, 47, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.S.T.; Freire, M.D.M.; Mazzafera, P.; Araujo, R.T.; Bueno, R.D.; Macedo, M.L.R. Insecticidal effect of labramin, a lectin-like protein isolated from seeds of the beach apricot tree, Labramia bojeri, on the Mediterranean flour moth, Ephestia kuehniella. J. Insect Sci. 2012, 12, 62. [Google Scholar] [CrossRef]
- Dunstand-Guzman, E.; Pena-Chora, G.; Hallal-Calleros, C.; Perez-Martinez, M.; Hernandez-Velazquez, V.M.; Morales-Montor, J.; Flores-Perez, F.I. Acaricidal effect and histological damage induced by Bacillus thuringiensis protein extracts on the mite Psoroptes cuniculi. Parasites Vectors 2015, 8, 285. [Google Scholar] [CrossRef]
- Nisbet, A.; Huntley, J.F. Progress and opportunities in the development of vaccines against mites, fleas and myiasis-causing flies of veterinary importance. Parasite Immunol. 2006, 28, 165–172. [Google Scholar] [CrossRef]
- Sandoval-Mojica, A.F.; Scharf, M.E. Gut genes associated with the peritrophic matrix in Reticulitermes flavipes (Blattodea: Rhinotermitidae): Identification and characterization. Arch. Insect Biochem. Physiol. 2016, 92, 127–142. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Sigrist, C.J.; De Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res. 2012, 41, D344–D347. [Google Scholar] [CrossRef]
- Nielsen, H.; Tsirigos, K.D.; Brunak, S.; von Heijne, G. A brief history of protein sorting prediction. Protein J. 2019, 38, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of protein subcellular localization using deep learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Katrine, T.; Schjoldager, B.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Jung, E.; Brunak, S. NetNGlyc 1.0 Server: Prediction of N-Glycosylation Sites in Human Proteins. DTU Bioinformatics. 2004. Available online: http://www.cbs.dtu.dk/services/NetNGlyc/ (accessed on 12 May 2022).
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Wakem, M.; Dijkman, G.; Alsarraj, M.; Nguyen, M. A practical approach to RT-qPCR—publishing data that conform to the MIQE guidelines. Methods 2010, 50, S1–S5. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).